Abstract
Persisters are small populations of quiescent bacterial cells that survive exposure to bactericidal antibiotics and are responsible for many persistent infections and posttreatment relapses. However, little is known about how to effectively kill persister bacteria. In the work presented here, we found that colistin, a membrane-active antibiotic, was highly active against Escherichia coli persisters at high concentrations (25 or 50 μg/ml). At a clinically relevant lower concentration (10 μg/ml), colistin alone had no apparent effect on E. coli persisters. In combination with other drugs, this concentration of colistin enhanced the antipersister activity of gentamicin and ofloxacin but not that of ampicillin, nitrofurans, and sulfa drugs in vitro. The colistin enhancement effect was most likely due to increased uptake of the other antibiotics, as demonstrated by increased accumulation of fluorescence-labeled gentamicin. Interestingly, colistin significantly enhanced the activity of ofloxacin and nitrofurantoin but not that of gentamicin or sulfa drugs in the murine model of urinary tract infection. Our findings suggest that targeting bacterial membranes is a valuable approach to eradicating persisters and should have implications for more effective treatment of persistent bacterial infections.
INTRODUCTION
Persisters are a small fraction of nonreplicating, metabolically quiescent bacteria that survive the lethal action of bactericidal antibiotic treatment and can potentially regrow after antibiotic removal, while remaining susceptible to the same antibiotic (1, 2). Persister cells are thought to be responsible for many persistent bacterial infections, such as tuberculosis (3), urinary tract infections (UTIs) (4), and biofilm infections (5). These infections require prolonged treatment, have a high risk of relapse, and can lead to genetic antibiotic resistance during prolonged and repeated treatment.
Although the persister phenomenon was first discovered in the 1940s (6, 7), only recently did we begin to understand the molecular mechanisms of persisters (1, 8, 9). Common antibiotics that inhibit macromolecule synthesis generally have poor activity against persister bacteria, and how the persister bacteria survive treatment with current antibiotics is still unclear. Theoretically, at least two possibilities exist: one is that the drugs cannot enter the persister cells, and the other is that the drugs enter the persister cells but cannot generate lethal effects as they do in growing cells. Work on Mycobacterium tuberculosis demonstrated that the intracellular concentrations of many antibiotics were considerably lower in nonreplicating bacteria than in replicating bacilli (10). Consistent with this observation, increasing the uptake of aminoglycosides by using mannitol (11) and silver (12) was shown to enhance the killing of bacterial persisters by gentamicin. Based on these observations, it is reasonable to postulate that lack of antibiotic entry may play an important role in the survival of persisters during antibiotic exposure and that a combination of cell membrane-active agents with cidal antibiotics may enhance the efficacy of the latter drugs against persisters. Colistin is a polypeptide antibiotic used in the treatment of Gram-negative bacterial infections (13) that acts through interaction with negatively charged lipopolysaccharide (LPS), thereby disrupting the outer membrane of the bacteria and altering the bacterial permeability (14). Here, we tested our hypothesis using the membrane-active antibiotic colistin in an in vitro persister model, as well as a mouse model of UTI infection, and found that colistin alone at high concentrations or combined with gentamicin could eradicate persisters very rapidly.
MATERIALS AND METHODS
Strains, culture conditions, antibiotics, and chemicals.
The Escherichia coli K-12 strain W3110 and the uropathogenic E. coli strain UTI89 were used in this work. Luria-Bertani (LB) broth or agar was used as the growth medium. All experiments were conducted at 37°C in LB broth with shaking at 200 rpm. The antibiotics ampicillin, ofloxacin, gentamicin, and colistin (catalog number C4461) and Texas red dye were obtained from Sigma Chemical Co. and were used at the concentrations described below. Mice were anesthetized with a mixture of saline (0.9% NaCl), ketamine, and acepromazine maleate at a ratio of 7:2:1.
Persister assays.
A single colony of the E. coli K-12 wild-type strain W3110 or the uropathogenic E. coli strain UTI89 was picked and grown in LB broth overnight, diluted 1:1,000 into fresh LB broth, grown for another 16 h to stationary phase, and used for persister assays. For the colistin concentration experiment, 1, 5, 10, 25, or 50 μg/ml colistin was added to a stationary culture and the culture incubated for various times. For the combination experiment, ampicillin (100 μg/ml), ofloxacin (5 μg/ml), and gentamicin (20 μg/ml) were added to the stationary culture alone or combined with colistin at 10 μg/ml. At different time points after the addition of antibiotics, 100 μl of culture was removed, washed with phosphate-buffered saline (PBS), 10-fold serially diluted, and plated for CFU counts on LB agar plates.
Synthesis of Texas red-conjugated gentamicin.
Gentamicin-Texas red conjugate (GTTR) was synthesized as described previously (11, 15). Briefly, gentamicin was dissolved in 100 mM K2CO3 buffer, pH 8.5, to a final concentration of 10 μg/ml. Texas red was dissolved in anhydrous N,N-dimethylformamide (Sigma Chemical Co.) to a concentration of 5 μg/ml and then slowly added to a 30 molar excess of gentamicin and incubated at 4°C overnight.
Flow cytometry analysis of gentamicin uptake in E. coli.
E. coli W3110 cultured in LB broth was treated with colistin (10 μg/ml), GTTR (10 μg/ml), or colistin combined with GTTR overnight. For the flow cytometry analysis, the antibiotic-treated bacteria were washed and diluted 1:100 in PBS to get a concentration of about 5.0 × 107/ml. The bacterial suspension was then stained with 1 mg/ml 4′,6-diamidino-2-phenylindole (DAPI) for 15 min, which allowed discrimination of the bacteria from debris and noise during the flow analysis. The uptake of GTTR in the presence and absence of colistin was analyzed using a MoFlo XDP cell sorter (Beckman Coulter, Inc.) equipped with a 100-mW, 561-nm laser and a 100-mW, 405-nm laser. Fluorescence detection of Texas red and DAPI was performed with 610/30 and 457/50 band pass filters.
UTI mouse model.
Experiments using the mouse model were performed as described previously (16), with slight modifications. Briefly, 7- to 8-week-old female C3HeN/Hsd mice (Harlan) were anesthetized via intraperitoneal injection of 120 μl of the ketamine mixture and then inoculated with a bacterial suspension of about 107 CFU of the 24-h static UTI89 culture in 50 μl saline by transurethral catheterization. Three days after inoculation, antibiotic treatment was given for 6 consecutive days. Ofloxacin (35 mg/kg of body weight/day) and nitrofurantoin (100 mg/kg/day) were given by oral gavage, gentamicin (10 mg/kg/day) and colistin (20 mg/kg/day) by the intraperitoneal route (17), and sulfamethoxazole and trimethoprim in the drinking water at 270 and 54 μg/ml, respectively. The drug-free control mice were given water by gavage. Mice were sacrificed 3 days after the 6-day antibiotic treatment. Bladders were harvested aseptically and homogenized in 1 ml PBS, and the bacterial titers were determined by plating serial dilutions of tissue homogenates on LB plates. All animal experiments were approved by the Johns Hopkins University Animal Care and Use Committee.
RESULTS
Colistin kills E. coli persisters in a concentration-dependent manner.
We first tested the activity of colistin against the stationary-phase culture of E. coli W3110, which is known to generate persisters. Fluoroquinolones are one of the few antibiotics known to have activity against nongrowing stationary-phase bacteria (18, 19), so ofloxacin was used as a control. The ofloxacin (5 μg/ml)-treated culture had more than 107 CFU/ml viable cells after 1 day of exposure. As shown by the results in Fig. 1, when 50 μg/ml colistin was added to the culture, the viable-cell count dropped from 109 CFU/ml to below the detection limit (10 CFU/ml) in just 8 h. At 25 μg/ml, colistin had a less potent effect than at the higher concentration but could still eradicate all the bacteria in just 1 day. However, colistin (10 μg/ml) had hardly any killing activity on stationary-phase bacteria, indicating that the antipersister activity of colistin is dependent on its concentration.
FIG 1.
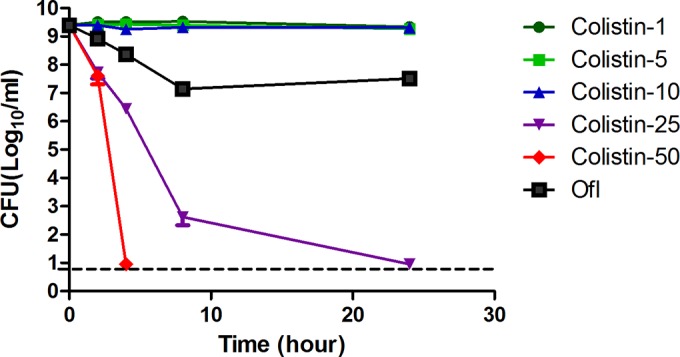
Concentration-dependent killing of E. coli persisters by colistin. E. coli strain W3110 was grown overnight to stationary phase, and then different concentrations of colistin (Col at 5, 10, 25, or 50 μg/ml) or ofloxacin (Ofl at 5 μg/ml) were added to the culture directly. At different times of drug exposure, the viability of the bacteria was determined by CFU counts. The horizontal dashed line represents the limit of detection. The data represent average values and plus-and-minus standard errors from three independent biological replicates.
Colistin enhances the activity of other antibiotics against E. coli persisters in vitro.
Since colistin acts on the outer membrane, we reasoned that colistin might facilitate the entry of other drugs into the cell, which in turn could kill the bacteria more effectively than those drugs alone. To test this hypothesis, we combined colistin at 10 μg/ml, a close to clinically relevant concentration that had no lethal effect on the stationary-phase bacteria alone, with other cidal antibiotics, such as gentamicin, ofloxacin, and ampicillin (Fig. 2). Indeed, colistin combined with gentamicin (20 μg/ml) eradicated all the stationary-phase bacteria in just 1 day, whereas cultures treated with gentamicin alone still had more than 108 CFU remaining. Colistin also enhanced the activity of ofloxacin (5 μg/ml), although the effect was not as strong as that with gentamicin, and the culture treated with the combination had a 10-fold-lower CFU count than was obtained with ofloxacin alone (Fig. 2). Intriguingly, colistin had little impact on the effect of ampicillin, as ampicillin alone did not kill the stationary-phase bacteria in 3 days of antibiotic exposure and its combination with colistin only marginally reduced the number of bacteria (Fig. 2). We were able to confirm the above-described findings obtained with the E. coli K-12 strain W3110 in experiments with the uropathogenic strain E. coli UTI89 (see Fig. S1 and Table S1 in the supplemental material). However, other drugs used to treat UTI in the clinic, such as nitrofurantoin or sulfamethoxazole and trimethoprim (SXT), showed little activity against the stationary-phase uropathogenic E. coli UTI89 either alone or in combination with colistin, indicating a selective enhancement effect of colistin for some antibiotics, including gentamicin and ofloxacin, but not other antibiotics, including ampicillin, nitrofurans, and sulfa drugs.
FIG 2.
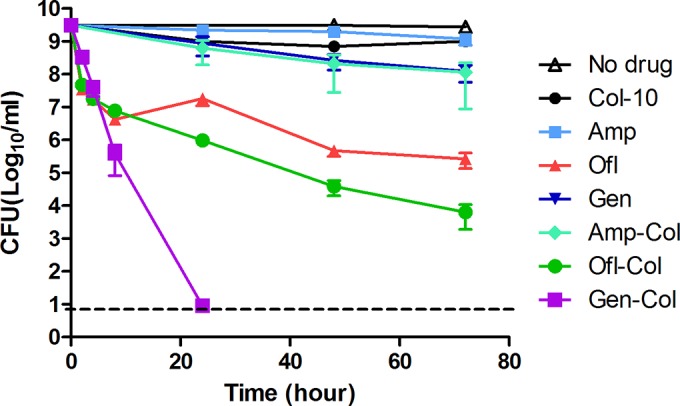
Colistin enhances the activity of other antibiotics. E. coli strain W3110 was grown overnight to stationary phase, followed by the addition of antibiotics to the culture directly. Colistin was used at 10 μg/ml and ampicillin, ofloxacin, and gentamicin were used at 100, 5, and 20 μg/ml, respectively. At different times of drug exposure, the viability of the bacteria was determined by CFU counts. Amp, ampicillin; Ofl, ofloxacin; Gen, gentamicin; Col, colistin. The horizontal dashed line represents the limit of detection. The data represent average values and plus-and-minus standard errors from three independent biological replicates.
Colistin promoted the entry of gentamicin into bacterial cells.
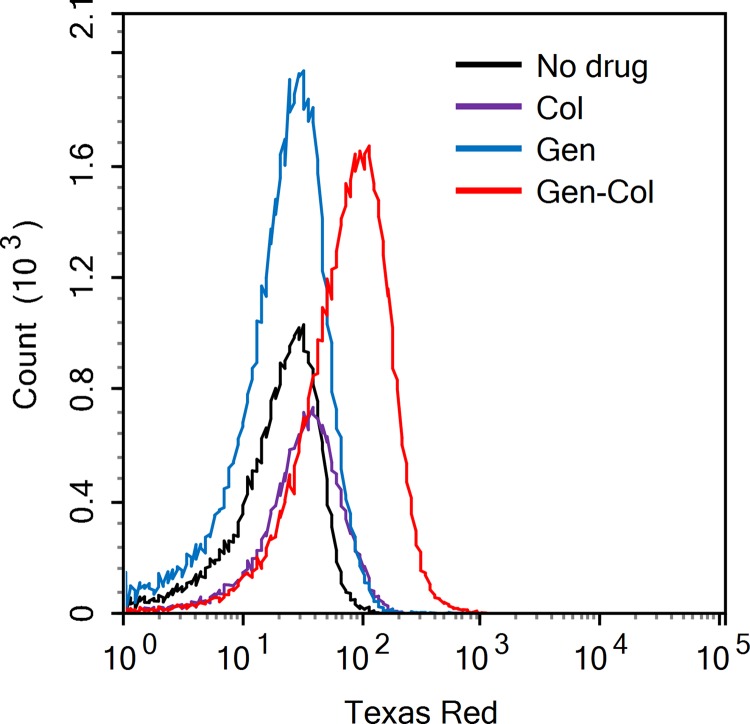
To confirm that the enhancement effect of colistin on gentamicin is due to greater intracellular accumulation of gentamicin in the presence of colistin, we labeled gentamicin with Texas red dye (GTTR) and used flow cytometry to analyze the intracellular drug concentration in the presence or absence of colistin. As shown by the results in Fig. 3, there was a clear shift of Texas red fluorescence intensity in the stationary-phase bacteria treated with colistin combined with GTTR, indicating more drug accumulation intracellularly in the presence of colistin. In contrast, the fluorescence intensity of gentamicin alone had no obvious difference from the drug-free control, indicating that gentamicin alone could hardly enter the nonreplicating stationary-phase bacteria in the absence of colistin. Therefore, these results suggest that the enhancement effect of colistin on gentamicin is due to increased uptake of gentamicin.
FIG 3.
Flow cytometry analysis shows increased accumulation of intracellular gentamicin in the presence of colistin. Stationary-phase culture of E. coli W3110 was treated with gentamicin-Texas red (GTTR) in the presence and absence of colistin overnight. Histogram overlay of detected GTTR showed significant increase of gentamicin as shown by elevated fluorescence in culture treated with gentamicin and colistin. Without the addition of colistin, the treatment with GTTR alone did not show measurable drug uptake with this assay.
Colistin enhances the efficacy of antibiotic treatment in the murine UTI model.
Since E. coli K-12 strain W3110 is a laboratory strain and is not pathogenic for mice, we used a clinically relevant and virulent UTI strain, UTI89, for the mouse experiment to validate the above-described results obtained with the W3110 strain. To determine whether the membrane-active drug colistin could also enhance the clearance of bacterial persisters by antibiotics in vivo, we tested the efficacy of colistin alone and in combination with commonly used UTI antibiotics in a mouse model of persistent uropathogenic E. coli infection (4). The current antibiotics commonly used to treat UTIs, such as beta-lactam antibiotics, fosfomycin, nitrofurantoin, the sulfa drug SXT, quinolones, and aminoglycosides (20), are not very effective for treating persistent uropathogenic E. coli infections, and relapses occur frequently (21). The bladders of mice were inoculated with 107 CFU of the uropathogenic E. coli UTI89 strain, and the infected mice were left for 3 days to establish the infection. Ofloxacin, gentamicin, nitrofurantoin, and the sulfa drug SXT, which are the standard antibiotics used to treat UTI in the clinic, were chosen for the treatment experiment. The mice were treated daily for 6 days, and 3 days after the final treatment, they were sacrificed and the bacterial titers in the bladders and kidneys were determined. The bacterial titers were significantly lower in all antibiotic-treated groups than in the untreated control group (Fig. 4), but none of the antibiotics could clear all the bacteria in the bladder of infected mice. Ofloxacin or colistin alone decreased the bladder bacterial titer to about 103 CFU, while the control group still had 106 CFU. Importantly, when ofloxacin was combined with colistin, the bacterial titer dropped to 102, statistically significantly different from the result for ofloxacin alone, where a titer of 103 bacteria was found (t test, P = 0.03), and the bacterial titer in one of the mice in the colistin-and-ofloxacin group showed complete eradication. Colistin had a similar effect on nitrofurantoin treatment, with borderline statistical significance (P = 0.05). However, colistin had no statistically significant effect on enhancing the activity of gentamicin or of the sulfa drug SXT, where the combination of gentamicin or SXT with colistin was only marginally better than either drug alone (Fig. 4).
FIG 4.
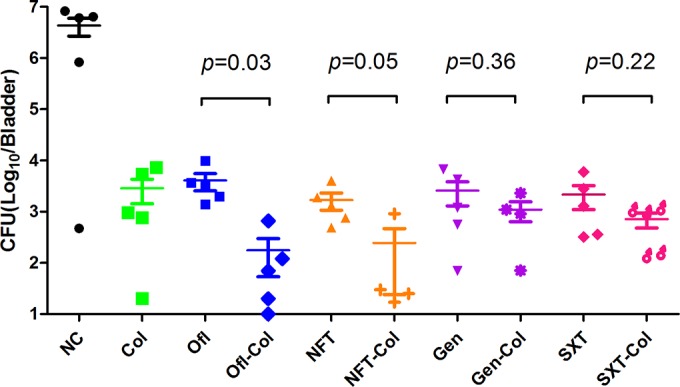
Colistin enhances the activity of ofloxacin and nitrofurantoin in the mouse UTI model. Mice were infected with uropathogenic E. coli UTI89 bacteria (107 CFU) via the transurethral route (see Materials and Methods), and the infection was allowed to become established for 3 days before antibiotic treatment. Ofloxacin (Ofl) (35 mg/kg/day), nitrofurantoin (NFT) (100 mg/kg/day), gentamicin (Gen) (10 mg/kg/day), colistin (Col) (20 mg/kg/day), and sulfamethoxazole and trimethoprim (SXT) (270 and 54 μg/ml, respectively [5:1]) were given daily for 6 days, and the negative-control (NC) mice were given water by gavage. Mice were sacrificed 3 days after the final antibiotic treatment, and the whole bladder of each was put into 1 ml PBS for homogenization and then serially diluted for CFU count (limit of detection, 10 bacteria). The CFU data for the UTI drugs alone and the same drugs combined with colistin were analyzed by t test using GraphPad Prism software. Median values are represented by short solid lines, and plus-and-minus standard errors by error bars.
DISCUSSION
Little is known about why antibiotics failed to kill nongrowing persister bacteria. Here, we showed that at least one reason is reduced antibiotic uptake in the nonreplicating stationary-phase bacteria. This was demonstrated by the direct strong cidal activity of the membrane-active agent colistin at high concentrations (Fig. 1) and the enhancement effect of colistin on gentamicin's activity against the stationary-phase bacteria (Fig. 2). Our flow cytometry analysis provides further direct evidence by demonstrating more accumulation of gentamicin in the bacteria in the presence of colistin than in its absence. Colistin also promoted the efficacy of ofloxacin for killing persisters, suggesting that the colistin enhancement effect is not restricted to aminoglycosides. However, colistin had little effect on the activity of ampicillin against stationary-phase bacteria (Fig. 2), implying that lack of antibiotic uptake is not the only reason for persister survival during drug exposure but, rather, that it is perhaps also due to lack of target activity, i.e., an absence of cell wall synthesis. Since we found that drugs could accumulate to a higher concentration in E. coli in the presence of colistin, it is likely that colistin may also have a similar effect on other Gram-negative bacteria. Further studies are needed to address this possibility in the future.
Colistin alone at high concentrations had very strong activity against stationary-phase E. coli bacteria, while most of the antibiotics tested had little or no effect on nonreplicating persister cells. This may be due to the unique way in which colistin kills the bacteria, through targeting the bacterial membranes. In keeping with this observation, we found in a recent separate study that colistin was more active than most common antibiotics against stationary-phase E. coli cells (19). Common antibiotics mainly target macromolecule biosynthesis in the cell, such as DNA, RNA, protein, and cell wall synthesis. However, the metabolic activity in persister cells is maintained at low levels, and synthesis of macromolecules is greatly reduced. When the target activity is low, inhibiting its function may be less lethal to the bacteria. However, colistin acts differently, by binding to the outer membrane of Gram-negative bacteria and disrupting the membrane integrity, leading to cell death (22). This type of killing is not dependent on the growth status of the bacteria, which may be the reason for its effective killing of nonreplicating persisters shown in this study (Fig. 1).
To further validate our in vitro data, we evaluated colistin for possible enhancement of antibiotic activity in the UTI mouse model. Interestingly, colistin in combination with ofloxacin almost completely eradicated the bacterial population in the bladder and was more active than ofloxacin or colistin alone (P < 0.05) (Fig. 4). Nitrofurantoin combined with colistin was also better than either drug alone (Fig. 4) (P = 0.05). However, it is worth noting that colistin had no apparent effect on enhancing nitrofurantoin activity in vitro but did so in vivo in the mouse model of UTI (Fig. 4). This is likely to be due to the difference in susceptibility of the bacteria to nitrofurans in vitro and in vivo, which could happen due to other stress conditions present in vivo but not in vitro that facilitate the activity of nitrofurans. Surprisingly, there was little difference between the groups treated with gentamicin combined with colistin and with gentamicin alone in the mouse model (Fig. 4). This is in contrast to the in vitro data, where colistin in combination with gentamicin had a strong effect on killing persisters. The failure of colistin to enhance the activity of gentamicin in vivo is most likely due to the inability of gentamicin to enter the bladder epithelial cells, where the persister cells reside in the form of intracellular bacterial communities (4), since gentamicin is known to act only on extracellular bacteria and not on intracellular bacteria. The mouse UTI model of persistence indeed showed that colistin could enhance the activity of other UTI antibiotics, such as ofloxacin and nitrofurantoin, but not that of gentamicin (Fig. 4). Our findings demonstrate the limitations of relying on in vitro results only and highlight the need to evaluate the in vitro results by performing animal studies.
Due to increasing antibiotic resistance, colistin is playing a more important role in fighting antibiotic-resistant infections and is used as the last resort for the treatment of multidrug-resistant infections caused by Gram-negative bacteria, such as Pseudomonas aeruginosa, Klebsiella pneumoniae, and Acinetobacter baumannii (23). It has been reported that polymyxin has a synergistic effect with carbapenems, rifampin, and azithromycin against antibiotic-resistant Acinetobacter baumannii in vitro (24). Indeed, colistin (6 mg/kg/day) in combination with vancomycin has been shown to improve the treatment of multidrug-resistant Acinetobacter baumannii infection in patients (25). Colistin also showed an enhancement effect with multiple antibiotics in a mouse foreign-body infection model (26). However, this is the first time that colistin was shown to have direct killing activity against nonreplicating UTI persisters and that its combination with ofloxacin and nitrofurantoin was shown to lead to increased killing of persisters and improved treatment of persistent UTI infection in the mouse model (Fig. 4). Our findings may have implications for improved treatment of persistent UTI infections in the clinic. Future clinical studies are needed to validate our findings from the mouse study.
In conclusion, we found that colistin has excellent activity against E. coli persisters and could enhance the activity of other UTI antibiotics against persisters both in vitro and in vivo. In particular, colistin significantly enhanced the activity of ofloxacin and nitrofurantoin in the murine model of urinary tract infection. These findings indicate that the bacterial membrane is a valuable target for eradicating bacterial persisters and may have implications for more effective treatment of persistent UTI and other bacterial infections. Further studies are needed to validate whether colistin in combination with ofloxacin or nitrofurantoin could improve the treatment of UTI in patients.
Supplementary Material
ACKNOWLEDGMENTS
We thank Matthew Mulvey for providing the UTI89 strain and Christopher Kizito for help with the animal work.
The work was supported in part by the Key Technologies Research and Development Program for Infectious Diseases of China (grant number 2013ZX10003008-003) and the National Natural Science Foundation of China (grant numbers 81101226 and 81471987). Y.Z. was supported by NIH grants AI99512 and AI108535.
Funding Statement
The work was supported in part by the Key Technologies Research and Development Program for Infectious Diseases of China (grant number 2013ZX10003008-003) and the National Natural Science Foundation of China (grant numbers 81101226 and 81471987). Y.Z. was supported by NIH grants AI99512 and AI108535.
Footnotes
Supplemental material for this article may be found at http://dx.doi.org/10.1128/AAC.01481-16.
REFERENCES
- 1.Zhang Y. 2014. Persisters, persistent infections and the yin-yang model. Emerg Microbes Infect 3:e3. doi: 10.1038/emi.2014.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Balaban NQ. 2011. Persistence: mechanisms for triggering and enhancing phenotypic variability. Curr Opin Genet Dev 21:768–775. doi: 10.1016/j.gde.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 3.Zhang Y, Yew WW, Barer MR. 2012. Targeting persisters for tuberculosis control. Antimicrob Agents Chemother 56:2223–2230. doi: 10.1128/AAC.06288-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blango MG, Mulvey MA. 2010. Persistence of uropathogenic Escherichia coli in the face of multiple antibiotics. Antimicrob Agents Chemother 54:1855–1863. doi: 10.1128/AAC.00014-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Beloin C, Renard S, Ghigo JM, Lebeaux D. 2014. Novel approaches to combat bacterial biofilms. Curr Opin Pharmacol 18:61–68. doi: 10.1016/j.coph.2014.09.005. [DOI] [PubMed] [Google Scholar]
- 6.Hobby GL, Meyer K, Chaffee E. 1942. Observations on the mechanism of action of penicillin. Proc Soc Exp Biol Med 50:281–285. doi: 10.3181/00379727-50-13773. [DOI] [Google Scholar]
- 7.Bigger J. 1944. Treatment of staphylococcal infections with penicillin by intermittent sterilisation. Lancet 244:497–500. doi: 10.1016/S0140-6736(00)74210-3. [DOI] [Google Scholar]
- 8.Orman MA, Brynildsen MP. 2015. Inhibition of stationary phase respiration impairs persister formation in E. coli. Nat Commun 6:7983. doi: 10.1038/ncomms8983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maisonneuve E, Gerdes K. 2014. Molecular mechanisms underlying bacterial persisters. Cell 157:539–548. doi: 10.1016/j.cell.2014.02.050. [DOI] [PubMed] [Google Scholar]
- 10.Sarathy J, Dartois V, Dick T, Gengenbacher M. 2013. Reduced drug uptake in phenotypically resistant nutrient-starved nonreplicating Mycobacterium tuberculosis. Antimicrob Agents Chemother 57:1648–1653. doi: 10.1128/AAC.02202-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allison KR, Brynildsen MP, Collins JJ. 2011. Metabolite-enabled eradication of bacterial persisters by aminoglycosides. Nature 473:216–220. doi: 10.1038/nature10069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Morones-Ramirez JR, Winkler JA, Spina CS, Collins JJ. 2013. Silver enhances antibiotic activity against gram-negative bacteria. Sci Transl Med 5:190ra181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwa A, Kasiakou SK, Tam VH, Falagas ME. 2007. Polymyxin B: similarities to and differences from colistin (polymyxin E). Expert Rev Anti Infect Ther 5:811–821. doi: 10.1586/14787210.5.5.811. [DOI] [PubMed] [Google Scholar]
- 14.Katz M, Tsubery H, Kolusheva S, Shames A, Fridkin M, Jelinek R. 2003. Lipid binding and membrane penetration of polymyxin B derivatives studied in a biomimetic vesicle system. Biochem J 375:405–413. doi: 10.1042/bj20030784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sandoval R, Leiser J, Molitoris BA. 1998. Aminoglycoside antibiotics traffic to the Golgi complex in LLC-PK1 cells. J Am Soc Nephrol 9:167–174. [DOI] [PubMed] [Google Scholar]
- 16.Hung CS, Dodson KW, Hultgren SJ. 2009. A murine model of urinary tract infection. Nat Protoc 4:1230–1243. doi: 10.1038/nprot.2009.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.DocoboPérez F, Nordmann P, Domínguez-Herrera J, López-Rojas R, Smani Y, Poirel L, Pachón J. 2012. Efficacies of colistin and tigecycline in mice with experimental pneumonia due to NDM-1-producing strains of Klebsiella pneumoniae and Escherichia coli. Int J Antimicrob Agents 39:251–254. doi: 10.1016/j.ijantimicag.2011.10.012. [DOI] [PubMed] [Google Scholar]
- 18.Zeiler HJ. 1985. Evaluation of the in vitro bactericidal action of ciprofloxacin on cells of Escherichia coli in the logarithmic and stationary phases of growth. Antimicrob Agents Chemother 28:524–527. doi: 10.1128/AAC.28.4.524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niu H, Cui P, Shi W, Zhang S, Feng J, Wang Y, Sullivan D, Zhang W, Zhu B, Zhang Y. 2015. Identification of anti-persister activity against uropathogenic Escherichia coli from a clinical drug library. Antibiotics 4:179–187. doi: 10.3390/antibiotics4020179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta K, Hooton TM, Naber KG, Wullt B, Colgan R, Miller LG, Moran GJ, Nicolle LE, Raz R, Schaeffer AJ, Soper DE. 2011. International clinical practice guidelines for the treatment of acute uncomplicated cystitis and pyelonephritis in women: a 2010 update by the Infectious Diseases Society of America and the European Society for Microbiology and Infectious Diseases. Clin Infect Dis 52:e103–e120. doi: 10.1093/cid/ciq257. [DOI] [PubMed] [Google Scholar]
- 21.Mulvey MA, Schilling JD, Martinez JJ, Hultgren SJ. 2000. Bad bugs and beleaguered bladders: interplay between uropathogenic Escherichia coli and innate host defenses. Proc Natl Acad Sci U S A 97:8829–8835. doi: 10.1073/pnas.97.16.8829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bialvaei AZ, Samadi Kafil H. 2015. Colistin, mechanisms and prevalence of resistance. Curr Med Res Opin 31:707–721. doi: 10.1185/03007995.2015.1018989. [DOI] [PubMed] [Google Scholar]
- 23.Pogue JM, Marchaim D, Kaye D, Kaye KS. 2011. Revisiting “older” antimicrobials in the era of multidrug resistance. Pharmacotherapy 31:912–921. doi: 10.1592/phco.31.9.912. [DOI] [PubMed] [Google Scholar]
- 24.Wareham DW, Bean DC. 2006. In-vitro activity of polymyxin B in combination with imipenem, rifampicin and azithromycin versus multidrug resistant strains of Acinetobacter baumannii producing OXA-23 carbapenemases. Ann Clin Microbiol Antimicrob 5:10. doi: 10.1186/1476-0711-5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ceccarelli G, Oliva A, d'Ettorre G, D'Abramo A, Caresta E, Barbara C, Mascellino M, Papoff P, Moretti C, Vullo V, Visca P, Venditti M. 2015. The role of vancomycin in addition with colistin and meropenem against colistin-sensitive multidrug resistant Acinetobacter baumannii causing severe infections in a paediatric intensive care unit. BMC Infect Dis 15:393. doi: 10.1186/s12879-015-1133-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Corvec S, Furustrand Tafin U, Betrisey B, Borens O, Trampuz A. 2013. Activities of fosfomycin, tigecycline, colistin, and gentamicin against extended-spectrum-β-lactamase-producing Escherichia coli in a foreign-body infection model. Antimicrob Agents Chemother 57:1421–1427. doi: 10.1128/AAC.01718-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.