Abstract
Twenty participants undergoing elective cataract surgery received 1% voriconazole eye drops (1 drop per eye) either 20, 40, 60, or 80 min before surgery. Median voriconazole concentrations of 1.9 to 3.2 mg/liter in aqueous humor samples were attained over the first 80 min, which were higher than in vitro MIC90 values for typical fungi that cause keratitis.
TEXT
Fungal keratitis remains one of the most difficult-to-treat corneal infections and accounts for approximately 50% of infectious keratitis cases in developing countries (1). The filamentous fungi Aspergillus and Fusarium spp. are the two most frequently encountered causative pathogens (2).
Voriconazole is increasingly used as a first-line treatment for ocular fungal infections (3) given its excellent in vitro activity against a wide variety of keratitis isolates (4), including less common fungi (e.g., Curvularia and Acremonium spp.). Topically administered 1% and 2% voriconazole eye drops have been documented to achieve good intraocular penetration in noninflamed (5, 6) and inflamed eyes (7). However, the voriconazole concentrations observed in the aqueous humor samples of patients receiving 2% voriconazole eye drops (6) were similar to that reported for the 1% voriconazole eye drops (5) when the same dosing frequency was used (i.e., total of four once-hourly doses). This suggests that increasing the concentration of voriconazole eye drops from 1% to 2% does not result in higher voriconazole concentrations in the aqueous humor of noninflamed human eyes. The extent of voriconazole clearance from the eye after topical administration might have been the key factor behind this observation, but this issue has largely remained unexplored, except in a recent ocular kinetic study by Senthilkumari et al. (8); therefore, data on this remain limited. The availability of such data is important for optimizing the dosing of voriconazole eye drops. Accordingly, we investigated the change in voriconazole concentrations in the aqueous humor of human eyes over time after topical administration of 1% voriconazole eye drops.
Between April 2009 and April 2011, participants who were ≥18 years old and were scheduled for elective cataract surgery at the Royal Victorian Eye and Ear Hospital (RVEEH) were recruited. Excluded were subjects with a history of inflammation of the eye (uveitis) to undergo surgery, with a history of kidney or liver failure, who were breastfeeding, pregnant, or trying to conceive, who had an allergy to voriconazole or benzalkonium chloride, or who were using medications known to interact with voriconazole. Written informed consent was obtained from participants prior to enrollment. The study was approved by the ethics committees of RVEEH and Monash University.
One-percent voriconazole eye drops were prepared aseptically from Vfend injections as previously described (6). All eye drops were stable for at least 14 weeks when stored at 2 to 8°C (9). Consenting participants each received a single drop (50 μl) of 1% voriconazole to the eye to undergo surgery 20 min (arm 1, n = 5), 40 min (arm 2, n = 5), 60 min (arm 3, n = 5), or 80 min (arm 4, n = 5) prior to the scheduled surgery (Table 1). The eye drops were administered by the nursing staff. The time of administration and any side effects experienced were recorded.
TABLE 1.
Patient characteristics and voriconazole concentrations in aqueous humors
| Study arm and patient no. (time before surgery) | Sex | Age (yr) | Concn. of voriconazole in aqueous humor (mg/liter) | Postdose sampling time (min) |
|---|---|---|---|---|
| Arm 1 (20 min) | ||||
| 1 | Male | 86 | 4.1 | 22 |
| 2 | Male | 44 | 3.2 | 20 |
| 3 | Female | 71 | 3.4 | 20 |
| 4 | Male | 79 | 1.9 | 25 |
| 5 | Male | 69 | 1.1 | 17 |
| Arm 2 (40 min) | ||||
| 1 | Male | 82 | 2.1 | 52 |
| 2 | Male | 75 | 2.6 | 44 |
| 3 | Male | 66 | 3.1 | 41 |
| 4 | Male | 72 | 3.3 | 39 |
| 5 | Male | 66 | 3.4 | 45 |
| Arm 3 (60 min) | ||||
| 1 | Female | 90 | 5.1 | 56 |
| 2 | Female | 74 | 1.7 | 58 |
| 3 | Male | 72 | 1.9 | 65 |
| 4 | Female | 77 | 3.3 | 55 |
| 5 | Female | 74 | 1.2 | 58 |
| Arm 4 (80 min) | ||||
| 1 | Male | 72 | 0.9 | 87 |
| 2 | Male | 68 | 2.3 | 80 |
| 3 | Male | 52 | 2.0 | 80 |
| 4 | Female | 51 | 2.0 | 78 |
| 5 | Female | 77 | 0.9 | 95 |
During surgery, 50 to 150 μl of aqueous humor was collected from each participant through a paracentesis site using a 30-gauge needle before proceeding with routine cataract surgery. The samples were then stored at 4°C and analyzed within 1 week using a validated high-performance liquid chromatography assay to determine the concentrations of voriconazole (5).
The median (range) voriconazole concentrations in the aqueous humor samples were 3.2 (1.1 to 4.1), 3.1 (2.1 to 3.4), 1.9 (1.2 to 5.1), and 2.0 (0.9 to 2.3) mg/liter, at 20, 40, 60, and 80 min, respectively, after voriconazole eye drop administrations (Table 1). The median (range) postdose sampling times were 20 (17 to 25), 44 (39 to 52), 58 (55 to 65), and 80 (78 to 95) min after voriconazole eye drop administration. The calculated elimination rate constant (ke) was 0.47 h−1, with an elimination half-life (t1/2) of 1.47 h (Fig. 1). The drops were generally well tolerated.
FIG 1.
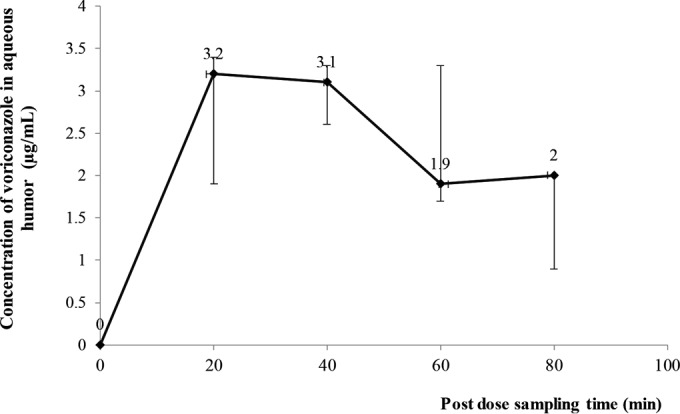
Median voriconazole aqueous humor concentration-time profile following a single 1% voriconazole eye drop. The elimination rate constant (ke) and elimination half-life (t1/2) were calculated using the following formula for first-order elimination (14): ke = −2.303[log(C2) − log(C1)]/(t2 − t1), such that t1/2 = 0.693/ke. The upper caps of the vertical black lines indicate third-quartile values, whereas the lower caps indicate first-quartile values.
In this study, the median voriconazole concentrations in the aqueous humor samples peaked between 20 (3.2 mg/liter) and 40 (3.1 mg/liter) min (Fig. 1), consistent with the findings from a single-dose kinetic study of topical 1% voriconazole by Senthilkumari et al. (8), in which the maximum mean voriconazole concentration in aqueous humor samples was 3.33 ± 1.61 mg/liter at 30 min (8). In addition, the authors of that study observed that increasing the concentration of voriconazole eye drops from 0.1% to 1% did not result in a 10-fold increase in the mean voriconazole concentrations in aqueous humor (8). Their data suggest nonlinear corneal absorption of voriconazole, which is consistent with the results of our previous studies that showed that the penetration of voriconazole via an intact cornea is unlikely to be concentration dependent for a concentration range between 1% and 2% (5, 6). The ocular kinetics of topical voriconazole seem to reflect the nonlinearity of the voriconazole pharmacokinetics profile when administered systemically (10). In addition, our results show a slower decline in voriconazole aqueous humor concentrations than was previously reported (elimination t1/2 = 0.82 h) by Senthilkumari et al. (8), who adopted a one-compartment model.
The findings from this study are consistent with those reported from previous studies (5, 11), specifically, that 1% voriconazole eye drops afforded voriconazole concentrations in the aqueous humor samples that were above the MIC90 (0.06 to 8 mg/liter) for most fungal species (15). With an estimated elimination half-life (t1/2) of 1.47 h, a dosing regimen of once every 2 h is likely to afford a voriconazole concentration in the aqueous humor that is sufficient to eradicate Aspergillus or Candida keratitis but not Fusarium keratitis (4). Poorer clinical resolution with topical 1% voriconazole has been reported for keratitis caused by Fusarium species in patients (12).
In a study by Lau et al. (5), the mean (±SD) measured voriconazole concentration in aqueous humor samples (1.90 ± 1.12 mg/liter) was at least three times lower than that reported by Vemulakonda et al. (6.49 ± 3.04 mg/liter) (11); both studies used 1% voriconazole eye drops. Drug accumulation from the different dosing frequencies (1 drop every hour for 4 h in the study by Lau et al. [5] versus 1 drop every 2 h for 24 h in the study by Vemulakonda et al. [11]) might explain the differences observed. Indeed, the differences might also be due to the differences in sampling times after the last dose (1.1 h for Lau et al. [5] versus 0.4 h for Vemulakonda et al. [11]). In the study by Senthilkumari et al. (8), a higher mean (±SD) voriconazole concentration of 7.47 ± 2.14 mg/liter was noted despite a multiple-dosing regimen (1 drop every hour for 5 h) and postdose sampling time (at least 1 h after the last dose) similar to those reported by Lau et al. (5). A reason for the observed difference might be the presence of preservative in the voriconazole eye drops used by Lau et al. (5). It has been postulated that the preservative benzalkonium chloride can either induce reflex tears, which causes voriconazole loss from the precorneal tear film, or interact with cyclodextrin derivatives in Vfend, which then decreases the intraocular penetration of voriconazole (8). A recent in vitro study that used freshly excised goat corneas found that the addition of 0.01% (wt/vol) benzalkonium chloride significantly increased transcorneal permeation of the voriconazole eye drops (13).
The voriconazole concentrations achieved in the human aqueous humor samples during the first 80 min after topical administration were consistently higher than the in vitro MIC90 of voriconazole against typical keratitis-causing fungi. Our data suggest that reducing the frequency of administration of voriconazole eye drops from every 1 h to every 2 h for maintenance therapy may be adequate for managing most cases of fungal keratitis but not those caused by Fusarium spp. Treatment with combined topical and systemic administration of voriconazole should be considered for treating keratitis due to less-susceptible fungi (7).
ACKNOWLEDGMENTS
We thank Lachlan Shaw and Catherine Rokahr from the RVEEH for technical and administrative support.
REFERENCES
- 1.Ozbek z, Kang S, Sivalingam J, Rapuano CJ, Cohen EJ, Hammersmith KM. 2006. Voriconazole in the management of Alternaria keratitis. Cornea 25:242–244. doi: 10.1097/01.ico.0000170692.05703.98. [DOI] [PubMed] [Google Scholar]
- 2.Thomas PA. 2003. Fungal infections of the cornea. Eye (Lond) 17:852–862. doi: 10.1038/sj.eye.6700557. [DOI] [PubMed] [Google Scholar]
- 3.Neoh CF, Daniell M, Chen SC, Stewart K, Kong DC. 2014. Clinical utility of caspofungin eye drops in fungal keratitis. Int J Antimicrob Agents 44:96–104. doi: 10.1016/j.ijantimicag.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 4.Marangon FB, Miller D, Giaconi JA, Alfonso EC. 2004. In vitro investigation of voriconazole susceptibility for keratitis and endophthalmitis fungal pathogens. Am J Ophthalmol 137:820–825. doi: 10.1016/j.ajo.2003.11.078. [DOI] [PubMed] [Google Scholar]
- 5.Lau D, Ferdinands M, Leung L, Fullinfaw R, Kong D, Davies G, Daniell M. 2008. Penetration of voriconazole, 1%, eyedrops into human aqueous humor: a prospective open-label study. Arch Ophthalmol 126:343–346. doi: 10.1001/archophthalmol.2007.71. [DOI] [PubMed] [Google Scholar]
- 6.Al-Badriyeh D, Leung L, Roydhouse T, Fullinfaw R, Daniell M, Davies GE, Stewart K, Kong DC. 2009. Prospective open-label study of the administration of two-percent voriconazole eye drops. Antimicrob Agents Chemother 53:3153–3155. doi: 10.1128/AAC.01700-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Thiel MA, zinkernagel AS, Burhenne J, Kaufmann C, Haefeli WE. 2007. Voriconazole concentration in human aqueous humor and plasma during topical or combined topical and systemic administration for fungal keratitis. Antimicrob Agents Chemother 51:239–244. doi: 10.1128/AAC.00762-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Senthilkumari S, Lalitha P, Prajna NV, Haripriya A, Nirmal J, Gupta P, Velpandian T. 2010. Single and multidose ocular kinetics and stability analysis of extemporaneous formulation of topical voriconazole in humans. Curr Eye Res 35:953–960. doi: 10.3109/02713683.2010.506968. [DOI] [PubMed] [Google Scholar]
- 9.Al-Badriyeh D, Li J, Stewart K, Kong DC, Leung L, Davies GE, Fullinfaw R. 2009. Stability of extemporaneously prepared voriconazole ophthalmic solution. Am J Health Syst Pharm 66:1478–1483. doi: 10.2146/ajhp080110. [DOI] [PubMed] [Google Scholar]
- 10.Al-Badriyeh D, Heng SC, Neoh CF, Slavin M, Stewart K, Kong DC. 2010. Pharmacoeconomics of voriconazole in the management of invasive fungal infections. Expert Rev Pharmacoecon Outcomes Res 10:623–636. doi: 10.1586/erp.10.69. [DOI] [PubMed] [Google Scholar]
- 11.Vemulakonda GA, Hariprasad SM, Mieler WF, Prince RA, Shah GK, Van Gelder RN. 2008. Aqueous and vitreous concentrations following topical administration of 1% voriconazole in humans. Arch Ophthalmol 126:18–22. doi: 10.1001/archophthalmol.2007.8. [DOI] [PubMed] [Google Scholar]
- 12.Sharma S, Das S, Virdi A, Fernandes M, Sahu SK, Kumar Koday N, Ali MH, Garg P, Motukupally SR. 2015. Reappraisal of topical 1% voriconazole and 5% natamycin in the treatment of fungal keratitis in a randomised trial. Br J Ophthalmol 99:1190–1195. doi: 10.1136/bjophthalmol-2014-306485. [DOI] [PubMed] [Google Scholar]
- 13.Malhotra S, Khare A, Grover K, Singh I, Pawar P. 2014. Design and evaluation of voriconazole eye drops for the treatment of fungal keratitis. J Pharm (Cairo) 2014:490595. doi: 10.1155/2014/490595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shargel L, Wu-Pong S, Yu A. 2012. Applied Biopharmaceutics and Pharmacokinetics, 6th ed McGraw-Hill Companies, Inc., New York, NY. [Google Scholar]
- 15.Gopinathan U, Garg P, Fernandes M, Sharma S, Athmanathan S, Rao GN. 2002. The epidemiological features and laboratory results of fungal keratitis: a 10-year review at a referral eye care center in South India. Cornea 21:555–559. [DOI] [PubMed] [Google Scholar]


