Abstract
With the increasing use of carbapenems, carbapenem-resistant Gram-negative bacteria have become a major concern in health care-associated infections. The present study was performed to evaluate the clinical and microbiological features of breakthrough Gram-negative bacteremia (GNB) during carbapenem therapy and to assess risk factors for development of breakthrough GNB. A case-control study was performed at a tertiary hospital from 2005 to 2014. Case patients were defined as individuals whose blood cultures grew Gram-negative bacteria while the patients were receiving carbapenems for at least 48 h before breakthrough GNB. Age-, sex-, and date-matched controls were selected from patients who received carbapenem for at least 48 h and did not develop breakthrough GNB during carbapenem treatment. A total of 101 cases of breakthrough GNB were identified and compared to 100 controls. The causative microorganisms for breakthrough GNB were Stenotrophomonas maltophilia (n = 33), Acinetobacter baumannii (n = 32), Pseudomonas aeruginosa (n = 21), and others (n = 15). Approximately 90% of S. maltophilia isolates were susceptible to levofloxacin and trimethoprim-sulfamethoxazole. The most common infection types were primary bacteremia (38.6%) and respiratory infections (35.6%). More than half of the patients died within a week after bacteremia, and the 30-day mortality rate was 70.3%. In a multivariate analysis, a longer hospital stay, hematologic malignancy, persistent neutropenia, immunosuppressant use, and previous colonization by causative microorganisms were significantly associated with breakthrough GNB. Our data suggest that S. maltophilia, A. baumannii, and P. aeruginosa are the major pathogens of breakthrough GNB during carbapenem therapy, in association with a longer hospital stay, hematologic malignancy, persistent neutropenia, immunosuppressant use, and previous colonization.
INTRODUCTION
Carbapenems, such as meropenem, imipenem, and doripenem, are the currently preferred agents for treating serious bacterial infections caused by multidrug-resistant (MDR) Gram-negative pathogens, such as members of the Enterobacteriaceae producing extended-spectrum β-lactamases or AmpC β-lactamase, Pseudomonas aeruginosa, and Acinetobacter baumannii (1). With the increasing use of carbapenems, however, carbapenem-resistant (CR) Gram-negative bacteria have become a major concern in health care-associated infections (2). In the United States, more than 23,000 infections caused by MDR A. baumannii, MDR P. aeruginosa, or CR Enterobacteriaceae are reported annually (3). In the Republic of Korea, the incidence of imipenem-resistant A. baumannii infections increased from 43.6% in 2006 to 82.5% in 2010 in intensive care unit (ICU) patients (4).
The term “breakthrough bacteremia” is defined as an episode of continuous or new-onset bacteremia in a patient receiving appropriate antibiotics for the microorganism recovered from blood cultures (5–11). In addition to the use of unsuitable antibiotics—due to either resistance of the microorganism (5, 8, 9, 11) or a suboptimal concentration of active drug at the target organ (5)—a particular type of microorganism (8, 9), inadequate control of the infectious source (5, 7, 8), and host immune status (5–11) may account for breakthrough bacteremia. Some reports have addressed breakthrough bacteremia in patients receiving any antibiotics (5–7), quinolones alone (8–10), or macrolides alone (11). However, little is known regarding breakthrough bacteremia during carbapenem treatment.
Some authors reported that breakthrough bacteremia was associated with poor outcomes compared to those for nonbreakthrough bacteremia (6, 7). Furthermore, because carbapenems are usually indicated for severe infections caused by MDR Gram-negative pathogens in severely ill or immunocompromised patients, breakthrough Gram-negative bacteremia (GNB) can be fatal. Therefore, this study was performed to evaluate the clinical and microbiologic features of breakthrough GNB during carbapenem therapy and to assess risk factors for development of breakthrough GNB.
MATERIALS AND METHODS
Study design and patient population.
A case-control study was performed at Samsung Medical Center, Seoul, Republic of Korea, a university-affiliated 1,950-bed tertiary care hospital, from September 2005 to July 2014. Patients who were older than 18 years were included in the study. We searched for initial eligible patients who had received a type II carbapenem (e.g., imipenem, meropenem, or doripenem) for at least 48 h and who developed GNB. Case patients were defined as individuals whose blood cultures grew Gram-negative bacteria and who had been receiving a type II carbapenem (e.g., imipenem, meropenem, or doripenem) for at least 48 h before breakthrough bacteremia. Patients who received ertapenem were not included in this study. Patients whose blood cultures yielded the same Gram-negative bacteria as those from a previous infection were excluded.
To establish the control group, we screened adults who received carbapenems for more than 48 h and had no breakthrough bacteremia during carbapenem therapy. The controls were selected and matched to case patients based on age, sex, and date of carbapenem therapy (within 1 month). One control patient was matched to each case patient. This study was performed and described in accordance with the STROBE guidelines for the reporting of cohort studies.
Data collection and definition.
Medical records were reviewed for the case and control groups. The following data were collected: age; sex; duration of hospital stay before carbapenem therapy; duration of carbapenem therapy; presence of polymicrobial infection; absolute neutrophil count (ANC); underlying diseases; severity of underlying illness; comorbid conditions; organ transplantation or hematopoietic stem cell transplantation; presence of a central venous catheter (CVC), urinary catheter, nasogastric tube, or percutaneous drainage; acquisition site for causative microorganisms; type of carbapenem used; primary focus of infection; and 7-day, 30-day, infection-related, and in-hospital mortality rates. Microbiological data were obtained from the database at our clinical microbiology laboratory.
The severity of the underlying illness was evaluated on the basis of McCabe and Jackson classification. Charlson's weighted index of comorbidity (WIC) was used to evaluate comorbid conditions (12). Neutropenia was defined as an ANC of <500 cells/mm3 or an ANC that was expected to decrease to <500 cells/mm3 during the next 48 h, according to the clinical practice guidelines of the Infectious Diseases Society of America (13). Clinical diagnosis was assessed by the attending physician, taking into account the clinical situation, laboratory results, and imaging studies. A catheter-related bloodstream infection (CRBSI) was diagnosed when the same organism grew from at least one percutaneous blood culture and from a culture of the catheter tip or when microbes from a blood sample drawn from a catheter hub were grown for at least 2 h before microbes from a blood sample from a peripheral vein (14). The severity of acute illness was assessed at the time of positive blood culture by using the Pitt bacteremia score as described previously (15). Previous colonization by causative microorganisms was defined as the isolation of causative microorganisms from the skin, mucous membranes, open wounds, or discharge in the absence of relevant signs or symptoms in the patients. We defined infection-related mortality as death that could be attributed to breakthrough GNB as the immediate cause. Infection-related death was considered to be related to breakthrough GNB if ≥1 of the following criteria were present: (i) blood cultures were positive for GNB at the time of death, (ii) death occurred before resolution of signs and symptoms of GNB, and (iii) death occurred ≤14 days after breakthrough GNB, without another explanation.
Microbiologic tests.
Blood samples for culture were taken from peripheral veins or central lines. Blood cultures were processed by use of a Bactec-9240 system (Becton Dickinson, Sparks, MD) or a BacT/Alert 3D system (bioMérieux Inc., Marcy l'Etoile, France). To identify microbes and to test their antimicrobial susceptibility, a Vitek II automated system (bioMérieux Inc.) was used with a standard identification card and the modified broth microdilution method. MIC breakpoints and quality control protocols were used according to standards established by the Clinical and Laboratory Standards Institute (CLSI) (16–19).
Statistical analysis.
Student's t test and the Mann-Whitney test were used to compare continuous variables, and the chi-square test and Fisher's exact test were used for comparison of categorical variables. To identify risk factors for breakthrough GNB, a logistic regression model was used to control for confounding variables. All P values were two-tailed, and P values of <0.05 were considered to be statistically significant. Variables that were statistically significant in the univariate analyses were candidates for multivariate analysis, in addition to the main variable of interest. SPSS for Windows/PASW, version 22.0 (IBM, Armonk, NY), was used for analysis.
RESULTS
Causative microorganisms for breakthrough GNB.
A total of 1,955 GNB episodes were initially identified. Among these episodes, 101 episodes were consistent with the definition of breakthrough GNB (5.2% [101/1,955 episodes]). The causative pathogens for breakthrough GNB are presented in Fig. 1. Stenotrophomonas maltophilia (n = 33; 32.7%) was the most common pathogen, followed by Acinetobacter baumannii (n = 32; 31.7%), Pseudomonas aeruginosa (n = 21; 20.8%), and others (n = 15; 14.9%). Among the 32 A. baumannii and 21 P. aeruginosa isolates, 27 (84.4%) and 17 (81.0%), respectively, were not susceptible to carbapenems. None of the A. baumannii and P. aeruginosa isolates were colistin resistant. Approximately 90% of S. maltophilia isolates were susceptible to levofloxacin and trimethoprim-sulfamethoxazole (Table 1).
FIG 1.
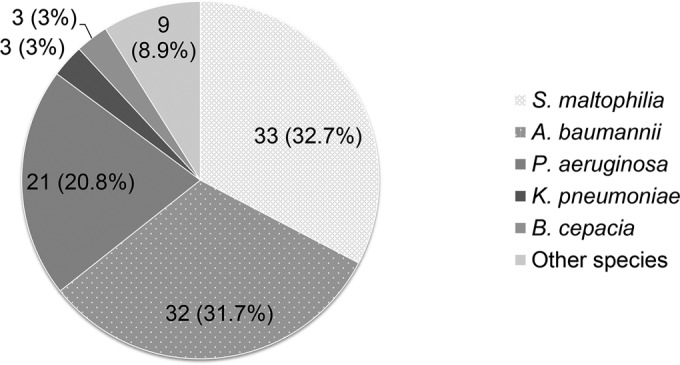
Distribution of pathogens of breakthrough Gram-negative bacteremia during carbapenem therapy.
TABLE 1.
Antimicrobial susceptibility results for causative microorganisms of breakthrough Gram-negative bacteremiaa
| Pathogen and antibiotic(s) | No. of susceptible isolates/total no. of isolates (%) |
|---|---|
| Stenotrophomonas maltophilia | |
| Levofloxacin | 30/33 (90.9) |
| Trimethoprim-sulfamethoxazole | 30/33 (90.9) |
| Ticarcillin-clavulanate | 6/12 (50.0) |
| Minocycline | 21/21 (100) |
| Acinetobacter baumannii | |
| Levofloxacin | 1/14 (7.1) |
| Amikacin | 9/32 (28.1) |
| Tigecycline | 0/3 (0.0) |
| Minocycline | 26/29 (89.7) |
| Colistin | 30/30 (100) |
| Trimethoprim-sulfamethoxazole | 5/29 (17.2) |
| Pseudomonas aeruginosa | |
| Levofloxacin | 6/13 (46.2) |
| Amikacin | 14/21 (66.7) |
| Colistin | 21/21 (100) |
| Ciprofloxacin | 12/21 (57.1) |
| Ceftazidime | 9/21 (42.9) |
| Cefepime | 11/21 (52.4) |
| Piperacillin-tazobactam | 8/21 (38.1) |
| Aztreonam | 5/21 (23.8) |
A Vitek II automated system (bioMérieux Inc.) was used with a standard identification card and the modified broth microdilution method to identify microbes and to test their susceptibility to antimicrobials, including colistin. MIC breakpoints and quality control protocols were used according to standards established by the Clinical and Laboratory Standards Institute (from 2005 to 2008, document M100-S13 [16]; from 2009 to January 2011, document M100-S18 [17]; from February 2011 to April 2012, document M100-S20 [18]; and from May 2012 to July 2014, document M100-S24 [19]).
Clinical outcomes for patients with breakthrough GNB.
Variables related to the clinical presentation of breakthrough GNB were analyzed and are presented in Table 2. Primary bacteremia and respiratory infections accounted for more than half of the cases of breakthrough GNB. None of the cases of breakthrough GNB resulted from urinary tract or central nervous system infection. Although primary bacteremia was the most common infection overall, respiratory infections were most common in patients with A. baumannii bacteremia. The outcomes of breakthrough GNB were poor, and more than half of the patients died within a week after bacteremia. The mortality rate was highest for A. baumannii infections, followed by infections with P. aeruginosa, S. maltophilia, and others. Mortality rates did not differ significantly between patients who received appropriate empirical antibiotics and those who did not (7-day mortality rates, 51.5% versus 61.8%, respectively; P > 0.05).
TABLE 2.
Clinical features and outcomes for 101 patients with breakthrough Gram-negative bacteremia during carbapenem therapy
| Variablea | No. (%) of patientsb |
|---|---|
| Type of infection | |
| Primary bacteremia | 39 (38.6) |
| Catheter-related infection | 12 (11.9) |
| Respiratory infection | 36 (35.6) |
| Urinary tract infection | 0 (0.0) |
| Hepatobiliary infection | 4 (4.0) |
| Intra-abdominal infection | 7 (6.9) |
| Skin and soft tissue infection | 2 (2.0) |
| Deep neck infection | 2 (2.0) |
| Severity of illness | |
| Pitt bacteremia score (median [IQR]) | 4.0 (2, 12) |
| ICU care | 70 (69.3) |
| Acute renal failure | 51 (50.5) |
| LOH after event (median [IQR]) | 7.0 (3.0, 23.0) |
| Duration of ICU care (days) (median [IQR]) | 3.0 (1.0, 7.0) |
| Mortality | |
| 7-day mortality | 55 (54.5) |
| 30-day mortality | 71 (70.3) |
| In-hospital mortality | 78 (77.2) |
| Infection-related mortality | 60 (59.4) |
| Time to death (days) (median [IQR]) | 5.0 (2.0, 18.5) |
Abbreviations: GNB, Gram-negative bacteremia; IQR, interquartile range; ICU, intensive care unit; LOH, length of hospital stay.
Unless specified otherwise.
Risk factor analysis for development of breakthrough GNB.
The baseline characteristics of patients with breakthrough GNB and of the control group are presented in Table 3. Both groups were predominantly male, and the prevalences of underlying diseases, including diabetes mellitus and cardiovascular, liver, pulmonary, renal, and neurologic diseases, were similar. The total durations of hospital stay were also similar. However, patients with breakthrough GNB had significantly longer hospital stays before carbapenem treatment (P < 0.001). Larger proportions of patients in the case group had hematologic malignancy, underwent hematopoietic stem cell transplantation (P < 0.05), and were in a neutropenic state during carbapenem therapy (P < 0.05). McCabe and Jackson scores were higher for the case group than for the control group (P < 0.05). Risk factors for development of breakthrough GNB are presented in Table 4. In the multivariate analysis using a logistic regression model, a longer hospital stay, hematologic malignancy, persistent neutropenia during carbapenem treatment, immunosuppressant use, and previous colonization by causative microorganisms were independent risk factors associated with breakthrough GNB during carbapenem therapy.
TABLE 3.
Clinical characteristics of the case and control groupsa
| Characteristic | Value |
P value | |
|---|---|---|---|
| Cases (n = 101) | Controls (n = 100) | ||
| Age (yr) (mean ± SD) | 50.5 ± 14.9 | 50.2 ± 14.6 | 0.900 |
| No. (%) of males | 65 (64.4) | 66 (66.0) | 0.800 |
| No. of HD (median [IQR]) | 50.0 (23.0, 86.0) | 43.5 (29.0, 80.0) | 0.419 |
| No. of HD until carbapenem treatment (median [IQR]) | 28.0 (18.0, 61.5) | 16.7 (10.7, 26.4) | <0.001 |
| No. (%) of patients receiving carbapenem | |||
| Meropenem | 85 (14.2) | 85 (85.0) | 1.000 |
| Imipenem | 12 (11.9) | 12 (12.0) | 1.000 |
| Doripenem | 4 (4.0) | 3 (3.0) | 0.712 |
| Duration of carbapenem treatment (days) (median [IQR]) | 8.0 (5.0, 12.0) | 11.0 (8.0, 14.0) | <0.001 |
| No. (%) of patients with underlying disease | |||
| Diabetes mellitus | 17 (16.8) | 19 (19.0) | 0.689 |
| Heart failure | 4 (4.5) | 14 (14.0) | 0.014 |
| Myocardial infarction | 3 (3.0) | 7 (7.0) | 0.214 |
| Cerebrovascular disease | 4 (4.0) | 5 (5.0) | 0.748 |
| Liver disease | 13 (12.9) | 13 (13.0) | 0.978 |
| Renal disease | 6 (5.9) | 5 (5.0) | 0.769 |
| Pulmonary disease | 13 (12.9) | 15 (15.0) | 0.663 |
| Solid tumor | 20 (19.8) | 25 (25.0) | 0.337 |
| Leukemia | 45 (44.6) | 33 (33.0) | 0.093 |
| Lymphoma | 19 (18.8) | 9 (9.0) | 0.045 |
| HSCT | 14 (13.9) | 5 (5.0) | 0.032 |
| SOT | 8 (7.9) | 12 (12.0) | 0.334 |
| Charlson's score (median [IQR]) | 2.0 (2.0, 3.0) | 3.9 (2.0, 5.0) | 0.172 |
| McCabe score (median [IQR]) | 3.0 (2.0, 3.0) | 2.0 (2.0, 3.0) | 0.006 |
| No. (%) of patients receiving ICU care | 52 (51.5) | 40 (40.0) | 0.102 |
| APACHE II score (median [IQR]) | 28.5 (24.0, 36.0) | 24.0 (20.0, 29.0) | 0.001 |
| No. (%) of patients with: | |||
| Mechanical ventilation | 36 (35.6) | 34 (34.0) | 0.807 |
| Renal replacement treatment | 23 (22.8) | 15 (15.0) | 0.159 |
| Neutropenia | 67 (66.3) | 43 (43.0) | 0.001 |
| Profound neutropenia | 27 (26.7) | 39 (39.0) | 0.064 |
| Prolonged neutropenia | 22 (21.8) | 34 (34.0) | 0.053 |
| Recovery from neutropenia during carbapenem treatment | 4 (4.0) | 30 (30.0) | <0.001 |
| Severe mucositisb | 16 (51.6) | 15 (32.6) | 0.095 |
| Cytotoxic chemotherapy | 63 (62.4) | 47 (47.0) | 0.029 |
| Corticosteroid use | 28 (27.7) | 24 (24.0) | 0.547 |
| Immunosuppressant use | 55 (54.5) | 18 (18.0) | <0.001 |
| Recent operation | 15 (14.9) | 27 (27.0) | 0.034 |
| Indwelling catheter | |||
| Urinary catheter | 58 (57.4) | 54 (54.0) | 0.625 |
| CVC | 93 (92.1) | 88 (88.0) | 0.334 |
| Nasogastric tube | 52 (51.5) | 42 (42.0) | 0.178 |
| Percutaneous drainage | 19 (18.8) | 26 (26.0) | 0.222 |
| Prior invasive procedure | 13 (12.9) | 22 (22.0) | 0.088 |
| Previous colonization by Gram-negative bacteria | 29 (28.7) | 17 (17.0) | 0.048 |
Abbreviations: SD, standard deviation; IQR, interquartile range; HD, hospital days; HSCT, hematopoietic stem cell transplant; SOT, solid organ transplant; APACHE II, Acute Physiology and Chronic Health Evaluation II; ICU, intensive care unit; CVC, central venous catheter.
Oral or gastrointestinal mucositis that interferes with swallowing or causes severe diarrhea during neutropenic period.
TABLE 4.
Risk factors associated with development of breakthrough Gram-negative bacteremiaa
| Factor | Univariate analysis |
Multivariate analysis |
||
|---|---|---|---|---|
| Odds ratio (95% CI) | P value | Odds ratio (95% CI) | P value | |
| Prolonged hospital stayb | 3.43 (1.86–6.34) | <0.001 | 2.84 (1.33–6.05) | 0.007 |
| Hematologic malignancy | 2.44 (1.41–4.40) | 0.002 | 6.65 (2.90–15.25) | <0.001 |
| HSCT | 3.06 (1.06–8.84) | 0.04 | 2.27 (0.46–11.29) | 0.32 |
| Persistent neutropeniac | 10.39 (3.50–30.84) | <0.001 | 24.83 (6.33–97.45) | <0.001 |
| Immunosuppressant use | 5.45 (2.86–10.36) | <0.001 | 2.75 (1.23–6.12) | 0.01 |
| Previous colonization by causative microorganisms | 1.97 (1.00–3.87) | 0.05 | 2.74 (1.18–6.38) | 0.02 |
Abbreviations: CI, confidence interval; HSCT, hematopoietic stem cell transplant.
More than 28 days prior to carbapenem treatment.
During carbapenem treatment.
DISCUSSION
This study demonstrated that S. maltophilia may be the main pathogen for breakthrough GNB during carbapenem therapy, along with MDR A. baumannii, MDR P. aeruginosa, and CR Enterobacteriaceae. Breakthrough GNB was independently associated with a prolonged hospital stay before carbapenem treatment, hematologic malignancy, persistent neutropenia during carbapenem treatment, and previous colonization with causative microorganisms. Although Escherichia coli and P. aeruginosa were the most common Gram-negative bacteria in breakthrough GNB in previous studies (5–8, 10), S. maltophilia was the most common pathogen in breakthrough GNB during carbapenem therapy in our study.
S. maltophilia is an important nosocomial pathogen with intrinsic multidrug resistance (20). Neutropenia, underlying malignancy, prolonged hospitalization, previous antibiotic use, steroid use, mechanical ventilator support, and indwelling central venous catheters have been reported as major risk factors for S. maltophilia bacteremia (20–23). Our study also showed that hematologic malignancy, persistent neutropenia, and prolonged hospitalization were risk factors for breakthrough GNB. However, only 4 patients (12%) with breakthrough S. maltophilia bacteremia had CRBSI in our study. Rather, respiratory infection and primary bacteremia were the main types of infection. Note, though, that more than half of patients with breakthrough GNB were in a neutropenic state, and it may be difficult to accurately distinguish between primary bacteremia and catheter-related infection in such patients. Micozzi et al. reported that more than 50% of S. maltophilia bacteremia cases presented as breakthrough infection, although no breakthrough bacteremia developed during carbapenem treatment (21). However, our study showed that S. maltophilia was the most common pathogen of breakthrough GNB during carbapenem treatment. This difference can be explained by the different study periods (1986 to 1996 versus 2005 to 2014) and by increased carbapenem use more recently. A previous study reported that increased carbapenem use was associated with an increased number of S. maltophilia isolates (24).
As expected, A. baumannii and P. aeruginosa were also major pathogens of breakthrough GNB during carbapenem treatment. Because the isolates were not available for further analysis, the mechanisms of carbapenem resistance in A. baumannii and P. aeruginosa isolates could not be evaluated. Prolonged hospitalization (25–27), previous carbapenem use (1, 25–30), ICU care (25, 27, 29), and the presence of medical devices (26–28) are considered risk factors for CR A. baumannii and CR P. aeruginosa colonization. The risk factors for CRGNB were similar to the risk factors for CR A. baumannii and CR P. aeruginosa colonization. In addition, prior colonization by CR A. baumannii was a risk factor for the development of GNB (26). In this study, prolonged hospitalization before carbapenem treatment and prior carriage of CR Gram-negative bacteria were risk factors for breakthrough GNB. However, the proportions of patients with ICU care, mechanical ventilation, indwelling catheters, and invasive procedures did not differ significantly between the case and control groups. Factors that affect host immunity, such as hematologic malignancies and neutropenia during carbapenem treatment, may play an important role in the development of GNB in patients previously colonized by MDR Gram-negative pathogens.
In previous studies of breakthrough bacteremia, the presence of CVCs was identified as an independent risk factor (5, 7, 8). However, the proportions of individuals with CVCs in this study did not differ significantly between the case and control groups. Patients with persistent bacteremia with the same pathogen were excluded because we intended to evaluate risk factors for bacteremia caused by a new pathogen during carbapenem therapy, with an appropriate source control for previous infection. The traditional definition may be suitable for studying breakthrough bacteremia during all antibiotic treatments for any pathogen (i.e., Gram-positive and -negative bacteria and fungi). Given the limited therapeutic options for uncontrolled Gram-negative bacterial infection during carbapenem treatment, physicians do their best to locate an uncontrolled source of infection or to administer appropriate antimicrobial therapy in cases of persistent GNB. Although we did not find the presence of a CVC to be an independent risk factor for breakthrough GNB, CVCs might be an important source. CRBSI was the third most common type of infection for breakthrough GNB.
We showed that previous colonization by MDR Gram-negative bacteria (mostly in the respiratory tract) was an independent risk factor for breakthrough GNB. Prolonged impairment of host immunity, including prolonged neutropenia and immunosuppressant use, as well as the selective antibiotic effect of carbapenems, may favor the development of breakthrough GNB in such patients.
According to the antibiotic susceptibility results in this study, there is no single antibacterial agent which is active against all three of the major pathogens for breakthrough GNB. As combination therapy, levofloxacin, trimethoprim-sulfamethoxazole, or minocycline with colistin may be an option for the selection of empirical antimicrobial therapy in patients who develop symptoms and signs of sepsis while receiving carbapenem therapy, particularly for patients at high risk of breakthrough GNB. The outcomes of breakthrough GNB were poor regardless of the use of appropriate antimicrobial therapy. Fatal underlying conditions, such as hematologic malignancy and prolonged neutropenia, as well as limited therapeutic options, may result in high mortality. Strategies to reduce colonization by MDR pathogens, such as proper infection control practices, antibiotic stewardship, environmental precautions, and routine screening cultures, should be applied to the care of patients at high risk for breakthrough GNB during carbapenem treatment (31). Further studies are necessary to find appropriate prophylactic methods for high-risk patients colonized by MDR pathogens. We also found that the duration of carbapenem therapy was longer for the control group. There may be several reasons for this, e.g., case patients died earlier regardless of appropriate therapy (the median time to death for the case group was 5 days) or there were earlier changes of carbapenem to other antibacterial agents once a carbapenem-resistant isolate was identified.
This study has several limitations. First, due to the retrospective study design, there might have been bias during data collection. Given that this study was an exploratory research study without an exact calculation of the sample size in a statistical manner, the study may have been underpowered to detect weaker but potentially clinically significant effects. Second, because we matched the control groups by age and sex, we could not evaluate the effects of age and sex as risk factors associated with development of breakthrough GNB. Third, while the study was conducted at a single medical center, the distribution of pathogens for breakthrough GNB may differ according to local epidemiology, especially in areas where carbapenemase-producing Enterobacteriaceae are highly prevalent. Further multicenter studies are needed to overcome this limitation. Finally, as this study was performed with the design of a case-control study, we could assess only the cumulative incidence of breakthrough GNB. A further study regarding this issue and including the incidence per day of carbapenem therapy may be warranted. Despite these limitations, this study may be the first and largest to evaluate the clinical presentation and risk factors of breakthrough GNB during carbapenem treatment, and we believe that the findings may provide useful information for clinicians who are caring for critically ill patients with carbapenem treatment.
Conclusions.
Our data suggest that the most likely pathogens are S. maltophilia, CR A. baumannii, and CR P. aeruginosa when breakthrough GNB occurs during carbapenem therapy. The independent risk factors associated with breakthrough GNB were a longer hospital stay before carbapenem administration, hematologic malignancy, persistent neutropenia during carbapenem treatment, immunosuppressant use, and previous colonization by causative microorganisms.
ACKNOWLEDGMENTS
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (grant HI12C0756).
Funding Statement
This study was supported by a grant from the Korean Health Technology R&D Project, Ministry of Health & Welfare, Republic of Korea (grant HI12C0756).
REFERENCES
- 1.Routsi C, Pratikaki M, Platsouka E, Sotiropoulou C, Papas V, Pitsiolis T, Tsakris A, Nanas S, Roussos C. 2013. Risk factors for carbapenem-resistant Gram-negative bacteremia in intensive care unit patients. Intensive Care Med 39:1253–1261. doi: 10.1007/s00134-013-2914-z. [DOI] [PubMed] [Google Scholar]
- 2.Huttner A, Harbarth S, Carlet J, Cosgrove S, Goossens H, Holmes A, Jarlier V, Voss A, Pittet D. 2013. Antimicrobial resistance: a global view from the 2013 World Healthcare-Associated Infections Forum. Antimicrob Resist Infect Control 2:31. doi: 10.1186/2047-2994-2-31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.CDC. 2013. Antibiotic resistance threats in the United States, 2013. CDC, Atlanta, GA. [Google Scholar]
- 4.Kwak YG, Cho YK, Kim JY, Lee MS, Kim HY, Kim YK, Park ES, Jin HY, Kim HB, Kim ES. 2011. Korean Nosocomial Infections Surveillance System, intensive care unit module report: data summary from July 2009 through June 2010. Korean J Nosocomial Infect Control 16:1–12. [Google Scholar]
- 5.Anderson ET, Young LS, Hewitt WL. 1976. Simultaneous antibiotic levels in “breakthrough” gram-negative rod bacteremia. Am J Med 61:493–497. doi: 10.1016/0002-9343(76)90328-4. [DOI] [PubMed] [Google Scholar]
- 6.Weinstein MP, Reller LB. 1984. Clinical importance of “breakthrough” bacteremia. Am J Med 76:175–180. [DOI] [PubMed] [Google Scholar]
- 7.Lopez Dupla M, Martinez JA, Vidal F, Almela M, Lopez J, Marco F, Soriano A, Richart C, Mensa J. 2005. Clinical characterization of breakthrough bacteraemia: a survey of 392 episodes. J Intern Med 258:172–180. doi: 10.1111/j.1365-2796.2005.01513.x. [DOI] [PubMed] [Google Scholar]
- 8.Spanik S, Trupl J, Kunova A, Drgona L, Salek T, Mardiak J, Kukuckova E, Studena M, Pichna P, Oravcova E, Grey E, Koren P, Svec J, Lacka J, Sufliarsky J, Krcmery V. 1997. Risk factors, aetiology, therapy and outcome in 123 episodes of breakthrough bacteraemia and fungaemia during antimicrobial prophylaxis and therapy in cancer patients. J Med Microbiol 46:517–523. doi: 10.1099/00222615-46-6-517. [DOI] [PubMed] [Google Scholar]
- 9.Van der Auwera P, Gerain J. 1993. Use of the quinolones in the prophylaxis and treatment of granulocytopenic immunocompromised cancer patients. Drugs 45(Suppl 3):S81–S90. [DOI] [PubMed] [Google Scholar]
- 10.Rangaraj G, Granwehr BP, Jiang Y, Hachem R, Raad I. 2010. Perils of quinolone exposure in cancer patients: breakthrough bacteremia with multidrug-resistant organisms. Cancer 116:967–973. doi: 10.1002/cncr.24812. [DOI] [PubMed] [Google Scholar]
- 11.Kelley MA, Weber DJ, Gilligan P, Cohen MS. 2000. Breakthrough pneumococcal bacteremia in patients being treated with azithromycin and clarithromycin. Clin Infect Dis 31:1008–1011. doi: 10.1086/318157. [DOI] [PubMed] [Google Scholar]
- 12.Charlson ME, Pompei P, Ales KL, MacKenzie CR. 1987. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis 40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 13.Freifeld AG, Bow EJ, Sepkowitz KA, Boeckh MJ, Ito JI, Mullen CA, Raad II, Rolston KV, Young JA, Wingard JR, Infectious Diseases Society of America. 2011. Clinical practice guideline for the use of antimicrobial agents in neutropenic patients with cancer: 2010 update by the Infectious Diseases Society of America. Clin Infect Dis 52:e56–e93. doi: 10.1093/cid/cir073. [DOI] [PubMed] [Google Scholar]
- 14.Mermel LA, Allon M, Bouza E, Craven DE, Flynn P, O'Grady NP, Raad II, Rijnders BJ, Sherertz RJ, Warren DK. 2009. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 update by the Infectious Diseases Society of America. Clin Infect Dis 49:1–45. doi: 10.1086/599376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chow JW, Yu VL. 1999. Combination antibiotic therapy versus monotherapy for gram-negative bacteraemia: a commentary. Int J Antimicrob Agents 11:7–12. doi: 10.1016/S0924-8579(98)00060-0. [DOI] [PubMed] [Google Scholar]
- 16.CLSI. 2003. Performance standards for antimicrobial susceptibility testing, 13th ed Document M100-S13. CLSI, Wayne, PA. [Google Scholar]
- 17.CLSI. 2008. Performance standards for antimicrobial susceptibility testing, 18th ed Document M100-S18. CLSI, Wayne, PA. [Google Scholar]
- 18.CLSI. 2010. Performance standards for antimicrobial susceptibility testing, 20th ed Document M100-S20. CLSI, Wayne, PA. [Google Scholar]
- 19.CLSI. 2014. Performance standards for antimicrobial susceptibility testing, 24th ed Document M100-S24. CLSI, Wayne, PA. [Google Scholar]
- 20.Lai CH, Chi CY, Chen HP, Chen TL, Lai CJ, Fung CP, Yu KW, Wong WW, Liu CY. 2004. Clinical characteristics and prognostic factors of patients with Stenotrophomonas maltophilia bacteremia. J Microbiol Immunol Infect 37:350–358. [PubMed] [Google Scholar]
- 21.Micozzi A, Venditti M, Monaco M, Friedrich A, Taglietti F, Santilli S, Martino P. 2000. Bacteremia due to Stenotrophomonas maltophilia in patients with hematologic malignancies. Clin Infect Dis 31:705–711. doi: 10.1086/314043. [DOI] [PubMed] [Google Scholar]
- 22.Boktour M, Hanna H, Ansari S, Bahna B, Hachem R, Tarrand J, Rolston K, Safdar A, Raad I. 2006. Central venous catheter and Stenotrophomonas maltophilia bacteremia in cancer patients. Cancer 106:1967–1973. doi: 10.1002/cncr.21846. [DOI] [PubMed] [Google Scholar]
- 23.Muder RR, Harris AP, Muller S, Edmond M, Chow JW, Papadakis K, Wagener MW, Bodey GP, Steckelberg JM. 1996. Bacteremia due to Stenotrophomonas (Xanthomonas) maltophilia: a prospective, multicenter study of 91 episodes. Clin Infect Dis 22:508–512. doi: 10.1093/clinids/22.3.508. [DOI] [PubMed] [Google Scholar]
- 24.Sanyal SC, Mokaddas EM. 1999. The increase in carbapenem use and emergence of Stenotrophomonas maltophilia as an important nosocomial pathogen. J Chemother 11:28–33. doi: 10.1179/joc.1999.11.1.28. [DOI] [PubMed] [Google Scholar]
- 25.Baran G, Erbay A, Bodur H, Onguru P, Akinci E, Balaban N, Cevik MA. 2008. Risk factors for nosocomial imipenem-resistant Acinetobacter baumannii infections. Int J Infect Dis 12:16–21. doi: 10.1016/j.ijid.2007.03.005. [DOI] [PubMed] [Google Scholar]
- 26.Sheng WH, Liao CH, Lauderdale TL, Ko WC, Chen YS, Liu JW, Lau YJ, Wang LH, Liu KS, Tsai TY, Lin SY, Hsu MS, Hsu LY, Chang SC. 2010. A multicenter study of risk factors and outcome of hospitalized patients with infections due to carbapenem-resistant Acinetobacter baumannii. Int J Infect Dis 14:e764–e769. doi: 10.1016/j.ijid.2010.02.2254. [DOI] [PubMed] [Google Scholar]
- 27.Voor In't Holt AF, Severin JA, Lesaffre EM, Vos MC. 2014. A systematic review and meta-analyses show that carbapenem use and medical devices are the leading risk factors for carbapenem-resistant Pseudomonas aeruginosa. Antimicrob Agents Chemother 58:2626–2637. doi: 10.1128/AAC.01758-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kang CI, Kim SH, Park WB, Lee KD, Kim HB, Kim EC, Oh MD, Choe KW. 2005. Risk factors for antimicrobial resistance and influence of resistance on mortality in patients with bloodstream infection caused by Pseudomonas aeruginosa. Microb Drug Resist 11:68–74. doi: 10.1089/mdr.2005.11.68. [DOI] [PubMed] [Google Scholar]
- 29.Lee SO, Kim NJ, Choi SH, Hyong Kim T, Chung JW, Woo JH, Ryu J, Kim YS. 2004. Risk factors for acquisition of imipenem-resistant Acinetobacter baumannii: a case-control study. Antimicrob Agents Chemother 48:224–228. doi: 10.1128/AAC.48.1.224-228.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vitkauskiene A, Dambrauskiene A, Cerniauskiene K, Rimdeika R, Sakalauskas R. 2013. Risk factors and outcomes in patients with carbapenem-resistant Acinetobacter infection. Scand J Infect Dis 45:213–218. doi: 10.3109/00365548.2012.724178. [DOI] [PubMed] [Google Scholar]
- 31.Ruhnke M, Arnold R, Gastmeier P. 2014. Infection control issues in patients with haematological malignancies in the era of multidrug-resistant bacteria. Lancet Oncol 15:e606–e619. doi: 10.1016/S1470-2045(14)70344-4. [DOI] [PubMed] [Google Scholar]


