Abstract
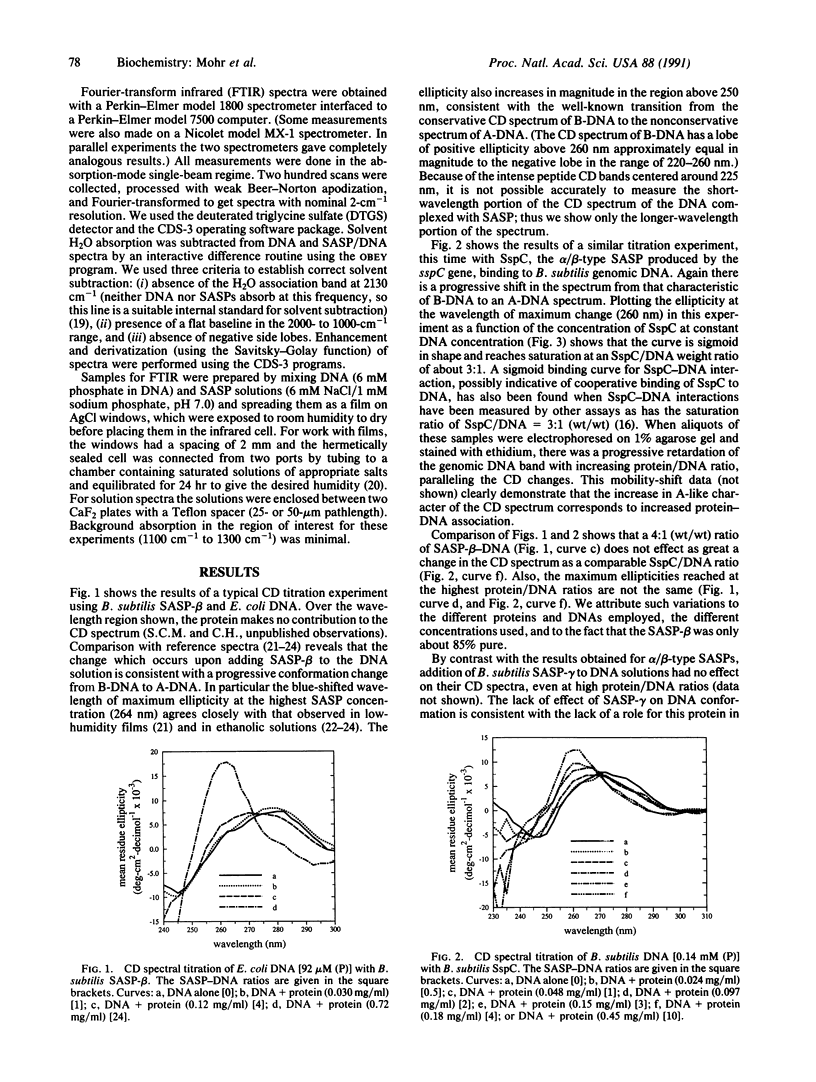
Small acid-soluble spore proteins (SASPs) appear 3-4 hr after the onset of sporulation in Gram-positive bacteria and constitute up to 20% of the protein of mature spores. Previous studies using Bacillus subtilis deletion mutants lacking SASP-alpha and -beta have shown that such mutations abolish the elevated resistance of spores to UV radiation. Analyses using circular dichroism and Fourier-transform infrared spectroscopy now demonstrate that binding alpha/beta-type SASPs to DNA in vitro causes a structural change in DNA, from the B to the A conformation. This may provide the basis whereby alpha/beta-type SASPs confer increased spore UV resistance in vivo--by changing spore DNA conformation, they alter DNA photochemistry such that UV irradiation produces spore photoproduct instead of the more lethal cyclobutane-type thymine dimers.
Full text
PDF
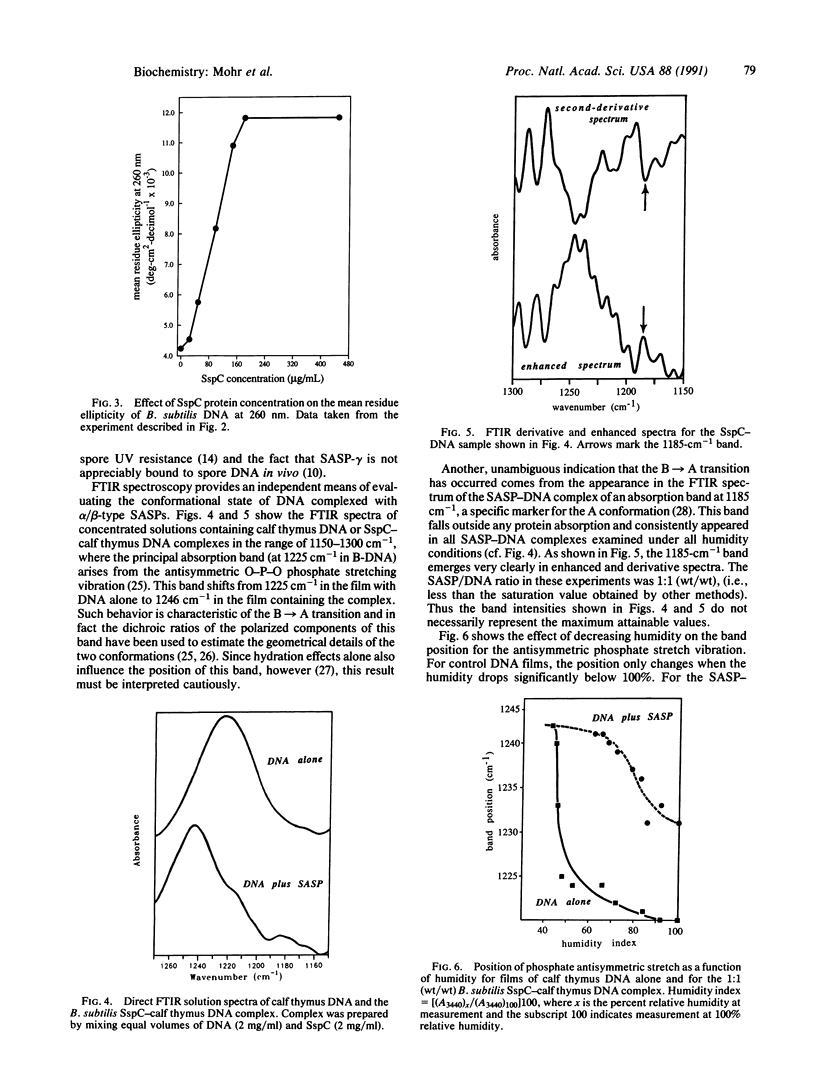

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
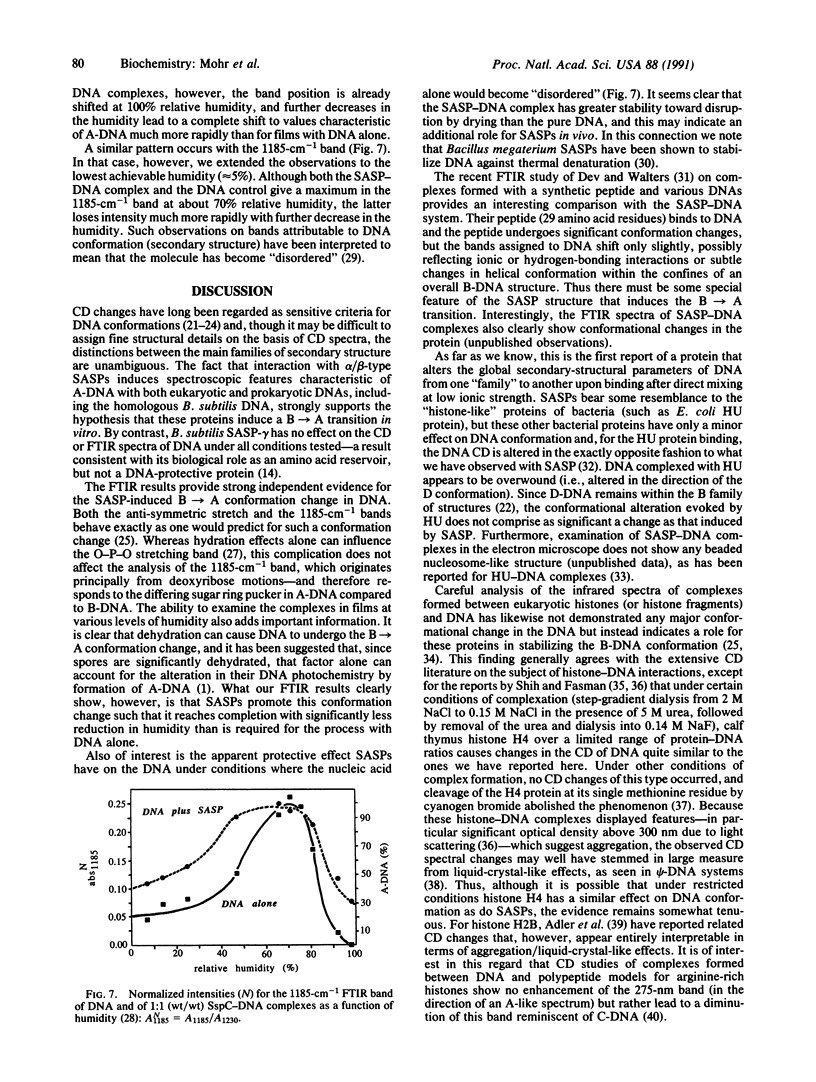
- Adler A. J., Fulmer A. W., Fasman G. D. Interaction of histone f2al fragments with deoxyribonucleic acid. Circular dichroism and thermal denaturation studies. Biochemistry. 1975 Apr 8;14(7):1445–1454. doi: 10.1021/bi00678a015. [DOI] [PubMed] [Google Scholar]
- Adler A. J., Ross D. G., Chen K., Stafford P. A., Woiszwillo M. J., Fasman G. D. Interaction of deoxyribonucleic acid with histone f2b and its half-molecules. Circular dichroism studies. Biochemistry. 1974 Jan 29;13(3):616–623. doi: 10.1021/bi00700a033. [DOI] [PubMed] [Google Scholar]
- Altschmied L., Hillen W. TET repressor.tet operator complex formation induces conformational changes in the tet operator DNA. Nucleic Acids Res. 1984 Feb 24;12(4):2171–2180. doi: 10.1093/nar/12.4.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becker M. M., Wang Z. Origin of ultraviolet damage in DNA. J Mol Biol. 1989 Dec 5;210(3):429–438. doi: 10.1016/0022-2836(89)90120-4. [DOI] [PubMed] [Google Scholar]
- Blazy B., Culard F., Maurizot J. C. Interaction between the cyclic AMP receptor protein and DNA. Conformational studies. J Mol Biol. 1987 May 5;195(1):175–183. doi: 10.1016/0022-2836(87)90334-2. [DOI] [PubMed] [Google Scholar]
- Broyles S. S., Pettijohn D. E. Interaction of the Escherichia coli HU protein with DNA. Evidence for formation of nucleosome-like structures with altered DNA helical pitch. J Mol Biol. 1986 Jan 5;187(1):47–60. doi: 10.1016/0022-2836(86)90405-5. [DOI] [PubMed] [Google Scholar]
- Butler A. P., Revzin A., von Hippel P. H. Molecular parameters characterizing the interaction of Escherichia coli lac repressor with non-operator DNA and inducer. Biochemistry. 1977 Nov 1;16(22):4757–4768. doi: 10.1021/bi00641a001. [DOI] [PubMed] [Google Scholar]
- Culard F., Maurizot J. C. Lac repressor - lac operator interaction. Circular dichroism study. Nucleic Acids Res. 1981 Oct 10;9(19):5175–5184. doi: 10.1093/nar/9.19.5175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dev S. B., Walters L. Fourier transform infrared spectroscopy for the characterization of a model peptide-DNA interaction. Biopolymers. 1990 Jan;29(1):289–299. doi: 10.1002/bip.360290131. [DOI] [PubMed] [Google Scholar]
- Donnellan J. E., Jr, Setlow R. B. Thymine Photoproducts but not Thymine Dimers Found in Ultraviolet-Irradiated Bacterial Spores. Science. 1965 Jul 16;149(3681):308–310. doi: 10.1126/science.149.3681.308. [DOI] [PubMed] [Google Scholar]
- Durand M., Maurizot J. C. Interaction of lac repressor with alternating poly d (A-T) and poly d (G-C). Circular dichroism studies. Biochimie. 1980;62(7):503–507. doi: 10.1016/s0300-9084(80)80070-8. [DOI] [PubMed] [Google Scholar]
- Fairall L., Martin S., Rhodes D. The DNA binding site of the Xenopus transcription factor IIIA has a non-B-form structure. EMBO J. 1989 Jun;8(6):1809–1817. doi: 10.1002/j.1460-2075.1989.tb03575.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray D. M., Ratliff R. L. Circular dichroism spectra of poly[d(AC):d(GT)], poly[r(AC):r(GU)], and hybrids poly[d(AC):r(GU)] and poly[r(AC):d(GT)] in the presence of ethanol. Biopolymers. 1975 Mar;14(3):487–498. doi: 10.1002/bip.1975.360140305. [DOI] [PubMed] [Google Scholar]
- Hackett R. H., Setlow P. Properties of spores of Bacillus subtilis strains which lack the major small, acid-soluble protein. J Bacteriol. 1988 Mar;170(3):1403–1404. doi: 10.1128/jb.170.3.1403-1404.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huey R., Mohr S. C. Condensed states of nucleic acids. III. psi(+) and psi(-) conformational transitions of DNA induced by ethanol and salt. Biopolymers. 1981 Dec;20(12):2533–2552. doi: 10.1002/bip.1981.360201205. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Schyolkina A. K., Poletayev A. I. Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism. Biopolymers. 1973;12(1):89–110. doi: 10.1002/bip.1973.360120109. [DOI] [PubMed] [Google Scholar]
- Johnson W. C., Tipper D. J. Acid-soluble spore proteins of Bacillus subtilis. J Bacteriol. 1981 Jun;146(3):972–982. doi: 10.1128/jb.146.3.972-982.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirpichnikov M. P., Yartzev A. P., Minchenkova L. E., Chernov B. K., Ivanov V. I. The absence of non-local conformational changes in OR3 operator DNA on complexing with the cro repressor. J Biomol Struct Dyn. 1985 Dec;3(3):529–536. doi: 10.1080/07391102.1985.10508440. [DOI] [PubMed] [Google Scholar]
- Mason J. M., Hackett R. H., Setlow P. Regulation of expression of genes coding for small, acid-soluble proteins of Bacillus subtilis spores: studies using lacZ gene fusions. J Bacteriol. 1988 Jan;170(1):239–244. doi: 10.1128/jb.170.1.239-244.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mason J. M., Setlow P. Essential role of small, acid-soluble spore proteins in resistance of Bacillus subtilis spores to UV light. J Bacteriol. 1986 Jul;167(1):174–178. doi: 10.1128/jb.167.1.174-178.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata N., Rupert C. S. Genetically controlled removal of "spore photoproduct" from deoxyribonucleic acid of ultraviolet-irradiated Bacillus subtilis spores. J Bacteriol. 1972 Jul;111(1):192–198. doi: 10.1128/jb.111.1.192-198.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicholson W. L., Setlow P. Dramatic increase in negative superhelicity of plasmid DNA in the forespore compartment of sporulating cells of Bacillus subtilis. J Bacteriol. 1990 Jan;172(1):7–14. doi: 10.1128/jb.172.1.7-14.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panou-Pomonis E., Sakarellos C., Sakarellos-Daitsiotis M. Circular dichroism studies on chromatin models. Interactions between DNA and sequential polypeptides containing arginine. Eur J Biochem. 1986 Nov 17;161(1):185–190. doi: 10.1111/j.1432-1033.1986.tb10140.x. [DOI] [PubMed] [Google Scholar]
- Patrick M. H., Gray D. M. Independence of photoproduct formation on DNA conformation. Photochem Photobiol. 1976 Dec;24(6):507–513. doi: 10.1111/j.1751-1097.1976.tb06867.x. [DOI] [PubMed] [Google Scholar]
- Pohle W., Fritzsche H. A new conformation-specific infrared band of A-DNA in films. Nucleic Acids Res. 1980 Jun 11;8(11):2527–2535. doi: 10.1093/nar/8.11.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pohle W., Zhurkin V. B., Fritzsche H. The DNA phosphate orientation. Infrared data and energetically favorable structures. Biopolymers. 1984 Nov;23(11 Pt 2):2603–2622. doi: 10.1002/bip.360231131. [DOI] [PubMed] [Google Scholar]
- Rahn R. O., Hosszu H. L. Influence of relative humidity on the photochemistry of DNA films. Biochim Biophys Acta. 1969 Sep 17;190(1):126–131. doi: 10.1016/0005-2787(69)90161-0. [DOI] [PubMed] [Google Scholar]
- Rambler M. B., Margulis L. Bacterial resistance to ultraviolet irradiation under anaerobiosis: implications for pre-phanerozoic evolution. Science. 1980 Nov 7;210(4470):638–640. doi: 10.1126/science.7001626. [DOI] [PubMed] [Google Scholar]
- Rouvière-Yaniv J., Yaniv M., Germond J. E. E. coli DNA binding protein HU forms nucleosomelike structure with circular double-stranded DNA. Cell. 1979 Jun;17(2):265–274. doi: 10.1016/0092-8674(79)90152-1. [DOI] [PubMed] [Google Scholar]
- Schnarr M., Durand M., Maurizot J. C. Nonspecific interaction of the lac repressor headpiece with deoxyribonucleic acid: fluorescence and circular dichroism studies. Biochemistry. 1983 Jul 19;22(15):3563–3570. doi: 10.1021/bi00284a005. [DOI] [PubMed] [Google Scholar]
- Setlow P. Purification and properties of some unique low molecular weight basic proteins degraded during germination of Bacillus megaterium spores. J Biol Chem. 1975 Oct 25;250(20):8168–8173. [PubMed] [Google Scholar]
- Setlow P. Small, acid-soluble spore proteins of Bacillus species: structure, synthesis, genetics, function, and degradation. Annu Rev Microbiol. 1988;42:319–338. doi: 10.1146/annurev.mi.42.100188.001535. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Fasman G. D. Circular dichroism studies of deoxyribonucleic acid complexes with arginine-rich histone IV (f2al). Biochemistry. 1971 Apr 27;10(9):1675–1683. doi: 10.1021/bi00785a027. [DOI] [PubMed] [Google Scholar]
- Shih T. Y., Fasman G. D. Circular dichroism studies of histone-deoxyribonucleic acid complexes. A comparison of complexes with histone I (f-1), histone IV (f2al), and their mixtures. Biochemistry. 1972 Feb 1;11(3):398–404. doi: 10.1021/bi00753a016. [DOI] [PubMed] [Google Scholar]
- Smith P. K., Krohn R. I., Hermanson G. T., Mallia A. K., Gartner F. H., Provenzano M. D., Fujimoto E. K., Goeke N. M., Olson B. J., Klenk D. C. Measurement of protein using bicinchoninic acid. Anal Biochem. 1985 Oct;150(1):76–85. doi: 10.1016/0003-2697(85)90442-7. [DOI] [PubMed] [Google Scholar]
- Stafford R. S., Donnellan J. E., Jr Photochemical evidence for conformation changes in DNA during germination of bacterial spores. Proc Natl Acad Sci U S A. 1968 Mar;59(3):822–828. doi: 10.1073/pnas.59.3.822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taillandier E., Taboury J., Liquier J., Gadenne M. C., Champagne M., Brahms J. Particular structural role of H1 in complexes with DNA and comparison with H2A- and H4-DNA complexes investigated by IR linear dichroism. Biopolymers. 1979 Aug;18(8):1877–1888. doi: 10.1002/bip.1979.360180805. [DOI] [PubMed] [Google Scholar]
- Tunis-Schneider M. J., Maestre M. F. Circular dichroism spectra of oriented and unoriented deoxyribonucleic acid films--a preliminary study. J Mol Biol. 1970 Sep 28;52(3):521–541. doi: 10.1016/0022-2836(70)90417-1. [DOI] [PubMed] [Google Scholar]
- Varghese A. J. 5-Thyminyl-5,6-dihydrothymine from DNA irradiated with ultraviolet light. Biochem Biophys Res Commun. 1970 Feb 6;38(3):484–490. doi: 10.1016/0006-291x(70)90739-4. [DOI] [PubMed] [Google Scholar]
- Wartell R. M., Adhya S. DNA conformational change in Gal repressor-operator complex: involvement of central G-C base pair(s) of dyad symmetry. Nucleic Acids Res. 1988 Dec 23;16(24):11531–11541. doi: 10.1093/nar/16.24.11531. [DOI] [PMC free article] [PubMed] [Google Scholar]



