Abstract
Phenotypic drug susceptibility testing is the current “gold standard” for detecting Mycobacterium tuberculosis susceptibility to antituberculous drugs. Pyrazinamide is one antituberculous drug for which the correlation between in vitro resistance and clinical outcomes remains unclear. Here we performed latent class analysis (LCA) to develop a consensus gold standard definition of pyrazinamide resistance using three paired standard pyrazinamide resistance assays. We then compared this consensus measure to the 2-month culture results for patients with multidrug-resistant tuberculosis (MDR-TB) who were treated for 2 months with first-line antituberculous drugs before their resistance results were known. Among 121 patients with MDR-TB, 60 (49.6%) were resistant to pyrazinamide by the Wayne method (L. G. Wayne, Am Rev Respir Dis 109:147–151, 1974), 71 (58.7%) were resistant by the Bactec MGIT 960 method, and 68 (56.2%) were resistant by pncA sequencing. LCA grouped isolates with positive results by at least two assays into a category which we considered the “consensus gold standard” for pyrazinamide resistance. The sensitivity and specificity for this consensus gold standard were 82.4% and 92.5%, respectively, for the Wayne method; 95.6% and 88.7%, respectively, for the Bactec MGIT 960 method; and 92.6% and 90.6%, respectively, for pncA sequencing. After we adjusted for other factors associated with poor outcomes, including age, sex, alcohol use, and baseline ethambutol resistance, patients whose isolates were resistant by the LCA-derived consensus gold standard were more likely to be culture positive at 2 months with an odds ratio of 1.95 (95% confidence interval, 0.74 to 5.11), but this result was not statistically significant. These findings underscore the need for improved diagnostics for routine use in programmatic settings.
INTRODUCTION
Phenotypic drug susceptibility testing (DST) is the current “gold standard” for detecting Mycobacterium tuberculosis susceptibility to antituberculous drugs. Despite its widespread use, there are limited high-quality data correlating phenotypic in vitro resistance with clinical outcomes for many of the available antituberculous drugs (1). Furthermore, the results of phenotypic DST may be discordant with those of genotypic DST (1–5), which hampers the development of sensitive genotypic methods to supplant the phenotypic gold standard. A notable example is the case of rifampin, a drug essential to first-line short-course chemotherapy for tuberculosis (TB) for which phenotypic DST fails to detect rpoB gene mutations associated with a poor clinical outcome (2, 6).
Pyrazinamide is one antituberculous drug for which the correlation between in vitro resistance and clinical outcomes remains unclear. There are three main reasons why evaluation of this association is difficult. First, phenotypic pyrazinamide DST suffers from poor reproducibility, which has led to considerable debate over its clinical significance (7, 8). This is because pyrazinamide is active against M. tuberculosis only at low pH, which inhibits the growth of bacilli, and small variations in pH due to technical error or the inoculum size can result in large differences in the measured MIC (9, 10). While current guidelines recommend an MIC breakpoint of 100 mg/liter (8, 11), values proposed to define pyrazinamide resistance have ranged from 64 to 900 mg/liter (12). Second, there is an apparent discrepancy between the low level of pyrazinamide activity in vitro and its high level of sterilizing activity in vivo. This may be due to differences between in vitro and in vivo environments (13). In mouse models of tuberculosis, the efficacy of pyrazinamide against M. tuberculosis varies with the level of granulomatous inflammation and hypoxia within lesions, suggesting that the role of pyrazinamide might vary with different in vivo microenvironments (14). Third, pyrazinamide is nearly always prescribed as part of a multidrug regimen, whether in short-course chemotherapy for tuberculosis or in second-line regimens for drug-resistant tuberculosis (15, 16). For patients receiving prolonged multidrug regimens, it is difficult to disentangle the contribution of individual drugs, resistance patterns, and adherence to final treatment outcomes.
Because none of the three diagnostic methods that we used represented a gold standard for pyrazinamide resistance, we performed latent class analysis (LCA) to arrive at a consensus definition of pyrazinamide resistance using the three paired assays. We then estimated the association between this “consensus” measure of pyrazinamide resistance and sputum culture status at 2 months among patients with unsuspected multidrug-resistant tuberculosis (MDR-TB) receiving standard first-line treatment.
MATERIALS AND METHODS
Setting.
We conducted this study in Lima, Peru, where the incidence of tuberculosis was estimated to be 95 cases per 100,000 population in 2012 (17). In this setting, tuberculosis is diagnosed and treated at community health centers run by the Ministry of Health according to guidelines from the Peruvian National Tuberculosis Program and the World Health Organization (WHO) (16, 18). National guidelines have specified universal first-line DST for all culture-positive patients since 2010 (19). Patients were started on standard short-course chemotherapy pending the results of the DST, unless they had documented prior resistance to first-line drugs, relapsed disease within 6 months of completing first-line treatment, or a history of two or more tuberculosis treatments within 2 years of completing the last treatment. Prior to 16 July 2010, national guidelines included the addition of streptomycin to short-course chemotherapy for patients with a previous tuberculosis treatment history (20); these were changed thereafter to specify that clinically stable patients with a history of tuberculosis treatment could be treated with standard short-course chemotherapy alone until DST results were available (19).
Study overview.
From 1 September 2009 through 29 August 2012, we invited all individuals age 16 years or older and diagnosed with microbiologically confirmed active tuberculosis at any of 92 participating Ministry of Health centers in Lima Ciudad and Lima Este, Peru, to enroll in the parent study. We then invited household contacts of these index patients to participate in a longitudinal study, through which we followed them for evidence of M. tuberculosis infection and/or disease over 12 months. For all patients diagnosed with active tuberculosis during the study period, we obtained sputum samples for smear and culture at baseline, at 2 months, and at treatment completion.
In the present study, we included index patients and household contacts who were initially treated with standard first-line antituberculous therapy and who were later diagnosed with MDR-TB on the basis of DST results obtained from sputum samples collected within 60 days of initiation of therapy. All patients had baseline pyrazinamide resistance testing by the method described by Wayne (21) (the Wayne method). We selected a subset of M. tuberculosis isolates that were resistant to both isoniazid and rifampin for pyrazinamide resistance testing by two additional assays, namely, the Bactec MGIT 960 method and pncA gene sequencing.
Data collection.
We obtained informed consent from the participants or their parents or guardians. We captured the following data at the time of diagnosis of TB in the index patients and secondary cases: clinical signs and symptoms; sociodemographic characteristics; and comorbidities, including human immunodeficiency virus (HIV) infection and diabetes mellitus. All patients were offered screening for HIV infection by the enzyme-linked immunosorbent assay followed by a confirmatory immunofluorescence or Western blot test.
Drug resistance testing.
We performed first-line DST in all patients with culture-positive tuberculosis using the indirect Löwenstein-Jensen proportion method for isoniazid, rifampin, and ethambutol. Rapid molecular testing for first-line drug resistance was not available for this study. For pyrazinamide resistance testing, we performed the Wayne method using Dubos agar, as recommended by the Peruvian National Reference Laboratory (21, 22); an M. tuberculosis H37Ra strain as a drug-susceptible positive control; and uninoculated test medium as a negative control. All study laboratories engaged in annual external quality assurance testing with the Peruvian National Reference Laboratory. The study laboratory that performed the majority of the ethambutol and the Wayne method testing scored 100% in College of American Pathologists (Northfield, IL) proficiency testing for ethambutol and pyrazinamide DST in 2012 and 2013. We obtained the MICs for pyrazinamide for all selected strains using the Bactec MGIT 960 method as recommended by the manufacturer (BD Diagnostics, Sparks, MD) by testing at the following pyrazinamide concentrations: 25 mg/liter, 50 mg/liter, 100 mg/liter, 120 mg/liter, 300 mg/liter, and 500 mg/liter. We classified strains with an MIC greater than 100 mg/liter to be resistant to pyrazinamide per WHO guidelines (11). We also performed whole-genome sequencing of M. tuberculosis isolates and classified isolates to be resistant to pyrazinamide if they exhibited any nonsynonymous pyrazinamidase mutation in the sequence of the coding region of the pncA gene compared to the M. tuberculosis H37Rv reference sequence. Some pncA mutations have been associated with pyrazinamide susceptible strains; therefore, we classified isolates with this set of pncA mutations to be susceptible to pyrazinamide (23, 24).
Analysis.
We performed latent class analysis (LCA) to arrive at a consensus gold standard for resistance to pyrazinamide. LCA is a useful method by which to estimate the test characteristics of imperfect diagnostic tests and the prevalence of true resistance when there is no gold standard (25). We modeled “true” pyrazinamide resistance to be an unobserved dichotomous variable (the latent variable) and used the predicted probability of membership in the resistant class to develop a consensus gold standard. We reported the LCA model results as the observed and predicted response categories of the pyrazinamide resistance assays, the predicted probability of membership in the pyrazinamide-resistant latent class, and the Bayesian information criterion (BIC) for the 2-class model compared to the 1-class model. We used a cutoff of a >80% predicted probability of membership in the pyrazinamide-resistant latent class to define the consensus gold standard. We reported the sensitivity, specificity, and area under the receiver operating characteristic (ROC) curve for each of the pyrazinamide resistance assays using the consensus gold standard as the reference.
We conducted univariate and multivariate logistic regression analyses to evaluate the association between “true” pyrazinamide resistance and 2-month culture status. We tested the following variables in univariate models of 2-month outcomes: age; sex; smoking; alcohol use; previous tuberculosis treatment; baseline smear status; cavitary disease; baseline ethambutol resistance; and baseline pyrazinamide resistance as measured by the Wayne method, the Bactec MGIT 960 method, pncA sequencing, and the consensus gold standard. We then constructed four multivariate models: one for each of the three pyrazinamide resistance assays and a fourth using the consensus gold standard as the dependent variable. We considered age, sex, and covariates with P values of <0.1 on univariate analysis for inclusion in the multivariate models. We also performed a sensitivity analysis in which we restricted the comparison to those patients whose MDR-TB isolates were also resistant to ethambutol at baseline. We conducted LCA with the randomLCA package for R, version 3.3.1 (26, 27), and all other analyses were performed using Stata/SE, version 14.1 (28).
Ethical approval.
The Research Ethics Committee of the Peruvian National Institute of Health (Lima, Peru) and the Committee on Human Studies at Harvard Medical School (Boston, MA) approved the study.
RESULTS
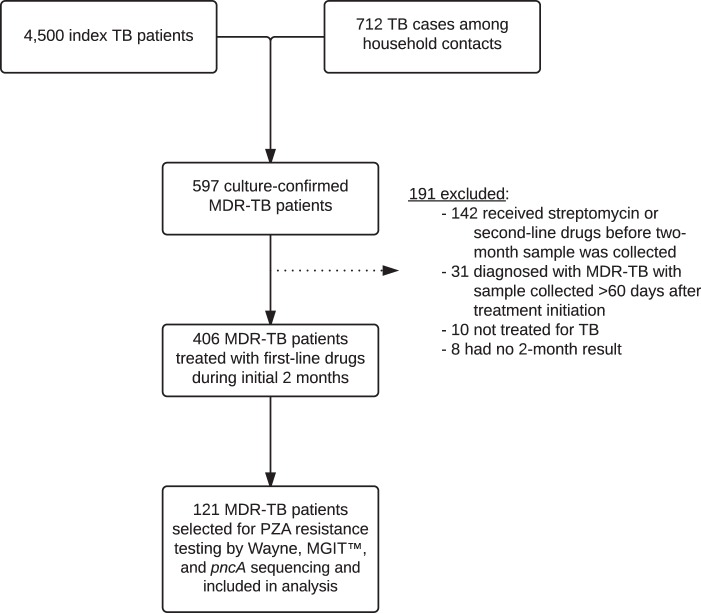
We enrolled 4,500 index patients and identified an additional 712 household contacts who developed active tuberculosis during the study period (Fig. 1). Of the combined 5,212 cases, 597 (11.5%) were confirmed to have MDR-TB. Of these, 406 (68.0%) received a first-line regimen during the initial 2 months of treatment and provided a sample at 2 months, before being switched to an appropriate second-line regimen when their DST results became available. We had access to 121 MDR-TB patients from this population who underwent testing by all three pyrazinamide resistance assays.
FIG 1.
Study flow. MDR, multidrug resistant; MGIT, mycobacteria growth indicator tube; PZA, pyrazinamide; TB, tuberculosis.
Table 1 shows the characteristics of the MDR-TB patients. Among the 121 patients selected for the study, most were male (59.5%), unemployed (64.5%), nonsmokers (98.3%), and nondrinkers (59.5%) and did not have a previous history of tuberculosis treatment (75.0%). Cavitary disease was present in 30 (25.4%) patients. A minority had recently been incarcerated (3.3%), had HIV coinfection (2.5%), or had self-reported diabetes (2.5%). Compared to the other MDR-TB patients enrolled, the selected patients were less likely to have a previous history of tuberculosis treatment (25.0% versus 37.6%; P = 0.010) and less likely to have had previous second-line tuberculosis treatment (0.8% versus 6.7%; P = 0.012).
TABLE 1.
Characteristics of MDR-TB patientsa
| Characteristic | Selected MDR-TB patients (n = 121) | Unselected MDR-TB patients (n = 476) | All MDR-TB patients (n = 597) |
|---|---|---|---|
| Mean (SD) age (yr) | 33.4 (16.8) | 31.3 (14.5) | 31.7 (15.0) |
| No. (%) of patients with the following characteristics: | |||
| Female sex | 49 (40.5) | 166 (34.9) | 215 (36.0) |
| Married (n = 594) | 43 (35.8) | 145 (30.6) | 188 (31.6) |
| Unemployed (n = 595) | 78 (64.5) | 297 (62.7) | 375 (63.0) |
| Incarceration in the past 5 yr (n = 592) | 4 (3.3) | 34 (7.2) | 38 (6.4) |
| Smoking status (n = 579) | |||
| Nonsmoker | 116 (98.3) | 439 (95.2) | 555 (95.9) |
| One cigarette per day | 1 (0.8) | 10 (2.2) | 11 (1.9) |
| >1 cigarette per day | 1 (0.8) | 12 (2.6) | 13 (2.2) |
| Alcohol use (n = 566) | |||
| Nondrinker | 69 (59.5) | 247 (54.9) | 316 (55.8) |
| <40 g or <3 drinks per day | 38 (32.8) | 147 (32.7) | 185 (32.7) |
| ≥40 g or ≥3 drinks per day | 9 (7.8) | 56 (12.4) | 65 (11.5) |
| HIV infection (n = 585) | 3 (2.5) | 19 (4.1) | 22 (3.8) |
| Diabetes (n = 593) | 3 (2.5) | 32 (6.8) | 35 (5.9) |
| Previous TB treatment (n = 596) | |||
| None | 90 (75.0) | 297 (62.4) | 387 (64.9) |
| First-line treatment | 29 (24.2) | 136 (28.6) | 165 (27.7) |
| Second-line treatment | 1 (0.8) | 32 (6.7) | 33 (5.5) |
| Treatment type unknown | 0 (0) | 11 (2.3) | 11 (1.9) |
| Mean (SD) BMI (kg/m2) (n = 586) | 21.7 (3.2) | 21.9 (3.4) | 21.9 (3.4) |
| No. (%) of patients with cavitary disease (n = 542) | 30 (25.4) | 120 (28.3) | 150 (27.7) |
BMI, body mass index; HIV, human immunodeficiency virus; MDR, multidrug resistant; TB, tuberculosis. Because of rounding and missing data, the sum of percentages may not equal 100%.
Figure 2 shows the results of the three pyrazinamide resistance assays. Sixty (49.6%) patients had isolates that were resistant to pyrazinamide as measured by the Wayne method, 71 (58.7%) had isolates that were resistant to pyrazinamide as measured by the Bactec MGIT 960 method, and 68 (56.2%) had isolates that were resistant to pyrazinamide as measured by pncA sequencing. Forty-eight (39.7%) patients had isolates that were resistant to pyrazinamide by all three assays, while 38 (31.4%) had isolates that were susceptible to pyrazinamide by all three assays. We found 30 unique nonsynonymous pncA mutations, which are listed in Table S1 in the supplemental material. One patient harbored an isolate with a mutation previously reported to be found exclusively in pyrazinamide-susceptible strains (Leu159Val) (24), and we classified this patient as having an infection with a pyrazinamide-susceptible isolate by pncA sequencing. Among the selected patients, 55 (45.5%) patients had M. tuberculosis isolates that were resistant to ethambutol at baseline. At 2 months, 71 (58.7%) of the patients tested were smear positive and 87 (75.0%) were culture positive. Five (4.1%) patients did not have a 2-month culture result.
FIG 2.

Venn diagram showing the results of the three pyrazinamide resistance assays. The number at the bottom right indicates the number of MDR-TB isolates susceptible by all three assays. MGIT, mycobacteria growth indicator tube.
Table 2 shows that the 2-class LCA model closely predicted the observed assay results. The BIC was 415.1 for the 2-class model and 512.1 for the 1-class model, showing that the 2-class model was the preferred model. All response categories with positive results by two or more pyrazinamide resistance assays had a >80% predicted probability of membership in the pyrazinamide-resistant latent class. Sixty-eight (56.2%) patients had positive results by two or more pyrazinamide resistance assays, which we classified as “true” resistance to pyrazinamide by the consensus gold standard. Table 3 shows that the sensitivity and specificity of each test against the consensus gold standard were 82.4% and 92.5%, respectively, for the Wayne method; 95.6% and 88.7%, respectively, for the Bactec MGIT 960 method; and 92.6% and 90.6%, respectively, for pncA sequencing.
TABLE 2.
Observed and predicted response categories to three PZA resistance assays and probability of PZA-resistant class membership by an LCA modela
| Response category |
No. (%) of patients in which the response was observedb | No. of patients with LCA-predicted response | LCA probability of PZAr class | ||
|---|---|---|---|---|---|
| Wayne method | MGIT | pncA sequencing | |||
| Resistant | Resistant | Resistant | 48 (39.7) | 47.999434 | 0.998824465 |
| Susceptible | Resistant | Resistant | 12 (9.9) | 12.000123 | 0.952536115 |
| Resistant | Resistant | Susceptible | 5 (4.1) | 5.000253 | 0.90243908 |
| Resistant | Susceptible | Resistant | 3 (2.5) | 3.000222 | 0.85509223 |
| Susceptible | Resistant | Susceptible | 6 (5.0) | 6.000156 | 0.179303685 |
| Susceptible | Susceptible | Resistant | 5 (4.1) | 5.000219 | 0.122326119 |
| Resistant | Susceptible | Susceptible | 4 (3.3) | 4.000193 | 0.06036289 |
| Susceptible | Susceptible | Susceptible | 38 (31.4) | 37.999399 | 0.001515012 |
LCA, latent class analysis; MGIT, mycobacteria growth indicator tube; PZA, pyrazinamide; PZAr, pyrazinamide resistant.
A total of 121 patients were evaluated.
TABLE 3.
PZA resistance assay characteristics among 121 MDR-TB patients, using a consensus gold standard derived from an LCA model as the comparatora
| PZA resistance assay | Sensitivity (%) | Specificity (%) | AUC of ROC |
|---|---|---|---|
| Wayne method | 82.4 (71.2–90.5) | 92.5 (81.8–97.9) | 0.87 (0.82–0.93) |
| MGIT | 95.6 (87.6–99.1) | 88.7 (77.0–95.7) | 0.92 (0.87–0.97) |
| pncA sequencing | 92.6 (83.7–97.6) | 90.6 (79.3–96.9) | 0.92 (0.87–0.97) |
AUC, area under the curve; LCA, latent class analysis; MDR-TB, multidrug-resistant tuberculosis; MGIT, mycobacteria growth indicator tube; PZA, pyrazinamide; ROC, receiver operating characteristic. Values in parentheses are 95% confidence intervals.
Table 4 shows that MDR-TB patients with pyrazinamide resistance were between 2 and 4 times as likely to experience poor outcomes at 2 months as MDR-TB patients whose isolates were susceptible, depending on the specific diagnostic assay used. We estimated the minimum detectable odds ratios (ORs) to be 3.64 for the Wayne method, 3.32 for the Bactec MGIT 960 method, and 3.54 for both pncA sequencing and the consensus gold standard, using a 2-sided test with a total sample of 116 subjects, a significance level of 5%, and 80% power. After we adjusted for age, sex, alcohol use, and baseline ethambutol resistance in the multivariate models, the only association that remained statistically significant was between resistance to pyrazinamide as measured by the Bactec MGIT 960 method and 2-month culture positivity (OR = 3.43; 95% confidence interval [CI], 1.28 to 9.15) (Table 5; Fig. 3). When we restricted the analysis to the 55 patients whose isolates were resistant to ethambutol at baseline, we obtained similar overall results (see Tables S2 to S4 in the supplemental material), with one notable exception. In multivariate analysis, we found that resistance to pyrazinamide by the Wayne method and pncA sequencing remained significantly associated with a higher odds of 2-month culture positivity (OR for the Wayne method = 12.4 [95% CI, 1.31 to 118.5]; OR for pncA sequencing = 8.57 [95% CI, 1.42 to 51.9]), while the consensus gold standard was associated with a higher odds of 2-month culture positivity which was not statistically significant (OR = 4.93; 95% CI, 0.89 to 27.3).
TABLE 4.
Univariate predictors of 2-month culture positivity among patients with unsuspected MDR-TB receiving standard first-line antituberculous therapya
| Variable | No. (%) of patientsb | Crude OR (95% CI)c |
|---|---|---|
| Age (yr) | 116 (100) | 1.03 (1.00–1.07) |
| Sex | ||
| Female | 47 (40.5) | 0.65 (0.28–1.53) |
| Male | 69 (59.5) | Reference |
| Smoking | ||
| Yes | 2 (1.7) | NA |
| No | 111 (95.7) | Reference |
| Unknown | 3 (2.6) | 0.67 (0.06–7.73) |
| Alcohol use | ||
| Yes | 46 (39.7) | 1.00 (0.42–2.37) |
| No | 65 (56.0) | Reference |
| Unknown | 5 (4.3) | NA |
| Previous TB treatment | ||
| Yes | 28 (24.1) | 3.36 (0.93–12.1) |
| No | 87 (75.0) | Reference |
| Unknown | 1 (0.9) | NA |
| Baseline smear status | ||
| Positive | 106 (91.4) | 1.32 (0.32–5.47) |
| Negative | 10 (8.6) | Reference |
| Cavitary disease | ||
| Yes | 29 (25.0) | 0.67 (0.27–1.71) |
| No | 86 (74.1) | Reference |
| Unknown | 1 (0.9) | NA |
| Baseline EMB resistance | ||
| Yes | 54 (46.6) | 3.69 (1.43–9.54) |
| No | 62 (53.4) | Reference |
| Baseline PZA resistance (Wayne method) | ||
| Yes | 58 (50.0) | 2.87 (1.17–7.00) |
| No | 58 (50.0) | Reference |
| Baseline PZA resistance (MGIT) | ||
| Yes | 69 (59.5) | 4.00 (1.65–9.73) |
| No | 47 (40.5) | Reference |
| Baseline PZA resistance (pncA sequencing) | ||
| Yes | 66 (56.9) | 2.81 (1.18–6.70) |
| No | 50 (43.1) | Reference |
| Baseline PZA resistance (consensus) | ||
| Yes | 66 (56.9) | 2.81 (1.18–6.70) |
| No | 50 (43.1) | Reference |
CI, confidence interval; EMB, ethambutol; MDR, multidrug resistant; MGIT, mycobacteria growth indicator tube; NA, not applicable; OR, odds ratio; PZA, pyrazinamide; TB, tuberculosis. Data are for a total of 116 patients. Two (1.7%) patients had a missing 2-month culture result due to a contaminated sample, and three (2.5%) patients had a missing 2-month culture result for an unknown reason.
The sample size varies across covariates due to missing data. Because of rounding, the sum of percentages may not equal 100%.
P values of <0.05 are shown in bold, and P values of ≥0.05 and <0.1 are shown in italics.
TABLE 5.
Multivariate comparison of 2-month culture positivity among patients with unsuspected MDR-TB receiving standard first-line antituberculous therapy by PZA resistance assay and the consensus gold standarda
| Variable | Adjusted OR (95% CI)b |
|---|---|
| Baseline PZA resistance (Wayne method) | |
| Yes | 2.34 (0.90–6.08) |
| No | Reference |
| Baseline PZA resistance (MGIT) | |
| Yes | 3.43 (1.28–9.15) |
| No | Reference |
| Baseline PZA resistance (pncA sequencing) | |
| Yes | 2.12 (0.79–5.72) |
| No | Reference |
| Baseline PZA resistance (consensus) | |
| Yes | 1.95 (0.74–5.11) |
| No | Reference |
CI, confidence interval; MDR-TB, multidrug-resistant tuberculosis; MGIT, mycobacteria growth indicator tube; OR, odds ratio; PZA, pyrazinamide. Data are for a total of 116 patients. Two (1.7%) patients had a missing 2-month culture result due to a contaminated sample, and three (2.5%) patients had a missing 2-month culture result for an unknown reason.
P values of <0.05 are shown in bold, and P values of ≥0.05 and <0.1 are shown in italics. All multivariate models are adjusted for age, sex, alcohol use, and baseline EMB resistance.
FIG 3.

Scatterplot of adjusted OR estimates and 95% CIs for 2-month culture positivity among patients with unsuspected MDR-TB receiving standard first-line antituberculous therapy, by pyrazinamide resistance assay and the consensus gold standard. The vertical dashed line indicates an odds ratio of 1 (no association). MGIT, mycobacteria growth indicator tube.
DISCUSSION
Here, we developed a consensus gold standard definition of pyrazinamide resistance using three paired resistance assays, and we evaluated the association between pyrazinamide resistance and 2-month treatment outcomes. We found that the Wayne method, the Bactec MGIT 960 method, and pncA sequencing were highly sensitive and specific for the consensus gold standard definition of two or more positive pyrazinamide resistance assays derived from an LCA model. To our knowledge, we are the first to use LCA to evaluate the performance of available pyrazinamide resistance assays. After adjusting for potential confounders, we found that pyrazinamide resistance, as measured by the three assays and the LCA-derived consensus gold standard, was associated with positive 2-month sputum cultures. However, our power was insufficient to conclusively find a difference at the standard alpha value of 0.05. In a sensitivity analysis, we found that restricting our analysis to patients whose isolates had additional ethambutol resistance yielded similar results.
Pyrazinamide is one of several antituberculous drugs that have been reported to have discrepant activity in vitro and in vivo. In the case of rifampin, a prior study found that phenotypic DST does not capture some clinically relevant resistance conferred by disputed rpoB mutations that had previously been considered of indeterminate significance; these were associated with a rate of failure or relapse of first-line retreatment of 63% (6). For pyrazinamide, only a few studies have directly evaluated the correlation between in vitro resistance with clinical outcomes (12, 29–32). One of these reported that patients whose isolates had an MIC breakpoint of over 50 mg/liter had poor sputum conversion rates (12). Another was a meta-analysis of individual patient data that found that patients treated with pyrazinamide had improved final MDR-TB and extensively drug-resistant tuberculosis (XDR-TB) treatment outcomes if their baseline M. tuberculosis isolates were susceptible to pyrazinamide (29). In this meta-analysis, however, most patients received individualized regimens that were appropriately adjusted on the basis of their isolates' drug resistance profiles, and a direct comparison of outcomes among patients with and without in vitro pyrazinamide resistance could not be made. In the present study, we leveraged the unfortunate fact that many patients did not receive immediate DST results to directly compare the 2-month culture status for patients with and without pyrazinamide-resistant isolates.
Using treatment outcomes to validate the results of phenotypic or genotypic drug susceptibility testing entails the methodological challenge of disentangling the effect of in vitro pyrazinamide resistance from other factors that contribute to poor treatment outcomes. These factors include resistance to other drugs, since pyrazinamide is always prescribed as part of a multidrug regimen, as well as any risk factors for poor outcomes, such as comorbidities like HIV coinfection and diabetes, disease severity, and adherence. The imperfect correlation between treatment outcomes and in vitro susceptibility testing has previously been described as the “90-60” rule, whereby bacterial infections due to susceptible organisms respond to therapy about 90% of the time, while those caused by resistant organisms respond to therapy about 60% of the time (33). Here, we used LCA to arrive at a more accurate consensus definition of resistance and then estimated the association between this measure of pyrazinamide resistance and clinical outcomes. We used a proximate endpoint of 2-month culture status, which has been associated with the end-of-treatment outcome among HIV-negative MDR-TB patients (97.5% of our analysis cohort) (34). The confidence interval for the consensus definition crossed an odds ratio of 1 after adjustment for potential confounders. This finding implies that the association between pyrazinamide resistance, as measured by currently available assays, and MDR-TB treatment outcomes may be weaker than what is expected from the 90-60 rule and further highlights the discrepant activity of pyrazinamide in vitro and in vivo.
Since we restricted our analysis to MDR-TB patients who received standard first-line therapy before their DST results became available, these patients received a maximum of two potentially effective drugs (ethambutol and pyrazinamide). While high-dose isoniazid may overcome low-level isoniazid resistance (15, 35–37), the patients in this study received standardized doses of isoniazid and rifampin. Nonetheless, we cannot completely exclude the possibility that isoniazid or rifampin may have exerted a low level of activity in these MDR-TB patients and decreased the proportion of patients with 2-month sputum culture positivity. In addition, while our study laboratory scored 100% on ethambutol proficiency testing during the study, we acknowledge that in vitro ethambutol resistance testing is often unreliable (38).
The currently recommended pyrazinamide DST method is the Bactec MGIT 960 method with a critical concentration of 100 mg/liter (8, 11). Many national tuberculosis programs do not perform routine pyrazinamide DST due to the technical difficulties of the Bactec MGIT 960 method and the general perception that it is unreliable. We found that the Bactec MGIT 960 method had a sensitivity of 95.6% and a specificity of 88.7% against the consensus gold standard. While the Bactec MGIT 960 method was the most sensitive in the primary analysis, pncA sequencing was the most sensitive (sensitivity, 97.4%) when we restricted our analysis to patients with ethambutol-resistant MDR-TB. The Wayne method, which is currently in routine use in Peru, was more specific than the other assays and had specificities of 92.5% in the primary analysis and 100.0% among patients with ethambutol-resistant MDR-TB. Further research should aim to improve the performance of molecular testing by identifying specific pncA mutations associated with poor treatment outcomes as targets for novel molecular assays. In conclusion, given that there is a discrepancy between in vitro pyrazinamide resistance and in vivo response, we propose that patient outcomes be used to validate new assays for pyrazinamide drug resistance, after adjustment for host-related risk factors for poor treatment response.
Supplementary Material
ACKNOWLEDGMENTS
We thank the patients and their families who made this study possible. We also thank the health care workers at the participating health centers in Lima Ciudad and Lima Este.
Funding Statement
The contents are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health. The funding source had no role in the study design, in the collection, analysis, and interpretation of data, in the writing of the report, or in the decision to submit the report for publication.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.00632-16.
REFERENCES
- 1.Domínguez J, Boettger EC, Cirillo D, Cobelens F, Eisenach KD, Gagneux S, Hillemann D, Horsburgh R, Molina-Moya B, Niemann S, Tortoli E, Whitelaw A, Lange C, TBNET, RESIST-TB networks. 2016. Clinical implications of molecular drug resistance testing for Mycobacterium tuberculosis: a TBNET/RESIST-TB consensus statement. Int J Tuberc Lung Dis 20:24–42. doi: 10.5588/ijtld.15.0221. [DOI] [PubMed] [Google Scholar]
- 2.Rigouts L, Gumusboga M, de Rijk WB, Nduwamahoro E, Uwizeye C, de Jong B, Van Deun A. 2013. Rifampin resistance missed in automated liquid culture system for Mycobacterium tuberculosis isolates with specific rpoB mutations. J Clin Microbiol 51:2641–2645. doi: 10.1128/JCM.02741-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Somoskovi A, Deggim V, Ciardo D, Bloemberg GV. 2013. Diagnostic implications of inconsistent results obtained with the Xpert MTB/Rif assay in detection of Mycobacterium tuberculosis isolates with an rpoB mutation associated with low-level rifampin resistance. J Clin Microbiol 51:3127–3129. doi: 10.1128/JCM.01377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Dormandy J, Somoskovi A, Kreiswirth BN, Driscoll JR, Ashkin D, Salfinger M. 2007. Discrepant results between pyrazinamide susceptibility testing by the reference BACTEC 460TB method and pncA DNA sequencing in patients infected with multidrug-resistant W-Beijing Mycobacterium tuberculosis strains. Chest 131:497–501. doi: 10.1378/chest.06-1899. [DOI] [PubMed] [Google Scholar]
- 5.Pang Y, Wang Z, Zheng H, Song Y, Wang Y, Zhao Y. 2015. Pyrazinamide resistance determined by liquid culture at low pH better correlates with genetic mutations in MDR tuberculosis isolates. J Microbiol Methods 119:142–144. doi: 10.1016/j.mimet.2015.10.014. [DOI] [PubMed] [Google Scholar]
- 6.Van Deun A, Aung KJM, Bola V, Lebeke R, Hossain MA, de Rijk WB, Rigouts L, Gumusboga A, Torrea G, de Jong BC. 2013. Rifampin drug resistance tests for tuberculosis: challenging the gold standard. J Clin Microbiol 51:2633–2640. doi: 10.1128/JCM.00553-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang Y, Chiu Chang K, Leung CC, Wai Yew W, Gicquel B, Fallows D, Kaplan G, Chaisson RE, Zhang W. 2012. ‘ZS-MDR-TB’ versus ‘ZR-MDR-TB’: improving treatment of MDR-TB by identifying pyrazinamide susceptibility. Emerg Microbes Infect 1:e5. doi: 10.1038/emi.2012.18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization. 2014. Companion handbook to the WHO guidelines for the programmatic management of drug-resistant tuberculosis. World Health Organization, Geneva, Switzerland. [PubMed] [Google Scholar]
- 9.Salfinger M, Heifets LB. 1988. Determination of pyrazinamide MICs for Mycobacterium tuberculosis at different pHs by the radiometric method. Antimicrob Agents Chemother 32:1002–1004. doi: 10.1128/AAC.32.7.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang Y, Mitchison D. 2003. The curious characteristics of pyrazinamide: a review. Int J Tuberc Lung Dis 7:6–21. [PubMed] [Google Scholar]
- 11.World Health Organization. 2012. Updated interim critical concentrations for first-line and second-line DST (as of May 2012). World Health Organization, Geneva, Switzerland. [Google Scholar]
- 12.Gumbo T, Chigutsa E, Pasipanodya J, Visser M, van Helden PD, Sirgel FA, McIlleron H. 2014. The pyrazinamide susceptibility breakpoint above which combination therapy fails. J Antimicrob Chemother 69:2420–2425. doi: 10.1093/jac/dku136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Somoskovi A, Wade MM, Sun Z, Zhang Y. 2004. Iron enhances the antituberculous activity of pyrazinamide. J Antimicrob Chemother 53:192–196. doi: 10.1093/jac/dkh042. [DOI] [PubMed] [Google Scholar]
- 14.Driver ER, Ryan GJ, Hoff DR, Irwin SM, Basaraba RJ, Kramnik I, Lenaerts AJ. 2012. Evaluation of a mouse model of necrotic granuloma formation using C3HeB/FeJ mice for testing of drugs against Mycobacterium tuberculosis. Antimicrob Agents Chemother 56:3181–3195. doi: 10.1128/AAC.00217-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Caminero JA, Sotgiu G, Zumla A, Migliori GB. 2010. Best drug treatment for multidrug-resistant and extensively drug-resistant tuberculosis. Lancet Infect Dis 10:621–629. doi: 10.1016/S1473-3099(10)70139-0. [DOI] [PubMed] [Google Scholar]
- 16.Falzon D, Jaramillo E, Schünemann HJ, Arentz M, Bauer M, Bayona J, Blanc L, Caminero JA, Daley CL, Duncombe C, Fitzpatrick C, Gebhard A, Getahun H, Henkens M, Holtz TH, Keravec J, Keshavjee S, Khan AJ, Kulier R, Leimane V, Lienhardt C, Lu C, Mariandyshev A, Migliori GB, Mirzayev F, Mitnick CD, Nunn P, Nwagboniwe G, Oxlade O, Palmero D, Pavlinac P, Quelapio MI, Raviglione MC, Rich ML, Royce S, Rüsch-Gerdes S, Salakaia A, Sarin R, Sculier D, Varaine F, Vitoria M, Walson JL, Wares F, Weyer K, White RA, Zignol M. 2011. WHO guidelines for the programmatic management of drug-resistant tuberculosis: 2011 update. Eur Respir J 38:516–528. doi: 10.1183/09031936.00073611. [DOI] [PubMed] [Google Scholar]
- 17.World Health Organization. 2013. Global tuberculosis report 2013. Report WHO/HTM/TB/2013.11. World Health Organization, Geneva, Switzerland. [Google Scholar]
- 18.World Health Organization. 2010. Treatment of tuberculosis: guidelines, 4th ed. Report WHO/HTM/TB/2009.420 World Health Organization, Geneva, Switzerland. [Google Scholar]
- 19.Ministerio de Salud. 2010. Actualización del sub numeral 7. Tratamiento de la tuberculosis de la NTS no. 041-MINSA/DFSP-V.01 Norma técnica de salud para el control de la tuberculosis aprobada por R.M. no. 383-2006/MINSA. Dirección General de Salud de las Personas, Estrategia Sanitaria Nacional para la Prevención y Control de la Tuberculosis, Lima, Peru. [Google Scholar]
- 20.Ministerio de Salud. 2006. Norma técnica de salud para el control de la tuberculosis. Dirección General de Salud de las Personas, Estrategia Sanitaria Nacional para la Prevención y Control de la Tuberculosis, Lima, Peru. [Google Scholar]
- 21.Wayne LG. 1974. Simple pyrazinamidase and urease tests for routine identification of mycobacteria. Am Rev Respir Dis 109:147–151. [DOI] [PubMed] [Google Scholar]
- 22.Morlock GP, Crawford JT, Butler WR, Brim SE, Sikes D, Mazurek GH, Woodley CL, Cooksey RC. 2000. Phenotypic characterization of pncA mutants of Mycobacterium tuberculosis. Antimicrob Agents Chemother 44:2291–2295. doi: 10.1128/AAC.44.9.2291-2295.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miotto P, Cabibbe AM, Feuerriegel S, Casali N, Drobniewski F, Rodionova Y, Bakonyte D, Stakenas P, Pimkina E, Augustynowicz-Kopeć E, Degano M, Ambrosi A, Hoffner S, Mansjö M, Werngren J, Rüsch-Gerdes S, Niemann S, Cirillo DM. 2014. Mycobacterium tuberculosis pyrazinamide resistance determinants: a multicenter study. mBio 5:e01819-14. doi: 10.1128/mBio.01819-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ramirez-Busby SM, Valafar F. 2015. Systematic review of mutations in pyrazinamidase associated with pyrazinamide resistance in Mycobacterium tuberculosis clinical isolates. Antimicrob Agents Chemother 59:5267–5277. doi: 10.1128/AAC.00204-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Collins J, Huynh M. 2014. Estimation of diagnostic test accuracy without full verification: a review of latent class methods. Stat Med 33:4141–4169. doi: 10.1002/sim.6218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.R Core Team. 2016. R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. [Google Scholar]
- 27.Beath K. 2016. randomLCA: random effects latent class analysis. R package, version 1.0-9. https://CRAN.R-project.org/package=randomLCA. [Google Scholar]
- 28.StataCorp. 2015. Stata statistical software: release 14. Stata Corp LP, College Station, TX. [Google Scholar]
- 29.Bastos ML, Hussain H, Weyer K, Garcia-Garcia L, Leimane V, Leung CC, Narita M, Penã JM, Ponce-de-Leon A, Seung KJ, Shean K, Sifuentes-Osornio J, Van der Walt M, Van der Werf TS, Yew WW, Menzies D, Collaborative Group for Meta-Analysis of Individual Patient Data in MDR-TB. 2014. Treatment outcomes of patients with multidrug-resistant and extensively drug-resistant tuberculosis according to drug susceptibility testing to first- and second-line drugs: an individual patient data meta-analysis. Clin Infect Dis 59:1364–1374. doi: 10.1093/cid/ciu619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Franke MF, Becerra MC, Tierney DB, Rich ML, Bonilla C, Bayona J, McLaughlin MM, Mitnick CD. 2015. Counting pyrazinamide in regimens for multidrug-resistant tuberculosis. Ann Am Thorac Soc 12:674–679. doi: 10.1513/AnnalsATS.201411-538OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yuen CM, Kurbatova EV, Tupasi T, Caoili JC, Van der Walt M, Kvasnovsky C, Yagui MJA, Bayona J, Contreras C, Leimane V, Ershova J, Via LE, Kim H, Akksilp S, Kazennyy BY, Volchenkov GV, Jou R, Kliiman K, Demikhova OV, Vasilyeva IA, Dalton T, Cegielski JP. 2015. Association between regimen composition and treatment response in patients with multidrug-resistant tuberculosis: a prospective cohort study. PLoS Med 12:e1001932. doi: 10.1371/journal.pmed.1001932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cegielski JP, Kurbatova E, Van der Walt M, Brand J, Ershova J, Tupasi T, Caoili JC, Dalton T, Contreras C, Yagui MJA, Bayona J, Kvasnovsky C, Leimane V, Kuksa L, Chen MP, Via LE, Hwang SH, Wolfgang M, Volchenkov GV, Somova T, Smith SE, Akksilp S, Wattanaamornkiet W, Kim HJ, Kim C-K, Kazennyy BY, Khorosheva T, Kliiman K, Viiklepp P, Jou R, Huang AS-E, Vasilyeva IA, Demikhova OV, Global PETTS Investigators. 2016. Multidrug-resistant tuberculosis treatment outcomes in relation to treatment and initial versus acquired second-line drug resistance. Clin Infect Dis 62:418–430. doi: 10.1093/cid/civ910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rex JH, Pfaller MA. 2002. Has antifungal susceptibility testing come of age? Clin Infect Dis 35:982–989. doi: 10.1086/342384. [DOI] [PubMed] [Google Scholar]
- 34.Kurbatova EV, Cegielski JP, Lienhardt C, Akksilp R, Bayona J, Becerra MC, Caoili J, Contreras C, Dalton T, Danilovits M, Demikhova OV, Ershova J, Gammino VM, Gelmanova I, Heilig CM, Jou R, Kazennyy B, Keshavjee S, Kim HJ, Kliiman K, Kvasnovsky C, Leimane V, Mitnick CD, Quelapio I, Riekstina V, Smith SE, Tupasi T, Van der Walt M, Vasilyeva IA, Via LE, Viiklepp P, Volchenkov G, Walker AT, Wolfgang M, Yagui MJA, Zignol M. 2015. Sputum culture conversion as a prognostic marker for end-of-treatment outcome in patients with multidrug-resistant tuberculosis: a secondary analysis of data from two observational cohort studies. Lancet Respir Med 3:201–209. doi: 10.1016/S2213-2600(15)00036-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Katiyar SK, Bihari S, Prakash S, Mamtani M, Kulkarni H. 2008. A randomised controlled trial of high-dose isoniazid adjuvant therapy for multidrug-resistant tuberculosis. Int J Tuberc Lung Dis 12:139–145. [PubMed] [Google Scholar]
- 36.Chang K-C, Yew WW, Tam C-M, Leung CC. 2013. WHO group 5 drugs and difficult multidrug-resistant tuberculosis: a systematic review with cohort analysis and meta-analysis. Antimicrob Agents Chemother 57:4097–4104. doi: 10.1128/AAC.00120-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dooley KE, Mitnick CD, Ann DeGroote M, Obuku E, Belitsky V, Hamilton CD, Makhene M, Shah S, Brust JCM, Durakovic N, Nuermberger E, Efficacy Subgroup, RESIST-TB . 2012. Old drugs, new purpose: retooling existing drugs for optimized treatment of resistant tuberculosis. Clin Infect Dis 55:572–581. doi: 10.1093/cid/cis487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Van Deun A, Wright A, Zignol M, Weyer K, Rieder HL. 2011. Drug susceptibility testing proficiency in the network of supranational tuberculosis reference laboratories. Int J Tuberc Lung Dis 15:116–124. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.