Abstract
Peste des petits ruminants virus (PPRV) causes the acute, highly contagious disease peste des petits ruminants (PPR) that affects small domestic and wild ruminants. PPR is of importance in the small livestock-keeping industry in Tanzania, especially in rural areas as it is an important source of livelihood. Morbidity and case fatality rate can be as high as 80–100% in naïve herds; however, in endemic areas, morbidity and case fatality range between 10 and 100% where previous immunity, age, and species of infected animal determine severity of outcome. PPR was officially confirmed in domestic animals in the Ngorongoro district of Tanzania in 2008. It is now considered to be endemic in the domestic sheep and goat populations throughout Tanzania, but restricted to one or more areas in the small ruminant wildlife population. In this article, we review the history and the current status of PPR in Tanzania and neighboring countries. To control and eradicate PPR in the region, a joint effort between these countries needs to be undertaken. The effort must also secure genuine engagement from the animal holders to succeed.
Keywords: small ruminants, sheep, goats, morbillivirus, peste des petits ruminants, East Africa
Peste des petits ruminants virus (PPRV) causes the acute and highly contagious disease peste des petits ruminants (PPR) that affects small domestic and wild ruminants. The virus belongs to the genus Morbillivirus in the family Paramyxoviridae alongside measles virus, canine distemper virus, phocine distemper virus, cetacean morbillivirus, the newly discovered feline morbillivirus, and the recently eradicated rinderpest virus (1–3). PPRV was first described in 1942 in the Ivory Coast and was recognized as a separate member of the genus Morbillivirus in 1979 (4, 5). It exists in one serotype separated into four lineages (I–IV); these lineages are based on the genetic comparison of a fragment of the nucleoprotein or the fusion protein (6). Historically, the four lineages follow a geographic distribution where lineages I and II are found in western and central Africa, lineage III in eastern Africa and the southern part of the Middle East, and lineage IV in the Middle East and southern Asia. Lineage IV was considered to be primarily an Asian one, but since the 1990s, there has been a wider distribution of it within Africa (6, 7).
Clinical signs associated with PPR are severe pyrexia (40–41°C), followed by mucopurulent nasal and ocular discharges, cough, dyspnea, necrotic stomatitis, and diarrhea (Fig. 1). Painful sores in the oral mucous membranes prevent the animal from eating and, in combination with the watery diarrhea, lead to severe dehydration. This can result in the death of the animal within 10–12 days after the onset of pyrexia (6, 8). Postmortem results include congested lungs (especially affecting the cranial lobes), edematous, congested retropharyngeal and mesenteric lymph nodes, and linear hemorrhages in the intestinal mucosa (Fig. 2) (8). Clinical signs can be highly similar to, and easily confused with, other small ruminant diseases. Moreover, secondary infections can also intensify clinical signs, making PPR a disease difficult to characterize and diagnose under field conditions (9). Differential diagnoses include, but are not limited to, contagious caprine pleuropneumonia, pasteurellosis, bluetongue, and foot-and-mouth disease (10). PPR morbidity and case fatality rate can be as high as 80–100% in naïve herds; however, it can range from 10 to 100% in endemic areas due to factors such as previous immunity, age, and species of the infected animal (11). PPR is a disease with significant socioeconomic impact. The affected sheep and goats are mostly held by poor and vulnerable groups of people who depend on them for income. Eradication of PPRV is therefore highly relevant to poverty alleviation (12). After the successful eradication of the closely related rinderpest disease, the United Nations Food and Agriculture Organization (FAO) launched a PPR control and eradication strategy together with the World Organisation for Animal Health (OIE) in March–April 2015. The goal was to gain more information, control, and finally eradicate PPR by 2030 in a similar way that was done with rinderpest (13). The southern border of PPRV distribution in eastern Africa is currently considered to be Tanzania with a high risk of spread further south (14, 15). Here, we review the history and the current situation of PPR in Tanzania and the strategies to control and eradicate PPR in Tanzania and neighboring countries.
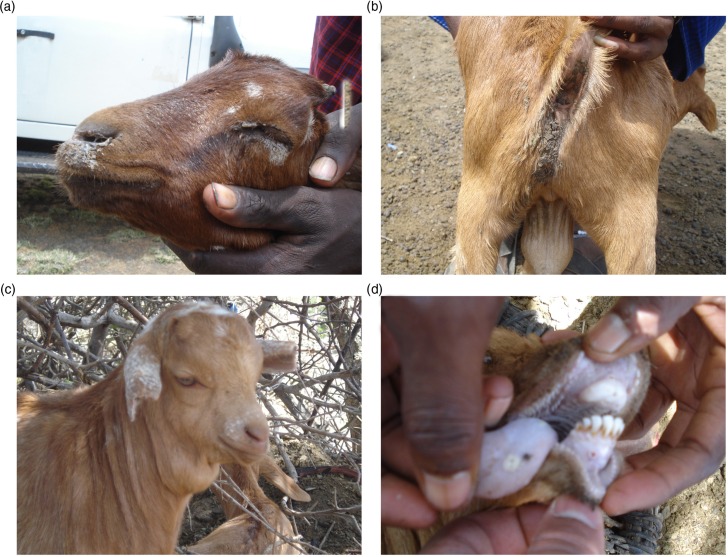
Fig. 1.
Clinical signs of peste des petits ruminants in goats of Ngorongoro, Tanzania. The pictures show oculonasal discharges and matting of eyelids (a) diarrhea soiling the perineum (b) submandibular edema (c), and sores and nodules on the gums and tongue (d).
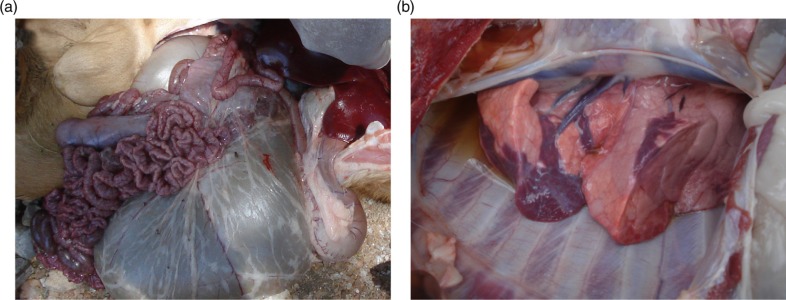
Fig. 2.
Postmortem findings in goats with peste des petits ruminants. Hemorrhages in the intestines (a) and pneumonia (b) in a goat confirmed with PPR in Ngorongoro, Tanzania.
History of PPR in Tanzania
Tanzania is located on the eastern coast of Africa and consists of the Tanzanian mainland and the island of Zanzibar. Countries neighboring Tanzania include, counterclockwise from the north, Kenya, Uganda, Rwanda, Burundi, the Democratic Republic of Congo (DRC), Zambia, Malawi, and Mozambique. In Kenya and Uganda, PPR was recognized in 2007, although PPRV antibodies had been detected already several years earlier (11, 16, 17). There are no reports of PPR outbreaks in Rwanda and Burundi, although PPR is suspected to be present (18). The DRC reported problems with PPR in 2012, and it is now considered to have areas where PPR is present (19). Zambia has recently reported PPRV antibodies, but no clinical cases of PPR or presence of PPRV (18). There have been no reports of PPR outbreaks from Malawi and Mozambique, but targeted surveillance is ongoing (18).
In Tanzania, the first serological screening was performed in 2000 (20). During the screening, 3,000 samples from all over Tanzania were tested with a competitive enzyme-linked immunosorbent assay (cELISA) for PPRV antibodies, and all samples were negative. No outbreak had been reported at that point, but there was serological evidence of infection in the neighboring countries of Uganda and Kenya (20). However, in December 2007, the Ngorongoro District Veterinary Officer suspected presence of PPR due to clinical signs in sheep and goats. High mortality rates in sheep and goat herds were observed in January to March 2008, but initial investigations yielded no conclusive results (21). The high mortality rate persisted so a new investigation was launched in June 2008 in the Arusha (Ngorongoro, Monduli, Karatu, and Longido), Manyara (Simanjiro), Kilimanjaro (Siha and Hai), and Mara regions (22). Samples from all districts, except Simanjiro, tested positive for PPRV in serological screening with a cELISA (45.4%, 703 of 1,549 samples), confirming the presence of PPR (Fig. 3 and Table 1) (22). Between September 2008 and July 2009, additional samples were taken in the regions Arusha, Kilimanjaro, Manyara, and Tanga to assess the magnitude of PPR and southward spread. Of the 3,478 serum samples collected from sheep and goats, 769 animals (22.1%) were positive for PPR antibodies. Six samples from sheep – one tissue sample (liver, spleen, heart, and mediastinal lymph nodes) and five whole blood samples – from the Ngorongoro district in Arusha region were tested by reverse transcription polymerase chain reaction (RT-PCR). Two were positive for PPRV, and phylogenetic analysis grouped the isolates into lineage III (21), the most abundant lineage in eastern Africa. The 2008 PPR outbreak was suspected to have been due to imported animals into Tanzania via live animal trade over the Tanzania–Kenya border (6, 21). A retrospective serological study in 2010 on 198 samples collected in the Ngorongoro district between 1998 and 2004 found antibodies against PPRV already present in 2004, indicating that PPR might have been present in Tanzania before it was confirmed in 2008 (23).
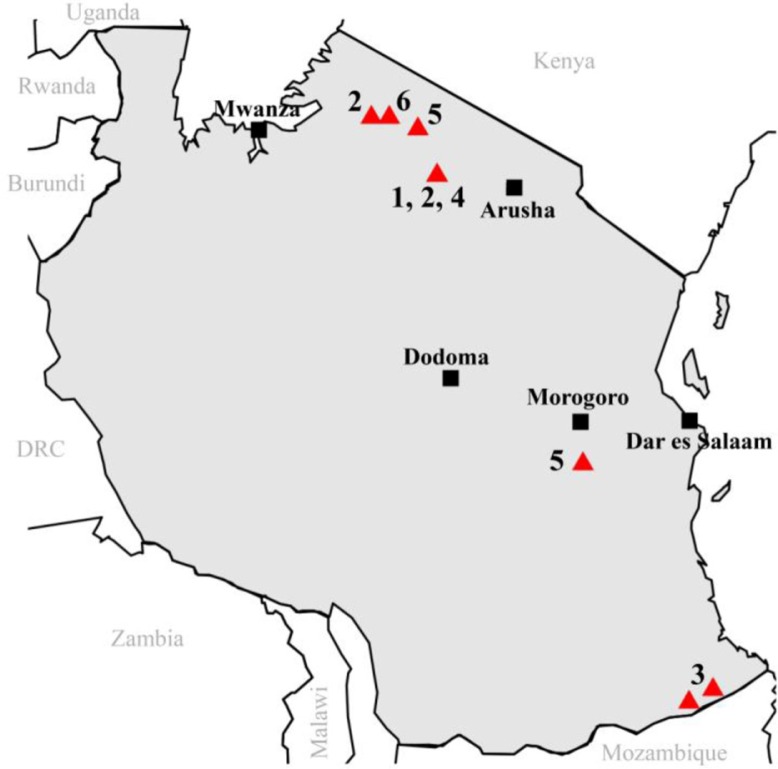
Fig. 3.
Sample collection sites from selected PPR studies in Tanzania. Triangles indicate sampling sites and numbers refer to the study cited (see Table 1).
Table 1.
Numbers indicate sample collection areas on the map in Fig. 3 and type of analyses
| Number on map | cELISA | RT-PCR | Reference |
|---|---|---|---|
| 1 | Positive | Not done | Karimuribo et al. (23) (samples from 2008) |
| 2 | Positive | Not done | Swai et al. (22) |
| 3 | Positive | Not done | Mbyuzi et al. (42) (samples from 2009) |
| 4 | Positive | Not done | Lembo et al. (28) |
| 5 | Not done | Positive | Kgotlele et al. (33) |
| 6 | Positive | Positive | Mahapatra et al. (29) |
cELISA =competitive enzyme-linked immunosorbent assay; RT-PCR=reverse transcription polymerase chain reaction.
A similar study was conducted in southern Tanzania in two districts bordering Mozambique due to a suspected outbreak of PPR during March to May 2011 (24). A total of 216 blood samples were collected and analyzed with cELISA and RT-PCR. A seroprevalence of 31% was detected, and in 53.6% of these samples, the PPRV genome was detected by RT-PCR (24). As the detection of PPR antibodies and genome showed evidence of PPR circulating in Tanzania, a retrospective study was performed on serum samples that had been collected in 2007 and 2009 as part of Rift Valley fever surveillance in the Mtwara and Lindi regions of southern Tanzania. The results showed antibodies against PPRV already in 2009 (22).
Although sheep and goats are the natural hosts of PPR, with goats the more susceptible of the two (25–27), cattle also develop subclinical infection and seroconvert. Screening in the Ngorongoro district in northern Tanzania showed seropositivity of 26.7% in cattle alive during the 2008 PPR outbreak and 5.9% in cattle born after (28). Wildlife in Tanzania have also been found to seroconvert, as shown by a study in the Serengeti Ecosystem (29). In 2014, blood samples and eye and nasal swabs were collected from wildlife dwelling close to resident livestock, or sharing pastures and water sources near domestic animals. Of these samples, 29 of 46 (63%) animals were seropositive for PPRV, and herd prevalence was 92% (12 of 13) (29). The only seronegative herd consisted of a single Thomson's gazelle, and the authors point out that this should not be seen as representing the entire species. In general, seropositivity increased with the age of the animals, which suggests a repeated exposure to the virus; however, the source of the virus – domestic animals or wildlife – is unclear (29). PPRV genome was detected in a single Grant's gazelle living in an area with an ongoing outbreak in sheep. Partial nucleoprotein sequence of PPRV from the sheep was generated and used for molecular characterization; it showed that the isolate belongs to lineage II (29).
Serological evidence of PPRV exposure has been demonstrated in other animal species, namely, camels in northern Tanzania. In a repository of serum samples collected in 2010 from seemingly clinically healthy camels in various administrative localities of northern Tanzania, 2.6% of the camels were seropositive for PPR (30). This is not unique to Tanzania; other African countries (e.g. Sudan, Ethiopia, and Nigeria) have also reported antibodies to PPRV in camels (31–33).
Current situation in Tanzania
PPRV is currently considered endemic in Tanzanian domestic sheep and goat populations (Fig. 4), whereas it is limited to one or more areas in the wildlife (18). So far the PPRV confirmed in Tanzania belongs to lineages II–IV. Lineage II has been described in northern and southern Tanzania (29, 34), lineage III in northern and eastern Tanzania (35), and lineage IV in southern Tanzania (34). Lineage III is the lineage most commonly found in eastern Africa, whereas lineage II is most commonly found in western and central Africa (6). Lineage IV was historically regarded as an Asian lineage, but since the 1990s it has spread in northern and eastern Africa replacing the other lineages (7, 35). The trend of mixed lineages present in a single country is not unique to Tanzania; other countries, including neighboring Uganda, have also reported this (36). The risk of further spread outside of Tanzania to neighboring countries, for example, Zambia, is high (14). Spread has already occurred to the Comoros Islands, a group of volcanic islands located east of Tanzania and Mozambique and northwest of Madagascar (37). In November 2012, a higher number of deaths than normal occurred in goat herds in the Comoros Islands. The cause was PPRV, and phylogenetic analysis showed that the virus belonged to lineage III (37). The virus was suspected to have been introduced with live goats imported from Tanzania (37).
Fig. 4.
Districts with seropositive cases for PPR, indicated in dark grey. Districts were sampled during a nationwide surveillance in 2008–2013; in one district (Mwanza), surveillance was done independently by a zonal veterinary center at the end of 2012. Source: Department of Veterinary Services, Tanzania.
Implications of PPR in Tanzania
Tanzania ranks third in Africa in livestock production (26% of the GDP) after Ethiopia and Sudan, with the dominant species being cattle, followed by goats, sheep, and pigs. The sheep population is estimated to be around 5 million and the goat population 15 million (38). Livestock keeping is an important industry across Africa, especially in rural areas where it is an essential source of livelihood. In Tanzania, about three out of five rural household incomes come from livestock activities, with livestock rearing earning an average of 22% of total household income (39). A study in the Tandahimba and Ulanga districts in southern Tanzania assessed the socioeconomic impact of PPR. The average value of small ruminants dropped by 10%, and the overall ability to use small ruminants as livelihood decreased by about 30% following the incursion of PPR (40). PPR impacts included a decrease of flock size and value, an inability of the flock to support household livelihood and a loss of potential income; the estimated total loss (including mortality and lost revenue) was TZS 735,820 (USD 490.6) per year. Cumulative loss due to PPR was around TZS 101.8 billion (USD 67.9 million) per year (40). With such high stakes and numbers of sheep and goats at risk, emergency vaccinations were carried out in 2009 in the northern districts around the foci of the 2008 PPR outbreak. In 2010, the vaccinations were done in the Morogoro and Mtwara regions. In total, these emergency vaccinations covered about 5.2 million goats and sheep in the affected and surrounding districts (41). In southern Tanzania, vaccinations were conducted in goat and sheep flocks in March 2011, with coverage of 57.0%; in March 2012, the vaccination coverage was increased to 71.0% (42). Despite these efforts, the disease continued to spread in the country due to uncontrolled livestock movements; inadequate surveillance, diagnostics, and reporting; low awareness among small stock farmers, traders, and transporters; and an incapacity to enforce regulations (15).
To control PPR in Tanzania and other Southern African Development Community (SADC), a ‘PPR Progressive Control and Eradication Strategy’ was set up to 1) prevent further introduction and spread of PPR in other areas of the country, 2) progressively control PPR in the infected zones, and 3) eradicate PPR from the infected countries by 2025 (15). At a global level, FAO and OIE experts together with other stakeholders formulated a ‘Global Strategy for the Control and Eradication of PPR’; this was launched in early 2015 (13). The global control strategy emphasizes harmonized and coordinated approaches with participation of countries sharing common ecosystems/borders and includes control of other small ruminant diseases and strengthening of veterinary services. The importance of engaging the affected groups (i.e. the animal owners) in the goal to eradicate PPR has also been emphasized (43). Most of the southern African countries are free from PPR, with the current southern border of confirmed presence of PPR being – from west to east – Angola, DRC, and Tanzania (18). During 2013–2015, FAO implemented a Technical Cooperation Project to prevent introduction of PPR into Malawi, Mozambique, and Zambia (44).
The regional strategies to control PPR, which are aligned with the FAO/OIE global control and eradication strategies and coordinated through the African Union Inter-African Bureau for Animal Resources (AU-IBAR), emphasize a harmonized and coordinated approach with participation of all member states (15). Surveillance has been recommended in high-risk countries. In the event of incursion, vaccination programs are recommended using the Nigeria 75/1 live vaccine produced by the Botswana Vaccine Institute, among others. Along the borders of Tanzania and the DRC, where PPRV is already present, vaccination is recommended in a 50-km-wide buffer zone (15). To control the disease in already infected countries, vaccination is the best choice. Stamping out is only a good option in smaller and well-defined infected populations, due to the high costs associated with compensation. The vaccination campaigns should take place every year to give a high enough herd immunity in populations like those of sheep and goats, which have a high turnover rate (15).
Conclusions
PPRV has probably been present in Tanzania since 2004, and the clinical episodes of 2008 were suspected to have been introduced into the northern part of the country from neighboring countries through uncontrolled animal movement. The disease spread to the southern parts in 2010/2011, and PPR is now considered endemic in the domestic sheep and goat populations in Tanzania. Legal and illegal movement of animals for trade is instrumental in the spread of PPR into and within Tanzania. The implications of PPRV are severe as small ruminant livestock production is an important income for a large group of the country's population. The FAO/OIE Global Strategy for the Control and Eradication of PPR has been formulated, and national and regional control strategies have to be aligned with it. This goal is supported by the AU-IBAR, which together with the SADC is working on strategies to control PPRV within Tanzania and other African countries. To implement these strategies and reach, support, and engage the sheep and goat holders, sufficient resources are needed for veterinary services to cover the whole country, including remote areas. The need to engage sheep and goat holders in the eradication of PPRV cannot be emphasized enough.
Acknowledgements
Research on PPRV in JJW, MB, and GM laboratories is supported by grants from the Swedish Research Council (Grant 348-2013-6402 and 348-2014-4293) and the Wellcome Trust (Grant WT087546MA) to the Southern African Centre for Infectious Disease Surveillance (SACIDS) at Sokoine University of Agriculture. The authors thank Emmanuel Sadikiel Macha for providing them pictures of goats with PPR taken during the 2013 outbreak in Ngorongoro. ET and TK are supported by the Swedish Research Council (Grant 348-2013-6402) and the Wellcome Trust (Grant WT087546MA).
Conflict of interest and funding
The authors declare no conflict of interest. Research on PPRV in JJW, MB and GM laboratories is supported by grants from the Swedish Research Council (Grant 348-2013-6402 and 348-2014-4293) and the Wellcome Trust (Grant WT087546MA).
References
- 1.Woo PC, Lau SK, Wong BH, Fan RY, Wong AY, Zhang AJ, et al. Feline morbillivirus, a previously undescribed paramyxovirus associated with tubulointerstitial nephritis in domestic cats. Proc Natl Acad Sci USA. 2012;109:5435–40. doi: 10.1073/pnas.1119972109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anderson J, Baron M, Cameron A, Kock R, Jones B, Pfeiffer D, et al. Rinderpest eradicated; what next? Vet Rec. 2011;169:10–11. doi: 10.1136/vr.d4011. [DOI] [PubMed] [Google Scholar]
- 3.Munir M, Zohari S, Berg M, editors. Molecular biology and pathogenesis of peste des petits ruminants virus. Heidelberg: Springer; 2013. Genome organization of peste des petits ruminants virus; pp. 1–22. [Google Scholar]
- 4.Gargadennec L, Lalanne A. La peste des petits ruminants. Bull Serv Zootech Epizoot Afr Occid Fr. 1942;5:15–21. [Google Scholar]
- 5.Gibbs E, Taylor W, Lawman M, Bryant J. Classification of peste des petits ruminants virus as the fourth member of the genus morbillivirus. Intervirology. 1979;11:268–74. doi: 10.1159/000149044. [DOI] [PubMed] [Google Scholar]
- 6.Banyard AC, Parida S, Batten C, Oura C, Kwiatek O, Libeau G. Global distribution of peste des petits ruminants virus and prospects for improved diagnosis and control. J Gen Virol. 2010;91(Pt 12):2885–97. doi: 10.1099/vir.0.025841-0. [DOI] [PubMed] [Google Scholar]
- 7.Kwiatek O, Ali YH, Saeed IK, Khalafalla AI, Mohamed OI, Obeida AA, et al. Asian lineage of peste des petits ruminants virus, Africa. Emerg Infect Dis. 2011;17:1223–31. doi: 10.3201/eid1707.101216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Munir M, Zohari S, Berg M, editors. Molecular biology and pathogenesis of peste des petits ruminants virus. Heidelberg: Springer; 2013. Pathophysiology and clinical assessment of peste des petits ruminants; pp. 33–48. [Google Scholar]
- 9.Couacy-Hymann E, Bodjo C, Danho T, Libeau G, Diallo A. Surveillance of wildlife as a tool for monitoring rinderpest and peste des petits ruminants in West Africa. Rev Sci Tech. 2005;24:869–77. [PubMed] [Google Scholar]
- 10.Roeder P, Obi T. Recognizing PPR a field manual, Rome: Food and Agriculture Organization. 1999. pp. 175–81. [Google Scholar]
- 11.Dundon WG, Kihu SM, Gitao GC, Bebora LC, John NM, Oyugi JO, et al. Detection and genome analysis of a lineage III peste des petits ruminants virus in Kenya in 2011. Transbound Emerg Dis. 2015 doi: 10.1111/tbed.12374. doi: http://dx.doi.org/10.1111/tbed.12374. [DOI] [PubMed] [Google Scholar]
- 12.FAO. Animal Production and Health Position Paper. Rome: Fao; 2013. Supporting livelihoods and building resilience through peste des petits ruminants (PPR) and small ruminant disease control. Available from: http://www.fao.org/publications/card/en/c/69a3a465-f2c7-5c92-b87a-0621c419e063/ [Google Scholar]
- 13.FAO, OIE. Global strategy for the control and eradication of PPR. 2015. Available from: http://www.fao.org/3/a-i4460e.pdf [cited 3 May 2016].
- 14.Chazya R, Muma JB, Mwacalimba KK, Karimuribo E, Mkandawire E, Simuunza M. A qualitative assessment of the risk of introducing peste des petits ruminants into northern Zambia from Tanzania. Vet Med Int. 2014;2014:202618. doi: 10.1155/2014/202618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.SADC. Southern African Development Community (SADC) control strategy for peste des petit ruminants (PPR) 2012. Available from: http://www.sadc.int/documents-publications/show/SADC%20Control%20Strategy%20for%20Peste%20de%20Petit%20Ruminants%20(PPR) [cited 04 September 2015].
- 16.Wamwayi HM, Rossiter PB, Kariuki DP, Wafula JS, Barrett T, Anderson J. Peste des petits ruminants antibodies in east Africa. Vet Rec. 1995;136:199–200. doi: 10.1136/vr.136.8.199. [DOI] [PubMed] [Google Scholar]
- 17.Luka PD, Erume J, Mwiine FN, Ayebazibwe C. Molecular characterization of peste des petits ruminants virus from the Karamoja region of Uganda (2007–2008) Arch Virol. 2012;157:29–35. doi: 10.1007/s00705-011-1135-4. [DOI] [PubMed] [Google Scholar]
- 18.OIE. World animal health information database (WAHIS) interface. 2016. Available from: http://www.oie.int/wahis_2/public/wahid.php/Wahidhome/Home [cited 14 September 2016].
- 19.FAO. Livestock epidemic causing havoc in Democratic Rebuplic of the Congo. 2012. Available from: http://www.fao.org/news/story/en/item/150317/icode/ [cited 30 September 2016].
- 20.Wambura P. Serological evidence of the absence of peste des petits ruminants in Tanzania. Vet Rec. 2000;146:473–4. doi: 10.1136/vr.146.16.473. [DOI] [PubMed] [Google Scholar]
- 21.Kivaria FM, Kwiatek O, Kapaga AM, Swai ES, Libeau G, Moshy W, et al. The incursion, persistence and spread of peste des petits ruminants in Tanzania: epidemiological patterns and predictions. Onderstepoort J Vet Res. 2013;80:1–10. doi: 10.4102/ojvr.v80i1.593. [DOI] [PubMed] [Google Scholar]
- 22.Swai ES, Kapaga A, Kivaria F, Tinuga D, Joshua G, Sanka P. Prevalence and distribution of peste des petits ruminants virus antibodies in various districts of Tanzania. Vet Res Commun. 2009;33:927–36. doi: 10.1007/s11259-009-9311-7. [DOI] [PubMed] [Google Scholar]
- 23.Karimuribo E, Loomu P, Mellau L, Swai E. Retrospective study on sero-epidemiology of peste des petits ruminants before its official confirmation in northern Tanzania in 2008. Res Opin Anim Vet Sci. 2011;1:184–7. [Google Scholar]
- 24.Muse EA, Karimuribo ED, Gitao GC, Misinzo G, Mellau LS, Msoffe PL, et al. Epidemiological investigation into the introduction and factors for spread of peste des petits ruminants, Southern Tanzania. Onderstepoort J Vet Res. 2012;79:49–54. doi: 10.4102/ojvr.v79i2.457. [DOI] [PubMed] [Google Scholar]
- 25.Aziz-ul-Rahman, Abubakar M, Rasool MH, Manzoor S, Saqalein M, Rizwan M, et al. Evaluation of risk factors for peste des petits ruminants virus in sheep and goats at the Wildlife-Livestock Interface in Punjab Province, Pakistan. BioMed Res Int 2016. 2016:7826245. doi: 10.1155/2016/7826245. doi: http://dx.doi.org/10.1155/2016/7826245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abubakar M, Jamal SM, Hussain M, Ali Q. Incidence of peste des petits ruminants (PPR) virus in sheep and goat as detected by immuno-capture ELISA (Ic ELISA) Small Ruminant Res. 2008;75:256–9. [Google Scholar]
- 27.Hussain M, Muneer R, Jahangir M, Awan A, Khokhar M, Zahur A, et al. Chromatographic strip technology: a pen-side test for the rapid diagnosis of pests des petits ruminants in sheep and goats. J Biol Sci. 2003;3:1–7. [Google Scholar]
- 28.Lembo T, Oura C, Parida S, Hoare R, Frost L, Fyumagwa R, et al. Peste des petits ruminants infection among cattle and wildlife in northern Tanzania. Emerg Infect Dis. 2013;19:2037. doi: 10.3201/eid1912.130973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahapatra M, Sayalel K, Muniraju M, Eblate E, Fyumagwa R, Shilinde S, et al. Spillover of Peste des petits ruminants virus from domestic to wild ruminants in the Serengeti ecosystem, Tanzania. Emerg Infect Dis. 2015;21:2230. doi: 10.3201/eid2112.150223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Swai E, Moshy W, Mbise E, Kaaya J, Bwanga S. Serological evidence of camel exposure to peste des petits ruminants virus in Tanzania. Res Opin Anim Vet Sci. 2011;1:325–9. [Google Scholar]
- 31.Abraham G, Sintayehu A, Libeau G, Albina E, Roger F, Laekemariam Y, et al. Antibody seroprevalences against peste des petits ruminants (PPR) virus in camels, cattle, goats and sheep in Ethiopia. Prev Vet Med. 2005;70:51–7. doi: 10.1016/j.prevetmed.2005.02.011. [DOI] [PubMed] [Google Scholar]
- 32.Khalafalla AI, Saeed IK, Ali YH, Abdurrahman MB, Kwiatek O, Libeau G, et al. An outbreak of peste des petits ruminants (PPR) in camels in the Sudan. Acta Trop. 2010;116:161–5. doi: 10.1016/j.actatropica.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 33.Kgotlele T, Macha E, Kasanga C, Kusiluka L, Karimuribo E, Van Doorsselaere J, et al. Partial genetic characterization of peste des petits ruminants virus from goats in northern and eastern Tanzania. Transbound Emerg Dis. 2014;61(Suppl. 1):56–62. doi: 10.1111/tbed.12229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woma TY, Kalla DJ, Ekong PS, Ularamu HG, Chollom SC, Lamurde II, et al. Serological evidence of camel exposure to peste des petits ruminants virus (PPRV) in Nigeria. Trop Anim Health Prod. 2015;47:603–6. doi: 10.1007/s11250-014-0747-6. [DOI] [PubMed] [Google Scholar]
- 35.Misinzo G, Kgotlele T, Muse E, Van Doorsselaere J, Berg M, Munir M. Peste des petits ruminants virus lineage II and IV from goats in southern Tanzania during and outbreak in 2011. British J Virol. 2015;2:1–4. [Google Scholar]
- 36.Libeau G, Diallo A, Parida S. Evolutionary genetics underlying the spread of peste des petits ruminants virus. Anim Front. 2014;4:14–20. [Google Scholar]
- 37.Cêtre-Sossah C, Kwiatek O, Faharoudine A, Soulé M, Moutroifi Y, Vrel M, et al. Impact and epidemiological investigations into the incursion and spread of peste des petits ruminants in the Comoros Archipelago: an increased threat to surrounding Islands. Transbound Emerg Dis. 2016;63:452–9. doi: 10.1111/tbed.12296. [DOI] [PubMed] [Google Scholar]
- 38.United Republic of Tanzania (URT) National sample census of agriculture, small holder agriculture. Volume III: Livestock sector – National Report. 2012. Available from: http://www.kilimo.go.tz/agricultural%20statistics/web/Nationa%20sample%20census%20of%20Agriculture%20200708%20vol.%20111/FINAL%20LIVESTOCK-%20NATIONAL%20Final%20Draft%2010%20March%202012.pdf [cited 07 September 2015].
- 39.Covarrubias K, Nsiima L, Zezza A. Livestock and livelihoods in rural Tanzania, a descriptive analysis of the 2009 National Panel Survey. 2012. Available from: http://openknowledge.worldbank.org/bitstream/handle/10986/17886/866280WP0Lives00Box385181B00PUBLIC0.pdf?sequence=1.
- 40.United Republic of Tanzania (URT) Working document, No 1. Nairobi, Kenya: FAO Animal Health and Production, ECTAD. 2013. Peste des petites ruminants (PPR): assessment of socio economic impacts in the Republic of Tanzania. [Google Scholar]
- 41.United Republic of Tanzania (URT) Peste des petits ruminants (PPR) progressive control and eradication strategy. 2013. [Google Scholar]
- 42.Mbyuzi AO, Komba EV, Kimera SI, Kambarage DM. Sero-prevalence and associated risk factors of peste des petits ruminants and contagious caprine pleuro-pneumonia in goats and sheep in the Southern Zone of Tanzania. Prev Vet Med. 2014;116:138–44. doi: 10.1016/j.prevetmed.2014.06.013. [DOI] [PubMed] [Google Scholar]
- 43.Fischer K, Chenais E, Torsson E, Wensman JJ. Where is the participation in participatory epidemiology? How engagement with social science could lead to improved understanding and control of peste des petits ruminants. British J Virol. 2016;3:105–14. [Google Scholar]
- 44.FAO. Capacity building to prevent peste des petit ruminants (PPR) introduction into Malawi. Mozambique and Zambia. 2013. Available from: http://www.fao.org/africa/programmes-and-projects/projects/en/ [cited 29 Agust 2016].