Abstract
The current study aimed to shed more light on the role of dopamine in temporal attention. To this end, we pharmacologically manipulated dopamine levels in a large sample of Parkinson’s disease patients (n=63) while they performed an attentional blink (AB) task in which they had to identify two targets (T1 and T2) presented in close temporal proximity among distractors. We specifically examined 1) differences in the magnitude of the AB between unmedicated Parkinson patients, who have depleted levels of striatal dopamine, and healthy controls, and 2) effects of two dopaminergic medications (L-DOPA and dopamine agonists) on the AB in the Parkinson patients at the group level and as a function of individual baseline performance. In line with the notion that relatively low levels of striatal dopamine may impair target detection in general, Parkinson patients OFF medications displayed overall poor target perception compared to healthy controls. Moreover, as predicted, effects of dopaminergic medication on AB performance critically depended on individual baseline AB size, although this effect was only observed for L-DOPA. L-DOPA generally decreased the size of the AB in patients with a large baseline AB (i.e., OFF medications), while L-DOPA generally increased the AB in patients with a small baseline AB. These findings may support a role for dopamine in the AB and temporal attention, more generally and corroborate the notion that there is an optimum dopamine level for cognitive function. They also emphasize the need for more studies that examine the separate effects of DA agonists and L-DOPA on cognitive functioning.
Keywords: Dopamine, Striatum, Attention, Attentional Blink, Parkinson’s Disease, L-dopa, Consciousness, Cognition
Introduction
The world around us changes continuously, bombarding our senses with more information than our brain can possibly process all the way up to the level of awareness. Attention is the generic term for those mechanisms, which dynamically prioritize processing of goal-relevant over irrelevant information and lead our experience to be dominated by one thing rather than another (Driver 2001). A key question concerns how attention influences the content of awareness. The attentional blink (AB) task is a powerful experimental paradigm used to investigate this question (Raymond et al. 1992). In this task, subjects view a rapidly presented sequence of visual stimuli and have to detect and identify two targets (T1 and T2) that are embedded in a stream of distracters. Subjects typically show high accuracy in identifying the first target (T1), but identification of the second target (T2) is much more variable and depends on its temporal succession to T1. When T2 appears more than 500 ms after T1, identification of T2 is high. However, if T2 occurs within 100–500 ms of T1, T2 is often not perceived (i.e., the AB; (Raymond et al. 1992)), an effect generally attributed to competition between stimuli for limited processing resources (Dux and Marois 2009; Martens and Wyble 2010).
Recent studies have linked striatal dopaminergic functioning to the AB (Colzato et al. 2008; Colzato et al. 2011; Slagter et al. 2012). For example, using positron emission tomography (PET), individual differences in D2-like receptor binding in the striatum were found to predict individual differences in AB size (Slagter et al. 2012). This is notable as while dopamine-mediated striato-frontal interactions have long been implicated in action selection, the striatum is connected through parallel loops to many frontal regions (Alexander and Crutcher 1990), and hence capable of modulating a wide range of frontal, and associated cognitive, processes. Indeed, a growing body of work supports the notion that striatal dopamine also plays a critical role in selecting information for representation in prefrontal working memory (Braver and Cohen 1999; Frank et al. 2001; Cools and D’Esposito 2011; Chatham and Badre 2015), and in switching the focus of attention (Van Schouwenburg et al. 2010). Together these findings indicate that striatal dopaminergic activity may not only contribute to action selection, but may also modulate the selection of goal-relevant or salient information for further cognitive processing.
The current study investigated the relationship between dopamine and the AB more directly by manipulating dopamine levels pharmacologically in a large sample of Parkinson’s disease patients. It specifically tested two predictions. Our first prediction was that Parkinson patients, who have depleted dopamine in the striatum, would display a bigger AB, and possibly also lower T1 accuracy, OFF medications compared to healthy controls due to a general target selection impairment. Secondly, we predicted that dopaminergic medications would modulate the AB in Parkinson patients as a function of their baseline (i.e., OFF medication) AB size. Based on work showing that baseline dopamine levels strongly determine effects of dopaminergic medications on cognitive performance (Cools and D’Esposito 2011; Wylie et al. 2012), we specifically predicted that dopaminergic medications would decrease the AB in individuals with a relatively large baseline AB, but increase the AB in individuals with a small baseline AB. We examined both the separate and combined effects of L-Dopa and dopamine agonists on AB performance, as little is known about differential effects of these medications on cognition in Parkinson’s disease.
MATERIALS AND METHODS
Participants
72 patients diagnosed with Parkinson’s disease and 33 healthy controls participated in the study. 9 patients and 2 controls were excluded from data analysis because they did not complete all sessions (6 patients) or scored poorly on a measure of global cognitive status (3 patients, 2 controls; see below). This resulted in 63 patients with Parkinson’s disease and 31 healthy controls being included in the data analyses. There were three groups of Parkinson patients: 1) patients currently prescribed both L-DOPA and a DA agonist (dual-therapy patients; n=23), 2) patients prescribed only L-DOPA (n=26), and 3) patients prescribed only a DA agonist (n=14). Monotherapy patients were tested twice, once OFF their medication and once ON their medication. Dual-therapy patients were tested four times, with each visit representing all combinations of medication states: ON L-DOPA and DA agonist, ON L-DOPA only, ON DA agonist only, and OFF both medications. This permitted us to study both the separate and combined effects of L-DOPA and DA agonists. Session order (e.g., ON, OFF medication) was counterbalanced across patients. Patients refrained from L-Dopa for minimum of 12 hours, DA Agonist for 36 hours, and were evaluated 1 hour after acute therapy in the optimal ON state. Patients were recruited from the patient population in the Movement Disorders Clinic at Vanderbilt University Medical Center. They all met the following inclusion criteria: no history of (i) other neurological condition besides Parkinson’s disease; (ii) bipolar affective disorder, schizophrenia, or other psychiatric condition known to compromise executive cognitive functions; and (iii) mood disorder (depression) or medical condition known to interfere with cognition (e.g., diabetes, pulmonary disease).
Participants were evaluated and diagnosed with idiopathic Parkinson’s disease by a movement disorder neurologist and were being treated with dopamine agonists and/or L-DOPA. DA agonists taken included Mirapex (4 DA agonist-only patients, 5 dual-therapy patients), Neupro (1 DA agonist-only patient, 3 dual-therapy patients), and Requip (8 DA agonist-only patients (3 of which took an extended release tablet), 15 dual-therapy patients (7 of which took an extended release tablet). Patients were excluded from analyses if they performed at a level on the Montreal Cognitive Assessment that revealed evidence of dementia (included scores > 21). The severity of their motor symptoms was graded using the Unified Parkinson Disease Rating Scale (UPDRS) motor subscore; additionally, they all received a Stage III rating or less using the Hoehn and Yahr scale (Hoehn & Yahr, 1967). Dosages for the dopamine medications were converted to levodopa equivalent daily dose (LEDD) values (Weintraub et al., 2006). During each visit, the patients performed several tasks, including the AB task (data of other tasks not reported here). Healthy controls, matched in age, gender and years of education, came to the lab once and performed the same battery of cognitive tasks. Controls were screened for using the same inclusion/exclusion criteria listed above. All participants had corrected-to-normal vision. They all provided informed consent before participating in the study in full compliance with the standards of ethical conduct in human investigation as regulated by the authors’ institutions. The demographic characteristics of the participants in the current study are listed in Table 1 separately for patient groups and healthy controls.
Table 1.
Sample characteristics. Standard deviation is noted between brackets. UPDRS: Unified Parkinson Disease Rating Scale; LEDD: levodopa equivalent daily dose; M: male; F: female.
| Healthy controls |
All patients |
L-DOPA only |
DA agonist only |
Dual- therapy |
|
|---|---|---|---|---|---|
| Sample size | 31 | 63 | 26 | 14 | 23 |
| Age (years) | 63,9 (5,1) | 63,7 (7,9) | 65,5 (9,0) | 62,8 (7,2) | 62,4 (7,0) |
| Sex (M:F) | 16:15 | 44:19 | 20:6 | 8:6 | 16:7 |
| Education (years) | 16,7 (2,5) | 15,7 (2,5) | 16,0 (2,4) | 16,1 (2,5) | 15,0 (2,5) |
| Disease duration (years) |
- | 6,4 (4,8) | 5,0 (2,5) | 6,0 (7,7) | 8,3 (4,0) |
| UPDRS motor score (OFF medication) |
- | 26,4 (13,0) | 24,4 (10,3) | 23,4 (10,8) | 30,4 (16,1) |
| Total LEDD (mg) | - | 717,5 (440) | 745,6 (429) | 242,9 (107) | 974,7 (342) |
Attentional blink task
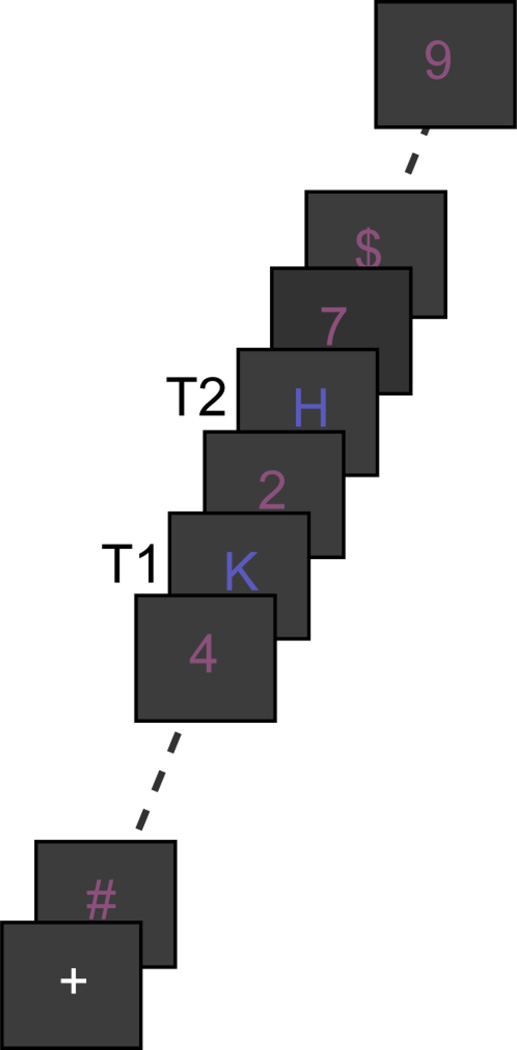
Participants performed an attentional blink task in which they had to detect two letters (T1 and T2) presented in a rapid stream of twenty-two distractor stimuli (Figure 1A). In a given trial, the two target letters were either both blue (RGB: 90, 90, 190) or both green (RGB: 30, 120, 55), while the distractor stimuli were always red (RGB: 140, 80, 125) (colors were isoluminant). Distractor stimuli were randomly drawn (without replacement) from the digits 2 to 9 and the following symbols: @ # $ %} < =. The target letters could be any capital letter from the alphabet, except I O Q S, as these letters resemble digits (1, 0 and 5). Each trial started with a 1000 ms fixation period during which a plus sign (font size 18, courier new) was displayed at the center of the screen. The subsequent stream consisted of 24 characters (font size 20, courier new), each presented at fixation for 67 ms followed by a 33 ms blank interval (i.e., 10 Hz presentation speed). T1 was randomly presented at temporal position 8 to 11. T2 followed T1 with equal probability at lag 1, 2, 3, 4, or 9 (i.e., after 0–3 or 8 intervening distractor stimuli). Participants were then shown two letters, one of which was T1, and asked to press a left button if they thought the letter on the left was T1, or a right button, if they thought the right letter was T1. They were then shown two different letters, one of which was T2, and again asked to press the button corresponding to the location of the letter that matched T2. Responses were forced choice and registered with response grips, as Parkinson patients often experience difficulty with, and/or are very slow in, typing in answers using a keyboard. There was no time limit imposed for responses. All stimuli were displayed on a grey background (RBG: 60,60,60) (display resolution: 640 by 480). The task was divided into 5 blocks of 30 trials each. In between blocks, participants could take short breaks. Participants first practiced the task for 18 trials. In the first 6 practice trials, the stream was presented at half speed (5Hz) to ease adaptation to the rapid pace of the stimulus stream.
Fig. 1.
The attentional blink (AB) task. Subjects had to detect two targets (T1 and T2; two letters) in a rapid stream of distractor stimuli. Shown is an example of a short T1-T2 interval trial.
Data analysis
For each participant, we calculated the percentage of trials (separately for each T1–T2 lag), in which both targets were correctly identified (T2/T1 correct), as well as the percentage of trials in which T1 was correctly identified irrespective of T2 accuracy (T1 correct). We also calculated each individual’s baseline (i.e. OFF medications) AB size as the difference in T2/T1 accuracy between Lag9 and the lag of maximal AB (Lag 2 or 3).
Parkinson patients OFF medication vs. healthy controls
To test our first prediction that Parkinson patients OFF medication will exhibit a larger AB as well as a more general target selection impairment, as indexed by lower T1 accuracy, compared to healthy controls, we computed a repeated measures ANOVA with T2/T1 accuracy or T1 accuracy serving as the dependent variable, Lag (1, 2, 3, 4, and 9) as a within-subject variable, and Group (Parkinson patients OFF medication(s), healthy controls) as a between-subject variable.
Effects of dopamine medication on the AB in Parkinson patients (subgroup analyses)
While our second prediction was that dopaminergic medication would modulate the size of the AB in Parkinson patients as a function of baseline AB size, we first examined the effects of dopaminergic medication on the AB at the group level (i.e., regardless of individual baseline AB size), as well as on T1 accuracy. Separate repeated measures ANOVAs were computed with Lag (1, 2, 3, 4 and 9) and Medication Condition as within-subject variables and, when data from different groups of patients were included, Therapy Group (L-DOPA only group, DA agonist only group, and/or L-DOPA+DA agonist group) as a between-subject variable. Medication Condition had two (i.e., ON L-DOPA (or agonist) vs. OFF) or four (i.e., ON L-DOPA, ON DA agonist, ON both, OFF both) levels depending on the therapy group under study. For example, when studying the effects of L-DOPA on AB size, Medication Condition had two levels (ON L-DOPA vs. OFF) and Therapy Group also had two levels (L-DOPA only group, L-DOPA+DA agonist group). This permitted examination of how specific DA medications affected performance.
The relationship between individual baseline AB size and effects of dopamine medication on AB size in Parkinson patients (individual scores analyses)
Next, we examined if effects of dopamine on AB size were driven by individual differences in baseline AB size. AB size was defined as the difference in T2/T1 accuracy between Lag9 and the lag of maximal AB. Note that even when no relationship exists between the baseline (x) and the change (y-x), the fact that x is present in both terms, leads to an expected spurious correlation of −.7 between x and y-x (Tu and Gilthorpe 2007). Therefore, instead of running a correlation between baseline AB size and the medication-induced change in AB size from baseline, we used a likelihood ratio chi-square test, like Mauchly’s test of sphericity, to test whether the co-variances between individual scores were significantly different between conditions (OFF medications, ON l-DOPA, ON DA agonist, ON both). Under the null hypothesis that dopaminergic medications have no effect on AB size, one would expect co-variances to be similar across conditions, assuming that measurement error is similar across conditions. If Mauchly’s sphericity test on the co-variance matrix indicates differences in co-variances across the different conditions (OFF, ON l-DOPA, ON DA agonist, ON both), this would suggest that dopaminergic medications affected either true AB size or measurement error size. The latter seems unlikely because the measurement conditions (the T1/T2 task conditions) were identical and the order of the conditions was counterbalanced. Therefore, rejection of the sphericity hypothesis should be attributable to a change in the underlying individual true AB size on dopaminergic medications. In case of rejection of the null hypothesis, we subsequently examined how variances of the covariances changed ON vs. OFF medications. Because the baseline-dependent changes predict certain relations between variances and covariances depending on whether the effect is due to one or the other medication or an interaction between the two, fitting and comparing different covariance structures to the data can analyze this.
Results
Parkinson patients vs. healthy controls: General gating impairment?
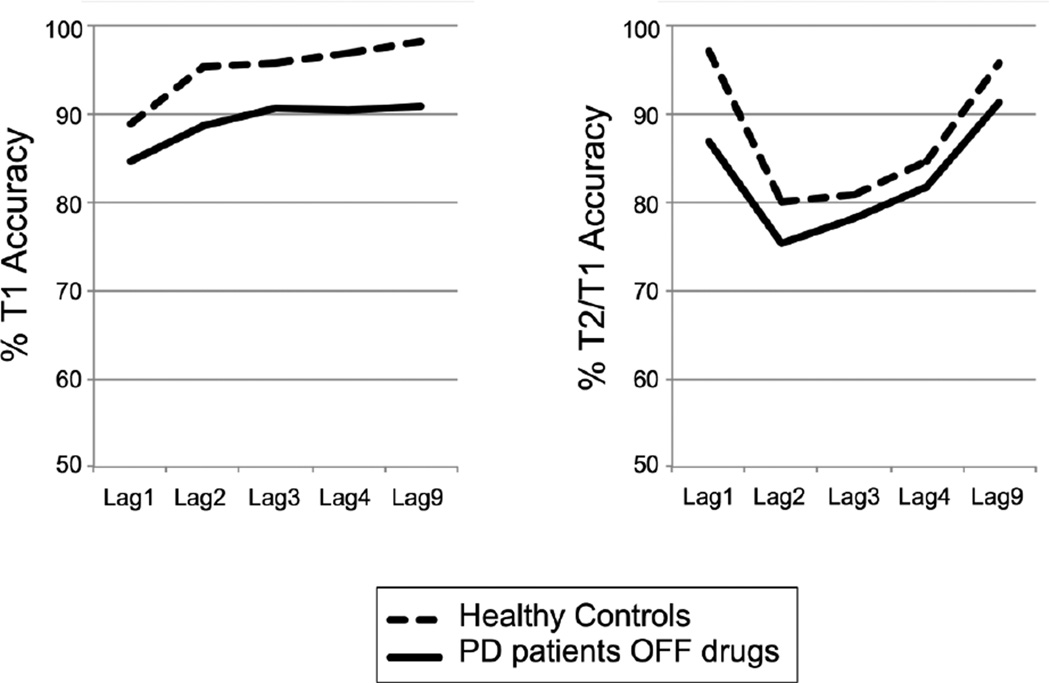
As can be seen in Figure 2, both Parkinson patients in an OFF medication state and healthy controls performed the task with high levels of overall T1 accuracy (PD: 89%; HC: 95%). Nevertheless, in line with the notion that low DA levels impair target identification generally, Parkinson patients OFF medication(s) performed significantly worse on the AB task compared to healthy controls, displaying both reduced overall T1 accuracy (main effect Group (patients vs. healthy controls): F(1,88)=8.45, p=.005) and T2/T1 accuracy (main effect Group: F(1,88)=5.19, p=.025). However, contrary to our first prediction, Parkinson patients OFF medication(s) did not display a bigger AB than healthy controls as the interaction between Group and Lag was not significant (F(2.99,262.92)=1.71, p=.16). Group differences in T1 accuracy were also not affected by Lag (F(3.06,260.04)=0.48, p=.70). Thus, Parkinson patients OFF medication(s) performed more poorly than matched healthy controls on both the T1 and the T2 task, regardless of Lag, possibly reflecting a general impairment in target detection.
Fig. 2.
This figure shows % T1 accuracy and T2/T1 accuracy as a function of lag, separately for healthy controls and Parkinson’s disease (PD) patients OFF medications. As can be seen, PD patients showed a general decrease in target detection accuracy.
Effects of dopamine on AB task performance within Parkinson patients
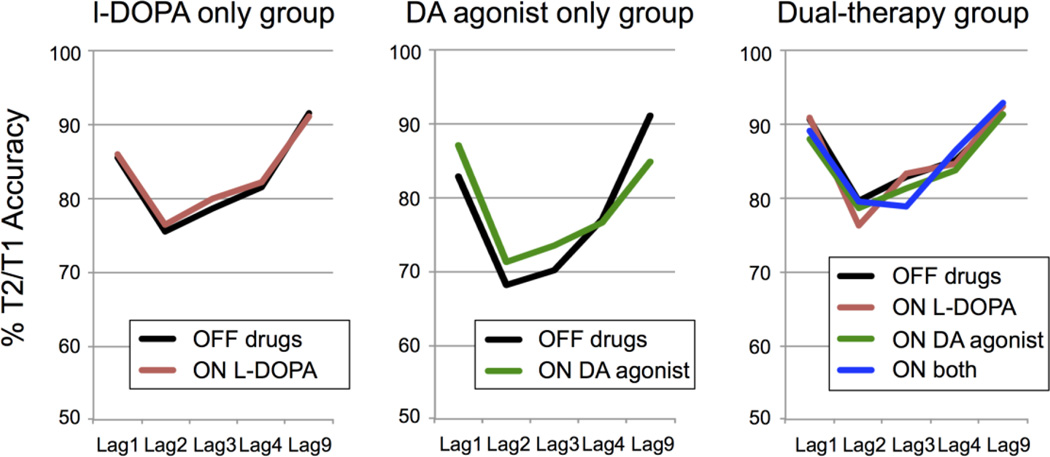
We next examined the effects of dopamine on the AB within the Parkinson patients. As can be seen in Figure 3, dopaminergic medications did not reduce the size of the AB in Parkinson patients as a group. This was true for both the L-DOPA only and DA agonist only conditions, as well as when these medications were combined. The absence of an effect of dopamine on AB size at the group level was confirmed statistically by the absence of an interaction between Lag and Medication Condition when comparing AB performance ON L-DOPA vs. OFF medication(s) in L-DOPA only and dual therapy patients (F(4,188)=0.32, p=.80), and ON DA agonist vs. OFF medication(s) in DA agonist-only and dual therapy patients (F(4,32)=0.94, p=.45). The interaction between Lag and Medication Condition (ON L-DOPA only, ON DA agonist only, ON both, OFF both) was not significant either in the dual therapy group (F(12,264)=1.13, p=.35). The lack of a medication-related modulation of the AB did not depend on medication status, as no significant interactions between Lag, Medication Condition and Therapy Group were observed (all p’s > .13). Thus, at the group level, contrary to our second prediction, effects of dopamine on the AB to T2 were not apparent. Dopamine also did not affect T1 accuracy, as indicated by non-significant effects of Medication Condition (all p’s > .19), interaction effects between Medication Condition and Lag (all p’s > .41), and between Therapy Group, Medication Condition and Lag (all p’s > .16).
Fig. 3.
This figure shows the (absence of) effects of dopaminergic medication on T2/T1 accuracy per Lag and Medication condition, separately for each Therapy Group.
Therapy groups (i.e., L-DOPA only, DA agonist only, dual therapy) did not differ significantly from each other in terms of age, education level or gender (all p’s >. 18) (see Table 1). UPDRS motor scores OFF medication also did not differ between medication groups, although disease duration was significantly longer for dual-therapy patients compared to L-DOPA-only patients (p=.001; no significant difference in disease duration between DA agonist-only patients and dual-therapy (p=.24) or L-DOPA only patients (p=.53). Total LEDD differed significantly between medication groups, with dual-therapy groups receiving the highest daily dose, then L-DOPA only patients, and DA agonist patients received the lowest daily does (dual-therapy patients vs. L-DOPA only (p=.046) and DA agonist only (p<.001) patients; L-DOPA only vs. DA agonist only patients (p<.001).
Individual differences in AB size predict effect of DA on the AB
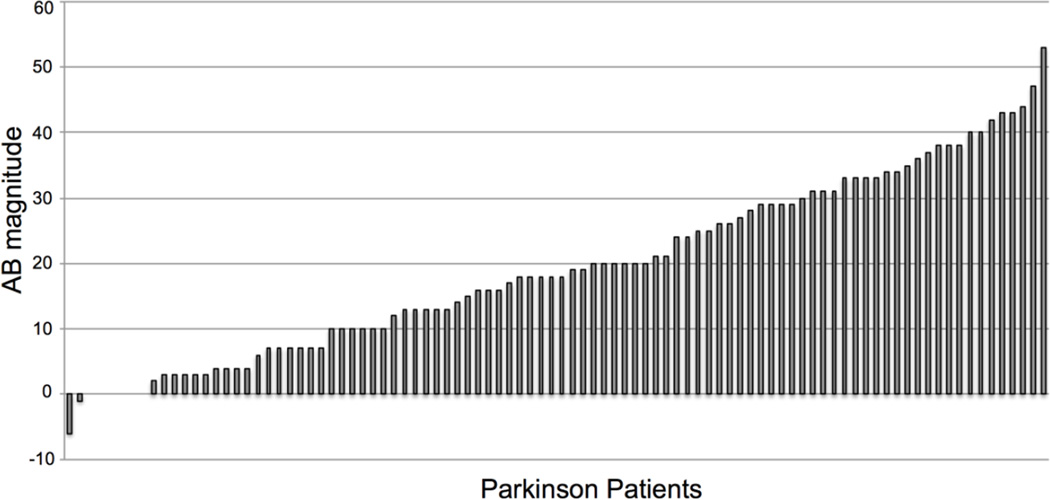
While AB size is stable across time within a given individual (Dale et al. 2013), it is well known that the magnitude of the AB differs substantially between individuals (Martens et al. 2006). Indeed, as can be seen in Figure 4, Parkinson patients OFF medication(s) also displayed a wide range of variability in AB size, with some patients almost never identifying T2, some almost always identifying T2, and the majority of patients falling somewhere in between these two extremes.
Fig. 4.
There was large variability in AB size across Parkinson patients (represented by the bars in the histogram).
Such individual differences in AB size may have masked effects of DA medications at the group level, as it is well known that effects of DA on cognitive performance depend on baseline DA levels and baseline cognitive performance (Cools and D’Esposito 2011). In fact, we specifically predicted that DA would reduce the AB in individuals with a relatively large baseline AB, and conversely increase the AB in individuals with a relatively small baseline AB. To test this prediction, we first examined if the co-variances between individual scores were significantly different between conditions (OFF medications, ON L-DOPA, ON DA agonist, ON both) using Mauchley’s sphericity test. One requirement for such a covariance structure analysis is that the covariance matrices are similar across groups. In this study patient groups were natural groups and not randomly assigned. Thus, it may well be that these groups have prior differences. We therefore first examined if the assumption of equal covariance structure was valid using multi-group covariance structure analysis in which an unstructured covariance matrix fitted to the data was constrained to be equal across groups. The likelihood ratio test (Box’s M test) indicated that the assumption of equal covariance matrices among the groups was untenable. Further tests showed that the DA agonist only and dual-therapy groups differed, but the L-DOPA and dual-therapy groups were similar. In the following, we therefore present the results from analyses with and without the patients in the DA agonist-only group included.
Using a likelihood ratio chi-square test (Mauchley’s sphericity test), we found that L-DOPA, but not DA agonists, modulated the AB as a function of individual baseline AB size. Specifically, the hypothesis that DA agonists had no effect on the underlying true AB sizes could not be rejected for being inconsistent with the observed covariance matrix (X2 = 3.404, df = 3, p = 0.333 with DA agonist only patients excluded; X2 = 3.88, df = 3, p = 0.275 with DA agonist only patients included), but the hypothesis that L-DOPA had no effect had to be rejected (X2 = 9.63, df = 3, p = 0.022) when the DA agonist only patients were left out of the analysis, and indeed the effect of L-DOPA was marginally significant if the DA agonist only patients were included in the analysis (X2 = 7.168, df = 3, p = 0.067). An interaction effect between medications (DA agonists and L-DOPA) appeared to be absent, whether (X2 = 10.56, df = 3, p = 0.159) or not (X2 = 10.09, df = 3, p = 0.184) the DA agonist only patients were excluded from the analysis. Thus, L-DOPA modulated the AB as a function of individual baseline AB size.
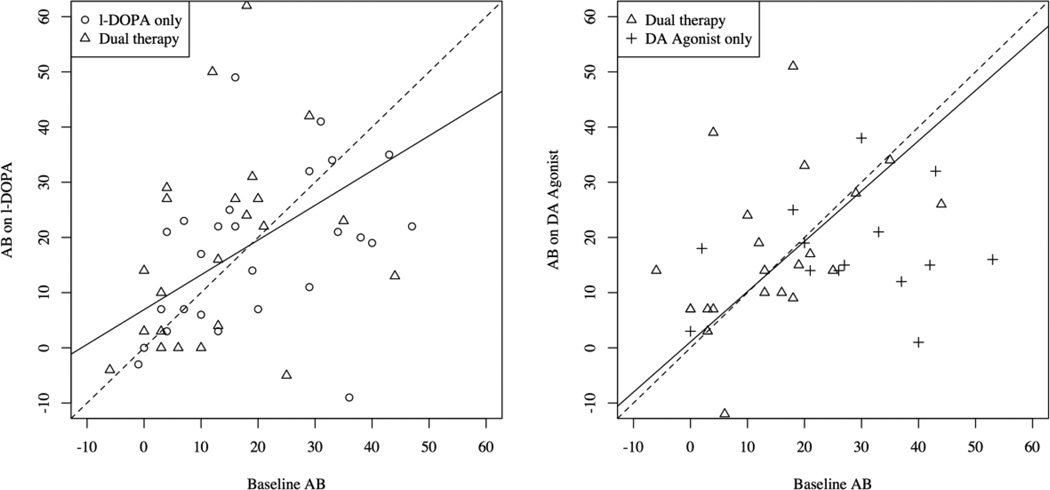
To determine how the AB was modulated by L-DOPA as a function of baseline AB size, we quantified the estimated effects by the intercept and slope of the linear relationship between L-DOPA condition and the baseline OFF medications condition that is implied by the covariance structure analysis. For comparison, we also did this for the DA agonist condition. The linear relationships are depicted as black solid lines in the scatter plots in Figure 5. The black dotted lines indicate the line y = x around which the data points would scatter if there were no effect at all. As can be seen in Figure 5 (right panel), the linear relationship found from the covariance structure analysis for the DA agonist condition is almost perfectly aligned with the dotted line (with all participants included: slope = 1.05, z=0.21, p=.83; without the DA agonist only participants: slope = .911, z=−0.43, p=.66), suggesting no modulation of the AB by DA agonists as a function of baseline AB size. However, the linear relationship derived from the covariance structure analysis for the L-DOPA condition is significantly slanted (with all participants included: slope = .617, z=−2.68, p=.008; without the DA agonist only participants: slope = .630, z=−2.43, p=.015). These observations indicate that, in line with our prediction, individuals with a small AB OFF medications performed worse on L-DOPA, whereas individuals with a large AB OFF medications performed better on L-DOPA. Yet, DA agonists did not modulate AB magnitude as a function of individual baseline performance.
Fig. 5.
Displayed is the cross-subject relationship between AB magnitude in the baseline condition (OFF medication) and the on L-DOPA (left panel) and on DA agonist only (right panel) conditions. L-DOPA tended to decrease the AB in patients with a large baseline AB and to increase the AB in patients with a small baseline AB. DA agonists did not modulate the AB as a function of baseline AB magnitude. Note that dual therapy patients are included in both plots, as they performed the AB task both on only L-DOPA and only on a DA agonist.
AB and patient characteristics
As one may expect, years since disease onset correlated significantly, although modestly, with UPDRS motor scores in the OFF medications condition across patients (r(61)=.27, p=.037), demonstrating more motor symptoms as a function of disease progression. We also explored the relationship between baseline AB size and these factors (years since disease onset, UPDRS motor score), but neither predicted AB size across subjects (all p’s > .64).
Discussion
The current study tested the hypothesis that DA plays a role in temporal attention by examining 1) differences in the magnitude of the AB between unmedicated Parkinson patients, who have depleted levels of striatal DA, and healthy controls, and 2) effects of dopaminergic medications on the AB in the Parkinson patients at the group level and as a function of individual baseline performance. In line with the notion that relatively low levels of striatal DA may impair target identification in general, Parkinson patients OFF medications displayed overall poor T1 and T2/T1 accuracy compared to healthy controls. These observations contrast with those of a previous study, which observed no differences in overall T1 and T2 accuracy (or AB size) between Parkinson patients ON medication and healthy controls (Vardy et al. 2003). Yet, in that study, Parkinson patients were only tested while medicated and individual differences in baseline (OFF medication) AB size were not taken into account.
Indeed, while at the group level, DA medications did not improve target accuracy in general, or modulate the size of the AB in the Parkinson patients, we found that, as predicted, the effects of DA medications on AB performance critically depended on individual baseline AB size, although this effect was only observed for L-DOPA. That is, L-DOPA generally decreased the size of the AB in patients with a large baseline AB (i.e., OFF medications), while L-DOPA generally increased the AB in patients with a small baseline AB. Given previous PET research which related the AB to striatal dopamine (Slagter et al. 2012), this observation may suggest that L-DOPA modulated the AB by modulating striatal dopamine levels. Yet, as discussed below, it is conceivable that effects of L-DOPA were not confined to the striatum. The current findings also emphasize the need for more studies that examine the separate effects of DA agonists and L-DOPA and combine this with neuroimaging to isolate the precise underlying neural mechanisms. While the DA precursor L-DOPA affects both D1 (exitatory) and D2 (inhibitory) receptor functioning, DA agonist effects are selective to D2 receptors (De Keyser et al. 1995; Seeman 2007). DA agonists have also been shown to affect ventral striatal activity (Voon et al. 2011), and lead to down-regulation of D2 receptors (Parish et al. 2002; Fasano et al. 2010). Thus, L-DOPA and DA agonists differentially affect dopaminergic neurotransmission, which may explain the selective effect of L-DOPA on the AB observed in the current study. Yet, the levodopa-equivalent daily dose was substantially lower in the DA agonist-only group. Thus, dosage differences may also have contributed to the observed differences between the two kinds of medications. Very few studies have directly compared the cognitive effects of L-DOPA and dopamine agonists, and more work is necessary to establish the extent to which these two types of medication affect performance in different cognitive domains in a similar or differential way. Our findings furthermore corroborate previous work showing that there is an optimum level of dopamine for cognitive function (Williams and Goldman-Rakic 1995; Zahrt et al. 1997; Arnsten 1998) and emphasize the need to take individual variation in baseline measures into account when isolating the effects of dopamine (Cools and D’Esposito 2011).
The here observed relationship between baseline AB performance and the effect of L-DOPA fits with a recently postulated model of the AB, which posits an U-shaped relationship between AB size and striatal dopamine levels, in that it suggests that the AB may result from both impaired gating (when striatal DA levels are too low) and inefficient gating (when striatal DA levels are too high) (Slagter et al. 2012). The observed inverted U-shaped relationship could also be responsible for the lack of a significant overall difference in AB size in Parkinson’s disease patients ON vs. OFF medications. The effects of dopaminergic medication were conceivably not confined to the striatum and could well differ from region to region and as a function of illness duration. For instance, replacement to an 'optimal' level in the motor system may lead to too much dopamine in other systems, in particular relatively intact or up-regulated brain areas, such as the ventromedial striatum (Gotham et al. 1988; Cools et al. 2001a). Dopamine can thus also impair cognitive functioning via overdosing. Individual differences in dopamine replacement in different brain regions and/or disease duration could thus also have influenced our results. Future studies combining neuroimaging with pharmacological manipulations are necessary to determine how dopamine may affect the AB.
The observed general impairment in target detection in Parkinson patients OFF dopaminergic medication relative to controls adds to previous reports that the depletion of central dopamine due to Parkinson’s disease produces deficits in working memory (Cools et al. 2010), cognitive flexibility (Cools et al. 2001b; Cools et al. 2003), interference and cognitive control during action selection (Wylie et al. 2009; Wylie et al. 2010), and cognitive reinforcement learning (Frank et al. 2004). Thus, depletion of striatal dopamine in Parkinson’s disease is associated with deficits in multiple cognitive domains, which can to some extent be remediated by dopaminergic medication. It has been postulated that one principle may underlie these diverse cognitive deficits as well as the characteristic motoric problems observed in Parkinson’s disease, namely the notion that reduced dopamine increases activity and causes long-term potentiation in the indirect pathway of the basal ganglia (Moustafa et al. 2008; Wiecki and Frank 2010; Maia and Frank 2011). The end result of activation of this pathway is increased inhibition of the thalamus and thereby of frontal cortex by the output structures of the basal ganglia. As the basal ganglia are connected via the thalamus to all regions of frontal cortex in parallel loops (Alexander and Crutcher 1990; Draganski et al. 2008), depletion of striatal dopamine in the basal ganglia can similarly affect these different loops and associated functions.
Yet, it should be emphasized that the observed effects of L-DOPA likely were not limited to the striatum, and that changes in prefrontal dopaminergic neurotransmission may have contributed to our findings (Cools et al. 2010). As patients were given a two-option choice at the end of each trial, this may have also influenced our findings. Moreover, other neurotransmitters, especially norepinephrine given its role in temporal attention (Aston-Jones and Cohen 2005), likely also contribute to the AB. For example, striatal dopamine may modulate the threshold for prefrontal gating, and norephinephrine may boost cortical target processing (Nieuwenhuis et al. 2005). As Parkinson’s disease is also associated with impairments in other neurotransmitter systems, including noradrenergic and cholinergic systems (Gratwicke et al. 2015), we thus cannot exclude the possibility that dysfunction in other systems contributed to observed effects. Nevertheless, together with previous findings (Colzato et al. 2008; Slagter et al. 2012) linking the AB to striatal dopamine, the current results, which reveal an effect of a dopaminergic medication on the AB as a function of individual AB size, suggest that dopamine may play a critical role in the attentional blink and temporal attention, more generally.
Neuroimaging studies of the AB have shown that only consciously perceived T2’s are associated with greater activity and sustained and recurrent interactions in a network of frontal, parietal and visual brain regions (e.g., Marois et al. 2000; Gross et al. 2004; Kranczioch et al. 2005; Slagter et al. 2010) in line with findings from neuroimaging studies using other paradigms (Rees et al. 2002; Haynes 2009; Dehaene and Changeux 2011; Lau and Rosenthal 2011; Aru et al. 2012; van Gaal and Lamme 2012). Based on this work, influential theories propose that conscious access is all or none and stems from a cognitive architecture with an evolved function: the flexible sharing of information throughout the cortex so that it can be used by various operations, such as manipulation in working memory (e.g., Baars, 1993; Dehaene and Naccache, 2001). Yet, this work leaves unresolved what determines whether a piece of information is ‘selected’ for global broadcasting and conscious access. Albeit speculative, through it’s ability to modulate thalamocortical interactions and thereby activity in the fronto-parietal network that gives rise to conscious experience, the striatum could be part of a subcortical network that provides a “gateway” to conscious experience, e.g., by switching the focus of attention (Van Schouwenburg et al. 2010) or by gating information for sustained representation in prefrontal working memory (Braver and Cohen 1999; Frank et al. 2001; Cools and D’Esposito 2011; Chatham and Badre 2015). However, other researchers have argued that we are conscious of much more information we can cognitively access and report on (Lamme 2010; Block 2011). Research furthermore indicates that visual consciousness and attention can be (neurally) dissociated (Koch and Tsuchiya 2007; Wyart and Tallon-Baudry 2008; Norman et al. 2015). Notably, not only AB studies (Slagter et al. 2010; Slagter et al. 2012), but also studies using simple backward masking tasks with only one stimulus (Christensen et al. 2006; Van Opstal et al. 2014; Bisenius et al. 2015), in which conscious access is not dependent on attentional selection as in the AB task (Dehaene and Changeux 2011), have reported greater activity in the striatum to consciously perceived stimuli. Future studies in humans that can measure striatal activity with high temporal precision are necessary to determine the precise contribution of the striatum to the AB and conscious perception, more generally.
Highlights.
We examined the effect of dopaminergic medication on temporal attention
Parkinson patients had to detect two temporally-close targets on and off medication
L-DOPA modulated second target perception based on baseline performance
Dopamine may play a role in temporal attention
L-DOPA and DA agonists have separate effects on cognitive functioning
Acknowledgments
This study was funded by a VIDI grant by the Netherlands Organization for Scientific Research (NWO) to HAS, a K23 NS080988 to DOC, and a National Institute on Aging grant (K23 AG028750) to SAW (the content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institute on Aging or the National Institutes of Health).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander GE, Crutcher MD. Functional architecture of basal ganglia circuits: neural substrates of parallel processing. Trends Neurosci. 1990;13:266–271. doi: 10.1016/0166-2236(90)90107-l. [DOI] [PubMed] [Google Scholar]
- Arnsten AF. Catecholamine modulation of prefrontal cortical cognitive function. Trends Cogn Sci (Regul Ed) 1998;2:436–447. doi: 10.1016/s1364-6613(98)01240-6. [DOI] [PubMed] [Google Scholar]
- Aru J, Bachmann T, Singer W, Melloni L. Distilling the neural correlates of consciousness. Neurosci Biobehav Rev. 2012;36:737–746. doi: 10.1016/j.neubiorev.2011.12.003. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: adaptive gain and optimal performance. Annu Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Baars BJ. A Cognitive Theory of Consciousness. Cambridge University Press; 1993. [Google Scholar]
- Bisenius S, Trapp S, Neumann J, Schroeter ML. Identifying neural correlates of visual consciousness with ALE meta-analyses. Neuroimage. 2015;122:177–187. doi: 10.1016/j.neuroimage.2015.07.070. [DOI] [PubMed] [Google Scholar]
- Block N. Perceptual consciousness overflows cognitive access. Trends Cogn Sci (Regul Ed) 2011;15:567–575. doi: 10.1016/j.tics.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Braver TS, Cohen JD. Dopamine, cognitive control, and schizophrenia: the gating model. Prog Brain Res. 1999;121:327–349. doi: 10.1016/s0079-6123(08)63082-4. [DOI] [PubMed] [Google Scholar]
- Chatham CH, Badre D. Multiple gates on working memory. Current Opinion in Behavioral Sciences. 2015;1:23–31. doi: 10.1016/j.cobeha.2014.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen MS, Ramsøy TZ, Lund TE, et al. An fMRI study of the neural correlates of graded visual perception. Neuroimage. 2006;31:1711–1725. doi: 10.1016/j.neuroimage.2006.02.023. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Slagter HA, de Rover M, Hommel B. Dopamine and the management of attentional resources: genetic markers of striatal D2 dopamine predict individual differences in the attentional blink. J Cogn Neurosci. 2011;23:3576–3585. doi: 10.1162/jocn_a_00049. [DOI] [PubMed] [Google Scholar]
- Colzato LS, Slagter HA, Spapé MMA, Hommel B. Blinks of the eye predict blinks of the mind. Neuropsychologia. 2008;46:3179–3183. doi: 10.1016/j.neuropsychologia.2008.07.006. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Enhanced or impaired cognitive function in Parkinson’s disease as a function of dopaminergic medication and task demands. Cereb Cortex. 2001a;11:1136–1143. doi: 10.1093/cercor/11.12.1136. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. Mechanisms of cognitive set flexibility in Parkinson’s disease. Brain. 2001b;124:2503–2512. doi: 10.1093/brain/124.12.2503. [DOI] [PubMed] [Google Scholar]
- Cools R, Barker RA, Sahakian BJ, Robbins TW. L-Dopa medication remediates cognitive inflexibility, but increases impulsivity in patients with Parkinson’s disease. Neuropsychologia. 2003;41:1431–1441. doi: 10.1016/s0028-3932(03)00117-9. [DOI] [PubMed] [Google Scholar]
- Cools R, D’Esposito M. Inverted-U-shaped dopamine actions on human working memory and cognitive control. Biol Psychiatry. 2011;69:e113–e125. doi: 10.1016/j.biopsych.2011.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cools R, Miyakawa A, Sheridan M, D’Esposito M. Enhanced frontal function in Parkinson’s disease. Brain. 2010;133:225–233. doi: 10.1093/brain/awp301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dale G, Dux PE, Arnell KM. Individual differences within and across attentional blink tasks revisited. Atten Percept Psychophys. 2013 doi: 10.3758/s13414-012-0415-8. [DOI] [PubMed] [Google Scholar]
- De Keyser J, De Backer JP, Wilczak N, Herroelen L. Dopamine agonists used in the treatment of Parkinson’s disease and their selectivity for the D1, D2, and D3 dopamine receptors in human striatum. Prog Neuropsychopharmacol Biol Psychiatry. 1995;19:1147–1154. doi: 10.1016/0278-5846(95)00232-4. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Changeux J-P. Experimental and theoretical approaches to conscious processing. Neuron. 2011;70:200–227. doi: 10.1016/j.neuron.2011.03.018. [DOI] [PubMed] [Google Scholar]
- Dehaene S, Naccache L. Towards a cognitive neuroscience of consciousness: basic evidence and a workspace framework. Cognition. 2001;79:1–37. doi: 10.1016/s0010-0277(00)00123-2. [DOI] [PubMed] [Google Scholar]
- Draganski B, Kherif F, Klöppel S, et al. Evidence for segregated and integrative connectivity patterns in the human Basal Ganglia. J Neurosci. 2008;28:7143–7152. doi: 10.1523/JNEUROSCI.1486-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driver J. A selective review of selective attention research from the past century. British Journal of Psychology. 2001;92:53–78. [PubMed] [Google Scholar]
- Dux PE, Marois R. The attentional blink: a review of data and theory. Atten Percept Psychophys. 2009;71:1683–1700. doi: 10.3758/APP.71.8.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fasano C, Kortleven C, Trudeau L-E. Chronic activation of the D2 autoreceptor inhibits both glutamate and dopamine synapse formation and alters the intrinsic properties of mesencephalic dopamine neurons in vitro. Eur J Neurosci. 2010;32:1433–1441. doi: 10.1111/j.1460-9568.2010.07397.x. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Loughry B, O’Reilly RC. Interactions between frontal cortex and basal ganglia in working memory: a computational model. Cogn Affect Behav Neurosci. 2001;1:137–160. doi: 10.3758/cabn.1.2.137. [DOI] [PubMed] [Google Scholar]
- Frank MJ, Seeberger LC, O’reilly RC. By carrot or by stick: cognitive reinforcement learning in parkinsonism. Science. 2004;306:1940–1943. doi: 10.1126/science.1102941. [DOI] [PubMed] [Google Scholar]
- Gotham AM, Brown RG, Marsden CD. “Frontal” cognitive function in patients with Parkinson’s disease “on” and “off” levodopa. Brain. 1988;111(Pt 2):299–321. doi: 10.1093/brain/111.2.299. [DOI] [PubMed] [Google Scholar]
- Gratwicke J, Jahanshahi M, Foltynie T. Parkinson’s disease dementia: a neural networks perspective. Brain. 2015;138:1454–1476. doi: 10.1093/brain/awv104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross J, Schmitz F, Schnitzler I, et al. Modulation of long-range neural synchrony reflects temporal limitations of visual attention in humans. Proc Natl Acad Sci USA. 2004;101:13050–13055. doi: 10.1073/pnas.0404944101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes J-D. Decoding visual consciousness from human brain signals. Trends Cogn Sci (Regul Ed) 2009;13:194–202. doi: 10.1016/j.tics.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Koch C, Tsuchiya N. Attention and consciousness: two distinct brain processes. Trends Cogn Sci (Regul Ed) 2007;11:16–22. doi: 10.1016/j.tics.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Kranczioch C, Debener S, Schwarzbach J, et al. Neural correlates of conscious perception in the attentional blink. Neuroimage. 2005;24:704–714. doi: 10.1016/j.neuroimage.2004.09.024. [DOI] [PubMed] [Google Scholar]
- Lamme VAF. How neuroscience will change our view on consciousness. Cogn Neurosci. 2010;1:204–220. doi: 10.1080/17588921003731586. [DOI] [PubMed] [Google Scholar]
- Lau H, Rosenthal D. Empirical support for higher-order theories of conscious awareness. Trends Cogn Sci (Regul Ed) 2011;15:365–373. doi: 10.1016/j.tics.2011.05.009. [DOI] [PubMed] [Google Scholar]
- Maia TV, Frank MJ. From reinforcement learning models to psychiatric and neurological disorders. Nat Neurosci. 2011;14:154–162. doi: 10.1038/nn.2723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marois R, Chun MM, Gore JC. Neural correlates of the attentional blink. Neuron. 2000;28:299–308. doi: 10.1016/s0896-6273(00)00104-5. [DOI] [PubMed] [Google Scholar]
- Martens S, Munneke J, Smid H, Johnson A. Quick minds don’t blink: electrophysiological correlates of individual differences in attentional selection. J Cogn Neurosci. 2006;18:1423–1438. doi: 10.1162/jocn.2006.18.9.1423. [DOI] [PubMed] [Google Scholar]
- Martens S, Wyble B. The attentional blink: past, present, and future of a blind spot in perceptual awareness. Neurosci Biobehav Rev. 2010;34:947–957. doi: 10.1016/j.neubiorev.2009.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moustafa AA, Sherman SJ, Frank MJ. A dopaminergic basis for working memory, learning and attentional shifting in Parkinsonism. Neuropsychologia. 2008;46:3144–3156. doi: 10.1016/j.neuropsychologia.2008.07.011. [DOI] [PubMed] [Google Scholar]
- Nieuwenhuis S, Gilzenrat MS, Holmes BD, Cohen JD. The role of the locus coeruleus in mediating the attentional blink: a neurocomputational theory. J Exp Psychol Gen. 2005;134:291–307. doi: 10.1037/0096-3445.134.3.291. [DOI] [PubMed] [Google Scholar]
- Norman LJ, Heywood CA, Kentridge RW. Exogenous attention to unseen objects? Conscious Cogn. 2015;35:319–329. doi: 10.1016/j.concog.2015.02.015. [DOI] [PubMed] [Google Scholar]
- Parish CL, Stanic D, Drago J, et al. Effects of long-term treatment with dopamine receptor agonists and antagonists on terminal arbor size. Eur J Neurosci. 2002;16:787–794. doi: 10.1046/j.1460-9568.2002.02132.x. [DOI] [PubMed] [Google Scholar]
- Raymond JE, Shapiro KL, Arnell KM. Temporary suppression of visual processing in an RSVP task: an attentional blink? J Exp Psychol Hum Percept Perform. 1992;18:849–860. doi: 10.1037//0096-1523.18.3.849. [DOI] [PubMed] [Google Scholar]
- Rees G, Kreiman G, Koch C. Neural correlates of consciousness in humans. Nat Rev Neurosci. 2002;3:261–270. doi: 10.1038/nrn783. [DOI] [PubMed] [Google Scholar]
- Seeman P. Antiparkinson therapeutic potencies correlate with their affinities at dopamine D2(High) receptors. Synapse. 2007;61:1013–1018. doi: 10.1002/syn.20453. [DOI] [PubMed] [Google Scholar]
- Slagter HA, Johnstone T, Beets IAM, Davidson RJ. Neural competition for conscious representation across time: an fMRI study. PLoS ONE. 2010;5:e10556. doi: 10.1371/journal.pone.0010556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slagter HA, Tomer R, Christian BT, et al. PET evidence for a role for striatal dopamine in the attentional blink: functional implications. J Cogn Neurosci. 2012;24:1932–1940. doi: 10.1162/jocn_a_00255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y-K, Gilthorpe MS. Revisiting the relation between change and initial value: a review and evaluation. Stat Med. 2007;26:443–457. doi: 10.1002/sim.2538. [DOI] [PubMed] [Google Scholar]
- van Gaal S, Lamme VAF. Unconscious high-level information processing: implication for neurobiological theories of consciousness. Neuroscientist. 2012;18:287–301. doi: 10.1177/1073858411404079. [DOI] [PubMed] [Google Scholar]
- Van Opstal F, Van Laeken N, Verguts T, et al. Correlation between individual differences in striatal dopamine and in visual consciousness. Curr Biol. 2014;24:R265–R266. doi: 10.1016/j.cub.2014.02.001. [DOI] [PubMed] [Google Scholar]
- Van Schouwenburg MR, Den Ouden HEM, Cools R. The Human Basal Ganglia Modulate Frontal-Posterior Connectivity During Attention Shifting. J Neurosci. 2010;30:9910–9918. doi: 10.1523/JNEUROSCI.1111-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vardy Y, Bradshaw JL, Iansek R. Dual target identification and the attentional blink in Parkinson’s disease. J Clin Exp Neuropsychol. 2003;25:361–375. doi: 10.1076/jcen.25.3.361.13811. [DOI] [PubMed] [Google Scholar]
- Voon V, Gao J, Brezing C, et al. Dopamine agonists and risk: impulse control disorders in Parkinson’s disease. Brain. 2011;134:1438–1446. doi: 10.1093/brain/awr080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiecki TV, Frank MJ. Neurocomputational models of motor and cognitive deficits in Parkinson’s disease. Prog Brain Res. 2010;183:275–297. doi: 10.1016/S0079-6123(10)83014-6. [DOI] [PubMed] [Google Scholar]
- Williams GV, Goldman-Rakic PS. Modulation of memory fields by dopamine D1 receptors in prefrontal cortex. Nature. 1995;376:572–575. doi: 10.1038/376572a0. [DOI] [PubMed] [Google Scholar]
- Wyart V, Tallon-Baudry C. Neural dissociation between visual awareness and spatial attention. J Neurosci. 2008;28:2667–2679. doi: 10.1523/JNEUROSCI.4748-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, Claassen DO, Huizenga HM, et al. Dopamine agonists and the suppression of impulsive motor actions in Parkinson disease. J Cogn Neurosci. 2012;24:1709–1724. doi: 10.1162/jocn_a_00241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, Ridderinkhof KR, Bashore TR, van den Wildenberg WPM. The effect of Parkinson’s disease on the dynamics of on-line and proactive cognitive control during action selection. J Cogn Neurosci. 2010;22:2058–2073. doi: 10.1162/jocn.2009.21326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wylie SA, van den Wildenberg WPM, Ridderinkhof KR, et al. The effect of Parkinson’s disease on interference control during action selection. Neuropsychologia. 2009;47:145–157. doi: 10.1016/j.neuropsychologia.2008.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahrt J, Taylor JR, Mathew RG, Arnsten AF. Supranormal stimulation of D1 dopamine receptors in the rodent prefrontal cortex impairs spatial working memory performance. J Neurosci. 1997;17:8528–8535. doi: 10.1523/JNEUROSCI.17-21-08528.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]