Abstract
Aging impairs skeletal muscle protein synthesis, leading to muscle weakness and atrophy. However, the underlying molecular mechanisms remain poorly understood. Here, we review evidence that mTORC1- and ATF4-mediated amino acid sensing pathways, triggered by impaired amino acid delivery to aged skeletal muscle, may play important roles in skeletal muscle aging. Interventions that alleviate age-related impairments in muscle protein synthesis, strength and/or muscle mass appear to do so by reversing age-related changes in skeletal muscle amino acid delivery, mTORC1 activity and/or ATF4 activity. An improved understanding of the mechanisms and roles of amino acid sensing pathways in skeletal muscle may lead to evidence-based strategies to attenuate sarcopenia.
Keywords: leucine, mTORC1, ATF4, ursolic acid, tomatidine, GCN2
Introduction
Aging reduces skeletal muscle strength and muscle quality (i.e., strength per unit muscle mass) and ultimately causes age-related skeletal muscle atrophy, also known as sarcopenia. The early stages of skeletal muscle aging are primarily marked by reduced strength, which becomes apparent around the age of 40, and can progressively impair normal activities and quality of life. The late stage of skeletal muscle aging, sarcopenia, has serious health consequences, including falls, immobility, loss of independent living, and increased mortality [1]. The cellular and molecular mechanisms of skeletal muscle aging are complex, just beginning to be revealed, and still poorly understood. Here, we review current evidence linking skeletal muscle aging to amino acid sensing mechanisms within skeletal muscle fibers.
Aging impairs delivery of dietary amino acids (AAs) to skeletal muscle
Muscle mass is ultimately controlled by the cellular processes of protein synthesis and breakdown (i.e., protein turnover). During conditions of muscle growth or hypertrophy, the rate of protein synthesis exceeds the rate of protein breakdown (i.e., muscle protein anabolism). However, muscle catabolism occurs when the rate of protein breakdown exceeds the rate of protein synthesis. In all types of skeletal muscle atrophy, including sarcopenia, the normal balance between protein synthesis and protein breakdown is disrupted, leading to a net loss of protein and muscle fiber size [2–4]. Although some early studies reported a basal (i.e., following an overnight fast) mismatch between muscle protein synthesis and breakdown in elderly humans (e.g., lower rates of synthesis and/or higher rates of breakdown), most studies have found that the rates of muscle protein turnover are not abnormal in elderly individuals under basal conditions [5–10]. These findings indicated that other factors that influence muscle protein turnover may be responsible for muscle atrophy during aging. Feeding and physical activity are two key factors that control daily muscle protein turnover. In young adults, feeding increases blood AA and insulin concentrations, which stimulates muscle protein anabolism by increasing the rate of protein synthesis and slightly reducing the rate of protein breakdown. On the other hand, muscle contraction from exercise or physical activity stimulates muscle protein anabolism independent of changes in circulating amino acids or insulin. Although aged skeletal muscle exhibits normal protein metabolism under basal conditions, it does not respond appropriately to low, physiologic doses of anabolic stimuli, such as insulin, exercise or nutrient intake. This phenomenon has been termed “anabolic resistance” [11].
The etiology of anabolic resistance is complex and multifactorial. One aspect involves a diminished response to protein or AA ingestion. For example, ingestion of an AA and carbohydrate mixture significantly increases muscle protein synthesis in young adults; however, it has no effect on muscle protein synthesis in older adults [12]. Removing carbohydrates and non-essential AAs from the mixture has no effect on the ability of essential AAs to stimulate muscle protein synthesis in young and older adults [13–14], indicating that the essential AAs are solely responsible for the nutrient induced muscle protein anabolic response. However, anabolic resistance is still detected in aging muscle when essential AAs alone are administered [15–16], and larger doses are required to stimulate muscle protein synthesis than those needed in younger individuals [8, 17]. The ability of larger doses of essential AAs to overcome anabolic resistance appears to be due to the content of leucine in the nutrient mixture. For example, a small dose of essential AAs (7 grams) is capable of stimulating muscle protein synthesis in young adults but not in older adults [16]. However, if the content of leucine is increased, then a dose of 7 grams of essential AAs can stimulate muscle protein synthesis in older adults [18]. This finding has led to the notion of a “leucine threshold” that must be met in order for AAs to maximize the rate of muscle protein synthesis. In young adults, this threshold appears to be ~2 grams of leucine whereas with aging the threshold is ~3 grams of leucine that must be ingested to adequately stimulate muscle protein synthesis [8, 18–19].
Another aspect of anabolic resistance involves an impaired muscle protein anabolic response to insulin or muscle contraction. Infusion of insulin to increase blood insulin to postprandial levels in glucose tolerant older adults does not stimulate muscle protein synthesis as it does in younger adults [20]. Hyperinsulinemia, above postprandial levels, is required to stimulate muscle protein synthesis in older adults, indicating a true resistance of muscle protein anabolism to insulin [21]. In addition, a bout of resistance exercise significantly increases muscle protein synthesis for up to 48 hours following exercise in young adults [22], whereas muscle protein synthesis is not increased in older adults post-exercise [23–24]. Therefore, three common stimuli (protein intake, insulin and physical activity) become less effective at enhancing muscle protein anabolism with advancing age.
Since anabolic resistance can be overcome by increasing the amount of protein ingested or increasing the infusion rate of insulin, the delivery of AAs to muscle tissue might be a rate-limiting factor in stimulating muscle protein synthesis in older adults. This hypothesis was tested via a pharmacological approach to block or induce vasodilation during exposure to AAs or insulin in humans. When insulin was directly infused into the leg of young adults with or without the nitric oxide synthase (NOS) inhibitor L-NMMA, the normal increase in blood flow, AA delivery and muscle protein synthesis were blocked when vasodilation was blocked with the NOS inhibitor [25]. In older adults, during an insulin infusion with or without a vasodilator (sodium nitroprusside), enhancing vasodilation during insulin infusion restored blood flow, AA delivery and muscle protein synthesis [26]. A similar experiment used sodium nitroprusside during AA ingestion and found that vasodilation during hyperaminoacidemia in older adults was capable of increasing blood flow, AA delivery and muscle protein synthesis to levels similar to that of their young counterparts [27]. These studies indicated that vasodilation of blood vessels in muscle is necessary for increasing AA delivery to muscle in order to increase muscle protein synthesis, and that impairments in AA delivery to muscle may be an underlying cause of anabolic resistance in aging.
Based on results such as these, some have speculated that anabolic resistance may be a central cause of sarcopenia [11]. Consistent with this notion, anabolic resistance is also observed during other conditions that cause skeletal muscle atrophy, such as inactivity, trauma, cancer cachexia and burn injury [28–30]. However, a causal relationship between anabolic resistance and skeletal muscle atrophy has not been definitively established. It also remains unclear if anabolic resistance is present during the early stages of skeletal muscle aging, when loss of strength and muscle quality are apparent, but a loss of muscle mass is not. The role of physical activity in anabolic resistance is also not completely understood, and it is not known whether anabolic resistance is a condition of aging per se or if physical inactivity is a primary cause. Importantly, all of the aforementioned characterizations of anabolic resistance have involved systemic administration of AAs or insulin, or exercise of whole limbs, rather than direct application of anabolic stimuli to muscle fibers. Thus, it remains unknown if anabolic resistance is a primary disorder of skeletal muscle fibers and/or other cell types within skeletal muscle. Indeed, as discussed below, accumulating evidence suggests that an important site of anabolic resistance may lie in the vasculature that provides insulin and AAs to skeletal muscle fibers.
Mechanisms of impaired AA delivery to aged skeletal muscle
As mentioned in the previous section, AA delivery to muscle is impaired with aging. Vascular tone in humans is determined via a balance between local vasoconstrictors and vasodilators produced by the endothelium [31]. Insulin stimulates NO production by activating endothelial NOS which results in vasodilation, increased muscle perfusion and capillary recruitment [25]. It is also well-known that aging is associated with reduced endothelial-derived vasodilation which appears to be due to an increase in circulating endothelin-1 (i.e., an endothelial-derived vasoconstrictor) [31]. In fact, it was previously shown that during anabolic resistance to insulin, endothelin-1 concentrations were much higher in older adults as compared to young adults at baseline and during the insulin infusion [20]. Since aerobic exercise has been shown to reduce blood endothelin-1 concentrations and improve endothelial function in both young and older adults, a subsequent study tested the impact of a 45-minute bout of aerobic exercise the evening prior to receiving an insulin infusion, on older adults. Aerobic exercise reduced blood endothelin-1 concentrations the following morning, which resulted in improved blood flow, AA delivery and muscle protein synthesis in response to insulin [32]. Furthermore, visualization and quantitation via contrast-enhanced ultrasound showed that capillary recruitment is a real indicator of endothelial function. Blocking endothelial-dependent vasodilation with L-NMMA in young adults prevented the normal increase in insulin-induced capillary recruitment and microvascular perfusion [25]. In contrast, when older adults receive an insulin infusion or ingest AAs concurrently with sodium nitroprusside, capillary recruitment and microvascular perfusion are restored to what is normally seen in young adults [26–27]. Finally, when a bout of aerobic exercise is performed the evening prior to ingesting a mixed meal, the feeding induced increase in capillary recruitment and microvascular perfusion is enhanced in older adults [33]. In all cases described above, restoration of microvascular perfusion in older adults allowed insulin and AAs to stimulate muscle protein synthesis and overcome anabolic resistance. This evidence strongly supports a role for endothelial dysfunction as an important underlying cause of anabolic resistance in aging.
AA delivery to muscle is enhanced when microvascular perfusion of muscle tissue is increased via endothelial-derived vasodilation, allowing the muscle tissue to be exposed to more insulin and AAs. However, insulin and AAs need to cross the endothelial cell and the interstitial space between the endothelial cells and the muscle sarcolemma before exerting their effects on skeletal muscle cells. Therefore, transport of insulin and AAs may be a rate-limiting factor in allowing anabolic stimuli to increase muscle protein synthesis. Stable isotopic tracers are used to measure the rate of transport of AAs from the blood into the muscle. In all the conditions described above wherein microvascular perfusion is increased, the delivery of AAs and the rate of AA transport into muscle cells is increased [25–27, 33]. This leads to the question as to whether there is a difference in AA transport rates across the endothelial cell versus AA transport into the muscle cell. Only one study [34] has attempted to look at this question by using a four-compartment stable isotope model (i.e., requiring sampling from the artery, vein, interstitium and muscle) to assess AA transport rates from the blood compartment into the interstitium, and comparing that rate to transport rates from the interstitium into muscle (i.e., muscle specific AA transport). The researchers found that AA transport rates were much lower across the endothelium, as compared to muscle transport [34], indicating that the rate-limiting process for AA transport is most likely regulated by endothelial function and microvascular perfusion, rather than muscle specific amino acid transport.
Although it is unclear whether AA transporters are involved in anabolic resistance, the mRNA expression of AA transporter is enhanced when insulin and AA availability is increased, or following a bout of exercise [35–36]. Basal expression of muscle AA transporters is not altered with aging, but a differential expression is seen following exercise and AA ingestion [6, 37]. Two primary limitations with the AA transporter literature include the lack of stable isotope studies measuring direct transport rates into muscle, and the lack of a good method to measure AA transporter translocation from the cytosol to the plasma membrane under different conditions. However, there are some intriguing data available, which suggest that AA transporter may be involved in anabolic resistance. For example, when older men are placed on bed rest for seven days, their muscle protein anabolic response to AA ingestion is impaired [38]. An interesting finding from this study was that, prior to bed rest, ingestion of a sufficient dose of AAs increased muscle AA transporter expression concurrently with muscle protein synthesis. However, following seven days of bed rest, the same AA ingestion was incapable of increasing muscle AA transporter expression or muscle protein synthesis [38]. In any event, despite the lack of definitive data for a regulatory role for AA transporters in anabolic resistance, endothelial function and microvascular perfusion are tightly linked to AA delivery and transport into muscle, which is a clear attribute of anabolic resistance.
Impaired AA delivery activates AA sensing mechanisms that reduce muscle protein synthesis
Because AAs are required for virtually every cellular process, impairments in skeletal muscle AA delivery could theoretically cause a catastrophic reduction in AA levels within skeletal muscle fibers. Fortunately, however, all mammalian cell types, including muscle fibers, possess evolutionarily ancient regulatory mechanisms that sense small changes in intracellular AA levels (see Box 1, Figure I). When intracellular AA levels begin to fall, these AA sensing pathways reduce protein synthesis (AA consumption) to maintain viable concentrations of intracellular AAs. Two of the best-characterized mammalian AA sensing pathways involve the mammalian/mechanistic target of rapamycin complex 1 (mTORC1) and activating transcription factor 4 (ATF4). Recent evidence suggests that both of these pathways contribute to reduced protein synthesis and ultimately atrophy in aged skeletal muscle.
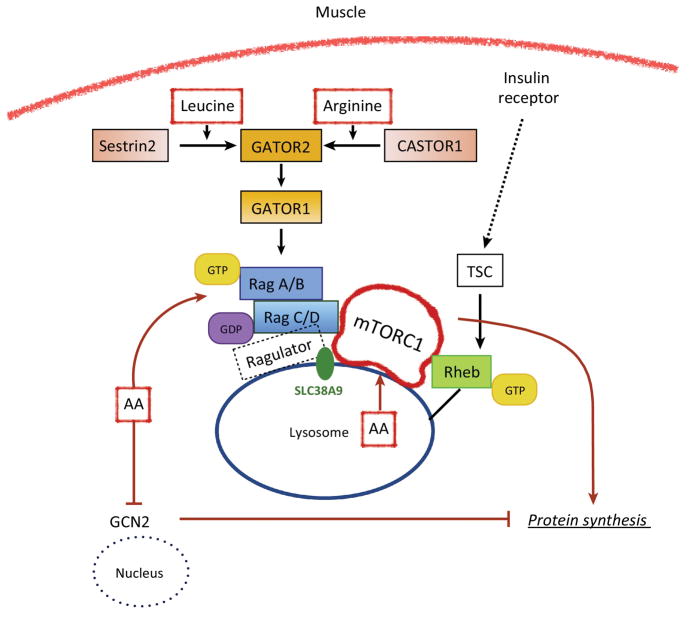
Box 1. Amino Acid Sensors in Cell.
It is well established that leucine is a potent stimulator of muscle protein synthesis and mTORC1 signaling in human skeletal muscle. However, the mechanisms underlying how muscle cells “sense” AAs have remained elusive. As shown schematically in Figure I below, recent work has discovered that during AA sufficiency, mTORC1 kinase activity is stimulated when it is translocated from the cytosol to the lysosomal membrane to interact with Rheb. Rag GTPases (RagA or RagB bound to RagC or RagD) respond to AAs within the cell by altering their nucleotide state and their interaction with Ragulator (a lysosomal scaffold). GATOR1 is a complex of three proteins and acts as a GAP (GTPase activating protein) for RagA/B, which inhibits mTORC1 activation. Upstream of GATOR1 is a five-protein complex known as GATOR2 that is considered a positive regulator of mTORC1 due to its ability to inhibit GATOR1. Exciting results from new studies, performed primarily in HEK293T cells, have identified at least three previously unknown AA sensors. The lysosomal AA transporter SLC38A9 has been linked to sensing arginine sufficiency within the lysosome, and interacts with Ragulator to activate mTORC1 [43]. More recently, a leucine sensor (Sestrin2) has been identified. Leucine blocks the interaction of Sestrin2 with the GATOR2 complex, which ultimately allows mTORC1 to be activated [44]. A similar mechanism exists for a newly identified arginine sensor (CASTOR1). In this case, arginine binding to CASTOR1 disrupts the GATOR2-CASTOR1 complex thereby allowing GATOR2 to inhibit GATOR1 and eventually leading to activation of mTORC1.
FIGURE I. Proposed amino acid sensing mechanisms in skeletal muscle fibers.
A hypothetical muscle fiber (adapted from reference 45) is shown that highlighting the recently identified AA sensing apparatus for leucine and arginine signaling to mTORC1. However, it is currently unknown whether leucine and arginine sensing in skeletal muscle cells is identical to what has been identified in kidney cells.
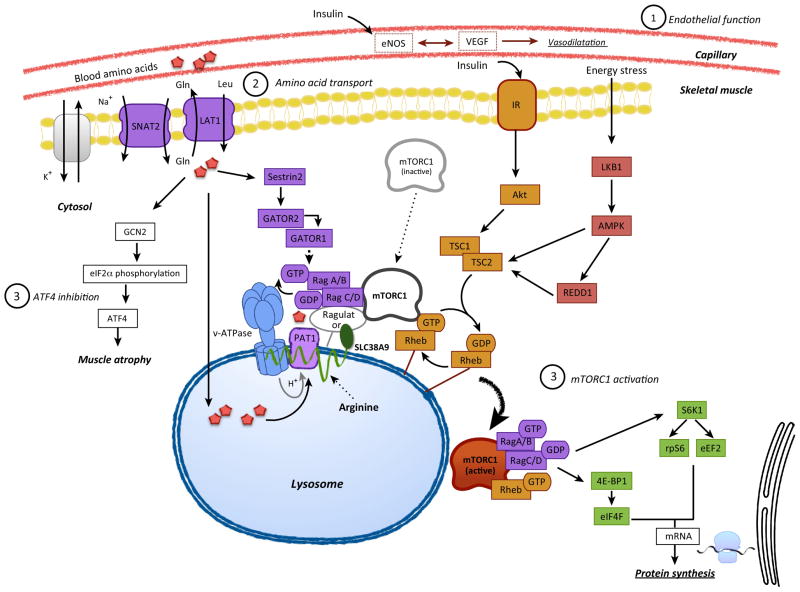
mTORC1 has kinase activity that plays an essential role in protein synthesis. As shown in Figure 1, activation of mTORC1 signaling results in enhanced translation initiation, elongation and an increase in protein synthesis [39]. In the skeletal muscle of young adults, mTORC1 activity is stimulated by AAs [36, 40–41], insulin [25] and muscle contraction [24, 42]. Most of these early studies in humans showed a correlation between mTORC1 activation and a subsequent increase in the rate of muscle protein synthesis. However, when rapamycin (a specific inhibitor of mTOR) is administered to young adults prior to ingesting essential AAs or performing a bout of resistance exercise, the ability to activate mTORC1 is blunted, and muscle protein synthesis is not stimulated [41–42]. These findings indicated that mTORC1 activation is required for AA and exercise induced increases in muscle protein synthesis in humans.
Figure 1. Increased AA delivery to muscle enhances AA sensing and activates protein synthesis.
We propose three main mechanisms that regulate AA sensing within muscle cells. 1) Vascular endothelial function is important for enhancing nutrient and hormone delivery to muscle tissue via vasodilation. 2) AA transport from the blood, across the endothelial cell, through the interstitial space, and eventually into muscle cells is dependent upon functional AA transporters. 3) AA sensing via activation of mTORC1 signaling and inhibition of ATF4 are important for fully activating muscle protein synthesis in response to AA sufficiency.
Aging is associated with a reduced ability to activate mTORC1 signaling. For example, infusion of insulin to postprandial levels increases mTORC1 signaling in young adults, but not in older adults [20]. A high dose of insulin is required to stimulate mTORC1 signaling in older adults [21]. To determine whether activation of mTORC1 was linked to AA delivery to muscle, an insulin infusion was performed in the presence of L-NMMA, in young adults. Preventing insulin-induced vasodilation and AA delivery to muscle resulted in smaller mTORC1 activation, as compared to the group receiving insulin only [25]. On the other hand, providing sodium nitroprusside during an insulin infusion in older adults, did restore muscle protein synthesis, as described previously, concurrently with an activation of mTORC1 signaling [26]. However, mTORC1 signaling was similar to the insulin only group. Similarly, when a mixed meal containing AAs and carbohydrate was provided to older adults, mTORC1 signaling was activated but protein synthesis was not, unless a bout of exercise was added to increase AA delivery by improving endothelial function [33]. These findings suggest that, in the condition of anabolic resistance, the administration of AAs or insulin can activate the mTORC1 signaling pathway, but protein synthesis is not fully stimulated until an adequate amount of intracellular AAs become available to support protein synthesis. On the other hand, anabolic resistance is clearly detected following a bout of resistance exercise in older adults [23–24]. In young adults, following a bout of high intensity resistance exercise, mTORC1 signaling is significantly activated in combination with an increase in muscle protein synthesis [24] for at least 24 hours post-exercise. In older adults, mTORC1 signaling is reduced and protein synthesis blunted [24]. As shown in Figure 1, insulin, AAs and muscle contraction all activate mTORC1 through three different and independent pathways. Whereas insulin activates mTORC1 through the insulin signaling pathway (i.e., Akt), our understanding of how cells sense AAs and how muscle contraction activates mTORC1 is only recently being uncovered [43–45]. In summary, the data presented above provides evidence that mTORC1 activation is partially dependent upon AA delivery and transport into muscle and, in order to sufficiently stimulate protein synthesis, a significant amount of AAs must enter the muscle cell.
In contrast to mTORC1, which stimulates protein synthesis via multiple mechanisms but is less active in response to anabolic stimul in aged skeletal muscle, ATF4 inhibits protein synthesis via multiple mechanisms and is more active in aged skeletal muscle. ATF4 is a protein in the basic leucine zipper (bZIP) transcription factor family, capable of interacting with a number of other bZIP proteins to form functional dimeric transcription factors [46]. In healthy young adult skeletal muscle fibers, ATF4 activity is low, but it rises during a variety of conditions that cause skeletal muscle atrophy, including starvation, muscle disuse and aging [47–49]. Increased ATF4 activity is sufficient to reduce protein synthesis and cause atrophy in the skeletal muscle fibers of young adult mice [48–49]. Conversely, mice lacking ATF4 activity in skeletal muscle fibers (muscle-specific ATF4 knockout mice) have elevated levels of muscle protein synthesis and maintain young adult levels of muscle strength, quality and mass, into old age [49]. ATF4 is currently the only known example of a skeletal muscle protein that is required for the loss of strength, muscle quality and muscle mass during aging (Figure 2). In addition to its essential role in age-related muscle weakness and atrophy, ATF4 is also partially required for skeletal muscle atrophy during starvation and limb immobilization [48, 50–51].
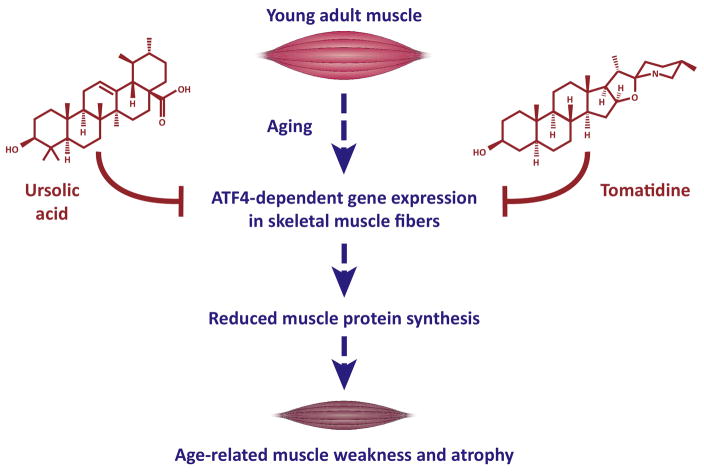
Figure 2. Impact of ATF4, ursolic acid, and tomatidine on skeletal muscle during aging.
The schematic shows a model of the effects of ATF4, ursolic acid, and tomatidine in skeletal muscle and with aging. ATF4 is required for the loss of skeletal muscle strength, quality and mass during aging [49]. Ursolic acid and tomatidine are natural compounds that blunt ATF4 activity in aged skeletal muscle, leading to reduced weakness and atrophy in aged skeletal muscle [49].
ATF4 regulates the expression of roughly 250 genes in skeletal muscle fibers [48–50]. At this point, only a few ATF4-dependent genes have been investigated at a functional level in skeletal muscle; however, three of those genes (Gadd45a, p21/Cdkn1a and 4E-BP1/Eif4ebp1) appear to play important roles in skeletal muscle aging. ATF4 is sufficient to induce the Gadd45a, p21 and 4E-BP1 genes in skeletal muscle fibers, leading to increased expression of Gadd45a, p21 and 4E-BP1 proteins [48–50]. Both Gadd45a and p21 are sufficient to induce skeletal muscle fiber atrophy in vivo [50–51]. Furthermore, Gadd45a reduces protein synthesis in cultured skeletal myotubes by a complex mechanism that includes inhibition of anabolic signaling [50]. 4E-BP1 is a potent and direct protein synthesis inhibitor whose activity is inhibited by mTORC1. These data suggest Gadd45a, p21 and 4E-BP1 as important downstream mediators of ATF4 in skeletal muscle aging. However, given the large size of the ATF4-dependent genetic program in skeletal muscle, additional ATF4-dependent mediators of skeletal muscle aging may remain to be discovered.
The upstream mechanism that controls ATF4 activity in aged skeletal muscle is not yet known. One well established mechanism for increasing ATF4 expression and activity is activation of eIF2α kinases [52]. Mammalian cells possess four eIF2α kinases that are activated by a variety of cellular stresses. Interestingly, one of these kinases, GCN2, is an AA sensor that is evolutionarily conserved from yeast to humans. In direct contrast to mTORC1, GCN2 is activated by low levels of AAs. When active, GCN2 phosphorylates the translation initiation factor eIF2α, leading to an overall reduction in protein synthesis. However, eIF2α phosphorylation also enhances synthesis of a few specific proteins, including ATF4. It is interesting to speculate that impaired AA delivery in aged skeletal muscle may coordinately decrease mTORC1 activity and increase GCN2 activity, and we believe this is another important area for future investigation.
Strategies to restore AA delivery, stimulate mTORC1 and/or inhibit ATF4 in aged skeletal muscle
Physical activity
Endothelial dysfunction is an attribute of aging. One bout of aerobic exercise can temporarily restore endothelial dysfunction, and aerobic exercise training can significantly improve endothelial function, in older adults [53]. In the studies described above acute aerobic exercise is effective at improving the anabolic action of insulin and AAs in aged human skeletal muscle [33], whereas bed rest in older adults reduces the anabolic action of AAs in muscle [38]. In addition, one study found that simply reducing the number of steps taken each day in younger adults also prevented AAs from stimulating muscle protein synthesis, indicating an important role for physical inactivity as an underlying cause of anabolic resistance [54]. Recently, blood flow restriction exercise has been found to be effective at improving endothelial function in older adults [55] and this may help explain its effectiveness in activating mTORC1 signaling and MPS in older adults [56]. It is not known whether chronic exercise training in older adults can abolish anabolic resistance.
Dietary protein and leucine
The acute studies described above demonstrate that increasing the amount of protein or leucine ingested is capable of overcoming anabolic resistance. A recent review of the literature has recommended that older adults should consume a slightly higher amount of protein to maintain muscle mass [57], and some evidence suggests that consuming a sufficient amount of protein at each meal (evenly distributed throughout the day) may be an effective strategy at maintaining muscle mass during aging [58–59]. The underlying rationale for increasing the amount of protein ingested at each meal is to ensure that the leucine threshold is met for older adults. It is not known whether a chronic increase in daily physical activity or exercise training is capable of lowering the leucine threshold in older adults.
Vasodilators
Endothelial function and the diminished delivery of AAs to muscle clearly impacts the ability of AAs to stimulate muscle protein synthesis in older adults [25–26, 33]. As mentioned above, providing a vasodilator (e.g., sodium nitroprusside) during an insulin infusion or AA ingestion restored muscle protein synthesis to these anabolic stimuli [26–27]. More recently, a small pilot study in five men (average age of 55 years) found that daily administration of 25 mg of sildenafil (i.e., a phosphodiesterase 5 inhibitor) for eight days stimulated muscle protein synthesis and reduced muscle fatigue [60]. Interestingly, one study [61] has shown that omega-3 (EPA/DHA) fatty acids can improve endothelial function in patients with chronic heart failure, and perhaps this can help explain the positive effect of fish oil on muscle protein anabolism in older adults [62]. These are intriguing data, however, it is not known whether chronic administration of a vasodilator will be an effective strategy at overcoming anabolic resistance.
Ursolic acid and tomatidine
Ursolic acid is a pentacyclic triterpene acid found in several edible herbs and fruits, including apples. Tomatidine is a steroidal alkaloid derived from tomato plants and green (unripe) tomatoes. The chemical structures of ursolic acid and tomatidine are shown in Figure 2. Recent studies in mice found that dietary supplementation with either ursolic acid or tomatidine blunts ATF4 activity in aged skeletal muscle. Consistent with this reduction in ATF4 activity, ursolic acid and tomatidine significantly reduced age-related deficits in strength, skeletal muscle quality and skeletal muscle mass [49] (Figure 2).
The mechanism by which ursolic acid and tomatidine reduce ATF4 activity in aged skeletal muscle is not yet known. Ursolic acid and tomatidine were originally identified in unbiased screens for small molecules whose collective effects on gene expression in human cell lines are roughly opposite to the changes in skeletal muscle gene expression that occur in humans during muscle atrophy [63–65]. As predicted, ursolic acid and tomatidine have broad effects on gene expression in aged skeletal muscle, can induce and repress many mRNAs, including some that are not ATF4-dependent [49]. Ursolic acid and tomatidine also reduce acute skeletal muscle atrophy in mouse models of starvation and muscle disuse [63–64]. Furthermore, when administered to young adult mice without muscle atrophy, both compounds stimulate skeletal muscle hypertrophy and increase strength, muscle quality and endurance exercise capacity [63–64, 66]. They also stimulate hypertrophy when directly applied to skeletal myotubes in culture, indicating that they act directly on skeletal muscle cells [63–64]. Moreover, ursolic acid and tomatidine stimulate hypertrophy of cultured myotubes from both humans and mice, indicating that both humans and mice contain receptors for ursolic acid and tomatidine ([64], in an mTORC1 dependent manner [63–65].
Thus far, in vivo human data on ursolic acid and tomatidine are limited to a randomized, placebo-controlled study that tested the effect of ursolic acid in healthy young adult humans undergoing resistance exercise training [67]. In that study, ursolic acid significantly increased muscle strength, as predicted by previous studies in preclinical models. It is not yet known if ursolic acid and tomatidine reduce the effects of aging in human skeletal muscle, but the existing data suggest them as promising candidates.
Concluding remarks and future perspectives
The underlying mechanisms of skeletal muscle aging are still poorly understood, however, reduced AA delivery to skeletal muscle and activation of mechanisms that sense low levels of AAs within skeletal muscle appear to play important roles. Furthermore, recent advances in aging and muscle biology research emphasize the need for a better understanding of the biochemical basis of anabolic resistance, including a more complete definition of the roles and relationships of aging, physical activity, endothelial dysfunction, mTORC1 activity and ATF4 activity. (see outstanding questions). In conclusion, the maintenance of skeletal muscle quality and strength appears to require the suppression of AA starvation mechanisms within skeletal muscle. Strategies designed to suppress those mechanisms should reduce the loss of muscle quality and function during aging.
Trends Box.
Aging impairs endothelial cell function in skeletal muscle, thereby reducing delivery of dietary amino acids to skeletal muscle fibers.
Aging promotes anabolic resistance by impairing the ability of amino acids, insulin or muscle contraction to increase protein synthesis in skeletal muscle.
Anabolic resistance may originate with endothelial dysfunction and impaired amino acid delivery to skeletal muscle fibers, thereby generating two distinct amino acid starvation responses (decreased mTORC1 activity and increased ATF4 activity) which reduce muscle protein synthesis, leading to muscle weakness and atrophy.
Potential therapeutic strategies include restoration of amino acid delivery to aged skeletal muscle via increased physical activity, dietary protein, pharmacologic vasodilators, and/or small molecules that stimulate mTORC1 and/or inhibit ATF4 in aged skeletal muscle fibers.
Acknowledgments
This work was supported by grants from the National Institute of Health (R56 AG051267, P30 AG024832, R43 AG044898, R43 AR069400, R41 AG047684), the Department of Veterans Affairs Biomedical Laboratory Research & Development Service (IBX000976A), the Department of Veterans Affairs Rehabilitation and Research Development Service (1I01RX001477) and the Fraternal Order of Eagles Diabetes Research Center at the University of Iowa. CMA and SME are inventors on patent applications related to ursolic acid and tomatidine, which have been filed by the University of Iowa Research Foundation and licensed to Emmyon, Inc. CMA is a founder and officer of Emmyon, Inc. SME is an employee of Emmyon, Inc. CMA and SME hold equity in Emmyon, Inc.
References
- 1.Cruz-Jentoft AJ, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing. 2010;39:412–423. doi: 10.1093/ageing/afq034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gordon BS, et al. Regulation of muscle protein synthesis and the effects of catabolic states. Int J Biochem Cell Biol. 2013;45:2147–2157. doi: 10.1016/j.biocel.2013.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bodine SC, et al. Skeletal muscle atrophy and the E3 ubiquitin ligases MuRF1 and MAFbx/atrogin-1. Am J Physiol Endocrinol Metab. 2014;307:E469–E484. doi: 10.1152/ajpendo.00204.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cohen S, et al. Muscle wasting in disease: molecular mechanisms and promising therapies. Nat Rev Drug Discov. 2015;14:58–74. doi: 10.1038/nrd4467. [DOI] [PubMed] [Google Scholar]
- 5.Volpi E, et al. Basal muscle amino acid kinetics and protein synthesis in healthy young and older men. JAMA. 2001;286:1206–1212. doi: 10.1001/jama.286.10.1206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dickinson JM, et al. Leucine-enriched amino acid ingestion after resistance exercise prolongs myofibrillar protein synthesis and amino acid transporter expression in older men. J Nutr. 2014;144:1694–1702. doi: 10.3945/jn.114.198671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wall BT, et al. Aging is accompanied by a blunted muscle protein synthetic response to protein ingestion. PLoS ONE. 2015;10:e0140903. doi: 10.1371/journal.pone.0140903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moore DR, et al. Protein ingestion to stimulate myofibrillar protein synthesis requires greater relative protein intakes in healthy older versus younger men. J Gerontol A Biol Sci Med Sci. 2015;70:57–62. doi: 10.1093/gerona/glu103. [DOI] [PubMed] [Google Scholar]
- 9.Bukhari SS, et al. Intake of low-dose leucine-rich essential amino acids stimulates muscle anabolism equivalently to bolus whey protein in older women at rest and after exercise. Am J Physiol Endocrinol Metab. 2015;308:E1056–E1065. doi: 10.1152/ajpendo.00481.2014. [DOI] [PubMed] [Google Scholar]
- 10.Markofski MM, et al. Effect of age on basal muscle protein synthesis and mTORC1 signaling in a large cohort of young and older men and women. Exp Gerontol. 2015;65:1–7. doi: 10.1016/j.exger.2015.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rennie MJ. Anabolic resistance: the effects of aging, sexual dimorphism, and immobilization on human muscle protein turnover. Appl Physiol Nutr Metab. 2009;34:377–381. doi: 10.1139/H09-012. [DOI] [PubMed] [Google Scholar]
- 12.Volpi E, et al. The response of muscle protein anabolism to combined hyperaminoacidemia and glucose-induced hyperinsulinemia is impaired in the elderly. J Clin Endocrinol Metab. 2000;85:4481–4490. doi: 10.1210/jcem.85.12.7021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tipton KD, et al. Nonessential amino acids are not necessary to stimulate net muscle protein synthesis in healthy volunteers. J Nutr Biochem. 1999;10:89–95. doi: 10.1016/s0955-2863(98)00087-4. [DOI] [PubMed] [Google Scholar]
- 14.Volpi E, et al. Essential amino acids are primarily responsible for the amino acid stimulation of muscle protein anabolism in healthy elderly adults. Am J Clin Nutr. 2003;78:250–258. doi: 10.1093/ajcn/78.2.250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cuthbertson D, et al. Anabolic signaling deficits underlie amino acid resistance of wasting, aging muscle. FASEB J. 2005;19:422–424. doi: 10.1096/fj.04-2640fje. [DOI] [PubMed] [Google Scholar]
- 16.Katsanos CS, et al. Aging is associated with diminished accretion of muscle proteins after the ingestion of a small bolus of essential amino acids. Am J Clin Nutr. 2005;85:1065–1073. doi: 10.1093/ajcn/82.5.1065. [DOI] [PubMed] [Google Scholar]
- 17.Paddon-Jones D, et al. Amino acid ingestion improves muscle protein synthesis in the young and elderly. Am J Physiol Endocrinol Metab. 2004;286:E321–E328. doi: 10.1152/ajpendo.00368.2003. [DOI] [PubMed] [Google Scholar]
- 18.Katsansos CS, et al. A high proportion of leucine is required for optimal stimulation of the rate of muscle protein synthesis by essential amino acids in the elderly. Am J Physiol Endocrinol Metab. 2006;291:E381–E387. doi: 10.1152/ajpendo.00488.2005. [DOI] [PubMed] [Google Scholar]
- 19.Reidy PT, et al. Role of ingested amino acids and protein in the promotion of resistance exercise-induced muscle protein anabolism. J Nutr. 2016;146:155–183. doi: 10.3945/jn.114.203208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rasmussen BB, et al. Insulin resistance of muscle protein metabolism in aging. FASEB J. 2006;20:768–769. doi: 10.1096/fj.05-4607fje. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujita S, et al. Supraphysiological hyperinsulinemia is necessary to stimulate skeletal muscle protein anabolism in older adults: evidence of a true age-related insulin resistance to muscle protein metabolism. Diabetologia. 2009;52:1889–1898. doi: 10.1007/s00125-009-1430-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Phillips SM, et al. Mixed muscle protein synthesis and breakdown after resistance exercise in humans. Am J Physiol. 1997;273:E99–E107. doi: 10.1152/ajpendo.1997.273.1.E99. [DOI] [PubMed] [Google Scholar]
- 23.Kumar V, et al. Age-related differences in the dose-response relationship of muscle protein synthesis to resistance exercise in young and old men. J Physiol. 2009;587:211–217. doi: 10.1113/jphysiol.2008.164483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fry CS, et al. Aging impairs contraction-induced human skeletal muscle mTORC1 signaling and protein synthesis. Skelet Muscle. 2011;1:11. doi: 10.1186/2044-5040-1-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Timmerman KL, et al. Insulin stimulates human skeletal muscle protein synthesis via an indirect mechanism involving endothelial-dependent vasodilation and mammalian target of rapamycin complex 1 signaling. J Clin Endocrinol Metab. 2010;95:3848–3857. doi: 10.1210/jc.2009-2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Timmerman KL, et al. Pharmacological vasodilation improves insulin-stimulated muscle protein anabolism but not glucose utilization in older adults. Diabetes. 2010;59:2764–2771. doi: 10.2337/db10-0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dillon EL, et al. Muscle protein metabolism responds similarly to exogenous amino acids in healthy younger and older adults during NO-induced hyperemia. Am J Physiol Regul Integr Comp Physiol. 2011;301:R1408–R1417. doi: 10.1152/ajpregu.00211.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Glover EI, et al. Immobilization induced anabolic resistance in human myofibrillar protein synthesis with low and high dose amino acid infusion. J Physiol. 2008;586:6049–6061. doi: 10.1113/jphysiol.2008.160333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rennie MJ. Anabolic resistance in critically ill patients. Crit Care Med. 2009;37:S398–S399. doi: 10.1097/CCM.0b013e3181b6ec1f. [DOI] [PubMed] [Google Scholar]
- 30.Porter C, et al. Amino acid infusion fails to stimulate skeletal muscle protein synthesis up to 1 year after injury in children with severe burns. J Trauma Acute Care Surg. 2013;74:1480–1485. doi: 10.1097/TA.0b013e3182921651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cardillo C, et al. Interactions between nitric oxide and endothelin in the regulation of vascular tone of human resistance vessels in vivo. Hypertension. 2000;35:1237–1241. doi: 10.1161/01.hyp.35.6.1237. [DOI] [PubMed] [Google Scholar]
- 32.Fujita S, et al. Aerobic exercise overcomes the age-related insulin resistance of muscle protein metabolism by improving endothelial function and Akt/mammalian target of rapamycin signaling. Diabetes. 2007;56:1615–1622. doi: 10.2337/db06-1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timmerman KL, et al. A moderate acute increase in physical activity enhances nutritive flow and the muscle protein anabolic response to mixed nutrient intake in older adults. Am J Clin Nutr. 2012;95:1403–1412. doi: 10.3945/ajcn.111.020800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Miller S, et al. In vivo muscle amino acid transport involves two distinct processes. Am J Physiol Endocrinol Metab. 2004;287:E136–E141. doi: 10.1152/ajpendo.00092.2004. [DOI] [PubMed] [Google Scholar]
- 35.Drummond MJ, et al. An increase in essential amino acid availability upregulates amino acid transporter expression in human skeletal muscle. Am J Physiol Endocrinol Metab. 2010;298:E1011–E1018. doi: 10.1152/ajpendo.00690.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Drummond MJ, et al. Skeletal muscle amino acid transporter expression is increased in young and older adults following resistance exercise. J Appl Physiol. 2011;111:135–142. doi: 10.1152/japplphysiol.01408.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dickinson JM, et al. Aging differentially affects human skeletal muscle amino acid transporter expression when essential amino acids are ingested after exercise. Clin Nutr. 2013;32:273–280. doi: 10.1016/j.clnu.2012.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drummond MJ, et al. Bed rest impairs skeletal muscle amino acid tranporter expression, mTORC1 signaling, and protein synthesis in response to essential amino acids in older adults. Am J Physiol Endocrinol Metab. 2012;302:E1113–E1122. doi: 10.1152/ajpendo.00603.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kimball SR. Integration of signals generated by nutrients, hormones, and exercise in skeletal muscle. Am J Clin Nutr. 2014;99:237S–242S. doi: 10.3945/ajcn.113.068387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fujita S, et al. Nutrient signaling in the regulation of human muscle protein synthesis. J Physiol. 2007;582:813–823. doi: 10.1113/jphysiol.2007.134593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dickinson JM, et al. Mammalian target of rapamycin complex 1 activation is required for the stimulation of human skeletal muscle protein synthesis by essential amino acids. J Nutr. 2011;141:856–862. doi: 10.3945/jn.111.139485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Drummond MJ, et al. Rapamycin administration in humans blocks the contraction-induced increase in skeletal muscle protein synthesis. J Physiol. 2009;587:1535–1546. doi: 10.1113/jphysiol.2008.163816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wang S, et al. Lysosomal amino acid transporter SLC38A9 signals arginine sufficiency to mTORC1. Science. 2014;347:188–194. doi: 10.1126/science.1257132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolfson RL, et al. Sestrin2 is a leucine sensor for the mTORC1 pathway. Science. 2016;351:43–48. doi: 10.1126/science.aab2674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chantranupong L, et al. The CASTOR proteins are arginine sensor for the mTORC1 pathway. Cell. 2016;165:153–164. doi: 10.1016/j.cell.2016.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ameri K, et al. Activating transcription factor 4. Int J Biochem Cell Biol. 2008;40:14–21. doi: 10.1016/j.biocel.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 47.Sacheck JM, et al. Rapid disuse and denervation atrophy involve transcriptional changes similar to those of muscle wasting during systemic diseases. FASEB J. 2007;21:140–155. doi: 10.1096/fj.06-6604com. [DOI] [PubMed] [Google Scholar]
- 48.Ebert SM, et al. The transcription factor ATF4 promotes skeletal myofiber atrophy during fasting. Mol Endocrinol. 2010;24:790–799. doi: 10.1210/me.2009-0345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ebert SM, et al. Identification and small molecule inhibition of an activating transcription factor 4 (ATF4)-dependent pathway to age-related skeletal muscle weakness and atrophy. J Biol Chem. 2015;290:25497–25511. doi: 10.1074/jbc.M115.681445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ebert SM, et al. Stress-induced skeletal muscle Gadd45a expression reprograms myonuclei and causes muscle atrophy. J Biol Chem. 2012;287:27290–27301. doi: 10.1074/jbc.M112.374777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fox DK, et al. p53 and ATF4 mediate distinct and additive pathways to skeletal muscle atrophy during limb immobilization. Am J Physiol Endocrinol Metab. 2014;307:E245–E261. doi: 10.1152/ajpendo.00010.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wek RC, et al. Coping with stress: eIF2 kinases and translational control. Biochem Soc Trans. 2006;34:7–11. doi: 10.1042/BST20060007. [DOI] [PubMed] [Google Scholar]
- 53.Seals DR, et al. You’re only as old as your arteries: translational strategies for preserving vascular endothelial function. Physiology (Bethesda) 2014;29:250–264. doi: 10.1152/physiol.00059.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Breen L, et al. Two weeks of reduced activity decreases leg lean mass and induces ‘anabolic resistance’ of myofibrillar protein synthesis in healthy elderly. J Clin Endocrinol Metab. 2013;98:2604–2612. doi: 10.1210/jc.2013-1502. [DOI] [PubMed] [Google Scholar]
- 55.Shimizu R, Hotta K, Yamamoto S, Matsumoto T, Kamiya K, Kato M, Hamazaki N, Kamekawa D, Akiyama A, Kamada Y, Tanaka S, Masuda T. Low-intensity resistance training with blood flow restriction improves vascular endothelial function and peripheral blood circulation in healthy elderly people. Eur J Appl Physiol. 2016 Apr;116(4):749–57. doi: 10.1007/s00421-016-3328-8. [DOI] [PubMed] [Google Scholar]
- 56.Fry CS, Glynn EL, Drummond MJ, Timmerman KL, Fujita S, Abe T, Dhanani S, Volpi E, Rasmussen BB. Blood flow restriction exercise stimulates mTORC1 signaling and muscle protein synthesis in older men. J Appl Physiol (1985) 2010 May;108(5):1199–209. doi: 10.1152/japplphysiol.01266.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Volpi E, et al. Is the optimal level of protein intake for older adults greater than the recommended dietary allowance? J Gerontol A Biol Sci Med Sci. 2013;68:677–681. doi: 10.1093/gerona/gls229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Paddon-Jones D, et al. Dietary protein recommendations and the prevention of sarcopenia. Curr Opin Clin Nutr Metab Care. 2009;12:86–90. doi: 10.1097/MCO.0b013e32831cef8b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Mamerow MM, et al. Dietary protein distribution positively influenes 24-h muscle protein synthesis in healthy adults. J Nutr. 2014;144:876–880. doi: 10.3945/jn.113.185280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Sheffield-Moore M, et al. Sildenafil increases muscle protein synthesis and reduced muscle fatigue. Clin Transl Sci. 2013;6:463–468. doi: 10.1111/cts.12121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Morgan DR, Dixon LJ, Hanratty CG, El-Sherbeeny N, Hamilton PB, McGrath LT, Leahey WJ, Johnston GD, McVeigh GE. Effects of dietary omega-3 fatty acid supplementation on endothelium-dependent vasodilation in patients with chronic heart failure. Am J Cardiol. 2006 Feb 15;97(4):547–51. doi: 10.1016/j.amjcard.2005.08.075. [DOI] [PubMed] [Google Scholar]
- 62.Smith GI, Atherton P, Reeds DN, Mohammed BS, Rankin D, Rennie MJ, Mittendorfer B. Dietary omega-3 fatty acid supplementation increases the rate of muscle protein synthesis in older adults: a randomized controlled trial. Am J Clin Nutr. 2011 Feb;93(2):402–12. doi: 10.3945/ajcn.110.005611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kunkel SD, et al. mRNA expression signatures of human skeletal muscle atrophy identify a natural compound that increases muscle mass. Cell Metab. 2011;13:627–638. doi: 10.1016/j.cmet.2011.03.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dyle MC, et al. Systems-based discovery of tomatidine as a natural small molecule inhibitor of skeletal muscle atrophy. J Biol Chem. 2014;289:14913–14924. doi: 10.1074/jbc.M114.556241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Adams CM, et al. Use of mRNA expression signatures to discover small molecule inhibitors of skeletal muscle atrophy. Curr Opin Clin Nutr Metab. 2015;18:263–268. doi: 10.1097/MCO.0000000000000159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kunkel SD, et al. Ursolic acid increases skeletal muscle and brown fat and decreases diet-induced obesity, glucose intolerance and fatty liver disease. PLoS ONE. 2012;7:e39332. doi: 10.1371/journal.pone.0039332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Bang HS, et al. Ursolic acid-induced elevation of serum irisin augments muscle strength during resistance training in men. Korean J Physiol Pharmacol. 2014;18:441–446. doi: 10.4196/kjpp.2014.18.5.441. [DOI] [PMC free article] [PubMed] [Google Scholar]