Abstract
Pancreatic ductal adenocarcinoma (PDA) is a devastating malignancy with limited and modest clinical treatments. High throughput technologies and accurate disease models now provide a comprehensive picture of the diverse molecular signaling pathways and cellular processes governing PDA tumorigenesis. Central among these is oncogenic KRAS, a mediator of cellular plasticity, metabolic reprogramming, and inflammatory and paracrine signaling required for tumor development and maintenance. Biological aggressiveness is further conferred by a highly fibrotic and immunosuppressive PDA microenvironment that also acts as a barrier to effective drug delivery. The regulation of these mechanisms and their implications for early cancer detection, chemoprevention and therapy are discussed.
Keywords: pancreatic cancer, KRAS, precision medicine, immunotherapy, tumor-stromal interactions
Pancreatic cancer is a highly recalcitrant malignancy
Pancreatic cancer has an overall 5-year survival rate of 7–8% and will account for 41,780 estimated deaths this year to become the 3rd leading cause of cancer mortality in the USA [1]. A majority (>85%) of pancreatic cancers are pancreatic ductal adenocarcinoma (PDA), histologically characterized by ductular differentiation and highly fibrotic (or desmoplastic) stromal reaction. PDA is highly resistant to current standard-of-care chemotherapy regimens, which offer only modest survival benefit for most patients [2]. PDA patients also commonly suffer from debilitating cachexia and other co-morbidities that negatively impact their performance status, further limiting treatment options [3]. With symptoms manifesting late and no reliable screening test, a majority of PDA patients (~80%) are diagnosed at an advanced, non-operable clinical stage. Even early-stage patients who undergo successful surgical resection are likely to succumb to recurrent regional or metastatic disease within 2–5 years [4].
After years of clinical trial failures, modest improvements in survival have been finally realized for advanced stage PDA through the use of multi-agent chemotherapy regimens such as FOLFIRINOX (fluorouracil, leucovorin, irinotecan and oxaliplatin) or gemcitabine plus nab-paclitaxel. Numerous additional clinical trials are addressing the efficacy of these and other promising combinations of chemotherapy in all (early and late) stages of disease [2, 5]. However, innovations in early cancer detection and systemic therapies are clearly needed to achieve robust and sustained treatment outcomes. In the context of these important clinical challenges, we highlight recent work detailing the molecular and cellular features governing pancreatic tumorigenesis.
Temporal evolution of PDA and opportunities for early detection
The temporal and stepwise accumulation of molecular and histologic alterations during dysplastic progression is well documented in patient samples and genetically engineered mouse models (GEMMs) [6]. Three well-described dysplastic lesions precede the development of PDA. Pancreatic intraepithelial neoplasia (PanIN) are microscopic ductular lesions that give rise to most invasive PDA. Macroscopic cystic or mass-forming ductular lesions involving or separate from the pancreatic ductal system include intraductal papillary mucinous neoplasms (IPMNs) and mucinous cystic neoplasm (MCNs), respectively. Although unproven, acinar-ductal metaplasia (ADM) and atypical flat duct lesions are additional proposed direct histologic precursors of invasive PDA [7].
Recent whole exome sequencing of patient-matched PDA primary and metastatic lesions from surgical and autopsy specimens has offered important insights into the evolution and progression of PDA. Subclonal heterogeneity in a primary tumor correlates with clonal evolution of metastatic lesions [8] and may be a source of treatment resistance via selective pressure. Modeling of subclonal genetic evolution based on cellular proliferation rates in human PDA estimates a timeline of more than 10 years between an initiating oncogenic event and a parental clone that gives rise to a primary tumor, as well as an additional 6–7 years before the development of metastatic foci [8]—a large window of potential opportunity to detect PDA at an earlier stage when treatment could be more effective. Initiatives are underway to identify sensitive and specific biomarkers for early cancer detection in stool, plasma or other biofluids [9]. Unfortunately, the low incidence of PDA precludes practical and cost-effective screening in the general population using present technologies. Therefore, early detection research has focused heavily on screening at-risk populations, including individuals with familial or hereditary forms of PDA, chronic pancreatitis, new-onset diabetes mellitus after age 50, or other composites of risk factors and clinical symptoms [9].
Whether early detection will significantly improve clinical outcomes is uncertain, particularly in view of data suggesting metastasis occurs early in PDA progression. A recent PDA GEMM study tracking fluorescently-tagged pancreatic epithelial cells finds they have already delaminated into pancreatic stroma and entered the circulation at an early stage of PanIN progression before development of frank PDA [10]. These disseminated cells expressed features of epithelial-mesenchymal transition (EMT) and cancer stemness [10]. Similarly, circulating epithelial cells can be detected in patients with pre-malignant cystic pancreatic lesions without invasive PDA [11]. Separate computational estimates of human PDA growth and dissemination rates based on radiographic and autopsy data predicts most small primary PDA tumors are associated with subclinical metastatic disease [12]. These findings are consistent with the high rate of metastatic relapse seen in early stage patients after margin-negative surgical resection. Therefore, an early detection biomarker may need to identify a high-risk precursor lesion prior to the onset of invasive PDA to be clinically useful [9]. Furthermore, given the high likelihood of clinically undetected microscopic metastatic disease, neoadjuvant systemic therapy may be warranted prior to surgery even in patients with early-stage, resectable PDA.
KRAS: master regulator of PDA development and maintenance
The literature overwhelmingly supports the notion that KRAS, a GTPase that performs key functions in normal tissue signaling, is the key oncogenic linchpin and a highly desirable therapeutic target for PDA.
KRAS in PDA progression
Oncogenic KRAS mutations are frequently detected in early PanINs and nearly ubiquitous in PDA (90–95%) [6]. In isolation, pancreas-specific expression of oncogenic Kras at its endogenous locus leads to the progressive accumulation of murine PanIN (mPanIN) lesions and occasional development of PDA after long latency [13]. Consistent with their appearance at later stages of PanIN progression, mutations in CDKN2A, TP53 and SMAD4/TGFβ signaling fail to initiate PDA on their own, but lead to the rapid development of PDA at high penetrance in combination with oncogenic Kras [14]. Of these, the KPC mouse (KrasLSLG12D/+;Tp53R172H/+;Pdx-Cre) is the most widely used for studies exploring disease biology and therapy [15]. Oncogenic Kras has also been repeatedly used as a requisite background for successful PDA GEMMs exploring associated signaling pathways and processes, as well as various GEMMs mechanistically linking PDA development to diet, pancreatitis and tumor-stromal interactions involving fibroblasts, inflammatory cells and other factors [14, 16–19].
KRAS and metabolic reprogramming
The diverse biochemical signaling effects and biological actions of KRAS in PDA tumor metabolism, cell proliferation, cell survival, cell migration, pro-tumorigenic inflammatory and paracrine signaling are detailed in recent reviews [20, 21]. Recent interest has focused on the critical role of KRAS and metabolic reprogramming in order to meet the anabolic growth demands of PDA [22, 23]. Oncogenic KRAS activates a transcriptional program in PDA, via activation of MEK signaling and MYC, that can stimulate glucose uptake, glycolysis, and altered glycolytic flux in support of anabolic metabolism[24]. Oncogenic KRAS also is critical for the maintenance of redox balance in PDA, shifting glutamine metabolism in favor of NADPH biosynthesis [25] and upregulating NRF2 and its antioxidant program [26]. Additionally, KRAS regulates autophagy and macropinocytosis scavenging pathways, each a source of metabolic substrates critical for PDA tumor growth and therefore also promising therapeutic targets [23]. RAS-transformed PDA cells utilize macropinocytosis as an important source of amino acids such as glutamine. Pharmacological inhibition of macropinocytosis inhibits PDA growth [27]. Likewise, autophagy is elevated in PDA and is critical for pancreatic tumor growth and progression. Hydroxychloroquine (HCQ), a well-tolerated inhibitor of autophagy, reduces growth of PDA in GEMMs and human xenografts [28, 29] and is now under evaluation in clinical trials for PDA and other malignancies.
KRAS-dependency in PDA
Elegant studies utilizing PDA GEMMs that reversibly induce oncogenic Kras expression demonstrate it is essential for the development and maintenance of all stages of PanIN and PDA, acting to regulate pro-tumorigenic inflammatory and stromal signaling [30] and reprogram cellular metabolism in favor of glycolytic and biosynthetic pathways [24]. Further studies exploring prolonged Kras extinction in these models identify subpopulations of PDA cells that survive Kras ablation and give rise to tumor relapse [31, 32]. PDA can circumvent Kras ablation through compensatory upregulation of downstream MEK/ERK activation or alternatively through upregulation of YAP1/TEAD2-mediated DNA proliferation and cell cycle [31], a finding consistent with a prior observation that YAP1 compensates for shRNA-mediated inhibition of KRAS in colon and pancreatic cancer cells [33]. In a separate study, Kras ablation-resistant cells had cancer stem cell features and relied upon mitochondrial respiration as opposed to anabolic glucose metabolism [32]. Therefore, anti-KRAS treatment approaches will need to further account for pre-existing (i.e., cancer stem cells) or evolving PDA cell subpopulations with disparate KRAS-dependency and metabolic sensitivities.
Cellular origin of pancreatic cancer
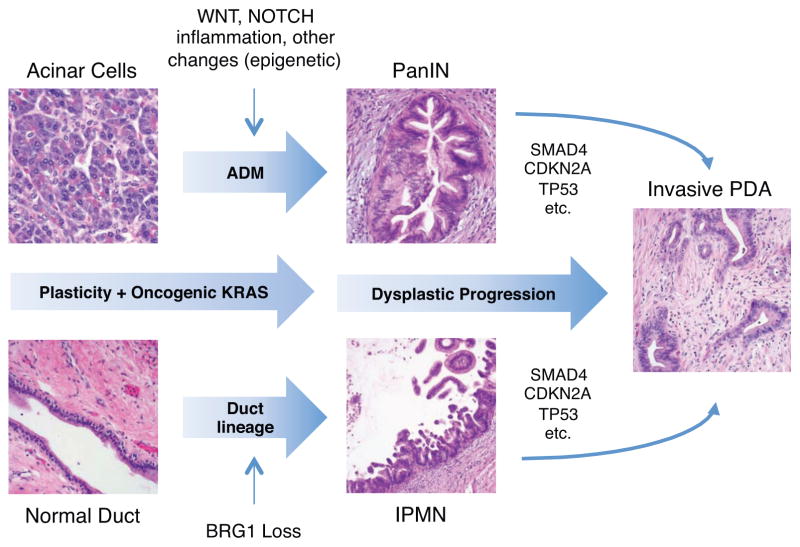
Multiple cell types harbor the capacity to initiate pancreatic tumorigenesis as revealed by GEMMs and organoid systems. A majority of lineage-tracing studies in Kras-driven GEMMs find PanIN-derived PDA arise from acinar cells that, in response to injury and/or oncogenic insults, undergo transdifferentiation through a process referred to as acinar-to-ductal metaplasia (ADM) [14]. In contrast, a recently published GEMM combining oncogenic Kras with deletion of the transcriptional activator Brg1 gives rise to IPMNs that arise from mature ductal cells undergoing partial dedifferentiation while retaining overall ductal morphology [34]. In fact, every cell type of the exocrine pancreas (duct, acinar and centroacinar cell) has been shown to harbor PDA tumor-initiating potential under the correct circumstances. There is also evidence to suggest that other rare stem cell-like populations in the adult pancreas may also initiate tumorigenesis [35]. These scenarios are not mutually exclusive and share the common thread that neoplastic progression is linked to cellular plasticity [36]. In order to maintain normal tissue homeostasis and adequate response to injury under physiologic circumstances, differentiated cells in the pancreas possess a high degree of cellular plasticity, a feature that can be exploited for regenerative medicine (i.e., diabetes mellitus). However, under the permissive influence of oncogenic KRAS and/or other predisposing factors (i.e., chronic inflammation, epigenetic alterations, altered developmental signaling, etc.), this plasticity is also a predisposing factor for malignant transformation [37] (Figure 1, Key Figure).
Figure 1 Key FIgure. Cellular origins of PDA.
Cellular plasticity in the exocrine pancreas in the context of oncogenic KRAS and/or further insults (i.e., cellular injury) leads to cellular reprogramming and oncogenic transformation. Acinar cells undergo cellular dedifferentiation and transdifferentiation towards ductal lineage in a process referred to as acinar-ductal metaplasia (ADM) with the further eventual rise of duct-like PanIN lesions. ADM and PanIN initiation are also facilitated by aberrant developmental signaling activity. Mature ductal cells may also undergo dedifferentiation to give rise to IPMN lesions in the context of loss of BRG1 and/or other genetic alterations. Both PanIN and IPMN lesions accumulate additional molecular alterations during progression to invasive adenocarcinoma. Cellular reprogramming and dysplastic progression are further facilitated by inflammation (chronic inflammation, macrophages, etc.) and cell-stromal paracrine interactions.
The molecular landscape of pancreatic cancer: new therapeutic opportunities
PDA is a molecularly diverse cancer as revealed by a growing number of next generation sequencing (NGS) studies [8, 38–43]. Individual PDA tumors harbor a small number of high frequency hallmark oncogenic mutations (KRAS, CDKN2A, TP53 and SMAD4) and a much larger number of heterogeneous alterations occurring at low prevalence. The initial landmark PDA sequencing study utilized integrated pathway analysis to distill this genetic heterogeneity into 12 core pathways altered in the majority of PDA [38]. Subsequent NGS studies have reaffirmed this observation while identifying additional core pathways genetically altered in at least subsets of PDA [8, 40, 42, 44] (TABLE 1). Although most individual alterations fail to predict clinical behavior, certain genetic hallmarks (i.e., increased chromosomal instability, amplification of MYC) do correlate with clinical outcomes [41, 42]. In addition to genetic alterations, transcriptional network analysis of global gene expression has been used to distinguish PDA subtypes associated with distinct histopathologic and clinical features, and is potentially a more robust means for predicting survival and response to therapy [39, 40, 45].
Table 1.
| Pathway/Processes | Select gene alterations | Clinical implications/drug approaches | Refs |
|---|---|---|---|
| Core Pathways
| |||
| RAS | KRAS, MAP2K4 | Combined MEK/PI3K inhibition, target KRAS directly or indirect (i.e., metabolic pathways) | [23, 75] |
| G1/S checkpoint | CDKN2A/B, TP53, RB | CDK4/6 inhibition, but may require further inhibition of MTOR, MEK or other pathways | [51] |
| TGFβ signaling | SMAD4, TGFBR1, ACVR1B | SMAD4 loss is a marker of poor prognosis | [76] |
| Wnt signaling | RNF43, AXIN1/2, GATA6 | Porcupine, tankyrase and FZD inhibitors, other direct and indirect inhibitors of Wnt | [53, 56] |
| NOTCH | JAG2, NOTCH1-4, MAML1 | Gamma-secretase/NOTCH inhibitors in trials | [43] |
| Hedgehog signaling | SMO, GLI1-3, LRP2 | Stromal targeting; SMO and GLI inhibitors | [18, 70] |
| Integrin | ITGA4, ITGA9, ILK, LAMA1/4/5 | Mediate tumor-stromal interactions, possible targets for stromal therapy | [77] |
| Small GTPase signaling | ARHGEF9, RP1, CDC42BPA | Important downstream effector pathway for KRAS that may warrant targeting | [20] |
| JNK signaling | MAP4K3, TNF, ATF2 | JNK inhibitors may inhibit PDA stem cells | [78] |
| DNA damage response | BRCA1/2, PALB2, ATM | Potential response to platinum agents, mitomycin C or PARP inhibitors | [41] |
| Invasion | ADAM11-12, PRSS23 | Cancer hallmark, general or specific inhibitors | [79] |
| Homophilic cell adhesion | CDH1-2, FAT, PCDH15 | EMT, invasion and metastasis | [79] |
| Apoptosis | CASP10, CAD, HIP1 | Cancer hallmark, general or specific inhibitors | [79] |
| Chromatin regulation | ARID1A/1B, PRBM1, KDM6A, SETD1A, MLL1-4 | ARID1A is prognostic; histone modifying agents, susceptibility to EZH2 inhibitor? | [42, 43] |
| Axon guidance | ROBO1/2, SLIT2, EPHA5/7, SEMA3A/3E/5A | Markers of poor prognosis, activate MET and WNT signaling (could alter drug response) | [44] |
|
| |||
| Additional Pathways or Actionable Targets
| |||
| RNA processing | RBM10, SF3B1, U2AF1 | RBM10 linked to prognosis based on KRAS alteration | [42] |
| HIPPO/FAT | FAT1-4, DCHS1-2, LATS1 | Potential resistance to KRAS inhibitor; direct/indirect inhibitors of YAP/TEAD? | [31, 33] |
| Mismatch repair | MLH1, MSH2 | Increased PDA risk with Lynch syndrome; marker of chemotherapy or anti-PD1 response? | [80] |
| MYC | MYC amplification | Worse prognosis/adenosquamous histology; use of CDK9 or BET inhibitors | [42, 43] |
| KRAS wild-type | PIK3CA, BRAF, STK11, GNAS, CHEK2 | Alternative oncogenic drivers; possible response to BRAF or EGFR inhibitors? | [42] |
In the most recent and comprehensive NGS study published to date, whole-genome and deep-exome sequencing analysis of over 450 tumor samples along with mutation functional interaction sub-network analysis identified a top list of 32 genes recurrently mutated in PDA. These were related to ten molecular processes, including KRAS, TGFβ, WNT, NOTCH, ROBO/SLIT, G1/S transition, SWI/SNF chromatin remodeling, histone modification, BRCA pathway, and RNA processing [40]. Druggable oncogenic mutations (i.e., BRAF, PIK3CA, HER2 amplification) while infrequently identified [40–42] could represent drug targets in tumors where they function as oncogenic drivers (i.e., KRAS-wild type PDA). More broadly applicable to potential treatment stratification are mutations that cluster to common molecular process with known or predicted pharmacologic sensitivities (Table 1).
KRAS
While KRAS has proven “undruggable” thus far, progress in a variety of approaches attempting to directly or indirectly target mutant KRAS or its downstream actions, as well as a recent National Cancer Institute initiative that commits further significant attention and resources to these efforts, raise hope for a viable anti-KRAS therapy [20, 21]. Offering proof-of-principle that mutant KRAS is a druggable target, prolonged in vivo delivery of siRNA targeting mutant KRAS improves PDA survival in xenograft models [46], and was tolerated and potentially efficacious in patients with locally advanced PDA [47]. Patient response to KRAS inhibition may prove more heterogeneous than expected. In addition to potential variable KRAS-dependency due to mechanisms detailed above with the inducible Kras GEMMs, differences in KRAS allelic frequency or codon mutation have now been linked to prognosis [42] and could differentially influence response to anti-KRAS therapy, as may other factors that alter levels of KRAS expression and activity[48].
BRCA and DNA Damage Response and Repair (DDR) Pathways
Prevalent germline mutations in familial PDA [49] are able to promote PDA in GEMMs [14], and genetic alterations in BRCA and DNA damage response and repair (DDR) pathways are observed in at least one-third of PDAs where they are linked to increased genomic instability and susceptibility to platinum therapy [41, 42]. The clinical utility of DNA damaging agents (i.e., platinum-based, mitomycin C) and poly ADP ribose polymerase (PARP) inhibitors are being extensively evaluated in clinical trials, the results of which will need to be interpreted in relation to different DDR genetic alterations and degree of underlying genomic instability [20, 50].
Retinoblastoma (RB) pathway and the G1/S transition
Frequent mutations in inhibitors of the tumor suppressor INK4 (CDKN2A/CDKN2B) or other RB pathway members promote cell cycle progression, justifying the use of cell cycle checkpoint inhibitors in PDA. Use of these inhibitors will need to account for potential compensatory responses leading to drug resistance or tumor growth exacerbation. For instance, pharmacologic inhibition of CDK4/6 leads to accumulation of mitochondria and reactive oxygen species (ROS) and activation of mTORC1 signaling in PDA cells, while enhanced cell cycle arrest or cytotoxicity can be achieved with the further addition of inhibitors for MTORC, MEK, BCL2 and/or ROS scavenging [51].
Wnt signaling
Genetic alterations tied to activation of Wnt/β-catenin-dependent (canonical) signaling are observed in 5–15% of PDA. Recent work from a variety of groups shows canonical Wnt signaling is required for pancreatic initiation and progression [52], mediates biological aggressiveness [53] and tumor-stromal paracrine interactions [54], confers cancer stem cell traits in susceptible cell populations [55], and is a viable therapeutic target in preclinical models [52, 53, 56]. Wnt inhibitors with favorable pharmacologic profiles are in various stages of preclinical and early clinical development. Of note, inactivating mutations in RNF43, an E3 ligase mediating the ubiquitylation and degradation of Frizzled receptors, predicts PDA Wnt growth dependency and response to LGK974 [56], an inhibitor of Wnt ligand secretion. Providing additional mechanisms by which a Wnt antagonist could inhibit PDA, β-catenin-independent (or non-canonical) Wnt signaling mediated by WNT2 increases metastatic potential and is linked to circulating tumor cell phenotype [57]. Additionally, oncogenic activity of KRAS in PDA is linked to its interaction with calmodulin that suppresses FZD8-mediated non-canonical Wnt/Ca2+ signaling. Disrupting this interaction with the orally-active protein kinase C activator prostatin represses tumorigenesis, a potential indirect approach for targeting KRAS [58].
NOTCH pathway
Amplification of NOTCH pathway components [42] is compatible with its activation in PDA [43]. Similar to Wnt, NOTCH signaling is critical for PDA initiation in the context of oncogenic KRAS [59] and further promotes stemness, EMT and chemoresistance in PDA [60]. Several PDA clinical trials are addressing the efficacy of inhibitors of gamma-secretase that block transmission of ligand-receptor signaling to the nucleus, as well as antibodies against NOTCH ligands [43].
Applying NGS to precision medicine
Experiences from the Individualized Molecular Pancreatic Cancer Therapy (IMPaCT) trial [61] and trials in other cancers [62] highlight several challenges faced in implementing precision medicine approaches based on information obtained from NGS or other high throughput technologies (i.e., proteomics, metabolomics). Issues surrounding statistical power, capacity and economic feasibility are particular stumbling blocks for a tumor as molecularly diverse as PDA. These concerns necessitate a shift away from clinical trial approaches traditionally applied to cytotoxic chemotherapy in the past. Improved clinical trial outcomes will likely hinge on greater utilization of: (1) “umbrella studies” that evaluate multiple treatment arms determined on a larger set of biomarkers; (2) “basket trials” that stratify patients to treatment arms based on specific biomarker profiles irrespective of tumor type (i.e., NCI Molecular Analysis for Therapy Choice Trial NCT02465060); (3) “tent protocols” that use initial comprehensive biomarker assessment to link a patient with any of a number of potential trial opportunities; and (4) “adaptive study designs” that are modified on the fly in response to accrued data [62].
Therapeutic opportunities in the pancreatic cancer microenvironment
In addition to the cancer cells themselves, significant biological obstacles and novel therapeutic opportunities are also presented by the pancreatic tumor microenvironment (TME) (Figure 2).
Figure 2. PDA tumor microenvironment (TME).
Low and high power images highlighting key components of the PDA pancreatic tumor microenvironment (TME) and a partial list of potentially relevant treatment approaches. The highly fibrotic extracellular matrix (ECM) contributes to high interstitial pressures that compromise tumor perfusion and drug delivery. The ECM includes abundant hyaluronic acid, the target of PEGPH20 now in human trials. Activated pancreatic stellate cells (PSCs) and related cancer-associated fibroblasts (CAFs) proliferate and secrete ECM through stromal cell activation. Stromal activation is mediated through reciprocal tumor-stromal-inflammatory cell-mediated paracrine interactions involving growth factors, cytokines and developmental signaling. Hedgehog (Hh) and Wnt pathways can be directly or indirectly targeted to block stromal signaling. Vitamin D reverses stromal activation of PSCs and CAFs and inhibits pancreatitis to improve response to chemotherapy in KPC mice and is now in human trials for PDA. Inset image of immune cells highlights a mixed peritumoral inflammatory infiltrate comprised of lymphocytes, plasma cells, macrophages and scattered neutrophils. Multiple preclinical and clinical trials are also exploring strategies to elicit a more robust anti-tumor immune response (i.e., cancer vaccines, IDO inhibitors), block tumor immunosuppression (i.e., immune checkpoint inhibitors such as anti-PD-1) and address immune-stromal crosstalk (i.e., CD40 agonist). Blood vessels and nerve can serve to further promote tumorigenesis in the PDA TME and are therefore additional potential targets. The complex interplay of factors and their sometimes diverse and paradoxical pro- and anti-tumor-promoting functions raises the potential need for combinatorial approaches in targeting the stroma and immune response.
Stromal therapy
PDA elicits a highly desmoplastic stromal reaction, which contributes to a poorly perfused and hypoxic TME able to facilitate tumor growth and metastasis, promote tumor immunosuppression, and function as a barrier to drug delivery [63]. As detailed in several recent excellent reviews, desmoplasia is the consequence of a pro-fibrotic stromal activation program dynamically regulated by multiple self-reinforcing and reciprocal interactions between tumor cells, extracellular matrix (ECM) elements and associated non-malignant cell types. These would include pancreatic stellate cells (PSCs) and other cancer-associated fibroblast-like cells (CAFs), inflammatory cells, blood vessels, lymphatics, and nerve [2, 5, 63, 64]. In some instances, these interactions can result in unexpected and paradoxical effects. For instance, chemotherapy can enhance pro-tumorigenic paracrine and pro-inflammatory signaling through induction of a senescence-associated secretory phenotype in CAFs in the pancreatic TME [65]. Stromal activation and an associated inflammatory response are critical factors in promoting the initiation and progression of PDA and its precursor lesions [16, 30]. PDA stromal activation correlates with worse patient survival [39], while biomarkers of stromal activation are potential diagnostic or predictive biomarkers for PDA early detection or treatment.
Stromal targeting strategies that deplete the ECM (i.e., PEGPH20, a pegylated form of recombinant hyaluronidase in clinical trials), inhibit CAF or PSC activation (i.e., vitamin D), or block paracrine signaling activity (i.e., SMO inhibitor IPI-926) each improve tumor perfusion, drug delivery, and overall survival in PDA GEMMs [18, 64, 66, 67]. Additional recent studies offer other promising targets for stromal therapy [63, 64]. For example, activation of tumor-infiltrating macrophages with a CD40 agonist depletes stroma in KPC mice [68]. Ablation of sensory neurons, which are abundant in the pancreatic TME and a potential conduit for tumor spread (i.e., perineural invasion), inhibits PDA initiation and progression in KPC mice through disruption of reciprocal neural-tumor interactions that promote growth factor and inflammatory signaling [69].
On the other hand, stromal disruption by certain pharmacologic and genetic approaches have also been shown to promote pancreatic tumorigenesis via unintended off-target effects on tumor cells and through disruption of growth restraints imposed by stromal components, leading to increased tumor cell proliferation, dedifferentiation, EMT, immunosuppression and angiogenesis [19, 70]. Clinical use of stromal targeting agents will require a comprehensive assessment of any such deleterious effects. In this regard, the aforementioned studies show immune checkpoint inhibitors or anti-angiogenic therapy may be appropriate combinatorial approaches able to mitigate these effects [19, 70].
Immunotherapy
As shown for a growing number of cancer types, strategies that harness an immune response to PDA could dramatically alter survival outcomes. However, immunotherapy faces several obstacles in PDA. The PDA TME is notable for a variety of inflammatory cell types and the expression of several pro-inflammatory and anti-inflammatory cytokines, many with established roles in promoting PDA initiation, progression, metastasis, and immune evasion [20]. In addition, estimates of tumor neoantigen repertoire derived from somatic mutation rates across cancer types indicate PDA is far less immunogenic than cancers that are clinically responsive to immune checkpoint inhibitors (i.e., melanoma and lung cancer) [71]. On the other hand, an immune response to PDA-specific antigens (i.e., mesothelin) is clearly detectable in patient tumors, which offers rationale for approaches seeking to prime or enhance effector cell activity in order to generate a stronger PDA-specific immunologic response. Promising approaches now being investigated in pre-clinical work and clinical trials include the use of costimulatory molecules, epitope-specific or whole cell cancer vaccines, dendritic cell therapy, and chimeric antigen receptor T cell therapy [72].
The success of cancer vaccines and other strategies that elicit an immune response to tumor may hinge upon the further addition of immune checkpoint inhibitors. The PDE TME is cumulatively immunosuppressive, the consequence of: (1) the aforementioned desmoplastic stroma that imparts restraints on immune effector cell trafficking; (2) molecular alterations in tumor cells (i.e., oncogenic KRAS) that drive gene expression patterns and signaling favoring immunosuppression; (3) a paucity of effector immune cells (i.e., natural killer cells, cytotoxic T cells) and preponderance of suppressive immune cells (i.e., myeloid-derived suppressor cells, T-regulatory cells, etc.); and (4) expression of additional co-inhibitory receptors and ligands (i.e., PD-1/PD-L1, CTLA-4, indoleamine 2,3-dioxygenase). Many of these factors correlate with or have been mechanistically linked to PDA aggressiveness and are being actively investigated in preclinical and clinical trials [2, 73].
Concluding remarks and future perspectives
An improved understanding of PDA biology, new technical innovations, and recent clinical strides in other cancers raise hope for forthcoming treatment breakthroughs for PDA. Genetically-engineered mouse models that closely recapitulate human PDA [14] and newly developed of human and mouse-tissue derived pancreatic organoids systems that model the full spectrum of PDA progression in vitro and in vivo [74] are proving invaluable for detailing the molecular and cellular drivers of PDA and evaluating treatment approaches. Application of NGS and other high throughput technologies to tissue or liquid biopsies (i.e., circulating tumor cells or cell-free DNA), offer opportunities for early cancer detection and personalized therapy. Finally, the accelerating development of systemic treatment approaches that not only account for tumor cell intrinsic susceptibilities and resistance mechanisms, but also leverage opportunities presented by the tumor microenvironment and immune system, raise hope that we are on the cusp of efficacious therapies that will reduce patient mortality for this devastating malignancy. Such therapeutic advances will depend heavily on further basic and translational research that addresses gaps in our understanding of the molecular and cellular basis of PDA (see outstanding questions), and more fully defines the activity of treatments to improve their overall efficacy while avoiding unwanted off-target activity and evolution of resistance.
Outstanding Questions.
Are KRAS inhibitors the magic bullet for PDA? How might qualitative or quantitative variations in oncogenic KRAS mutation influence therapeutic outcomes? Will mechanisms able to bypass KRAS dependency contribute to treatment resistance and can these be addressed with combinatorial therapies?
Will stromal inhibitor therapy enhance response to cytotoxic chemotherapy in patients? Are combinatorial approaches using anti-angiogenesis, immune modulators or other agents necessary to deliver anti-stromal agents, safely and effectively?
Is PDA susceptible to immunotherapy? Which combinatorial approaches are necessary to elicit a stronger, therapeutically beneficial immunologic response against PDA?
Which combination known or novel biomarkers can be used for early detection? Will clinical interventions based on earlier detection offer significant clinical benefits to PDA patients after accounting for lead-time bias and other factors?
TRENDS BOX.
PDA is a genetically diverse disease, and high throughput technologies now offering the opportunity to individually tailor patient treatment, based the identification of actionable molecular alterations or therapeutic susceptibilities.
Oncogenic KRAS is critical in PDA development and maintenance, playing key regulatory roles in cellular metabolism, inflammatory response and developmental and growth factor signaling.
Inflammation, stroma and other factors in the tumor microenvironment promote PDA and represent key obstacles and opportune targets for therapy.
A large window of opportunity exists for detecting PDA at an earlier stage when treatment interventions are likely to be more effective.
Acknowledgments
The authors acknowledge the many excellent and important studies that could not be cited due to the scope and format of this review. The authors were supported by funding from the Hirshberg Foundation for Pancreatic Cancer Research, American Cancer Society (RSG-12-083-01-TBG), and National Institutes of Health (NIH, P01CA163200 and P01DK098108) to DWD and funding from the Department of Energy and NIH (R01CA187678 and U01CA198846) to TRD.
Footnotes
Financial and conflict of interest disclosures: none
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.AmericanCancerSociety. Cancer Facts & Figures 2016. American Cancer Society; Atlanta: 2016. pp. 1–66. [Google Scholar]
- 2.Garrido-Laguna I, et al. Pancreatic cancer: from state-of-the-art treatments to promising novel therapies. Nat Rev Clin Oncol. 2015;12:319–334. doi: 10.1038/nrclinonc.2015.53. [DOI] [PubMed] [Google Scholar]
- 3.Tan CR, et al. Pancreatic cancer cachexia: a review of mechanisms and therapeutics. Front Physiol. 2014;5:88. doi: 10.3389/fphys.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ryan DP, et al. Pancreatic adenocarcinoma. N Engl J Med. 2014;371:2140–2141. doi: 10.1056/NEJMc1412266. [DOI] [PubMed] [Google Scholar]
- 5.Neuzillet C, et al. State of the art and future directions of pancreatic ductal adenocarcinoma therapy. Pharmacol Ther. 2015;155:80–104. doi: 10.1016/j.pharmthera.2015.08.006. [DOI] [PubMed] [Google Scholar]
- 6.Maitra A, et al. Pancreatic cancer. Annu Rev Pathol. 2008;3:157–188. doi: 10.1146/annurev.pathmechdis.3.121806.154305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Basturk O, et al. A Revised Classification System and Recommendations From the Baltimore Consensus Meeting for Neoplastic Precursor Lesions in the Pancreas. Am J Surg Pathol. 2015;39:1730–1741. doi: 10.1097/PAS.0000000000000533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yachida S, et al. Evolution and dynamics of pancreatic cancer progression. Oncogene. 2013;32:5253–5260. doi: 10.1038/onc.2013.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chari ST, et al. Early detection of sporadic pancreatic cancer: summative review. Pancreas. 2015;44:693–712. doi: 10.1097/MPA.0000000000000368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rhim AD, et al. EMT and dissemination precede pancreatic tumor formation. Cell. 2012;148:349–361. doi: 10.1016/j.cell.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rhim AD, et al. Detection of circulating pancreas epithelial cells in patients with pancreatic cystic lesions. Gastroenterology. 2014;146:647–651. doi: 10.1053/j.gastro.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Haeno H, et al. Computational modeling of pancreatic cancer reveals kinetics of metastasis suggesting optimum treatment strategies. Cell. 2012;148:362–375. doi: 10.1016/j.cell.2011.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hingorani SR, et al. Preinvasive and invasive ductal pancreatic cancer and its early detection in the mouse. Cancer Cell. 2003;4:437–450. doi: 10.1016/s1535-6108(03)00309-x. [DOI] [PubMed] [Google Scholar]
- 14.Perez-Mancera PA, et al. What we have learned about pancreatic cancer from mouse models. Gastroenterology. 2012;142:1079–1092. doi: 10.1053/j.gastro.2012.03.002. [DOI] [PubMed] [Google Scholar]
- 15.Hingorani SR, et al. Trp53R172H and KrasG12D cooperate to promote chromosomal instability and widely metastatic pancreatic ductal adenocarcinoma in mice. Cancer Cell. 2005;7:469–483. doi: 10.1016/j.ccr.2005.04.023. [DOI] [PubMed] [Google Scholar]
- 16.Guerra C, et al. Chronic pancreatitis is essential for induction of pancreatic ductal adenocarcinoma by K-Ras oncogenes in adult mice. Cancer Cell. 2007;11:291–302. doi: 10.1016/j.ccr.2007.01.012. [DOI] [PubMed] [Google Scholar]
- 17.Dawson DW, et al. High-fat, high-calorie diet promotes early pancreatic neoplasia in the conditional KrasG12D mouse model. Cancer Prev Res (Phila) 2013;6:1064–1073. doi: 10.1158/1940-6207.CAPR-13-0065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Olive KP, et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science. 2009;324:1457–1461. doi: 10.1126/science.1171362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ozdemir BC, et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell. 2014;25:719–734. doi: 10.1016/j.ccr.2014.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ying H, et al. Genetics and biology of pancreatic ductal adenocarcinoma. Genes Dev. 2016;30:355–385. doi: 10.1101/gad.275776.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeitouni D, et al. KRAS Mutant Pancreatic Cancer: No Lone Path to an Effective Treatment. Cancers (Basel) 2016;8 doi: 10.3390/cancers8040045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ward PS, et al. Metabolic reprogramming: a cancer hallmark even warburg did not anticipate. Cancer Cell. 2012;21:297–308. doi: 10.1016/j.ccr.2012.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kimmelman AC. Metabolic Dependencies in RAS-Driven Cancers. Clin Cancer Res. 2015;21:1828–1834. doi: 10.1158/1078-0432.CCR-14-2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ying H, et al. Oncogenic Kras maintains pancreatic tumors through regulation of anabolic glucose metabolism. Cell. 2012;149:656–670. doi: 10.1016/j.cell.2012.01.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Son J, et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature. 2013;496:101–105. doi: 10.1038/nature12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.DeNicola GM, et al. Oncogene-induced Nrf2 transcription promotes ROS detoxification and tumorigenesis. Nature. 2011;475:106–109. doi: 10.1038/nature10189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Commisso C, et al. Macropinocytosis of protein is an amino acid supply route in Ras-transformed cells. Nature. 2013;497:633–637. doi: 10.1038/nature12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang A, et al. Autophagy is critical for pancreatic tumor growth and progression in tumors with p53 alterations. Cancer Discov. 2014;4:905–913. doi: 10.1158/2159-8290.CD-14-0362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosenfeldt MT, et al. p53 status determines the role of autophagy in pancreatic tumour development. Nature. 2013;504:296–300. doi: 10.1038/nature12865. [DOI] [PubMed] [Google Scholar]
- 30.Collins MA, et al. Oncogenic Kras is required for both the initiation and maintenance of pancreatic cancer in mice. J Clin Invest. 2012;122:639–653. doi: 10.1172/JCI59227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kapoor A, et al. Yap1 activation enables bypass of oncogenic Kras addiction in pancreatic cancer. Cell. 2014;158:185–197. doi: 10.1016/j.cell.2014.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Viale A, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shao DD, et al. KRAS and YAP1 converge to regulate EMT and tumor survival. Cell. 2014;158:171–184. doi: 10.1016/j.cell.2014.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.von Figura G, et al. The chromatin regulator Brg1 suppresses formation of intraductal papillary mucinous neoplasm and pancreatic ductal adenocarcinoma. Nat Cell Biol. 2014;16:255–267. doi: 10.1038/ncb2916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bailey JM, et al. DCLK1 marks a morphologically distinct subpopulation of cells with stem cell properties in preinvasive pancreatic cancer. Gastroenterology. 2014;146:245–256. doi: 10.1053/j.gastro.2013.09.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Roy N, et al. Regulation of Cellular Identity in Cancer. Dev Cell. 2015;35:674–684. doi: 10.1016/j.devcel.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris JPt, et al. KRAS, Hedgehog, Wnt and the twisted developmental biology of pancreatic ductal adenocarcinoma. Nat Rev Cancer. 2010;10:683–695. doi: 10.1038/nrc2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones S, et al. Core signaling pathways in human pancreatic cancers revealed by global genomic analyses. Science. 2008;321:1801–1806. doi: 10.1126/science.1164368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Moffitt RA, et al. Virtual microdissection identifies distinct tumor- and stroma-specific subtypes of pancreatic ductal adenocarcinoma. Nat Genet. 2015;47:1168–1178. doi: 10.1038/ng.3398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Bailey P, et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature. 2016 doi: 10.1038/nature16965. [DOI] [PubMed] [Google Scholar]
- 41.Waddell N, et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature. 2015;518:495–501. doi: 10.1038/nature14169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Witkiewicz AK, et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat Commun. 2015;6:6744. doi: 10.1038/ncomms7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Knudsen ES, et al. Genetic Diversity of Pancreatic Ductal Adenocarcinoma and Opportunities for Precision Medicine. Gastroenterology. 2016;150:48–63. doi: 10.1053/j.gastro.2015.08.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Biankin AV, et al. Pancreatic cancer genomes reveal aberrations in axon guidance pathway genes. Nature. 2012;491:399–405. doi: 10.1038/nature11547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Collisson EA, et al. Subtypes of pancreatic ductal adenocarcinoma and their differing responses to therapy. Nat Med. 2011;17:500–503. doi: 10.1038/nm.2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zorde Khvalevsky E, et al. Mutant KRAS is a druggable target for pancreatic cancer. Proc Natl Acad Sci U S A. 2013;110:20723–20728. doi: 10.1073/pnas.1314307110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Golan T, et al. RNAi therapy targeting KRAS in combination with chemotherapy for locally advanced pancreatic cancer patients. Oncotarget. 2015;6:24560–24570. doi: 10.18632/oncotarget.4183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.di Magliano MP, et al. Roles for KRAS in pancreatic tumor development and progression. Gastroenterology. 2013;144:1220–1229. doi: 10.1053/j.gastro.2013.01.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Roberts NJ, et al. Whole Genome Sequencing Defines the Genetic Heterogeneity of Familial Pancreatic Cancer. Cancer Discov. 2016;6:166–175. doi: 10.1158/2159-8290.CD-15-0402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sahin IH, et al. Genomic instability in pancreatic adenocarcinoma: a new step towards precision medicine and novel therapeutic approaches. Expert Rev Gastroenterol Hepatol. 2016:1–13. doi: 10.1586/17474124.2016.1153424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Franco J, et al. Metabolic Reprogramming of Pancreatic Cancer Mediated by CDK4/6 Inhibition Elicits Unique Vulnerabilities. Cell Rep. 2016;14:979–990. doi: 10.1016/j.celrep.2015.12.094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Zhang Y, et al. Canonical Wnt Signaling Is Required for Pancreatic Carcinogenesis. Cancer Res. 2013;73:4909–4922. doi: 10.1158/0008-5472.CAN-12-4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.White BD, et al. Dysregulation of Wnt/beta-catenin signaling in gastrointestinal cancers. Gastroenterology. 2012;142:219–232. doi: 10.1053/j.gastro.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Froeling FE, et al. Retinoic Acid-Induced Pancreatic Stellate Cell Quiescence Reduces Paracrine Wnt-beta-Catenin Signaling to Slow Tumor Progression. Gastroenterology. 2011 doi: 10.1053/j.gastro.2011.06.047. [DOI] [PubMed] [Google Scholar]
- 55.Ilmer M, et al. RSPO2 Enhances Canonical Wnt Signaling to Confer Stemness-Associated Traits to Susceptible Pancreatic Cancer Cells. Cancer Res. 2015;75:1883–1896. doi: 10.1158/0008-5472.CAN-14-1327. [DOI] [PubMed] [Google Scholar]
- 56.Jiang X, et al. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc Natl Acad Sci U S A. 2013;110:12649–12654. doi: 10.1073/pnas.1307218110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yu M, et al. RNA sequencing of pancreatic circulating tumour cells implicates WNT signalling in metastasis. Nature. 2012;487:510–513. doi: 10.1038/nature11217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang MT, et al. K-Ras Promotes Tumorigenicity through Suppression of Non-canonical Wnt Signaling. Cell. 2015;163:1237–1251. doi: 10.1016/j.cell.2015.10.041. [DOI] [PubMed] [Google Scholar]
- 59.De La OJ, et al. Notch and Kras in pancreatic cancer: at the crossroads of mutation, differentiation and signaling. Cell Cycle. 2009;8:1860–1864. doi: 10.4161/cc.8.12.8744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wang Z, et al. Acquisition of epithelial-mesenchymal transition phenotype of gemcitabine-resistant pancreatic cancer cells is linked with activation of the notch signaling pathway. Cancer Res. 2009;69:2400–2407. doi: 10.1158/0008-5472.CAN-08-4312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chantrill LA, et al. Precision Medicine for Advanced Pancreas Cancer: The Individualized Molecular Pancreatic Cancer Therapy (IMPaCT) Trial. Clin Cancer Res. 2015;21:2029–2037. doi: 10.1158/1078-0432.CCR-15-0426. [DOI] [PubMed] [Google Scholar]
- 62.Biankin AV, et al. Patient-centric trials for therapeutic development in precision oncology. Nature. 2015;526:361–370. doi: 10.1038/nature15819. [DOI] [PubMed] [Google Scholar]
- 63.Erkan M, et al. The role of stroma in pancreatic cancer: diagnostic and therapeutic implications. Nat Rev Gastroenterol Hepatol. 2012;9:454–467. doi: 10.1038/nrgastro.2012.115. [DOI] [PubMed] [Google Scholar]
- 64.Stromnes IM, et al. Stromal reengineering to treat pancreas cancer. Carcinogenesis. 2014;35:1451–1460. doi: 10.1093/carcin/bgu115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Toste PA, et al. Chemotherapy-induced Inflammatory Gene Signature and Pro-tumorigenic Phenotype in Pancreatic CAFs via Stress-associated MAPK. Mol Cancer Res. 2016 doi: 10.1158/1541-7786.MCR-15-0348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Sherman MH, et al. Vitamin d receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell. 2014;159:80–93. doi: 10.1016/j.cell.2014.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Provenzano PP, et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell. 2012;21:418–429. doi: 10.1016/j.ccr.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beatty GL, et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science. 2011;331:1612–1616. doi: 10.1126/science.1198443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Saloman JL, et al. Ablation of sensory neurons in a genetic model of pancreatic ductal adenocarcinoma slows initiation and progression of cancer. Proc Natl Acad Sci U S A. 2016 doi: 10.1073/pnas.1512603113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Rhim AD, et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell. 2014;25:735–747. doi: 10.1016/j.ccr.2014.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Schumacher TN, et al. Neoantigens in cancer immunotherapy. Science. 2015;348:69–74. doi: 10.1126/science.aaa4971. [DOI] [PubMed] [Google Scholar]
- 72.Kotteas E, et al. Immunotherapy for pancreatic cancer. J Cancer Res Clin Oncol. 2016 doi: 10.1007/s00432-016-2119-2. [DOI] [PubMed] [Google Scholar]
- 73.Kunk PR, et al. From bench to bedside a comprehensive review of pancreatic cancer immunotherapy. J Immunother Cancer. 2016;4:14. doi: 10.1186/s40425-016-0119-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Boj SF, et al. Organoid models of human and mouse ductal pancreatic cancer. Cell. 2015;160:324–338. doi: 10.1016/j.cell.2014.12.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Collisson EA, et al. A central role for RAF-->MEK-->ERK signaling in the genesis of pancreatic ductal adenocarcinoma. Cancer Discov. 2012;2:685–693. doi: 10.1158/2159-8290.CD-11-0347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Shugang X, et al. Prognostic Value of SMAD4 in Pancreatic Cancer: A Meta-Analysis. Transl Oncol. 2016;9:1–7. doi: 10.1016/j.tranon.2015.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Mahadevan D, et al. Tumor-stroma interactions in pancreatic ductal adenocarcinoma. Mol Cancer Ther. 2007;6:1186–1197. doi: 10.1158/1535-7163.MCT-06-0686. [DOI] [PubMed] [Google Scholar]
- 78.Okada M, et al. The novel JNK inhibitor AS602801 inhibits cancer stem cells in vitro and in vivo. Oncotarget. 2016 doi: 10.18632/oncotarget.8395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Hanahan D, et al. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 80.Riazy M, et al. Mismatch repair status may predict response to adjuvant chemotherapy in resectable pancreatic ductal adenocarcinoma. Mod Pathol. 2015;28:1383–1389. doi: 10.1038/modpathol.2015.89. [DOI] [PubMed] [Google Scholar]