Abstract
Polymyxin B is increasingly used as a treatment of last resort for multidrug-resistant Gram-negative infections. Despite being available as a mixture of several structurally related analogues, the properties are commonly reported as an aggregate of the individual components. We compared the pharmacokinetics of individual polymyxin B components in an animal model and in humans. There were no considerable differences observed in the pharmacokinetics among major components of polymyxin B. Combining different components for pharmacokinetic analysis appeared reasonable.
TEXT
Polymyxin B has emerged as the drug of last resort for multidrug-resistant (MDR) Gram-negative infections (1, 2). Polymyxin B is a cationic, polypeptide antibiotic, commercially available as a mixture of closely related cyclic structural analogues. The major components of the mixture are polymyxin B1, B2, B3, and isoleucine B1 (3). Although available as a mixture, the pharmacological/toxicological properties of polymyxin B are commonly reported as an aggregate of the individual components (4, 5). Our previous work reported that there was no significant difference in in vitro potencies of various components against several clinical isolates of MDR bacteria (6). Despite being used clinically for decades, there is still a significant knowledge gap in the current understanding of its pharmacokinetic properties. A fundamental question remains as to whether these components have similar pharmacokinetic characteristics (7). Therefore, in this study we compared the pharmacokinetic profiles of different polymyxin B components in an animal model as well as in human subjects.
Polymyxin B (USP) was purchased from Sigma-Aldrich (St. Louis, MO) and X-GEN Pharmaceuticals, Inc. (Northport, NY). Blank human and rat sera were obtained from Equitech-Bio, Inc. (Kerrville, TX). Carbutamide was purchased from Aldrich (Milwaukee, WI). Liquid chromatography-mass spectrometry (LC-MS)-grade acetonitrile and water were obtained from Mallinckrodt Baker (Philipsburg, NJ). LC-MS-grade formic acid was purchased from Fluka Analytical (St. Louis, MO).
A total of 17 female Sprague-Dawley rats (225 to 250 g) (Harlan, Indianapolis, IN) were used. The animals received food and water ad libitum. Jugular veins were cannulated to facilitate intravenous drug administration. All animals were cared for in accordance with the highest humane and ethical standards, as approved by the Institutional Animal Care and Use Committee (IACUC) of the University of Houston. Prior to each experiment, polymyxin B powder was dissolved in sterile water for injection and diluted to the desired concentration. The animals were administered a single dose of polymyxin B (3 mg/kg of body weight as a slow intravenous bolus dose over 10 min). Blood samples were obtained via jugular vein cannula or cardiac puncture (if it was a terminal sacrifice) at 1, 1.5, 3, 4.5, 6, and 7.5 h postdose and allowed to clot on ice. Serum samples were obtained by centrifugation (6,000 × g for 15 min at 4°C) and stored at −80°C until drug analysis. The average concentration at each time point was used for pharmacokinetic analysis.
Two patients with suspected/documented Gram-negative bacterial infections given polymyxin B were also examined. The first patient was a 61-year-old male (body weight, 64 kg; estimated creatinine clearance, 50 ml/min) given 1.5 mg/kg of polymyxin B based on actual body weight. The second patient was a 73-year-old male (112 kg; estimated creatinine clearance, 81 ml/min) given 2.2 mg/kg of polymyxin B. In both cases, polymyxin B was administered as daily intravenous infusions over 3 h. After written informed consent was obtained, four serum samples were obtained from each patient at steady state over the fourth dosing interval (prior to dosing, 2 h postdose, 8 to 12 h postdose, and before the next dose) to determine the concentrations of major polymyxin B components. The study was approved by the Institutional Review Boards (IRBs) of the New York University Langone Medical Center (New York, NY) and the University of Houston.
A validated ultraperformance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS) method was used to determine the concentrations of polymyxin B in rat and human sera (8). The serum concentrations of each component were quantified individually and subsequently reported collectively as the total polymyxin B concentration. The pharmacokinetics of polymyxin B were derived using two different approaches. In the first approach, we used the total polymyxin B concentrations in serum. In contrast, the concentration-time profiles of each individual polymyxin B component were used in the second approach. The dose fractions of the individual components were based on their relative abundance in the USP mixture (previously estimated to be 0.612, 0.254, 0.056, and 0.077 for polymyxins B1, B2, B3, and isoleucine B1, respectively). In both scenarios, a one-compartment linear model with a zero-order input was used to fit the concentration-time profiles using ADAPT 5 (University of Southern California, Los Angeles, CA).
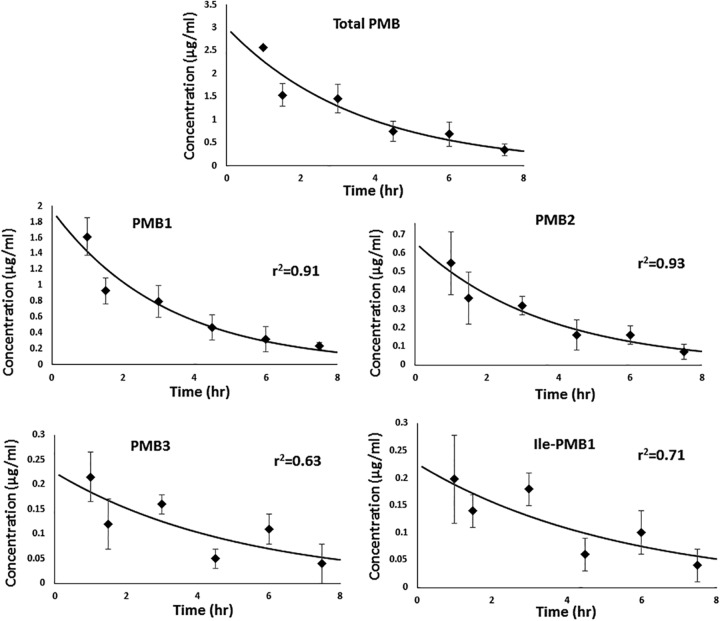
For the rats, a median of 4 samples (range, 2 to 8) were obtained at each time point. The overall model fit to the data was satisfactory, as shown in Fig. 1. In addition, the best-fit pharmacokinetic parameters are shown in Table 1. Strictly speaking, the pharmacokinetic parameters (e.g., clearance and volume of distribution) of the individual components were not identical, but most the differences were within a 2-fold range. As indicated in Table 1, the half-life of each component was in close agreement since half-life was attributed to the rate (ratio) of decline and not directly dependent on the absolute dose values. On the contrary, the volume of distribution was more variable among the components, which could be a manifestation of batch-to-batch variation in the dose proportion of components. The weighted average pharmacokinetic parameters using individual components were in reasonably close agreement with those derived directly from the total polymyxin B concentrations. Moreover, for the rats, the area under the concentration-time curve from 0 h to infinity (AUC0–∞) observed for total polymyxin B was 10.7 mg h liter−1, which was comparable to that derived from adding the AUC0–∞ of individual polymyxin B components in the mixture (10.9 mg h liter−1). A similar trend was also observed in the patient samples, as shown in Table 1. The areas under the concentration-time curve from 0 to 24 h at steady state (AUC0–24 ss) for total polymyxin B for patient 1A and patient 1D observed were 48.2 and 64.5 mg h liter−1, respectively. These values were comparable to the summed AUC0–24 ss of individual polymyxin B components in the USP mixture (48.0 and 64.6 mg h liter−1, respectively).
FIG 1.
Model fitting of total polymyxin B (PMB) and individual components. The data points represent the average serum concentrations at each time point; error bars depict the standard deviations, and solid lines represent the best-fit lines.
TABLE 1.
Comparison of the best-fit pharmacokinetic parameters for rats and patients 1A and 1D
| Parametera | Result for rats or patientb |
|||||
|---|---|---|---|---|---|---|
| CL for rats (liters/kg/h) or patient (liters/h) | V (liters/kg) | t1/2 (h) | kel (h−1) | AUC0–∞ for rats or AUC0–24 ss for patient (mg h liter−1) | r2 | |
| Rats | ||||||
| Total PMB | 0.28 | 1.00 | 2.47 | 0.28 | 10.71 | 0.90 |
| PMB1 | 0.30 | 0.95 | 2.21 | 0.31 | 6.17 | 0.91 |
| PMB2 | 0.32 | 1.16 | 2.50 | 0.28 | 2.37 | 0.93 |
| PMB3 | 0.15 | 0.75 | 3.59 | 0.19 | 1.16 | 0.63 |
| Ile-PMB1 | 0.19 | 1.02 | 3.76 | 0.18 | 1.23 | 0.71 |
| Weighted avg | 0.29 | 1.00 | 2.48 | 0.29 | ||
| Sum of individual components | 10.93 | |||||
| Patient 1A | ||||||
| Total PMB | 1.97 | 33.01 | 11.61 | 0.06 | 48.22 | 0.96 |
| PMB1 | 1.90 | 31.35 | 11.46 | 0.06 | 30.66 | 0.95 |
| PMB2 | 4.20 | 74.16 | 12.25 | 0.06 | 5.76 | 0.90 |
| PMB3 | 0.90 | 15.87 | 12.16 | 0.06 | 5.91 | 0.99 |
| Ile-PMB1 | 1.31 | 21.76 | 11.56 | 0.06 | 5.61 | 0.85 |
| Weighted avg | 2.38 | 40.62 | 11.70 | 0.06 | ||
| Sum of individual components | 47.95 | |||||
| Patient 1D | ||||||
| Total PMB | 3.72 | 61.16 | 11.39 | 0.06 | 64.46 | 0.96 |
| PMB1 | 3.55 | 58.93 | 11.51 | 0.06 | 41.37 | 0.96 |
| PMB2 | 7.96 | 105.00 | 9.15 | 0.08 | 7.67 | 0.94 |
| PMB3 | 1.81 | 32.55 | 12.44 | 0.06 | 7.45 | 1.00 |
| Ile-PMB1 | 2.28 | 41.72 | 12.68 | 0.05 | 8.11 | 0.90 |
| Weighted avg | 4.47 | 67.82 | 11.05 | 0.06 | ||
| Sum of individual components | 64.61 | |||||
PMB, polymyxin B. In each case, the weighted average is based on the relative abundance of different polymyxin B components.
CL, clearance; V, volume of distribution; kel, elimination rate constant; t1/2, elimination half-life; AUC0–∞, area under the concentration-time curve from 0 h to infinity; AUC0–24 ss, area under the concentration-time curve over 24 h at steady state.
To our knowledge, this is the first study providing specific insights into the comparative pharmacokinetic profiling of individual polymyxin B components. In addition to studying single-dose pharmacokinetics in experimental animals, we have also provided data from humans at steady state to strengthen our observations. Furthermore, two different approaches to derive total AUC were compared. Potential limitations of this study included a small number of subjects examined and the potential batch-to-batch variation among products from different manufacturers. Our results in rats and human subjects indicated the pharmacokinetics of the individual components were not considerably different from each other. Our laboratory has previously reported that AUC/MIC ratio of polymyxin B was the pharmacokinetic/pharmacodynamic index most closely linked to bactericidal activity (9). Taking various study/experimental variations (e.g., sampling, interbatch relative abundance or assay variance) into consideration, it appeared reasonable to use the summed concentrations of individual components (i.e., total polymyxin B concentration) in pharmacokinetic studies to estimate overall drug exposure of polymyxin B.
Funding Statement
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
REFERENCES
- 1.Curcio D. 2014. Multidrug-resistant Gram-negative bacterial infections: are you ready for the challenge? Curr Clin Pharmacol 9:27–38. doi: 10.2174/15748847113089990062. [DOI] [PubMed] [Google Scholar]
- 2.Vergidis PI, Falagas ME. 2008. Multidrug-resistant Gram-negative bacterial infections: the emerging threat and potential novel treatment options. Curr Opin Investig Drugs 9:176–183. [PubMed] [Google Scholar]
- 3.Orwa JA, Govaerts C, Busson R, Roets E, Van Schepdael A, Hoogmartens J. 2001. Isolation and structural characterization of polymyxin B components. J Chromatogr A 912:369–373. doi: 10.1016/S0021-9673(01)00585-4. [DOI] [PubMed] [Google Scholar]
- 4.Abdelraouf K, He J, Ledesma KR, Hu M, Tam VH. 2012. Pharmacokinetics and renal disposition of polymyxin B in an animal model. Antimicrob Agents Chemother 56:5724–5727. doi: 10.1128/AAC.01333-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sandri AM, Landersdorfer CB, Jacob J, Boniatti MM, Dalarosa MG, Falci DR, Behle TF, Bordinhao RC, Wang J, Forrest A, Nation RL, Li J, Zavascki AP. 2013. Population pharmacokinetics of intravenous polymyxin B in critically ill patients: implications for selection of dosage regimens. Clin Infect Dis 57:524–531. doi: 10.1093/cid/cit334. [DOI] [PubMed] [Google Scholar]
- 6.Tam VH, Cao H, Ledesma KR, Hu M. 2011. In vitro potency of various polymyxin B components. Antimicrob Agents Chemother 55:4490–4491. doi: 10.1128/AAC.00119-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tam VH, Hou J, Kwa AL, Prince RA. 2009. Comment on: Development and validation of a reversed-phase high-performance liquid chromatography assay for polymyxin B in human plasma. J Antimicrob Chemother 63:627–629. doi: 10.1093/jac/dkn483. [DOI] [PubMed] [Google Scholar]
- 8.He J, Gao S, Hu M, Chow DS, Tam VH. 2013. A validated ultra-performance liquid chromatography-tandem mass spectrometry method for the quantification of polymyxin B in mouse serum and epithelial lining fluid: application to pharmacokinetic studies. J Antimicrob Chemother 68:1104–1110. doi: 10.1093/jac/dks536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tam VH, Schilling AN, Vo G, Kabbara S, Kwa AL, Wiederhold NP, Lewis RE. 2005. Pharmacodynamics of polymyxin B against Pseudomonas aeruginosa. Antimicrob Agents Chemother 49:3624–3630. doi: 10.1128/AAC.49.9.3624-3630.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]