Abstract
Background
Klebsiella pneumoniae is a bacterial pathogen that has developed resistance to multiple antibiotics and is a major cause of nosocomial infections worldwide. Carbapenemase-producing Klebsiella pneumoniae have been isolated in many hospitals in Venezuela, but they have not been well-studied. The aim of this study was to characterize carbapenem-resistant Klebsiella pneumoniae isolates from the pediatric service of a hospital located in Anzoategui State, in the eastern part of Venezuela.
Methods
Nineteen Klebsiella pneumoniae strains isolated in the hospital from April to July 2014 were evaluated phenotypically and molecularly for the presence of carbapenemases blaKPC, blaIMP and blaVIM. Molecular epidemiology was performed with Repetitive Extragenic Palindromic-PCR (REP-PCR) and Multilocus Sequence Typing (MLST). They were also studied for phenotypic and molecular resistance to a quaternary ammonium compound (QAC) disinfectant.
Results
All 19 isolates contained both bla VIM-2 and bla KPC-2 genes, and the bla KPC-2 gene was associated with Tn4401b. All isolates were phenotypically sensitive to QACs and contained qacΔE and addA2 genes typical of class 1 integrons. Analysis by REP-PCR and MLST showed that all isolates had identical profiles characteristic of sequence type ST833.
Conclusion
All 19 strains are bla VIM-2 and bla KPC-2−producing ST833 K. pneumoniae sensitive to QACs. This analysis may help to understand the routes of dissemination and confirms that QAC disinfectants can be used to help control their spread.
Electronic supplementary material
The online version of this article (doi:10.1186/s12879-016-1927-y) contains supplementary material, which is available to authorized users.
Keywords: Klebsiella pneumoniae, carbapenem, resistance, ST833
Background
The emergence of plasmid-mediated carbapenem hydrolyzing β-lactamases and their spread amongst Gram-negative bacteria, especially Klebsiella pneumoniae, has become a serious threat for hospitalized patients worldwide. The two principal types of acquired carbapenemases are the molecular class B metallo-β-lactamases (MBLs) and the molecular class A K. pneumoniae carbapenemases (KPCs), both of which have been detected in many countries [1–4]. The bla VIM gene encodes a transferable carbapenemase and is generally found within class 1 integrons. Although originally identified in Pseudomonas aeruginosa, VIM enzymes are now endemic within Enterobacteriaceae [5, 6]. The bla KPC genes are usually located within Tn4401 [7], a Tn3-based transposon harbored on plasmids or in the chromosome. In recent years carbapenem-resistant K. pneumoniae has become a frequent cause of nosocomial infections in several hospitals in different Venezuelan cities [8], but the epidemiology, molecular epidemiology, clinical impact and carbapenemase genes of Venezuelan carbapenemase-producing K. pneumoniae have not been described. In this study we characterize 19 carbapenemase-producing K. pneumoniae isolates associated with nosocomial infections centered in the pediatric service of a large Venezuelan tertiary-care public hospital. All 19 isolates contained both bla VIM-2 and bla KPC-2 genes, were phenotypically sensitive to QACs and contained qacΔE and addA2 genes typical of class 1 integrons. Analysis by REP-PCR and MLST showed that all isolates had identical profiles characteristic of sequence type ST833.
Methods
Hospital setting
The “Rafael Tobías Guevara” Pediatrics Department is part of the “Dr. Luis Razetti” Hospital, a large tertiary-care public hospital with 502 beds, located in Anzoátegui State, in the eastern part of Venezuela. The “Rafael Tobías Guevara” Pediatrics Department has 73 beds in the neonatal unit, 3 delivery rooms, 28 beds in the neonatal intermediate care unit and 12 beds in the neonatal intensive care unit. In 2014 nearly 26,000 women gave birth in this hospital, 30 % of whom were adolescents.
Study population
The study was conducted from April to July 2014 and included all carbapenem-resistant Klebsiella pneumoniae strains isolated from pediatric patients hospitalized in the “Rafael Tobías Guevara” Pediatrics Department during this period. If there were more than one isolate from a patient, only the first was included in the study. Information on the source of the clinical specimen, the patient’s age, gender and hospital location was obtained from specimen records in the bacteriology laboratory and made irreversibly anonymous. The Bioethics Commission of the Instituto Venezolano de Investigaciones Científicas determined that the study did not require its approval.
Bacterial strains and antibiotic susceptibility testing
The study included 19 Klebsiella pneumoniae strains isolated from April to July 2014 in the bacteriology laboratory of the “Rafael Tobías Guevara” Pediatrics Department. They all showed intermediate or full resistance to carbapenems according to the Clinical and Laboratory Standards Institute (CLSI) 2014 cutoff points [9]. The broader antimicrobial susceptibility profile of the Klebsiella pneumoniae strains was determined by the broth dilution method according to the CLSI guidelines for the following antibiotics: amikacin, tetracycline, chloramphenicol, amoxicillin/clavulanic acid, ampicillin, ampicillin/sulbactam, cefalotin, cefoxitin, ceftazidime, cefepime, piperacillin, piperacillin/tazobactam, aztreonam, cefoperazone/sulbactam, imipenem, meropenem, ciprofloxacin, and trimethoprim/sulfamethoxazole. Carbapenemase activity in all 19 isolates was confirmed by the Hodge test [9]. The disk diffusion assay with ertapenem and 3-aminophenyl boronic acid (400 μg) was used to confirm the presence of KPC-type β-lactamases [10], and with ertapenem and EDTA to confirm the presence of metallo- β-lactamases in all 19 isolates.
Detection of genes encoding β-lactamases and their genetic environment, integron cassettes and efflux pumps
Bacterial strains were grown on MacConkey agar and incubated overnight at 37 °C. One colony was resuspended in 100 μl of sterile distilled water and the bacteria were lysed by heating at 100 °C for 10 min. Cellular debris was removed by centrifugation at 13,000 g for 10 min and the supernatant was used as template DNA for PCR amplifications [11].
The carbapenemase genes bla KPC [12] bla IMP and bla VIM [13] were amplified by PCR using the primers listed in Additional file 1: Table S1. The genetic environment of the bla KPC genes was determined by PCR using previously described primers [14] specific for the Tn4401 transposon (Additional file 1: Table S1). To detect the qacΔE gene encoding a putative efflux pump often found in class 1 integrons [15] as well as other integron cassettes [16], PCR was performed using primers specific for the 5′ and 3′ conserved integrons segments (Additional file 1: Table S1). PCR was also used for to detect putative efflux pumps qacA, qacB [15] and qacC genes [17] (Additional file 1: Table S1). The positive controls for the PCR reactions were characterized by phenotypic testing and PCR followed by sequencing of the relevant genes: a KPC-producing K. pneumoniae strain carrying a bla KPC gene; a IMP-producing P. aeruginosa strain carrying a bla IMP gene; a P. aeruginosa strain carrying a bla VIM gene; and 4 isolates of A. baumannii resistant to QACs disinfectans carrying qacA, qacB, qacC and qacΔE genes, respectively.
After PCR amplification, amplified fragments from the 19 clinical isolates were purified with Qiaquick PCR Spin columns (Qiagen) and sequenced in both forward and reverse directions (Macrogen, Korea) with the same primers used for PCR amplification. The sequences were compared with the National Center for Biotechnology Information (NCBI) (https://blast.ncbi.nlm.nih.gov/Blast.cgi) and Lahey databases (http://www.lahey.org/Studies/).
Disinfectant susceptibility test in Klebsiella pneumoniae isolates
Susceptibility to Quaternary Ammonium Compounds (QACs) was determined using the quantitative suspension test for bactericidal activity, according to the protocol of Kawamura-Sato [18, 19]. The test was performed with the commercial disinfectant most commonly used in Venezuelan hospitals [20], which contains 10 % lauryl dimethyl benzyl ammonium bromide as the active QAC agent.
Molecular genotyping
The genetic relationships amongst the 19 carbapenem-resistance isolates were determined by Repetitive Element Palindromic-PCR (REP-PCR) and Multi-Locus Sequence Typing (MLST). REP-PCR was performed using previously described primers REP1 and REP2 [21] (Additional file 1: Table S1). MLST was performed using the methodology described by Diancourt et al. [22] (Additional file 1: Table S1). After PCR amplification, the fragments were sequenced with the amplification primers in the forward and reverse directions by Macrogen, Korea. Allele numbers and sequence types (STs) were assigned by the Klebsiella pneumoniae MLST web site (http://bigsdb.pasteur.fr/klebsiella/klebsiella.html).
Plasmid analysis
Plasmid DNA was extracted by the Kieser extraction method [23] and analyzed by gel electrophoresis in 0.7 % agarose.
Results
Clinical and epidemiological characteristics
We analyzed 19 carbapenem-resistant K. pneumoniae strains isolated from clinical specimens of an equal number of patients in the “Rafael Tobías Guevara” Pediatrics Department located in the “Dr. Luis Razetti” Teaching Hospital. The patients’ demographic and clinical characteristics are summarized in Table 1. All infections were health care associated. Fourteen patients (n = 14/19, 73.7 %) were newborns and 12 (n = 12/19, 63.1 %) were male. The most common infection sites were bloodstream (n = 15/19, 79 %) and bronchial secretions (n = 2/19, 10.5 %) (Table 1).
Table 1.
Clinical characteristics of patients infected by carbapenem-resistant K. pneumoniae isolates from a Venezuelan Hospital
| No. (%) of isolates | |
|---|---|
| Characteristic | Total no. |
| Gender | |
| Female | 7 (36.9 %) |
| Male | 12 (63.1 %) |
| Age | |
| Newborn (0–6 days) | 14 (73.7 %) |
| Lower infant (1–12 months) | 2 (10.5 %) |
| Higher infant (1–2 years) | 2 (10.5 %) |
| School (5–10 years) | 1 (5.3 %) |
| Infection site | |
| Bloodstream | 15 (79 %) |
| Bronchial secretion | 2 (10.4 %) |
| Catheter | 1 (5.3 %) |
| Lesion secretion | 1 (5.3 %) |
| Hospitalization área | |
| Neonatal | 12 (63.2 %) |
| Internal medicine | 2 (10.5 %) |
| Intensive care | 2 (10.5 %) |
| Surgery | 3 (15.8 %) |
Antibiotic susceptibility testing of K. pneumoniae isolates
All 19 isolates were resistant to trimethoprim-sulfamethoxazole, imipenem and meropenem, but testing for resistance to other antibiotics revealed seven resistance profiles. Profiles 2 and 5 were the most common with four isolates each, followed by profiles 1 and 4 with three isolates each (Table 2). The isolates in profile 7, the most resistant, were not sensitive to any of antibiotics tested, while profile 2 isolates were the most sensitive, showing resistance only to ciprofloxacin (Table 2). The percentages of isolates resistant to other antibiotics were: amikacin, 63.2 %; ciprofloxacin, 94.7 %; tetracycline, 84.2 %; chloramphenicol, 42.1 %; and tigecycline 15.8 %.
Table 2.
Antibiotic susceptibility profiles of carbapenem-resistant K. pneumoniae isolates from a Venezuelan Hospital
| Profile:Isolates | CM | AK | CIP | TE | TYG | % of isolates belonging profile |
|---|---|---|---|---|---|---|
| 1: ANZ2, ANZ4, ANZ6 | S | S | R | S | S | 15.8 |
| 2: ANZ8, ANZ11, ANZ12, ANZ13 | S | S | R | R | S | 21.0 |
| 3: ANZ10 | S | R | S | R | S | 5.3 |
| 4: ANZ1, ANZ3, ANZ19 | S | R | R | R | S | 15.8 |
| 5: ANZ5, ANZ7, ANZ9, ANZ14 | R | R | R | R | S | 21.0 |
| 6: ANZ16 | R | R | R | R | - | 5.3 |
| 7: ANZ17, ANZ18, ANZ21 | R | R | R | R | R | 15.8 |
All strains were resistant to imipenem, meropenem, and trimethoprim-sulfamethoxazole
CM chloramphenicol, AK amikacin, CIP Ciprofloxacin, TE tetracycline, TGC tigecycline, (−) no data
Detection of genes encoding β–lactamases
Using PCR, both the bla KPC-2 and bla VIM-2 genes were detected in 100 % (n = 19/19) of the isolates, while the blaIMP gene could not be amplified from any isolate.
Genetic environment of blaKPC gene
The bla KPC gene is generally associated with a Tn3-based transposon, Tn4401, composed of a transposase gene, a resolvase gene, the bla KPC gene and two insertion sequences (ISKpn6 and ISKpn7) [7]. Six isoforms of Tn4401 have been identified which differ by various deletions upstream of the bla KPC gene [24, 25]. Amplification of the regions flanking the bla KPC-2 genes with Tn4401 specific primers [14] produced a 703-bp PCR fragment from all 19 isolates, consistent with the Tn4401 variant “b” [14] (Fig. 1). The ISKpn6 and tnpA genes were also amplified separately from all 19 isolates, but the inverted repeat sequences of Tn4401 could not be amplified with primers specific for this region [7, 14, 26] (Additional file 1: Table S1).
Fig. 1.
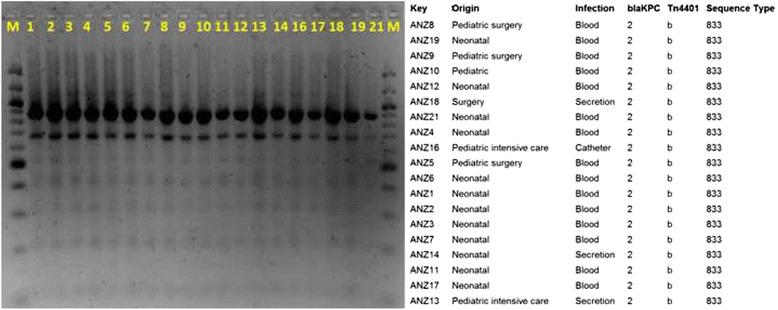
REP-PCR profiles of 19 isolates of bla VIM-2 and bla KPC-2-coharboring K. pneumoniae from a Public Venezuelan Hospital. Bands were visualized with ethidium bromide staining on a 1.0 % TBE agarose gel. M: Molecular Weight Marker 100 bp ladder (New England Biolabs®), line 1: ANZ1, line 2: ANZ2, line 3: ANZ3, line 4: ANZ4, line 5: ANZ5, line 6: ANZ6, line 7: ANZ7, line 8: ANZ8, line 9: ANZ9, line 10: ANZ10, line 11: ANZ11, line 12: ANZ12, line 13: ANZ13, line 14: ANZ14, line 16: ANZ16, line 17: ANZ17, line 18: ANZ18, line 19: ANZ19, line 21: ANZ21. The table to the right illustrates results from the bla KPC sequence analysis, MLST and sequence analysis of the nonconserved region of the Tn4401 element
Detection of genes located in class 1 integrons
Amplification and sequencing with primers specific for class 1 integrons detected qacΔE and addA2 genes in all 19 isolates. The addA2 gene encodes an aminoglycoside adenyltransferase that confers streptomycin resistance, while qacΔE gene encodes a truncated Quaternary Ammonium Compounds resistance protein.
Disinfectant susceptibility and molecular detection of the qacA, qacB, and qacC genes
All 19 isolates were susceptible to a disinfectant containing QAC agent lauryl dimethyl benzyl ammonium bromide. The qacA, qacB and qacC genes, implicated in resistance to this type of disinfectants, could not be amplified from any of the isolates.
Molecular genotyping
The REP-PCR technique produced very similar patterns from all 19 K. pneumoniae isolates. PCR amplification and sequencing of the seven genes [22] used for MLST produced identical profiles from all 19 strains. Comparison with the Klebsiella pneumoniae MLST website (http://bigsdb.pasteur.fr/klebsiella/klebsiella.html) revealed that this MLST profile is characteristic of sequence type, ST833 (100 %, n = 19/19) (Fig. 1).
Plasmid analysis
Plasmid DNA was isolated from all 19 isolates, and the plasmid profiles are shown in Fig. 2. It appears that there are some similarly sized plasmids that are present in almost all of the 19 isolates. These shared plasmids could carry the located bla KPC-2, bla VIM-2, addA2 and qacΔE genes, which have been commonly found in class 1 integrons within transposons carried on plasmids [27–30].
Fig. 2.
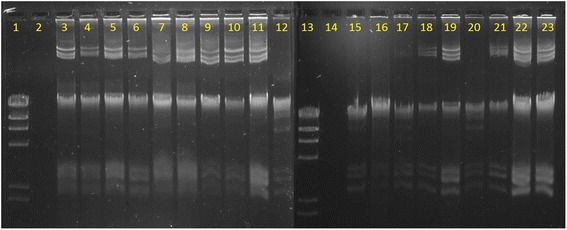
Plasmidic DNA profile of 19 isolates from a Public Venezuelan Hospital. Bands were visualized with ethidium bromide staining on a 0.7 % TBE agarose gel. Line 1: 100 bp ladder (New England Biolabs®), line 2: Escherichia coli XL1-Blue (negative control), line 3: ANZ1, line 4: ANZ2, line 5: ANZ3, line 6: ANZ4, line 7: ANZ5, line 8: ANZ6, line 9: ANZ7, line 10: ANZ8, line 11: ANZ9, line 12: ANZ10, line 13: 100 bp ladder (New England Biolabs®), line 14: Escherichia coli XL1-Blue (negative control), line 15: ANZ11, line 16: ANZ12, line 17: ANZ13, line 18: ANZ14, line 19: ANZ16, line 20: ANZ17, line 21: ANZ18, line 22: ANZ19, line 23: ANZ21
Discussion
We report 19 carbapenem reistant K. pneumoniae strains isolated from the pediatrics service of a hospital in Venezuela that co-harbor both bla KPC and bla VIM genes. All 19 strains appear to have identical Rep-PCR profiles, and by MLST all appear to belong to ST833, suggesting that this resistant strain has become endemic in the pediatric service, especially in the neonatal units of this hospital, where 12 of the strains were isolated. The limited patient information available and irreversibly anonymous nature of the strains did not permit retrospective analysis of the patients to exclude the possibility that some represented colonization or transient carrier states with KPC-producing K. pneumoniae. This seems unlikely, as 15 of the 19 strains were isolated from blood cultures, suggesting they were associated with severe infections. Cross contamination also seems unlikely as the isolates showed seven different profiles of resistance to other antibiotics.
There are no official data available about epidemiological monitoring of bacteria causing nosocomial infections in Venezuelan hospitals. The only surveillance program that provides data about antimicrobial resistance in Venezuela is PROVENRA (http://provenra.cloudapp.net/), a private initiative with information on antibiotic resistance from 1998 to 2012. The information is collected from microbiology laboratories in 46 hospitals nationwide, but does not include the “Dr. Luis Razetti” hospital studied in the present report. According to data reported for 2012 on the PROVENRA webpage, 72 % of K. pneumoniae isolated in neonatology services were resistant to ertapenem and 65 % were resistant to meropenem and imipenem (http://provenra.cloudapp.net/). This high prevalence of resistance was confirmed in our study.
Other studies have described carbapenem resistance in Venezuela. Fernández-Canigia and Dowziki in 2012 [31] presented data from the Latin American region on Gram-negative isolates used in the Tigecycline Evaluation and Surveillance Trial (T.E.S.T.). They describe decreasing susceptibility to carbapenems among ESBL producing K. pneumoniae in the Latin America countries studied, but found that 90.3 % of Venezuelan strains were sensitive to meropenem. Jones et al. [32] reported the results of a resistance surveillance program monitoring antimicrobial susceptibility patterns in Latin America in which only 15 % of Klebsiella isolates were carbapenem-resistant. Finally, Kazmierczak et al. [33] analyzed Gram-negative pathogens collected from 40 countries, including Venezuela, as part of a global surveillance study in 2012–2014. Carbapenem non-susceptible Enterobacteriaceae were characterized for bla genes encoding MBLs and serine β-lactamases variants with PCR and sequencing. In the strains from Venezuela there was one isolate of K. pneumoniae containing NDM-1 and one isolate of P. aeruginosa carrying VIM-2 [33].
The KPC-2 allele, one of 21 variants of the bla KPC gene, was found in all our 19 K. pneumoniae isolates and is one of the most extensively distributed worldwide [34], including in the South American countries of Colombia [35, 36] Brazil [37–40] Argentina [41, 42]. In Venezuela [43, 44], a KPC-2-producing K. oxytoca was isolated from a pediatric patient in the state of Mérida [44], but to our knowledge, KPC genes have not been previously reported in Venezuelan K. pneumoniae isolates.
In addition to the KPC-2 gene, all of our 19 isolates also carried the VIM-2-carbapenemase. The bla VIM gene is extensively distributed worldwide, with VIM-2 the most widespread variant [45]. Endemicity of VIM enzymes has been reported in Greece, Taiwan, and Japan [46, 47], although outbreaks and single strains of VIM producers have been reported in many other countries including the Latin American countries of México, Argentina, Colombia and Venezuela [45]. In Venezuela the bla VIM gene was previously found in clinical isolates of Pseudomonas aeruginosa [48, 49], and Marcano et al. [50] reported VIM-producing carbapenem-resistant K. pneumoniae isolated from the urine of a 7-year-old girl hospitalized at the “Hospital de Niños J. M. de los Ríos” in Caracas, Venezuela.
Strains carrying both bla KPC and bla VIM carbapenemases have been previously reported in Greece [1, 51–54] Colombia [55], Germany [56], Italy [6], and Spain [57]. In Venezuela the two carbapenemases have only been reported together in a multiply resistant strain of Enterobacter cloacae [58] isolated from the urine of a 83-year-old patient in a hospital in the city of Cumaná, which is only about 90 miles from hospital we studied. It might be interesting to know whether both the E. cloacae and K. pneumoniae isolates could be carrying these carbapenemase genes within the same transferable genetic context.
Class 1 integrons are the most common integron type present in clinical isolates of the Enterobacteriaceae, and are increasingly detected in isolates of K. pneumoniae. The gene cassettes most frequently identified within class 1 integrons in Enterobacteriaceae are those encoding resistance to streptomycin (aadA) and trimethoprim (dfrA) [59, 60]. By PCR we found that 19 K. pneumoniae strains contained the addA2 gene within the 5′CS-3′CS region of the integron. We did not look for the presence of the dfrA gene, but all 19 strains were resistant to trimethoprim-sulfamethoxazole. The qacΔE gene, which has also been associated with class 1 integrons [61] was amplified from all 19 isolates.
KPC genes are often found within a transposon-associated element, Tn4401 [7]. Tn4401 possesses genes encoding a transposase (tnpA) and a resolvase (tnpR), and has been characterized as an active transposon that is able to mobilize the bla KPC genes at high frequency without target specificity [7, 25]. In all 19 strains in this study the bla KPC-2 genes appeared to be within a Tn3-based structure consistent with the Tn4401 isoform ‘b’ [62], which has been observed in Greece [63], Colombia [14], Brazil [64] and the USA. Similar to the report by Pereira et al. [64] the inverted repeat sequences of the flanking regions were not amplified in our isolates, suggesting that their insertion sites may be different from those of K. pneumoniae YC described by Naas et al. [7].
The KPC and VIM genes are generally found on plasmids [65], and the plasmids profiles from all 19 strains contained similar bands, suggesting that the integron containing the carbapenemase genes could be present within a transposable element on a common plasmid, but further studies are needed to confirm this possibility.
All 19 isolates evaluated in this study demonstrated similar patterns with REP-PCR analysis, and by MLST all belonged to ST833, a genotype that has only been reported in Israel [28] and Trieste, Italy [29]. Interestingly, the ST833 K. pneumoniae strain isolated in the Trieste Pediatric Hospital was from a blood culture of a 3-year-old patient transferred from a Venezuelan hospital to undergo marrow transplantation [29].
We have not found any previous studies describing MLST characterization of K. pneumoniae strains isolated in Venezuela, but we have used MLST to analyze the strains of KPC-producing K. pneumoniae in a few other hospitals in the country (manuscripts submitted). Although the other hospitals always contained a variety of sequence types, we found isolates belonging to ST833 in hospitals in two other Venezuelan states--Zulia and the Capital District. These are in the west and center of the country, respectively, and distant from hospital studied in the current report, which is located in the eastern state of Anzoátegui. From our albeit limited sampling, it appears that the carbapenem resistant K. pneumoniae ST833 strain could be extensively distributed throughout the country. Perhaps a Venezuelan patient acquired the ST833 strain as an infection or colonization while hospitalized in Israel and then returned to Venezuela, where it subsequently disseminated throughout the country with the movements of patients and health care staff.
ST833 is part of the 258 clonal complex, and the ST833 allelic profile (3-3-1-1-1-1-12) differs from ST258 only in the tonB allele. The clonal complex CC258 is the dominant and most successful KPC producing strains as has spread widely and rapidly across the world [30, 66], although the reasons for its apparent advantage have yet to be completely explained facilitated by its production of proteins involved in cell motility, secretion and DNA repair and modification [67–69].
Disinfectants containing QACs are commonly used in Venezuelan hospitals, and therefore the presence of QAC resistance would complicate efforts to reduce the prevalence of this ST833 strain. Fortunately, all strains were phenotypically sensitive to the QAC disinfectant and we could not amplify the qacA, qacB [15] or qacC genes [16, 17] which have all been associated with QAC resistance. Nevertheless, the use of QAC disinfectants is clearly only a minor part of the measures that are required to control nosocomial infections.
Venezuelan medical personnel are keenly aware that carbapenem-resistant K. pneumoniae have become a frequent cause of nosocomial infections in several hospitals in different Venezuelan cities [8]. We hope that the molecular epidemiology provided by this study can aid in tracking the presence and dissemination of the strains involved, and thereby contribute to the vigilance and surveillance required to effectively treat them and to reduce their presence.
Conclusions
Molecular characterization of 19 carbapenem-resistant K. pneumoniae strains isolated from pediatric patients in a large public hospital in Anzoátegui, Venezuela, revealed that all contained the bla KPC-2 and bla VIM-2 genes, encoding serine and metallo carbapenemases, respectively. MLST analysis showed that all 19 strains belong to ST833, suggesting an alarming endemic presence of this strain throughout the hospital’s pediatric service, but particularly in the neonatal units.
Acknowledgments
We thank the team of curators of the Institut Pasteur MLST and whole genome MLST for collating the databases and making them publicly available at http://bigsdb.pasteur.fr/klebsiella/klebsiella.html”. We also thank to Henry Sosa, Loma Abdulkhalek and Rohan Pinto for initiating this study as an undergraduate thesis.
Funding
This work was supported by research grants from Instituto Venezolano de Investigaciones Científicas (Number 1237), Caracas, Venezuela.
Availability of data and materials
Contained within the manuscript.
Authors’ contributions
AF, YR, EF and AG performed the experiments; AF, EF, AG, HT contributed reagents/materials/analysis tools; AF and HT conceived and designed the experiments, analysed the data and wrote and revised the manuscript. All authors read and approved the final manuscript.
Competing interests
The authors declare that they have no competing interests.
Consent for publication
Not applicable.
Ethics statement
Not required.
Abbreviations
- AK
Amikacin
- bp
Base-pair
- CC
Clonal complex
- CIP
Ciprofloxacin
- CLSI
Clinical and Laboratory Standards Institute
- CM
Chloramphenicol
- CS
Conserved-segment
- DNA
Deoxyribonucleic acid
- Dr
Doctor
- IMP
Imienemase
- IS
Insertion sequences
- KPC
Klebsiella pneumoniae Carbapenemase
- MBL
Metallo-β-lactamase
- MLST
Multi-Locus Sequence Typing
- NCBI
National Center for Biotechnology Information
- NDM
New Delhi Metallo-β-lactamase
- PCR
Polymerase chain reaction
- QAC
Quaternary Ammonium Compounds
- REP-PCR
Repetitive Element Palindromic - Polymerase Chain Reaction
- ST
Sequence type
- TE
Tetracycline
- TGC
Tigecycline
- Tn
Transposon
- UPMG
Unweighted pair group method
- VIM
Verona Integron-ecoded Metallo-β-lactamase
Additional file
Oligonucleotides used for PCR of carbapenemase-resistant Klebsiella pneumoniae isolates from a Hospital in Venezuela. (DOC 77 kb)
Contributor Information
Aura Falco, Email: afalco@ivic.gob.ve.
Yusibeska Ramos, Email: yusibeska@gmail.com.
Esther Franco, Email: colile@yahoo.com.
Alegría Guzmán, Email: guzman.alegria@gmail.com.
Howard Takiff, Email: htakiff@ivic.gob.ve.
References
- 1.Meletis G, Tzampaz E, Protonotariou E, Sofianou D. Emergence of Klebsiella pneumoniae carrying bla(VIM) and bla(KPC) genes. Hippokratia. 2010;14(2):139–40. [PMC free article] [PubMed] [Google Scholar]
- 2.Nordmann P, Cuzon G, Naas T. The real threat of Klebsiella pneumoniae carbapenemase-producing bacteria. Lancet Infect Dis. 2009;9(4):228–36. doi: 10.1016/S1473-3099(09)70054-4. [DOI] [PubMed] [Google Scholar]
- 3.Cuzon G, Naas T, Demachy MC, Nordmann P. Plasmid-mediated carbapenem-hydrolyzing beta-lactamase KPC-2 in Klebsiella pneumoniae isolate from Greece. Antimicrob Agents Chemother. 2008;52(2):796–7. doi: 10.1128/AAC.01180-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pournaras S, Protonotariou E, Voulgari E, Kristo I, Dimitroulia E, Vitti D, Tsalidou M, Maniatis AN, Tsakris A, Sofianou D. Clonal spread of KPC-2 carbapenemase-producing Klebsiella pneumoniae strains in Greece. J Antimicrob Chemother. 2009;64(2):348–52. doi: 10.1093/jac/dkp207. [DOI] [PubMed] [Google Scholar]
- 5.Patel G, Bonomo RA. Status report on carbapenemases: challenges and prospects. Expert Rev Anti Infect Ther. 2011;9(5):555–70. doi: 10.1586/eri.11.28. [DOI] [PubMed] [Google Scholar]
- 6.Perilli M, Bottoni C, Grimaldi A, Segatore B, Celenza G, Mariani M, Bellio P, Frascaria P, Amicosante G. Carbapenem-resistant Klebsiella pneumoniae harbouring blaKPC-3 and blaVIM-2 from central Italy. Diagn Microbiol Infect Dis. 2013;75(2):218–21. doi: 10.1016/j.diagmicrobio.2012.10.008. [DOI] [PubMed] [Google Scholar]
- 7.Naas T, Cuzon G, Villegas MV, Lartigue MF, Quinn JP, Nordmann P. Genetic structures at the origin of acquisition of the beta-lactamase bla KPC gene. Antimicrob Agents Chemother. 2008;52(4):1257–63. doi: 10.1128/AAC.01451-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marcano D, De Jesus A, Hernandez L, Torres L. Frequency of enzymes associated with reduced sensitivity to beta-lactam antibiotics in enterobacteria isolates, Caracas, Venezuela. Rev Panam Salud Publica. 2011;30(6):529–34. [PubMed] [Google Scholar]
- 9.CLSI . 24th International Supplement M100-S24, Clinical and Laboratory Standards Institute. Wayne: CLSI; 2014. Performance standards for antimicrobial susceptibility testing; pp. 50–60. [Google Scholar]
- 10.Tsakris A, Poulou A, Themeli-Digalaki K, Voulgari E, Pittaras T, Sofianou D, Pournaras S, Petropoulou D. Use of boronic acid disk tests to detect extended- spectrum beta-lactamases in clinical isolates of KPC carbapenemase-possessing enterobacteriaceae. J Clin Microbiol. 2009;47(11):3420–6. doi: 10.1128/JCM.01314-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wang L, Gu H, Lu X. Rapid low-cost detection of Klebsiella pneumoniae carbapenemase genes by internally controlled real-time PCR. J Microbiol Methods. 2012;91(3):361–3. doi: 10.1016/j.mimet.2012.09.009. [DOI] [PubMed] [Google Scholar]
- 12.Bradford PA, Bratu S, Urban C, Visalli M, Mariano N, Landman D, Rahal JJ, Brooks S, Cebular S, Quale J. Emergence of carbapenem-resistant Klebsiella species possessing the class A carbapenem-hydrolyzing KPC-2 and inhibitor-resistant TEM-30 beta-lactamases in New York City. Clin Infect Dis. 2004;39(1):55–60. doi: 10.1086/421495. [DOI] [PubMed] [Google Scholar]
- 13.Poirel L, Walsh TR, Cuvillier V, Nordmann P. Multiplex PCR for detection of acquired carbapenemase genes. Diagn Microbiol Infect Dis. 2011;70(1):119–23. doi: 10.1016/j.diagmicrobio.2010.12.002. [DOI] [PubMed] [Google Scholar]
- 14.Cuzon G, Naas T, Truong H, Villegas MV, Wisell KT, Carmeli Y, Gales AC, Venezia SN, Quinn JP, Nordmann P. Worldwide diversity of Klebsiella pneumoniae that produce beta-lactamase blaKPC-2 gene. Emerg Infect Dis. 2010;16(9):1349–56. doi: 10.3201/eid1609.091389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang C, Cai P, Guo Y, Mi Z. Distribution of the antiseptic-resistance genes qacEDelta1 in 331 clinical isolates of Pseudomonas aeruginosa in China. J Hosp Infect. 2007;66(1):93–5. doi: 10.1016/j.jhin.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 16.Levesque C, Piche L, Larose C, Roy PH. PCR mapping of integrons reveals several novel combinations of resistance genes. Antimicrob Agents Chemother. 1995;39(1):185–91. doi: 10.1128/AAC.39.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mayer S, Boos M, Beyer A, Fluit AC, Schmitz FJ. Distribution of the antiseptic resistance genes qacA, qacB and qacC in 497 methicillin-resistant and -susceptible European isolates of Staphylococcus aureus. J Antimicrob Chemother. 2001;47(6):896–7. doi: 10.1093/jac/47.6.896. [DOI] [PubMed] [Google Scholar]
- 18.Kawamura-Sato K, Wachino J, Kondo T, Ito H, Arakawa Y. Reduction of disinfectant bactericidal activities in clinically isolated Acinetobacter species in the presence of organic material. J Antimicrob Chemother. 2008;61(3):568–76. doi: 10.1093/jac/dkm498. [DOI] [PubMed] [Google Scholar]
- 19.Ramos Y, Alonso G. Evaluación de la resistencia a agentes desinfectantes en bacterias aisladas de ambientes naturales. Rev Soc Venez Microbiol. 2011;31(2):130–7. [Google Scholar]
- 20.Chalbaud A, Ramos Y, Alonso G. Microbes in applied research: current advances and challenges. Spain: World Scientific Publishing Co. Pte. Ltd; 2012. Antibiotic and disinfectant resistance in Acinetobacter baumannii genotyped isolates from the Caracas University Hospital; pp. 481–5. [Google Scholar]
- 21.Versalovic J, Koeuth T, Lupski JR. Distribution of repetitive DNA sequences in eubacteria and application to fingerprinting of bacterial genomes. Nucleic Acids Res. 1991;19(24):6823–31. doi: 10.1093/nar/19.24.6823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Diancourt L, Passet V, Verhoef J, Grimont PA, Brisse S. Multilocus sequence typing of Klebsiella pneumoniae nosocomial isolates. J Clin Microbiol. 2005;43(8):4178–82. doi: 10.1128/JCM.43.8.4178-4182.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kieser T. Factors affecting the isolation of CCC DNA from Streptomyces lividans and Escherichia coli. Plasmid. 1984;12(1):19–36. doi: 10.1016/0147-619X(84)90063-5. [DOI] [PubMed] [Google Scholar]
- 24.Naas T, Cuzon G, Truong HV, Nordmann P. Role of ISKpn7 and deletions in blaKPC gene expression. Antimicrob Agents Chemother. 2012;56(9):4753–9. doi: 10.1128/AAC.00334-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bryant KA, Van Schooneveld TC, Thapa I, Bastola D, Williams LO, Safranek TJ, Hinrichs SH, Rupp ME, Fey PD. KPC-4 Is encoded within a truncated Tn4401 in an IncL/M plasmid, pNE1280, isolated from Enterobacter cloacae and Serratia marcescens. Antimicrob Agents Chemother. 2013;57(1):37–41. doi: 10.1128/AAC.01062-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pereira CA, Marra AR, Camargo LF, Pignatari AC, Sukiennik T, Behar PR, Medeiros EA, Ribeiro J, Girao E, Correa L, et al. Nosocomial bloodstream infections in Brazilian pediatric patients: microbiology, epidemiology, and clinical features. PLoS One. 2013;8(7):e68144. doi: 10.1371/journal.pone.0068144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nobari S, Shahcheraghi F, Rahmati Ghezelgeh F, Valizadeh B. Molecular characterization of carbapenem-resistant strains of Klebsiella pneumoniae isolated from Iranian patients: first identification of blaKPC gene in Iran. Microb Drug Resist. 2014;20(4):285–93. doi: 10.1089/mdr.2013.0074. [DOI] [PubMed] [Google Scholar]
- 28.Baraniak A, Izdebski R, Fiett J, Sadowy E, Adler A, Kazma M, Salomon J, Lawrence C, Rossini A, Salvia A, et al. Comparative population analysis of Klebsiella pneumoniae strains with extended-spectrum beta-lactamases colonizing patients in rehabilitation centers in four countries. Antimicrob Agents Chemother. 2013;57(4):1992–7. doi: 10.1128/AAC.02571-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Garbari L, Busetti M, Dolzani L, Petix V, Knezevich A, Bressan R, Gionechetti F, Tonin EA, Lagatolla C. pKBuS13, a KPC-2 encoding plasmid from Klebsiella pneumoniae Sequence Type 833, carrying Tn4401b inserted into a Xer site-specific recombination locus. Antimicrob Agents Chemother. 2015;59:5226–31. doi: 10.1128/AAC.04543-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Qi Y, Wei Z, Ji S, Du X, Shen P, Yu Y. ST11, the dominant clone of KPC-producing Klebsiella pneumoniae in China. J Antimicrob Chemother. 2011;66(2):307–12. doi: 10.1093/jac/dkq431. [DOI] [PubMed] [Google Scholar]
- 31.Fernandez-Canigia L, Dowzicky MJ. Susceptibility of important Gram-negative pathogens to tigecycline and other antibiotics in Latin America between 2004 and 2010. Ann Clin Microbiol Antimicrob. 2012;11:29. doi: 10.1186/1476-0711-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jones RN, Guzman-Blanco M, Gales AC, Gallegos B, Castro AL, Martino MD, Vega S, Zurita J, Cepparulo M, Castanheira M. Susceptibility rates in Latin American nations: report from a regional resistance surveillance program (2011) Braz J Infect Dis. 2013;17(6):672–81. doi: 10.1016/j.bjid.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kazmierczak KM, Rabine S, Hackel M, McLaughlin RE, Biedenbach DJ, Bouchillon SK, Sahm DF, Bradford PA. Multiyear, multinational survey of the incidence and global distribution of metallo-beta-lactamase-producing Enterobacteriaceae and Pseudomonas aeruginosa. Antimicrob Agents Chemother. 2015;60(2):1067–78. doi: 10.1128/AAC.02379-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Munoz-Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, Cormican M, Cornaglia G, Garau J, Gniadkowski M, Hayden MK, et al. Clinical epidemiology of the global expansion of Klebsiella pneumoniae carbapenemases. Lancet Infect Dis. 2013;13(9):785–96. doi: 10.1016/S1473-3099(13)70190-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mojica MF, Correa A, Vargas DA, Maya JJ, Montealegre MC, Rojas LJ, Ruiz SJ, Quinn JP, Villegas MV. Molecular correlates of the spread of KPC-producing Enterobacteriaceae in Colombia. Int J Antimicrob Agents. 2012;40(3):277–9. doi: 10.1016/j.ijantimicag.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 36.Villegas MV, Lolans K, Correa A, Suarez CJ, Lopez JA, Vallejo M, Quinn JP. First detection of the plasmid-mediated class A carbapenemase KPC-2 in clinical isolates of Klebsiella pneumoniae from South America. Antimicrob Agents Chemother. 2006;50(8):2880–2. doi: 10.1128/AAC.00186-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gales AC, Castanheira M, Jones RN, Sader HS. Antimicrobial resistance among Gram-negative bacilli isolated from Latin America: results from SENTRY Antimicrobial Surveillance Program (Latin America, 2008–2010) Diagn Microbiol Infect Dis. 2012;73(4):354–60. doi: 10.1016/j.diagmicrobio.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 38.Monteiro J, Santos AF, Asensi MD, Peirano G, Gales AC. First report of KPC-2-producing Klebsiella pneumoniae strains in Brazil. Antimicrob Agents Chemother. 2009;53(1):333–4. doi: 10.1128/AAC.00736-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Seki LM, Pereira PS, de Souza Mda P, Conceicao Mde S, Marques EA, Porto CO, Colnago EM, Alves Cde F, Gomes D, Assef AP, et al. Molecular epidemiology of KPC-2- producing Klebsiella pneumoniae isolates in Brazil: the predominance of sequence type 437. Diagn Microbiol Infect Dis. 2011;70(2):274–7. doi: 10.1016/j.diagmicrobio.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 40.Chagas TP, Seki LM, da Silva DM, Asensi MD. Occurrence of KPC-2-producing Klebsiella pneumoniae strains in hospital wastewater. J Hosp Infect. 2011;77(3):281. doi: 10.1016/j.jhin.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 41.Pasteran FG, Otaegui L, Guerriero L, Radice G, Maggiora R, Rapoport M, Faccone D, Di Martino A, Galas M. Klebsiella pneumoniae Carbapenemase-2, Buenos Aires, Argentina. Emerg Infect Dis. 2008;14(7):1178–80. doi: 10.3201/eid1407.070826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gomez SA, Pasteran FG, Faccone D, Tijet N, Rapoport M, Lucero C, Lastovetska O, Albornoz E, Galas M, Melano RG, et al. Clonal dissemination of Klebsiella pneumoniae ST258 harbouring KPC-2 in Argentina. Clin Microbiol Infect. 2011;17(10):1520–4. doi: 10.1111/j.1469-0691.2011.03600.x. [DOI] [PubMed] [Google Scholar]
- 43.Villegas MV, Kattan JN, Quinteros MG, Casellas JM. Prevalence of extended-spectrum beta-lactamases in South America. Clin Microbiol Infect. 2008;14(Suppl 1):154–8. doi: 10.1111/j.1469-0691.2007.01869.x. [DOI] [PubMed] [Google Scholar]
- 44.Labrador I, Araque M. First description of KPC-2-producing klebsiella oxytoca isolated from a pediatric patient with nosocomial pneumonia in Venezuela. Case Rep Infect Dis. 2014;2014:434987. doi: 10.1155/2014/434987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Nordmann P, Naas T, Poirel L. Global spread of Carbapenemase-producing Enterobacteriaceae. Emerg Infect Dis. 2011;17(10):1791–8. doi: 10.3201/eid1710.110655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Queenan AM, Bush K. Carbapenemases: the versatile beta-lactamases. Clin Microbiol Rev. 2007;20(3):440–58. doi: 10.1128/CMR.00001-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walsh TR, Toleman MA, Poirel L, Nordmann P. Metallo-beta-lactamases: the quiet before the storm? Clin Microbiol Rev. 2005;18(2):306–25. doi: 10.1128/CMR.18.2.306-325.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mendes RE, Castanheira M, Garcia P, Guzman M, Toleman MA, Walsh TR, Jones RN, Program SAS. First isolation of bla(VIM-2) in Latin America: report from the SENTRY Antimicrobial Surveillance Program. Antimicrob Agents Chemother. 2004;48(4):1433–4. doi: 10.1128/AAC.48.4.1433-1434.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Sánchez D, Marcano D, Spadola E, León L, Payares D, Ugarte C, Salgado N, Maggi G, Guevara A, Torres S, et al. Metaloenzimas tipo VIM Detectadas en Aislamientos Clínicos en Pseudomonas aeruginosa en Cuatro Hospitales en Venezuela. Revista Cientifica del Instituto Nacional de HIgiene “Rafael Rangel”. In Press.
- 50.Marcano D, Pasteran F, Rapoport M, Faccone D, Ugarte C, Salgado N, Payares D, Spadola E, Lopez Y, Maggi G, et al. First isolation of a VIM-producing Klebsiella pneumoniae from a seven-year-old child in Venezuela. J Infect Dev Ctries. 2008;2(3):241–4. doi: 10.3855/jidc.270. [DOI] [PubMed] [Google Scholar]
- 51.Giakkoupi P, Pappa O, Polemis M, Vatopoulos AC, Miriagou V, Zioga A, Papagiannitsis CC, Tzouvelekis LS. Emerging Klebsiella pneumoniae isolates coproducing KPC-2 and VIM-1 carbapenemases. Antimicrob Agents Chemother. 2009;53(9):4048–50. doi: 10.1128/AAC.00690-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zioga A, Miriagou V, Tzelepi E, Douzinas E, Tsakiri M, Legakis NJ, Daikos GL, Tzouvelekis LS. The ongoing challenge of acquired carbapenemases: a hospital outbreak of Klebsiella pneumoniae simultaneously producing VIM-1 and KPC-2. Int J Antimicrob Agents. 2010;36(2):190–1. doi: 10.1016/j.ijantimicag.2010.04.002. [DOI] [PubMed] [Google Scholar]
- 53.Pournaras S, Poulou A, Voulgari E, Vrioni G, Kristo I, Tsakris A. Detection of the new metallo-beta-lactamase VIM-19 along with KPC-2, CMY-2 and CTX-M-15 in Klebsiella pneumoniae. J Antimicrob Chemother. 2010;65(8):1604–7. doi: 10.1093/jac/dkq190. [DOI] [PubMed] [Google Scholar]
- 54.Papagiannitsis CC, Giakkoupi P, Vatopoulos AC, Tryfinopoulou K, Miriagou V, Tzouvelekis LS. Emergence of Klebsiella pneumoniae of a novel sequence type (ST383) producing VIM-4, KPC-2 and CMY-4 beta-lactamases. Int J Antimicrob Agents. 2010;36(6):573–4. doi: 10.1016/j.ijantimicag.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 55.Montealegre MC, Correa A, Briceno DF, Rosas NC, De La Cadena E, Ruiz SJ, Mojica MF, Camargo RD, Zuluaga I, Marin A, et al. Novel VIM metallo-beta-lactamase variant, VIM-24, from a Klebsiella pneumoniae isolate from Colombia. Antimicrob Agents Chemother. 2011;55(5):2428–30. doi: 10.1128/AAC.01208-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Steinmann J, Kaase M, Gatermann S, Popp W, Steinmann E, Damman M, Paul A, Saner F, Buer J, Rath P. Outbreak due to a Klebsiella pneumoniae strain harbouring KPC-2 and VIM-1 in a German university hospital, July 2010 to January 2011. Euro Surveill. 2011;16:33. [PubMed] [Google Scholar]
- 57.Pena I, Picazo JJ, Rodriguez-Avial C, Rodriguez-Avial I. Carbapenemase-producing Enterobacteriaceae in a tertiary hospital in Madrid, Spain: high percentage of colistin resistance among VIM-1-producing Klebsiella pneumoniae ST11 isolates. Int J Antimicrob Agents. 2014;43(5):460–4. doi: 10.1016/j.ijantimicag.2014.01.021. [DOI] [PubMed] [Google Scholar]
- 58.Martinez D, Marcano D, Rodulfo H, Salgado N, Cuaical N, Rodriguez L, Cana L, Medina B, Guzman M, De Donato M. KPC and VIM producing Enterobacter cloacae strain from a hospital in northeastern Venezuela. Invest Clin. 2015;56(2):182–7. [PubMed] [Google Scholar]
- 59.Li B, Hu Y, Wang Q, Yi Y, Woo PC, Jing H, Zhu B, Liu CH. Structural diversity of class 1 integrons and their associated gene cassettes in Klebsiella pneumoniae isolates from a hospital in China. PLoS One. 2013;8(9):e75805. doi: 10.1371/journal.pone.0075805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.DeLappe N, O’Halloran F, Fanning S, Corbett-Feeney G, Cheasty T, Cormican M. Antimicrobial resistance and genetic diversity of Shigella sonnei isolates from western Ireland, an area of low incidence of infection. J Clin Microbiol. 2003;41(5):1919–24. doi: 10.1128/JCM.41.5.1919-1924.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Santos C, Caetano T, Ferreira S, Ramalheira E, Mendo S. A novel complex class 1 integron found in a Klebsiella pneumoniae isolate from Portugal. Clin Microbiol Infect. 2011;17(7):1036–9. doi: 10.1111/j.1469-0691.2010.03416.x. [DOI] [PubMed] [Google Scholar]
- 62.Cuzon G, Naas T, Nordmann P. Functional characterization of Tn4401, a Tn3-based transposon involved in blaKPC gene mobilization. Antimicrob Agents Chemother. 2011;55(11):5370–3. doi: 10.1128/AAC.05202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Giakkoupi P, Papagiannitsis CC, Miriagou V, Pappa O, Polemis M, Tryfinopoulou K, Tzouvelekis LS, Vatopoulos AC. An update of the evolving epidemic of blaKPC-2-carrying Klebsiella pneumoniae in Greece (2009–10) J Antimicrob Chemother. 2011;66(7):1510–3. doi: 10.1093/jac/dkr166. [DOI] [PubMed] [Google Scholar]
- 64.Pereira PS, de Araujo CF, Seki LM, Zahner V, Carvalho-Assef AP, Asensi MD. Update of the molecular epidemiology of KPC-2-producing Klebsiella pneumoniae in Brazil: spread of clonal complex 11 (ST11, ST437 and ST340) J Antimicrob Chemother. 2013;68(2):312–6. doi: 10.1093/jac/dks396. [DOI] [PubMed] [Google Scholar]
- 65.Endimiani A, Carias LL, Hujer AM, Bethel CR, Hujer KM, Perez F, Hutton RA, Fox WR, Hall GS, Jacobs MR, et al. Presence of plasmid-mediated quinolone resistance in Klebsiella pneumoniae isolates possessing blaKPC in the United States. Antimicrob Agents Chemother. 2008;52(7):2680–2. doi: 10.1128/AAC.00158-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhou T, Zhang Y, Li M, Yu X, Sun Y, Xu J. An outbreak of infections caused by extensively drug-resistant Klebsiella pneumoniae strains during a short period of time in a Chinese teaching hospital: epidemiology study and molecular characteristics. Diagn Microbiol Infect Dis. 2015;82(3):240–4. doi: 10.1016/j.diagmicrobio.2015.03.017. [DOI] [PubMed] [Google Scholar]
- 67.Chmelnitsky I, Shklyar M, Hermesh O, Navon-Venezia S, Edgar R, Carmeli Y. Unique genes identified in the epidemic extremely drug-resistant KPC-producing Klebsiella pneumoniae sequence type 258. J Antimicrob Chemother. 2013;68(1):74–83. doi: 10.1093/jac/dks370. [DOI] [PubMed] [Google Scholar]
- 68.Yang J, Ye L, Guo L, Zhao Q, Chen R, Luo Y, Chen Y, Tian S, Zhao J, Shen D, et al. A nosocomial outbreak of KPC-2-producing Klebsiella pneumoniae in a Chinese hospital: dissemination of ST11 and emergence of ST37, ST392 and ST395. Clin Microbiol Infect. 2013;19(11):E509–15. doi: 10.1111/1469-0691.12275. [DOI] [PubMed] [Google Scholar]
- 69.Chen L, Mathema B, Chavda KD, DeLeo FR, Bonomo RA, Kreiswirth BN. Carbapenemase-producing Klebsiella pneumoniae: molecular and genetic decoding. Trends Microbiol. 2014;22(12):686–96. doi: 10.1016/j.tim.2014.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Contained within the manuscript.


