Abstract
Objectives
Despite well-known fitness advantages to males who produce and maintain high endogenous testosterone levels, such phenotypes may be costly if testosterone-mediated investment in reproductive effort trade-off against investment in somatic maintenance. Previous studies of androgen-mediated trade-offs in human immune function find mixed results, in part because most studies either focus on a few indicators of immunity, are confounded by phenotypic correlation, or are observational. Here the association between male endogenous testosterone and 13 circulating cytokines are examined before and after ex vivo antigen stimulation with phytohaemagglutinin (PHA) and lipopolysaccharides (LPS) in a high pathogen population of Bolivian forager-horticulturalists.
Materials and Methods
A Milliplex 13-plex cytokine panel measured cytokine concentration in whole blood samples from 109 Tsimane men aged 40–89 (median=50 years) before and after antigen stimulation with PHA and LPS. Urinary testosterone was measured via enzyme immunoassay; demographic and anthropometric data were collected as part of the Tsimane Health and Life History Project.
Results
Higher endogenous testosterone was associated with down-regulated responses in all cytokines after PHA stimulation (but significantly in only 2/13 cytokines), controlling for age and body mass index. In contrast, testosterone was not significantly associated with down-regulation of cytokines after LPS stimulation. MANOVAs indicate that men with higher testosterone showed reduced cytokine responses to PHA compared to LPS (p=0.0098).
Discussion
Endogenous testosterone appears to be immunomodulatory rather than immunosuppressive. Potentially costlier forms of immune activation like those induced by PHA (largely T-cell biased immune activation) are down-regulated in men with higher testosterone, but testosterone has less impact on potentially less costly immune activation following LPS stimulation (largely B-cell mediated immunity).
Keywords: Immunocompetence Handicap Hypothesis, phytohaemagglutinin (PHA), lipopolysaccharides (LPS), testosterone, Tsimane
Trade-offs between reproduction and longevity are among the most comprehensively researched areas of life history theory (Boddy et al. 2015; Kirkwood and Rose 1991; Muehlenbein and Bribiescas 2005). Testosterone and other steroid hormones play critical roles in the development and maintenance of many sexually dimorphic traits in males resulting from sexual selection (Andersson 1994), but at a cost; ceteris paribus, energetic investments in reproductive effort trade-off against investments in somatic maintenance (Folstad and Karter 1992; Muehlenbein and Bribiescas 2005). Since fitness gains of investment in reproduction earlier in life often outweigh the gains from somatic repair, reproduction is usually prioritized over longevity. Natural and controlled experiments provide evidence in favor of testosterone-mediated male survival, with castrated males living longer than intact males across many species including humans (Min et al. 2012).
One of the costlier forms of somatic investment in adulthood is immune function (Straub et al. 2010; Zuk and Stoehr 2002). Immune activation increases resting metabolism in humans and other species by 8–56%, and can incur caloric costs of up to 2000 kj/day −1 (Demas et al. 2011; Derting and Compton 2003; Muehlenbein et al. 2010; Straub et al. 2010). The Immunocompetence Handicap Hypothesis (ICHH) rose out of classical life-history theory and decades of research into costly signaling to become a dominant framework in the evolutionary ecology and behavioral endocrinology literature. It proposes that testosterone – as a critical mediator of male reproductive effort – suppresses immune function, and thus elaborate androgen-based traits represent an honest signal of male quality since only high quality males can afford to incur such costs (Folstad and Karter 1992; Furman et al. 2013; Zuk and Stoehr 2002). While the ICHH is a dominant theoretical model in the life history literature, evidence in favor of this hypothesis is limited, and other lines of research have suggested alternative models to explain associations between androgens and immune function (Foo et al. 2016; Nunn et al. 2009).
The immunosuppressive effects of exogenously administered testosterone are well documented from experimental studies of nonhuman animal models (Duffy et al. 2000; Peters 2000; Poiani et al. 2000; Yao et al. 2003). However, recent studies and meta-analyses find mixed evidence that endogenous testosterone is an active immunosuppressant in free-living mammals (Foo et al. 2016; Nunn et al. 2009; Roberts et al. 2004). In fact, naturalistic studies often show positive associations between endogenous testosterone and induced antibody response, suggesting that males in good condition can afford to maintain both high testosterone levels and robust immune responses (i.e. positive phenotypic correlations) (Peters 2000; Rantala et al. 2012). These findings are bolstered by experimental work in a reptile model showing that immune function is enhanced when exogenous testosterone is paired with food supplementation, but without food supplementation exogenous testosterone results in decreased innate immune function (Ruiz et al. 2010). Importantly, testosterone is down-regulated following infection and tissue injury, making it difficult to isolate the effects of testosterone on immune function in cross-sectional studies (Christeff et al. 1988; Spratt et al. 1993; Spratt et al. 2006). While meta-analyses suggest that testosterone is overall immunosuppressive (Foo et al. 2016), there is still ambiguity depending on which aspects of immune function are studied, and whether the impacts of testosterone on immune function are direct or indirect.
Another major issue is that most studies assessing trade-offs between immune function and testosterone rely on only a single biomarker of immune function, precluding the ability to examine a potential immuno-modulatory role of testosterone. Immune function involves multiple coordinated responses, each with their own costs, benefits and interactions with other immune and endocrine responses. Numerous cytokines, for example, play critical roles in immune cell signaling and lymphocyte differentiation and have varying impacts on physiology and varying energetic costs (Table 1). As an alternative to the ICHH, it has been proposed that testosterone is immuno-modulatory rather than immunosuppressive, that is, testosterone regulates trade-offs between different types of immune response (Muehlenbein and Bribiescas 2005; Simmons and Roney 2009). If only the energetically costlier forms of immunity are downregulated in higher testosterone males, then energetic availability may underlie some of the associations reported in the literature. Indeed, evidence suggests that energetic costs (e.g. from illness, fasting) can impact both testosterone levels and immune function (Scrimshaw and SanGiovanni 1997; Spratt et al. 1993; Trumble et al. 2010). The ability to mount a rapid response to local infection or tissue injury is of particular utility, as high testosterone males more frequently engage in aggressive physical competition with other males (Archer 2006). In mandrills, male-male competition in the form of physical aggression is a common route for spreading simian immunodeficiency virus (SIV) (Nerrienet et al. 1998), while physically aggressive interactions between wild rodent males increase transmission rates of hantavirus (Glass et al. 1988). Innate immune responses, which include pro- and anti-inflammatory cytokines, not only are crucial rapid responses to injury, but also play a role in subsequent wound healing (Werner and Grose 2003). In free-ranging baboons, where success in male-male physical conflict determines males’ reproductive access to females, high status males – who have higher testosterone than subordinate males (Gesquiere et al. 2011) – heal faster than subordinates (Archie et al. 2012), perhaps because of positive phenotypic correlation. Thus, while there may be trade-offs between testosterone and some more energetically costly aspects of immune function (Best and Hoyle 2013), one would not expect that testosterone would down-regulate all aspects of immune function equally.
Table 1.
Cytokine response to antigen type, see figure 1.
| Response to Mitogen | Cytokine | Produced By | Role |
|---|---|---|---|
| IL-2 | T cells | Triggers T-cells to become effector cells, Expansion of T-cell clones | |
| Stronger PHA Cytokine Response | IL-4 | Basophils may be initial effector, Th2 cells (auto) | Differentiates B cells to plasma cells, B-cell IgE class switching |
| IL-13 | T cells | Induce IgE secretion, physiological changes in parasitized organs | |
| IL-5 | T cells, Eosinophils | B-cell growth, Ig secretion, Eosinophil activation | |
|
| |||
| IL-7 | Dendritic cells, NOT lymphocytes | Proliferation of all lymphocytes | |
| INF-γ | NK and NKT cells, Th1 CD4 and CD8 effector cells | Activates macrophages, inhibits viral replication | |
| Intermediate Response to both PHA and LPS | GM-CSF | Macropahges, T cells, Mast Cells, NK Cells | Granulocyte and monocyte production |
| IL-8 | Macrophages | Neutrophil chemotaxis | |
| IL-6 | T cells, macrophages | energy mobilization, temperature increase, B cell growth, T-reg antagonism | |
|
| |||
| IL-12p70 | Dendritic cells, macrophages, immature B cells | T cell growth, UP IFN-g, TNF-a from T and NK, down IL-4. Increases toxicity of NK and CD8. IL-2 ups IL-12 receptors, enhancing the effect | |
| IL-10 | Monocytes, Th2 (less), Treg, cytotoxic T-cells to inhibit NK in response to virus | Negative feedback on self, down regulates MHC II, Enhances B cell, down regulates IFN-γ, IL-2,IL-3, TNF-α, GM-CSF | |
| Stronger LPS Cytokine Response | TNF-α | Macrophages, CD4, NK | Fever, inflammation, apoptosis |
| IL-1β | Activated macrophages | Lymphocyte activator | |
Studying the role of testosterone in modulating immune function under naturalistic conditions is notoriously difficult in humans, where ethical concerns limit the use of various experimental protocols commonly used in nonhuman animal models. Observational studies demonstrate that testosterone decreases immediately following illness or injury (Christeff et al. 1988; Muehlenbein et al. 2010; Spratt et al. 1993; Spratt et al. 2008), which is consistent with a life history framework which posits that energetic stress shunts caloric resources from investment in reproductive effort (often proxied by a high testosterone phenotype) toward immune function. While it is clear that any energetic stress, whether due to immune activation from infection (Muehlenbein et al. 2010) or caloric restriction (Trumble et al. 2010), results in decreased testosterone production, it is less clear that endogenous testosterone actively down-regulates human immune function. Indeed, a longitudinal study of Filipino males found a positive association between testosterone and immunoglobulin A (sIgA), a marker of mucosal immunity (Gettler et al. 2014).
Study goals and hypotheses
This study contributes to the literature with 1) data from a free-living energy limited population living a relatively traditional lifestyle, 2) use of multiple measures of immune activation, and 3) measurement of biomarkers at baseline and following an ex vivo challenge. The analyses herein focus on 109 older adult men (aged 40–89 years, median age=50 years) in a population of forager horticulturalists facing high pathogen burden (Gurven et al. 2008). In this immunologically stressed population, we expect energetic trade-offs between testosterone and immune function to be stronger than that observed among energetically replete industrialized populations with lower infectious burden (Blackwell et al. 2015; Gurven et al. 2008). Additionally, industrialized populations have significantly higher levels of testosterone at younger ages compared to subsistence populations, and steeper age-related declines (Ellison et al. 2002; Trumble et al. 2012).
Most prior observational studies in humans have only measured circulating concentrations of immune markers under baseline conditions, as opposed to the immune response to challenge. Recent studies indicate that immune response to challenge is far costlier than baseline immune function, both in nonhuman animal models (Demas et al. 2011; Derting and Compton 2003) and humans (Muehlenbein et al. 2010). Given behavioral effects of androgens (e.g. (Archer 2006)), individuals with higher testosterone may also engage in behavior that increases the likelihood of wounding or encountering pathogens (e.g. increased male-male competition) (Klein 2000).
In this study, levels of urinary testosterone are examined in relation to 13 circulating cytokines following ex vivo whole blood antigen stimulation with a T-cell mitogen, phytohaemagglutinin (PHA) and a B-cell mitogen, lipopolysaccharides (LPS). PHA is a commonly used mitogen that activates the division and replication of T-cells, while LPS is a cell wall component of gram negative bacteria that binds to toll-like receptor 4 (TLR4) and initiates B-cell division and differentiation into plasma cells as well as activation of macrophages, monocytes, and dendritic cells (Ceuppens et al. 1988; Heumann and Roger 2002; Wheelock 1965). This study can therefore examine androgen mediated immuno-modulation in response to several common immune challenges facing humans. This experimental approach has major advantages; first, we are able to stimulate an immune response ex vivo which permits intra- and inter-individual comparisons in cytokine response to the same challenge controlling for baseline cytokine levels and other potential confounders. Additionally, while under normal physiological conditions circulating testosterone rapidly decreases following infection or tissue injury (Christeff et al. 1988; Simmons and Roney 2009; Spratt et al. 1993; Spratt et al. 2008), using an ex vivo design we can examine the relationship between baseline testosterone and cytokine response to stimulation without any potential for steroidal down regulation post-infection.
Given the advantages of this experimental design over previous observational studies, we hypothesize that we will have a level of contrast necessary to differentiate between broad immunosuppression versus immunomodulation of specific aspects of immune function. We hypothesize that higher endogenous testosterone will be associated with more down-regulation of energetically costly aspects of immune function, such as T-cell mediated immune responses, but not broad, generalized immunosuppression.
MATERIALS AND METHODS
Ethics statement
For all protocols institutional (UCSB and UNM) IRB approval was granted, as was informed consent at three levels: 1) Tsimane government that oversees projects, 2) village leadership, and 3) study participants.
Bio-specimen collection and preparation
Tsimane men (n=122) came to the Tsimane Health and Life History Project’s (THLHP) clinic in San Borja, Beni, Bolivia to participate in THLHP protocols (e.g. economic and demographic interviews, biospecimen collection), and to receive a routine medical exam as part of the project’s behavioral-biomedical surveillance. All Tsimane aged 40+ were invited to participate in this and other studies regardless of their health status, and approximately 85% of adults participated. Prior to the medical exam and interviews, men provided a first morning void urine specimen. Fasting blood was drawn, both with and without heparin as an anti-coagulant. One vacutainer of blood without anti-coagulant was allowed to clot, and then serum was separated via centrifugation (1500g × 10 minutes) and frozen in liquid nitrogen. Multiple 100 μL aliquots of heparinized whole blood were immediately added to separate round bottom microtiter wells in a sterile 96-well plate. The first aliquot received 100 μL of 20 μg/mL phytohaemagglutinin (PHA, Sigma cat. 61764) diluted in RPMI-1640, for a final concentration of 10 μg/mL PHA. The second aliquot received 100 μL of 20 μg/mL Lipopolysaccharides (LPS. Sigma cat. L2630) diluted in RPMI-1640, for a final concentration of 10 μg/mL LPS. A third aliquot was mixed with 100 μL RPMI-1640 without mitogens. In all three cases, RPMI was supplemented with 100 IU/ml penicillin and 100 μg/ml streptomycin (Sigma cat. P0781) to prevent contamination. In the absence of a CO2 incubator in the San Borja clinic, the microtiter plate was sealed in a glass Tupperware with a lit candle, which burned the O2 in the container, thus enriching the CO2 concentration (May et al. 2009). The sealed plates were incubated at 37°C for 72 hours. Following this, the supernatant was removed and frozen in liquid nitrogen for transport to UNM. Specimens were transported on dry ice and stored at −80°C for up to two years before assay.
Biomarkers of immune activation
Immediately following the blood draw, leukocyte counts were measured with a QBC Autoread Plus (QBC Diagnostics). For both baseline (unstimulated) serum specimens and post-stimulation whole blood specimens, 13 cytokines (TNF-α, INF-γ, IL-1β, Il-2, IL-4, IL-5, IL-6, IL-7, IL-8, IL-10, IL-12p70, IL-13, and GM-CSF) were measured with a Milliplex MAP High-Sensitivity Human Cytokine Panel (HSCYTO-60, Millipore Corp., Billerica, MA) on the Luminex MagPix, at the Hominoid Reproductive Ecology Laboratory at the University of New Mexico. Following manufacturer recommendation, calibrators were prepared with a serum matrix for baseline specimens, and with RPMI for antigen-stimulated samples, and specimens below the limits of detection were assigned the lower limit of detection. Quality control specimens were within expected ranges provided by the manufacturer.
C-Reactive Protein (CRP) was measured at the UCSB Human Biodemography lab via enzyme immunoassay (Brindle et al. 2010). Within and between plate coefficients of variation (CVs) were 7.3% and 10.2% for the high and 5.3% and 9.2% for the low controls.
2.4 Urinary testosterone
Though both urine and serum samples were obtained, we elected to assess testosterone in urine because urine samples reflect testosterone production over a longer period and are less susceptible to transient fluctuations. Since a large proportion of testosterone in urine is present in its conjugated form of testosterone glucuronide, we first deconjugated samples with beta-glucuronidase (Helix pomatia, Calbiochem, <2% aryl sulfatase activity) and ether extraction. Testosterone was measured with an in-house enzyme linked immunosorbent assay (ELISA) using a polyclonal antibody (R156/7, provided by C. Munro at the University of California-Davis) which cross reacts 100% with testosterone, 57.4% with 5α-dihydrotestosterone, 0.27% with androstenedione, and <0.05% with other androgens (Muir et al. 2001). Within and between plate CVs were 4.7% and 12.2% for the high and 4.7% and 14.9% for the low controls. Urinary testosterone was corrected for specific gravity (Miller et al. 2004).
Statistical Methods
From the initial sample of 122, males with acute infections (CRP >10 mg/L, n=12), were removed prior to analyses. Additionally, one individual was missing testosterone data. Three individuals were missing PHA stimulation data and six individuals LPS stimulation data, due to contamination of samples during field procedures. Final sample sizes were 103 for LPS analyses, 106 for PHA analyses, and 109 for combined analyses. Cytokines (baseline and ex vivo stimulated) and testosterone values were logged to normalize distributions unless otherwise noted, and converted to Z-scores. Cytokines were normalized across all stimulation conditions to place all cytokines on a similar scale, and allow for simultaneous testing of effects across multiple cytokines. A second set of z-scores were also normalized only within a particular stimulation type, to allow comparison of relative cytokine changes under particular mitogen stimulation conditions. Models reported in the paper indicate which z-scores were utilized for a particular analysis. Mitogen stimulations were run in batches on a given day, and each batch was grouped together and treated as a random effect to control for potential non-independence due to field conditions (e.g. temperature fluctuations) or plate characteristics. Linear mixed model analyses examined associations between mitogen type, cytokine type, testosterone, and cytokine response, controlling for age, BMI, CRP, and baseline cytokines as fixed effects, and subject and batch as random effects, using lme in the nlme package in R. Models were fit with restricted maximum likelihood (REML). We then used anova on our lme models to assess significance of multilevel factors (Pinheiro and Bates 2006). Additional exploratory linear mixed effects regression models examined associations between testosterone and each individual cytokine response to both PHA and LPS stimulation (z-scored), controlling for fixed effects of age, BMI, CRP and baseline cytokine, and a random effect of batch. We utilize an α= 0.05 as the cutoff for statistical significance. P-values are not Bonferroni corrected as the tests are not independent (cytokines interact and covary), violating the independence requirement of a Bonferroni correction. Interaction terms (e.g. testosterone by BMI) were assessed, but did not achieve statistical significance and are thus omitted. Statistical analyses were conducted in R 3.2.2 (https://cran.r-project.org/).
RESULTS
3.1 Testosterone and baseline characteristics
Urinary testosterone was not associated with baseline immune parameters, including overall baseline serum cytokines (β = 0.026, t = 0.44, p = 0.658) or any individual cytokine level (all p’s > 0.19). Baseline total leukocyte (β = −0.011, t = −0.05, p = 0.962) counts were not associated with testosterone level.
Cytokine response to T- and B-cell mitogens
Cytokine responses to each of the mitogens are categorized in Figure 1A and Tables 2–6 and S1–4. Comparing across mitogens, cytokine responses differ significantly (p < 0.001) between PHA and LPS stimulation (Table 3; Figure 1A), with PHA characterized by high IL-2, IL-4, IL-5, and IL-13, and LPS characterized by high IL-12p70, IL-10, TNFα, and IL-1 β. The remaining cytokines (IL-7, INF-γ, GM-CSF, IL-8, IL-6) showed intermediate responses to both mitogens (Figure 1A). There was no clear trend for the RPMI only stimulation condition (Figure 1A).
Figure 1.
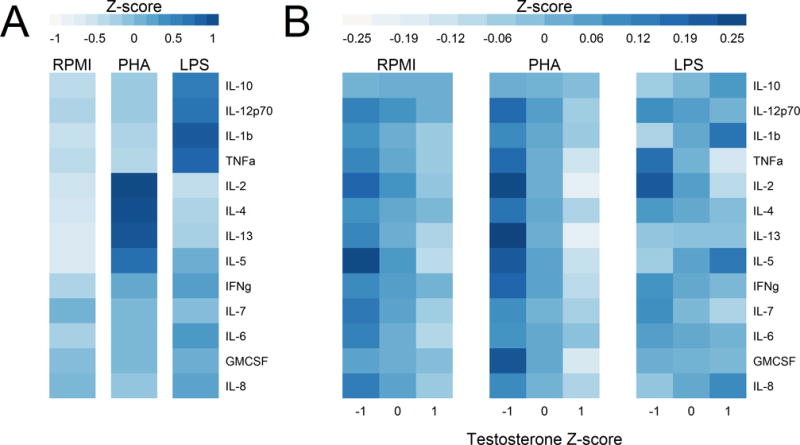
Mean normalized cytokine levels under each stimulation condition relative to normalized testosterone. Conditions include medium only (RPMI) or stimulation with a B-cell and monocyte (LPS) or a T-cell mitogen (PHA). A) Mean cytokine levels for each stimulation condition. Each cytokine is normalized across all conditions in order to show variation across stimulation types, while eliminating the absolute differences in level between different cytokines. B) Mean cytokine levels within each stimulation condition, relative to testosterone level. Cytokines are normalized within each mitogen condition to show only variation associated with testosterone. Higher testosterone is generally associated with lower cytokine production after PHA stimulation and in RPMI medium alone. All z-scores were calculated by first logging cytokine or testosterone values, and then subtracting the mean and dividing by the standard deviation. Both A and B show the predicted values from the mixed models shown in Table 3, controlling for BMI, baseline serum cytokine levels, baseline CRP, and analysis batch.
Table 2.
Associations between testosterone and overall cytokine response for each mitogen controlling for phenotypic variables.
| RPMI Only | LPS Only | PHA Only | |||||||
|---|---|---|---|---|---|---|---|---|---|
|
|
|
||||||||
| DF | F-value | p-value | DF | F-value | p-value | DF | F-value | p-value | |
| Intercept | 1/1205 | 0.95 | 0.329 | 1/1200 | 0.30 | 0.581 | 1/1237 | 0.16 | 0.688 |
| Age | 1/57 | 0.03 | 0.856 | 1/59 | 1.54 | 0.219 | 1/59 | 0.10 | 0.753 |
| BMI | 1/57 | 5.40 | 0.024 | 1/59 | 0.90 | 0.346 | 1/59 | 0.21 | 0.649 |
| Baseline Serum Cytokine | 1/1205 | 65.69 | <.0001 | 1/1200 | 37.67 | <0.001 | 1/1237 | 22.68 | <0.001 |
| Log CRP | 1/57 | 5.47 | 0.023 | 1/59 | 6.10 | 0.016 | 1/59 | 4.67 | 0.035 |
| Cytokine Type | 12/1205 | 0.04 | 1.000 | 12/1200 | 0.05 | 1.000 | 12/1237 | 0.03 | 1.000 |
| Testosterone | 1/57 | 4.32 | 0.042 | 1/59 | 0.00 | 0.953 | 1/59 | 4.04 | 0.049 |
| Cytokine Type × Testosterone | 12/1205 | 0.31 | 0.988 | 12/1200 | 0.93 | 0.514 | 12/1237 | 0.53 | 0.895 |
Models also contain random effects for batch and subject. Degrees of freedom are estimated by lme and do not reflect sample size due to non-independence of observations clustered by a random effect term for batch (Pinheiro & Bates 2006). Cytokines are z-scored within mitogen condition. Baseline serum cytokines and testosterone are both z-scores. F and p-values are the Wald test statistics for the terms in the model, entered sequentially, so main effects are calculated prior to including interaction terms. Bold indicates statistical significance (p<0.05).
Table 6.
Effect of testosterone on unstimulated cytokines in RPMI alone, controlling for baseline cytokines, CRP, age and BMI for 110 Tsimane men. Each cytokine is modeled separately. Bold indicates statistical significance (p<0.05).
| Outcome | Testosterone (Z) | Baseline Cytokine (Z) | Log CRP | Age | BMI | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| β | P | β | P | β | P | β | P | β | P | |
| GM-CSF | −0.083 | 0.257 | 0.045 | 0.555 | −0.122 | 0.096 | −0.006 | 0.413 | −0.064 | 0.041 |
| IL-1β | −0.025 | 0.715 | 0.175 | 0.012 | −0.139 | 0.044 | 0.002 | 0.796 | −0.027 | 0.354 |
| IL-2 | −0.105 | 0.175 | 0.215 | 0.010 | −0.240 | 0.002 | −0.001 | 0.899 | −0.073 | 0.022 |
| IL-7 | −0.012 | 0.897 | 0.388 | <0.001 | −0.064 | 0.486 | 0.008 | 0.412 | −0.035 | 0.345 |
| IL-6 | −0.144 | 0.089 | 0.592 | <0.001 | −0.124 | 0.126 | −0.007 | 0.379 | −0.021 | 0.530 |
| IL-8 | −0.114 | 0.070 | 0.049 | 0.437 | −0.118 | 0.062 | −0.008 | 0.240 | −0.025 | 0.360 |
| TNF-α | −0.088 | 0.354 | 0.281 | 0.004 | −0.079 | 0.391 | −0.003 | 0.778 | −0.071 | 0.059 |
| INF-γ | −0.062 | 0.424 | 0.337 | <0.001 | −0.034 | 0.651 | −0.006 | 0.470 | −0.043 | 0.178 |
| IL-12p70 | −0.119 | 0.122 | 0.731 | <0.001 | 0.032 | 0.660 | 0.008 | 0.287 | −0.002 | 0.950 |
| IL-4 | −0.141 | 0.086 | −0.168 | 0.066 | −0.055 | 0.529 | 0.007 | 0.389 | −0.080 | 0.020 |
| IL-5 | −0.043 | 0.588 | 0.205 | 0.015 | 0.034 | 0.669 | 0.010 | 0.205 | 0.002 | 0.955 |
| IL-13 | −0.146 | 0.072 | −0.044 | 0.643 | −0.215 | 0.008 | −0.001 | 0.921 | −0.090 | 0.009 |
| IL-10 | −0.090 | 0.308 | 0.199 | 0.049 | −0.112 | 0.194 | −0.001 | 0.935 | −0.063 | 0.082 |
Table 3.
Associations between testosterone and cytokine response across mitogen conditions controlling for phenotypic variables.
| Z-scores Standardized Across Mitogen Conditions
|
Z-scores Standardized Within Mitogen Condition
|
|||||
|---|---|---|---|---|---|---|
| DF | F-value | p-value | DF | F-value | p-value | |
| Intercept | 1/2548 | 0.27 | 0.604 | 1/2548 | 0.24 | 0.622 |
| Age | 1/62 | 0.03 | 0.860 | 1/62 | 0.03 | 0.857 |
| BMI | 1/62 | 0.45 | 0.504 | 1/62 | 0.51 | 0.478 |
| Baseline Cytokine | 1/2548 | 44.58 | <0.001 | 1/2548 | 50.20 | <0.001 |
| Log CRP | 1/62 | 5.88 | 0.018 | 1/62 | 5.88 | 0.018 |
| Cytokine Type | 12/2548 | 0.06 | 1.000 | 12/2548 | 0.06 | 1.000 |
| Mitogen Type | 1/2548 | 0.25 | 0.614 | 1/2548 | 0.01 | 0.932 |
| Testosterone | 1/62 | 1.25 | 0.267 | 1/62 | 1.04 | 0.311 |
| Cytokine Type × Mitogen Type | 12/2548 | 81.92 | <0.001 | 12/2548 | 0.02 | 1.000 |
| Mitogen Type × Testosterone | 1/2548 | 6.70 | 0.010 | 1/2548 | 6.81 | 0.009 |
| Cytokine Type × Testosterone | 12/2548 | 0.67 | 0.779 | 12/2548 | 0.95 | 0.499 |
Models only include PHA and LPS as stimulation types, so do not include RPMI only samples. Models also contain random effects for batch and subject. Degrees of freedom are estimated by lme and do not reflect sample size due to non-independence of observations clustered by a random effect term for batch (Pinheiro & Bates 2006). Serum cytokines and testosterone are both z-scores. Models equate to Figure 1A and 1B, respectively. F and p-values are the Wald test statistics for the terms in the model, entered sequentially, so main effects are calculated prior to including interaction terms. Bold indicates statistical significance (p<0.05).
Impact of endogenous testosterone on the cytokine response to T- and B-cell mitogens
Testosterone was a significant predictor of overall cytokine response to PHA (β = −0.131, t = −2.01, p = 0.049), although the effect is not specific to particular cytokines (no significant interaction with cytokine type; Table 2). Testosterone was not a significant predictor of overall cytokine response to LPS (β = −0.003, t = −0.06, p = 0.479). These results were unchanged when controlling for leukocyte count (Table S1).
Testosterone levels interacted with mitogen stimulation type; there was a negative testosterone by PHA interaction (Table 3). Higher testosterone is generally associated with lower cytokine production after PHA stimulation and in RPMI medium alone. Normalizing for stimulation type illustrates this further (Figure 1B), by illuminating only the testosterone-associated variance in cytokine levels.
Testosterone and individual cytokine response to ex vivo antigen stimulation
Our models suggested that effects of testosterone are on overall cytokine response to PHA, and not on particular cytokines. To verify this we performed exploratory analyses examining each cytokine individually (Tables 4–6). Controlling for baseline cytokine level, age, CRP, and BMI, testosterone was negatively associated with all 13 PHA-stimulated cytokines, significantly so with only GM-CSF and IL-8, but with suggestive trends for IL-2, IL-7, and IL-13 (Table 4). Ten of 13 cytokines had standardized parameter estimates < −0.1, with only IL-1β, IL-6, and IL-4 showing little evidence of association. Testosterone was not significantly associated with any cytokine following LPS stimulation (Table 5), and with LPS stimulation, only two cytokines had standardized parameters < −0.1 or > 0.1: IL-1β was positively associated with testosterone (β = 0.148, p = 0.132) and IL-2 was negatively associated (β = −0.125, p = 0.165). These results were unchanged when controlling for leukocyte count (Tables S2–S4).
Table 4.
Effect of testosterone on PHA-stimulated cytokines, controlling for baseline cytokines, CRP, age and BMI for 110 Tsimane men. Each cytokine is modeled separately. Bold indicates statistical significance (p<0.05).
| Outcome | Testosterone (Z) | Baseline Cytokine (Z) | Log CRP | Age | BMI | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| β | P | β | P | β | P | β | P | β | P | |
| GM-CSF | −0.217 | 0.027 | 0.098 | 0.285 | −0.189 | 0.033 | −0.005 | 0.558 | −0.022 | 0.552 |
| IL-1β | −0.095 | 0.343 | 0.005 | 0.955 | −0.051 | 0.563 | −0.007 | 0.466 | −0.036 | 0.350 |
| IL-2 | −0.205 | 0.062 | −0.022 | 0.825 | −0.156 | 0.109 | −0.001 | 0.937 | 0.014 | 0.732 |
| IL-7 | −0.111 | 0.091 | 0.218 | 0.001 | −0.013 | 0.828 | −0.004 | 0.532 | −0.017 | 0.502 |
| IL-6 | −0.069 | 0.514 | −0.161 | 0.154 | −0.057 | 0.590 | 0.009 | 0.405 | −0.029 | 0.473 |
| IL-8 | −0.180 | 0.035 | 0.004 | 0.962 | −0.242 | 0.002 | −0.003 | 0.677 | −0.066 | 0.049 |
| TNF-α | −0.149 | 0.135 | 0.232 | 0.012 | −0.064 | 0.474 | 0.003 | 0.727 | 0.001 | 0.981 |
| INF-γ | −0.111 | 0.161 | 0.107 | 0.145 | −0.158 | 0.031 | 0.000 | 0.959 | −0.029 | 0.350 |
| IL-12p70 | −0.102 | 0.313 | 0.330 | <0.001 | −0.054 | 0.554 | 0.005 | 0.614 | −0.041 | 0.283 |
| IL-4 | −0.077 | 0.477 | 0.178 | 0.078 | −0.061 | 0.526 | 0.004 | 0.729 | 0.040 | 0.316 |
| IL-5 | −0.140 | 0.144 | 0.449 | <0.001 | −0.043 | 0.620 | 0.000 | 0.995 | 0.025 | 0.475 |
| IL-13 | −0.202 | 0.064 | 0.071 | 0.484 | −0.138 | 0.154 | 0.000 | 0.979 | 0.005 | 0.892 |
| IL-10 | −0.104 | 0.247 | 0.263 | 0.004 | −0.260 | 0.002 | 0.001 | 0.923 | −0.048 | 0.164 |
Table 5.
Effect of testosterone on LPS stimulated cytokines, controlling for baseline cytokines, CRP, age and BMI for 110 Tsimane men. Each cytokine is modeled separately. Bold indicates statistical significance (p<0.05).
| Outcome | Testosterone (Z) | Baseline Cytokine (Z) | Log CRP | Age | BMI | |||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| β | P | β | P | β | P | β | P | β | P | |
| GM-CSF | −0.046 | 0.601 | 0.109 | 0.242 | −0.159 | 0.066 | −0.002 | 0.807 | −0.048 | 0.169 |
| IL-1β | 0.148 | 0.132 | −0.006 | 0.951 | −0.057 | 0.534 | −0.003 | 0.722 | 0.027 | 0.468 |
| IL-2 | −0.125 | 0.165 | 0.331 | <0.001 | −0.132 | 0.120 | 0.001 | 0.864 | −0.084 | 0.015 |
| IL-7 | −0.010 | 0.866 | 0.200 | 0.002 | −0.027 | 0.649 | 0.009 | 0.138 | 0.010 | 0.687 |
| IL-6 | −0.003 | 0.977 | −0.197 | 0.103 | −0.013 | 0.902 | 0.003 | 0.778 | −0.016 | 0.691 |
| IL-8 | 0.003 | 0.971 | −0.021 | 0.832 | −0.128 | 0.114 | −0.007 | 0.402 | −0.047 | 0.159 |
| TNF-α | −0.042 | 0.561 | 0.189 | 0.013 | −0.071 | 0.318 | 0.003 | 0.721 | 0.022 | 0.444 |
| INF-γ | 0.022 | 0.716 | 0.145 | 0.028 | −0.052 | 0.399 | −0.002 | 0.765 | −0.002 | 0.945 |
| IL-12p70 | −0.045 | 0.666 | 0.195 | 0.049 | −0.030 | 0.753 | −0.013 | 0.177 | −0.044 | 0.249 |
| IL-4 | −0.049 | 0.529 | 0.285 | 0.001 | −0.103 | 0.170 | 0.000 | 0.979 | −0.017 | 0.561 |
| IL-5 | 0.061 | 0.463 | 0.568 | <0.001 | −0.076 | 0.342 | −0.014 | 0.081 | −0.001 | 0.978 |
| IL-13 | −0.046 | 0.613 | 0.293 | 0.002 | −0.245 | 0.005 | −0.008 | 0.360 | −0.064 | 0.071 |
| IL-10 | 0.055 | 0.504 | 0.134 | 0.127 | −0.220 | 0.007 | 0.002 | 0.782 | −0.003 | 0.936 |
DISCUSSION
This study tested trade-offs among older Tsimane men between androgens and immune activation biomarkers by examining associations between endogenous testosterone and mitogen-stimulated cytokine levels. Our results indicate that endogenous testosterone is associated with immuno-modulation, or at least selectively suppressive as opposed to broadly immunosuppressive. Testosterone is associated with reduced cytokine responses following stimulation with a T-cell mitogen, PHA, while testosterone has no significant association with response to stimulation with a B-cell and monocyte mitogen, LPS. These results fit within a larger life-history and ecological immunology literature suggesting that men with higher levels of testosterone downregulate some, but not all aspects of immune activation. In this case we find an association suggesting that cytokine responses to T-cell mitogens are downregulated in higher testosterone males. Unlike B-cells, which can continue to produce relatively long lasting antibodies, cytotoxic T-cells must be continually produced and clonally expanded in large numbers. From an energetic perspective, T-cell mediated immune activation may be more costly than B-cell mediated immune activation due to this need to produce many cells (Lochmiller and Deerenberg 2000). Thus testosterone appears to selectively downregulate the most energetically expensive forms of immune activation, which is expected if testosterone serves an adaptive immuno-modulatory function, especially in energy-limited subsistence populations.
Stimulation with PHA results in an immune response similar to that expected with exposure to viral infection (Ceuppens et al. 1988; Wheelock 1965), and tends to cause greater changes in IL-2, IL-4, IL-5, and IL-13 (Table 1, Figure 1A). In animal models, treatment with testosterone results in reduced response to viral infections, as measured by higher viral titers (Lindström et al. 2001). Research on humans indicates that men are more susceptible to viral infections than women, and also that testosterone is down-regulated in men infected with diverse viruses ranging from influenza vaccinations to HIV (Grinspoon et al. 1996; Klein 2000; Simmons and Roney 2009). If men with higher testosterone have a decreased cytokine response to viral infection, then reducing levels of testosterone following infection would be an optimal response. Indeed, studies find decreases in testosterone following illness and tissue injury (e.g (Christeff et al. 1988; Muehlenbein and Bribiescas 2005; Spratt et al. 1993; Spratt et al. 2008)). Testosterone can be lowered by decreasing hypothalamic-pituitary-gonadal production, or by increasing aromatization of testosterone to estrogen (Spratt et al. 2006). Changes in testosterone and other biomarkers during and following illness may underlie some sickness behaviors like reduced physical activity and other depressive symptoms (Shattuck and Muehlenbein 2015; Stieglitz et al. 2015).
Contrary to the results from PHA stimulation, cytokines produced by stimulation with LPS were not associated with testosterone. LPS stimulates B cells as well as macrophages, monocytes, and dendritic cells. Macrophages in particular may be important in response to injury, and so the maintenance of their activity with elevated testosterone might be important. However, B-cells are much more abundant in Tsimane blood compared to industrialized populations, while monocytes are very rare (Blackwell et al. 2016), so much of the LPS response is likely a B-cell response. Unlike T-cells that are lysed when destroying infections, B-cells that remain inactive are relatively low cost reservoirs that can be activated to produce antibodies as needed (McDade et al. 2016). While the developmental costs of producing B-cells and immunoglobulins can be high, the maintenance and activation costs are relatively low, as are the collateral costs in terms of tissue damage when activated (McDade et al. 2016). For a 10 kg human infant, the total cost of producing immunoglobulin G (IgG) is approximately 0.043% of their daily protein budget, while the costs of B-cell proliferation during infection is estimated to be 0.00048% of this budget (McDade et al. 2016; Waterlow 1984). Immunoglobulins have a half-life of approximately 25 days, and thus once produced, antibodies have protective effects that require little additional energetic maintenance or input (Mankarious et al. 1988). On a-cell-by-cell cost, B-cells and T-cells likely have similar costs. However, T-cells are 2–3 times more common in the blood, and for this reason alone may require more overall resources (Bisset et al. 2004; Blackwell et al. 2016). Thus from an energetic perspective, T-cell mediated immune activation may be energetically costlier than B-cell mediated immune activation. Testosterone perhaps may be down-regulating the most energetically expensive forms of immune activation, which is expected if testosterone serves an adaptive immuno-modulatory function.
There are well-characterized impacts of testosterone on both T-cells and B-cells. Evidence suggests that testosterone can bind to, and actively inhibit T-cell differentiation and proliferation (Benten et al. 1999; Kissick et al. 2014; McMurray et al. 2001). B-cells do not express surface androgen receptors (Benten et al. 2002), and testosterone does not appear to be associated with differences in B-cell proliferation (McMurray et al. 2001), though treatment with exogenous testosterone does decrease antibody production in animal models (Kanda et al. 1996). Previous studies report positive associations between androgens and anti-inflammatory cytokines (Liva and Voskuhl 2001; Malkin et al. 2004). While the results in the present study do not replicate these findings, there are several confounding factors that make direct comparisons difficult. First, those studies used exogenous testosterone administration, which while an excellent way to isolate the impact of testosterone on cytokines may not be ecologically valid, as many aspects of physiology other than just androgens are modified when androgens naturally increase. Other studies using exercise to naturalistically manipulate testosterone do not find the same associations between testosterone and IL-10 (Benini et al. 2015). Secondly, Tsimane have significantly lower levels of age-matched testosterone than males living in industrialized populations (Trumble et al. 2012), and thus the low levels seen in this population, and perhaps throughout much of human evolution, may potentially have had less of an impact on anti-inflammatory cytokines than the relatively high levels seen in men in industrialized populations and men taking exogenous testosterone.
Our study finds associations between endogenous testosterone and cytokine production. However, there may be multiple mechanisms through which this effect could occur. Endogenous testosterone is present in the blood samples used for stimulation, and may act directly on leukocytes at the time of stimulation. Alternatively, long term differences in testosterone may have effects on leukocyte phenotypes that affect responsiveness to stimulation, irrespective of levels in the blood at the particular time of stimulation. Androgen receptors are present on T-cells (Benten et al. 1999), but their numbers might be dynamically regulated in response to baseline androgen levels and other feedback mechanisms. Additionally, developmental changes at early ages may have led to differences between individuals in both testosterone and immune function, leading to associations that are not necessarily causal, but rather correlates of other phenotypic variables.
These hypotheses are not mutually exclusive, and we think it likely that all three of these pathways might be important. Our results suggest that testosterone is not associated with baseline cytokines or leukocyte counts, yet there are other ways immune function might vary as a function of disease or developmental history. Future studies will be needed to separate these causal mechanisms more carefully.
There are several reasons why our results may differ from other studies of the ICHH. The immune system is multifaceted and these studies differ in the type of immune activation being studied. While a recent meta-analysis found support for testosterone down-regulating many aspects of immune function, it notes significantly more variation and less consistency across 19 studies examining impacts of testosterone on cytokines compared to other markers of immune function (cell-mediated, humoral, parasitic white blood cell based studies) (Foo et al. 2016). Cytokines are just one aspect of immunity, and while the results reported here are consistent with the notion that testosterone is immuno-modulatory in contrast to the ICHH as originally formulated, further research is needed. A more direct test of immuno-modulation would involve manipulation of testosterone levels, though such an experimental design would have reduced ecological validity. Additionally, the participants in this study were on average older than some previous studies (see limitation section below).
Trade-offs between reproduction and survival are one of the most studied life-history trade-offs. Quasi-experimental studies like the one conducted here suggest that ceteris paribus, higher testosterone males show down-regulated immune response to some, but not all, components of immune function, implicating testosterone as a potential immuno-suppressant to some of the energetically costlier aspects of human immune function. That said, there is no single global measure of immune function; instead millions of years of evolution have shaped a system to fight multiple pathogens in myriad ways through numerous physiological pathways. So while higher endogenous levels of testosterone may down-regulate some aspects of immune function (e.g. cytokine response to T-cell mitogens), that does not mean that testosterone is generally immuno-suppressive; indeed cytokine responses to B-cell mitogens were largely unaffected by testosterone. Additionally, relatively long-lived humans invest more in some aspects of immune function and survival compared to species with faster life histories (i.e murine models) which prioritize early reproduction (Lee 2006). Thus while we find little evidence of the ICHH in humans, these results may not be applicable to other species with faster life history strategies.
Much of the previous research into immune-testosterone links have been conducted in seasonally breeding birds or mice, which may not offer a good model of human immune function (Greenman et al. 2005; Warren et al. 2014). In seasonal breeders, more than just circulating testosterone changes during the mating season; major changes are observed in energy expenditure, sleep, body composition, social behavior, and food consumption, all of which can alter immune function (Greenman et al. 2005). Tsimane are exposed to high pathogen loads (Blackwell et al. 2015; Gurven et al. 2008) which also vary seasonally and may predispose their immune system to be less reactive to minor insults like antigen stimulation (McDade et al. 2010), or alternatively primed to respond to any type of insult. These alternative hypotheses require further testing. Because Tsimane men have lower levels of testosterone compared to men in industrialized populations (Ellison et al. 2002; Trumble et al. 2012), Tsimane men may not experience the kind of generalized immunosuppression observed when superphysiological levels of testosterone are exogenously applied to nonhuman animal models (Duffy et al. 2000; Peters 2000; Poiani et al. 2000; Yao et al. 2003). Previous research among the Tsimane also found no evidence that disease symptoms (e.g. diarrhea, respiratory illness, injuries) from clinical exams were associated with testosterone in a small sample of men (Trumble et al. 2013).
Strengths and Limitations
A strength of our study design is that we tested the impact of two different mitogens across 13 cytokines; had we just focused on only PHA or LPS we would have had different results and interpretations. While ex vivo whole blood antigen stimulation only provides a small window into the role of testosterone in modulating immune function, it is also a powerful tool that allows us to examine immune responses that would otherwise be unethical or impossible to study in non-laboratory settings. This experimental protocol also avoids confounding by ensuring that all specimens receive the same pathogenic exposure, and that androgens do not down-regulate following illness, both of which are critical for understanding the role of these steroids in affecting immune function. While antigen stimulation is beneficial for isolating the role of steroids on specific aspects of immune activation, it is not guaranteed that the response from ex vivo stimulation will match the myriad potential additional responses in a living human. Nonetheless this demonstrates the importance of field-friendly experimental manipulations; had this been a purely observational study of baseline cytokines and testosterone we would have observed no relationship between testosterone and circulating cytokines (see results above). This study is also limited by focusing solely on males, and on older males over age 40; the immuno-modulatory effects of testosterone could potentially be stronger in younger males who are investing more energy in reproductive effort. Additionally, given changes in immune function with age among the Tsimane (Blackwell et al. 2016), it is possible that baseline cytokines, or cytokine responsiveness may differ at younger ages. This does not diminish the results of this study; with modal ages of death in the 70s, a 45 year old Tsimane male can expect to live an additional 25.6 years of life (Gurven et al. 2007), and thus trade-offs between androgens and immune function still have important consequences even at later ages. With the rise of testosterone replacement therapies in industrialized populations (Gan et al. 2013), these results could also be of interest to the wider biomedical community as more older men are exposed to higher doses of testosterone. Some might argue that results from populations like the Tsimane are not generalizable to industrialized populations, but we argue that they represent a crucial step toward understanding human immune and endocrine physiology, which evolved under very different conditions than those observed in modern settings with less pathogen exposure.
CONCLUSIONS
Testosterone is more strongly associated with cytokine down-regulation in response to PHA stimulation, which induces a largely T-cell mediated response; stimulation with LPS (largely biased toward B-cell immunity) did not result in generalized immunosuppression. It is clear from these results that steroid hormones are associated with differential cytokine responses to different mitogen types, a result that would not have been found focusing solely on one mitogen or one biomarker of immune function, or if relying only upon (unstimulated) baseline circulatory cytokines. Future studies will focus on the differential impacts of testosterone and other steroid hormones on cytokine response to additional mitogen types and using a wider age range and across sexes, to better understand the role of such hormones in modulating immune function. Parasites, bacterial infections, and viruses were all major selective pressures throughout human evolution, and understanding the impact of steroids on immune responses to each of these infections can provide greater understanding of trade-offs between reproductively beneficial androgens and longevity.
Acknowledgments
We thank the Tsimane for participating, and Tsimane Health and Life History personnel, as well as the associate editor and two anonymous reviewers. Funding comes from the National Institutes of Health/National Institute on Aging (NIA R01AG024119, R56AG024119, R01AG024119). JS acknowledges support from the Agence Nationale de la Recherche (ANR) – Labex IAST. Funding sources had no direct involvement in study design, data collection, analysis, interpretation of data, or manuscript preparation or submission.
LITERATURE CITED
- Andersson MB. Sexual selection. Princeton, N.J.: Princeton University Press; 1994. p. xx, 599. [Google Scholar]
- Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neurosci Biobehav Rev. 2006;30(3):319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Archie EA, Altmann J, Alberts SC. Social status predicts wound healing in wild baboons. Proceedings of the National Academy of Sciences. 2012;109(23):9017–9022. doi: 10.1073/pnas.1206391109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benini R, Prado Nunes PR, Orsatti CL, Barcelos LC, Orsatti FL. Effects of acute total body resistance exercise on hormonal and cytokines changes in men and women. J Sports Med Phys Fitness. 2015;55(4):337–344. [PubMed] [Google Scholar]
- Benten WPM, Lieberherr M, Giese G, Wrehlke C, Stamm O, Sekeris CE, Mossmann H, Wunderlich F. Functional testosterone receptors in plasma membranes of T cells. The FASEB Journal. 1999;13(1):123–133. doi: 10.1096/fasebj.13.1.123. [DOI] [PubMed] [Google Scholar]
- Benten WPM, Stephan C, Wunderlich F. B cells express intracellular but not surface receptors for testosterone and estradiol. Steroids. 2002;67(7):647–654. doi: 10.1016/s0039-128x(02)00013-2. [DOI] [PubMed] [Google Scholar]
- Best A, Hoyle A. The evolution of costly acquired immune memory. Ecology and Evolution. 2013;3(7):2223–2232. doi: 10.1002/ece3.611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bisset LR, Lung TL, Kaelin M, Ludwig E, Dubs RW. Reference values for peripheral blood lymphocyte phenotypes applicable to the healthy adult population in Switzerland. European journal of haematology. 2004;72(3):203–212. doi: 10.1046/j.0902-4441.2003.00199.x. [DOI] [PubMed] [Google Scholar]
- Blackwell A, Trumble B, Maldonado Suarez I, Stieglitz J, Beheim B, Snodgrass J, Kaplan H, Gurven M. Immune Function in Amazonian Horticulturalists. Ann Hum Biol In Press. 2016 doi: 10.1080/03014460.2016.1189963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell AD, Tamayo MA, Beheim B, Trumble BC, Stieglitz J, Hooper PL, Martin M, Kaplan H, Gurven M. Helminth infection, fecundity, and age of first pregnancy in women. Science. 2015;350(6263):970–972. doi: 10.1126/science.aac7902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddy AM, Kokko H, Breden F, Wilkinson GS, Aktipis CA. Cancer susceptibility and reproductive trade-offs: a model of the evolution of cancer defences. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2015;370(1673):20140220. doi: 10.1098/rstb.2014.0220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brindle E, Fujita M, Shofer J, O’Connor KA. Serum, plasma, and dried blood spot high-sensitivity C-reactive protein enzyme immunoassay for population research. Journal of Immunological Methods. 2010;362(1–2):112–120. doi: 10.1016/j.jim.2010.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ceuppens JL, Baroja ML, Lorre K, Van Damme J, Billiau A. Human T cell activation with phytohemagglutinin. The function of IL-6 as an accessory signal. The Journal of Immunology. 1988;141(11):3868–3874. [PubMed] [Google Scholar]
- Christeff N, Benassayag C, Carli-Vielle C, Carli A, Nunez EA. Elevated oestrogen and reduced testosterone levels in the serum of male septic shock patients. Journal of steroid biochemistry. 1988;29(4):435–440. doi: 10.1016/0022-4731(88)90254-3. [DOI] [PubMed] [Google Scholar]
- Demas G, Greives T, Chester E, French S. The energetics of immunity. Ecoimmunology. 2011:259. [Google Scholar]
- Derting T, Compton S. Immune Response, Not Immune Maintenance, Is Energetically Costly in Wild White-Footed Mice (Peromyscus leucopus) Physiological and Biochemical Zoology. 2003;76(5):744–752. doi: 10.1086/375662. [DOI] [PubMed] [Google Scholar]
- Duffy DL, Bentley GE, Drazen DL, Ball GF. Effects of testosterone on cell-mediated and humoral immunity in non-breeding adult European starlings. Behavioral Ecology. 2000;11(6):654–662. [Google Scholar]
- Ellison PT, Bribiescas RG, Bentley GR, Campbell BC, Lipson SF, Panter-Brick C, Hill K. Population variation in age-related decline in male salivary testosterone. Hum Reprod. 2002;17(12):3251–3253. doi: 10.1093/humrep/17.12.3251. [DOI] [PubMed] [Google Scholar]
- Folstad I, Karter AJ. Parasites, bright males, and the immunocompetence handicap. American Naturalist. 1992:603–622. [Google Scholar]
- Foo YZ, Nakagawa S, Rhodes G, Simmons LW. The effects of sex hormones on immune function: a meta-analysis. Biological Reviews. 2016 doi: 10.1111/brv.12243. [DOI] [PubMed] [Google Scholar]
- Furman D, Hejblum BP, Simon N, Jojic V, Dekker CL, Thiébaut R, Tibshirani RJ, Davis MM. Systems analysis of sex differences reveals an immunosuppressive role for testosterone in the response to influenza vaccination. Proceedings of the National Academy of Sciences. 2013 doi: 10.1073/pnas.1321060111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan EH, Pattman S, HS Pearce S, Quinton R. A UK epidemic of testosterone prescribing, 2001–2010. Clinical Endocrinology. 2013;79(4):564–570. doi: 10.1111/cen.12178. [DOI] [PubMed] [Google Scholar]
- Gesquiere LR, Learn NH, Simao MCM, Onyango PO, Alberts SC, Altmann J. Life at the Top: Rank and Stress in Wild Male Baboons. Science. 2011;333(6040):357–360. doi: 10.1126/science.1207120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gettler LT, McDade TW, Agustin SS, Feranil AB, Kuzawa CW. Testosterone, immune function, and life history transitions in Filipino males (Homo sapiens) International Journal of Primatology. 2014:1–18. [Google Scholar]
- Glass GE, Childs JE, Korch GW, LeDuc JW. Association of intraspecific wounding with hantaviral infection in wild rats (Rattus norvegicus) Epidemiology and Infection. 1988;101(2):459–472. doi: 10.1017/s0950268800054418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenman C, Martin LI, Hau M. Reproductive State, but Not Testosterone, Reduces Immune Function in Male House Sparrows (Passer domesticus) Physiological and Biochemical Zoology. 2005;78(1):60–68. doi: 10.1086/425194. [DOI] [PubMed] [Google Scholar]
- Grinspoon S, Corcoran C, Lee K, Burrows B, Hubbard J, Katznelson L, Walsh M, Guccione A, Cannan J, Heller H. Loss of lean body and muscle mass correlates with androgen levels in hypogonadal men with acquired immunodeficiency syndrome and wasting. The Journal of Clinical Endocrinology & Metabolism. 1996;81(11):4051–4058. doi: 10.1210/jcem.81.11.8923860. [DOI] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Supa AZ. Mortality experience of Tsimane Amerindians of Bolivia: Regional variation and temporal trends. Am J Hum Biol. 2007;19(3):376–398. doi: 10.1002/ajhb.20600. [DOI] [PubMed] [Google Scholar]
- Gurven M, Kaplan H, Winking J, Finch C, Crimmins EM. Aging and Inflammation in Two Epidemiological Worlds. J Gerontol A Biol Sci Med Sci. 2008;63(2):196–199. doi: 10.1093/gerona/63.2.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heumann D, Roger T. Initial responses to endotoxins and Gram-negative bacteria. Clinica Chimica Acta. 2002;323(1–2):59–72. doi: 10.1016/s0009-8981(02)00180-8. [DOI] [PubMed] [Google Scholar]
- Kanda N, Tsuchida T, Tamaki K. Testosterone inhibits immunoglobulin production by human peripheral blood mononuclear cells. Clinical & Experimental Immunology. 1996;106(2):410–415. doi: 10.1046/j.1365-2249.1996.d01-842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood TBL, Rose MR. Evolution of Senescence: Late Survival Sacrificed for Reproduction. Philosophical Transactions of the Royal Society of London Series B: Biological Sciences. 1991;332(1262):15–24. doi: 10.1098/rstb.1991.0028. [DOI] [PubMed] [Google Scholar]
- Kissick HT, Sanda MG, Dunn LK, Pellegrini KL, On ST, Noel JK, Arredouani MS. Androgens alter T-cell immunity by inhibiting T-helper 1 differentiation. Proceedings of the National Academy of Sciences. 2014;111(27):9887–9892. doi: 10.1073/pnas.1402468111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neuroscience & Biobehavioral Reviews. 2000;24(6):627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- Lee KA. Linking immune defenses and life history at the levels of the individual and the species. Integrative and Comparative Biology. 2006;46(6):1000–1015. doi: 10.1093/icb/icl049. [DOI] [PubMed] [Google Scholar]
- Lindström KM, Krakower D, Lundström JO, Silverin B. The effects of testosterone on a viral infection in greenfinches (Carduelis chloris): an experimental test of the immunocompetence-handicap hypothesis. 2001:207–211. doi: 10.1098/rspb.2000.1352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liva SM, Voskuhl RR. Testosterone Acts Directly on CD4+ T Lymphocytes to Increase IL-10 Production. The Journal of Immunology. 2001;167(4):2060–2067. doi: 10.4049/jimmunol.167.4.2060. [DOI] [PubMed] [Google Scholar]
- Lochmiller RL, Deerenberg C. Trade-offs in evolutionary immunology: just what is the cost of immunity? Oikos. 2000;88(1):87–98. [Google Scholar]
- Malkin CJ, Pugh PJ, Jones RD, Kapoor D, Channer KS, Jones TH. The Effect of Testosterone Replacement on Endogenous Inflammatory Cytokines and Lipid Profiles in Hypogonadal Men. The Journal of Clinical Endocrinology & Metabolism. 2004;89(7):3313–3318. doi: 10.1210/jc.2003-031069. [DOI] [PubMed] [Google Scholar]
- Mankarious S, Lee M, Fischer S, Pyun K, Ochs H, Oxelius V, Wedgwood R. The half-lives of IgG subclasses and specific antibodies in patients with primary immunodeficiency who are receiving intravenously administered immunoglobulin. The Journal of laboratory and clinical medicine. 1988;112(5):634–640. [PubMed] [Google Scholar]
- May L, Van Bodegom D, Kuningas M, Meij J, De Craen A, Frölich M, Westendorp R. Performance of the whole-blood stimulation assay for assessing innate immune activation under field conditions. Cytokine. 2009;45(3):184–189. doi: 10.1016/j.cyto.2008.12.010. [DOI] [PubMed] [Google Scholar]
- McDade TW, Georgiev AV, Kuzawa CW. Trade-offs between acquired and innate immune defenses in humans. Evolution, Medicine, and Public Health. 2016;2016(1):1–16. doi: 10.1093/emph/eov033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDade TW, Rutherford J, Adair L, Kuzawa CW. Early origins of inflammation: microbial exposures in infancy predict lower levels of C-reactive protein in adulthood. Proceedings of the Royal Society B: Biological Sciences. 2010;277(1684):1129–1137. doi: 10.1098/rspb.2009.1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMurray RW, Suwannaroj S, Ndebele K, Jenkins JK. Differential Effects of Sex Steroids on T and B Cells: Modulation of Cell Cycle Phase Distribution, Apoptosis and bcl-2 Protein Levels. Pathobiology. 2001;69(1):44–58. doi: 10.1159/000048757. [DOI] [PubMed] [Google Scholar]
- Miller RC, Brindle E, Holman DJ, Shofer J, Klein NA, Soules MR, O’Connor KA. Comparison of specific gravity and creatinine for normalizing urinary reproductive hormone concentrations. Clinical chemistry. 2004;50(5):924–932. doi: 10.1373/clinchem.2004.032292. [DOI] [PubMed] [Google Scholar]
- Min K-J, Lee C-K, Park H-N. The lifespan of Korean eunuchs. Current Biology. 2012;22(18):R792–R793. doi: 10.1016/j.cub.2012.06.036. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP, Bribiescas RG. Testosterone-mediated immune functions and male life histories. American Journal of Human Biology. 2005;17(5):527–558. doi: 10.1002/ajhb.20419. [DOI] [PubMed] [Google Scholar]
- Muehlenbein MP, Hirschtick JL, Bonner JZ, Swartz AM. Toward quantifying the usage costs of human immunity: Altered metabolic rates and hormone levels during acute immune activation in men. Am J Hum Biol. 2010;22(4):546–556. doi: 10.1002/ajhb.21045. [DOI] [PubMed] [Google Scholar]
- Muir C, Spironello-Vella E, Pisani N, deCatanzaro D. Enzyme immunoassay of 17 beta-estradiol, estrone conjugates, and testosterone in urinary and fecal samples from male and female mice. Horm Metab Res. 2001;33(11):653–658. doi: 10.1055/s-2001-18692. [DOI] [PubMed] [Google Scholar]
- Nerrienet E, Amouretti X, Müller-Trutwin M, Poaty-Mavoungou V, Bedjebaga I, Nguyen HT, Dubreuil G, Corbet S, Wickings E, Barre-Sinoussi F. Phylogenetic analysis of SIV and STLV type I in mandrills (Mandrillus sphinx): indications that intracolony transmissions are predominantly the result of male-to-male aggressive contacts. AIDS research and human retroviruses. 1998;14(9):785–796. doi: 10.1089/aid.1998.14.785. [DOI] [PubMed] [Google Scholar]
- Nunn CL, Lindenfors P, Pursall ER, Rolff J. On sexual dimorphism in immune function. Philosophical Transactions of the Royal Society B: Biological Sciences. 2009;364(1513):61–69. doi: 10.1098/rstb.2008.0148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters A. Testosterone treatment is immunosuppressive in superb fairy–wrens, yet free–living males with high testosterone are more immunocompetent. Proceedings of the Royal Society of London Series B: Biological Sciences. 2000;267(1446):883–889. doi: 10.1098/rspb.2000.1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinheiro J, Bates D. Mixed-effects models in S and S-PLUS. Springer Science & Business Media; 2006. [Google Scholar]
- Poiani A, Goldsmith AR, Evans MR. Ectoparasites of house sparrows (Passer domesticus): an experimental test of the immunocompetence handicap hypothesis and a new model. Behavioral Ecology and Sociobiology. 2000;47(4):230–242. [Google Scholar]
- Rantala MJ, Moore FR, Skrinda I, Krama T, Kivleniece I, Kecko S, Krams I. Evidence for the stress-linked immunocompetence handicap hypothesis in humans. Nature communications. 2012;3:694. doi: 10.1038/ncomms1696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts ML, Buchanan KL, Evans MR. Testing the immunocompetence handicap hypothesis: a review of the evidence. Animal Behaviour. 2004;68(2):227–239. [Google Scholar]
- Ruiz M, French SS, Demas GE, Martins EP. Food supplementation and testosterone interact to influence reproductive behavior and immune function in Sceloporus graciosus. Hormones and Behavior. 2010;57(2):134–139. doi: 10.1016/j.yhbeh.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scrimshaw NS, SanGiovanni JP. Synergism of nutrition, infection, and immunity: an overview. The American journal of clinical nutrition. 1997;66(2):464S–477S. doi: 10.1093/ajcn/66.2.464S. [DOI] [PubMed] [Google Scholar]
- Shattuck EC, Muehlenbein MP. Human sickness behavior: Ultimate and proximate explanations. American Journal of Physical Anthropology. 2015;157(1):1–18. doi: 10.1002/ajpa.22698. [DOI] [PubMed] [Google Scholar]
- Simmons ZL, Roney JR. Androgens and energy allocation: quasi-experimental evidence for effects of influenza vaccination on men’s testosterone. Am J Hum Biol. 2009;21(1):133–135. doi: 10.1002/ajhb.20837. [DOI] [PubMed] [Google Scholar]
- Spratt DI, Cox P, Orav J, Moloney J, Bigos T. Reproductive axis suppression in acute illness is related to disease severity. The Journal of Clinical Endocrinology & Metabolism. 1993;76(6):1548–1554. doi: 10.1210/jcem.76.6.8501163. [DOI] [PubMed] [Google Scholar]
- Spratt DI, Kramer RS, Morton JR, Lucas FL, Becker K, Longcope C. Characterization of a prospective human model for study of the reproductive hormone responses to major illness. Am J Physiol Endocrinol Metab. 2008;295(1):E63–69. doi: 10.1152/ajpendo.00472.2007. [DOI] [PubMed] [Google Scholar]
- Spratt DI, Morton JR, Kramer RS, Mayo SW, Longcope C, Vary CPH. Increases in serum estrogen levels during major illness are caused by increased peripheral aromatization. Am J Physiol Endocrinol Metab. 2006;291(3):E631–638. doi: 10.1152/ajpendo.00467.2005. [DOI] [PubMed] [Google Scholar]
- Stieglitz J, Trumble BC, Thompson ME, Blackwell AD, Kaplan H, Gurven M. Depression as sickness behavior? A test of the host defense hypothesis in a high pathogen population. Brain, behavior, and immunity. 2015 doi: 10.1016/j.bbi.2015.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Straub RH, Cutolo M, Buttgereit F, Pongratz G. Energy regulation and neuroendocrine–immune control in chronic inflammatory diseases. Journal of Internal Medicine. 2010;267(6):543–560. doi: 10.1111/j.1365-2796.2010.02218.x. [DOI] [PubMed] [Google Scholar]
- Trumble B, Cummings D, Von Rueden C, O’Connor K, Smith E, Gurven M, Kaplan H. Physical competition increases testosterone among Amazonian forager-horticulturalists: a test of the ‘challenge hypothesis’. Proc R Soc B. 2012:1471–2954. doi: 10.1098/rspb.2012.0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumble BC, Brindle E, Kupsik M, O’Connor KA. Responsiveness of the reproductive axis to a single missed evening meal in young adult males. Am J Hum Biol. 2010;22(6):775–781. doi: 10.1002/ajhb.21079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trumble BC, Cummings DK, O’Connor KA, Holman DJ, Smith EA, Kaplan H, Gurven M. Age-independent increases in male salivary testosterone during physical activity among Tsimane forager horticulturalists. Evolution and Human Behavior. 2013;34(5):350–357. doi: 10.1016/j.evolhumbehav.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warren HS, Tompkins RG, Moldawer LL, Seok J, Xu W, Mindrinos MN, Maier RV, Xiao W, Davis RW. Mice are not men. Proceedings of the National Academy of Sciences. 2014 doi: 10.1073/pnas.1414857111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterlow J. Protein turnover with special reference to man. Quarterly Journal of Experimental Physiology. 1984;69(3):409–438. doi: 10.1113/expphysiol.1984.sp002829. [DOI] [PubMed] [Google Scholar]
- Werner S, Grose R. Regulation of Wound Healing by Growth Factors and Cytokines. Physiological Reviews. 2003;83(3):835–870. doi: 10.1152/physrev.2003.83.3.835. [DOI] [PubMed] [Google Scholar]
- Wheelock EF. Interferon-Like Virus-Inhibitor Induced in Human Leukocytes by Phytohemagglutinin. Science. 1965;149(3681):310–311. doi: 10.1126/science.149.3681.310. [DOI] [PubMed] [Google Scholar]
- Yao G, Liang J, Han X, Hou Y. In vivo modulation of the circulating lymphocyte subsets and monocytes by androgen. International Immunopharmacology. 2003;3(13–14):1853–1860. doi: 10.1016/j.intimp.2003.09.002. [DOI] [PubMed] [Google Scholar]
- Zuk M, Stoehr A. Immune Defense and Host Life History. The American Naturalist. 2002;160(S4):S9–S22. doi: 10.1086/342131. [DOI] [PubMed] [Google Scholar]


