Abstract
Background
Fatigue is one of the most debilitating side effects of cancer therapy. Identifying biomarkers early during cancer therapy may help us understand the biologic underpinnings of the persistence of fatigue following therapy.
Objective
We aimed to identify early biomarkers of fatigue by examining correlations of levels of cytokines during external beam radiation therapy (EBRT) with persistence of fatigue one year following treatment completion in men with non-metastatic prostate cancer (NM-PC).
Methods
A sample of 34 men with NM-PC scheduled to receive EBRT were followed at baseline (T1), midpoint of EBRT (T2), and one year following EBRT (T3). Demographic and clinical data were obtained by chart review. The Functional Assessment of Cancer Therapy-Fatigue (FACT-F) was administered to measure fatigue levels. Plasma cytokine levels were determined at T1 and T2 using the Bio-Rad Bio-Plex Cytokine Assay Kits.
Results
Significant correlations were observed between levels of IL-3, IL-8, IL-9, IL-10, IL-16, IP10, IFNα2, IFNγ, and SDF1α at T2 with worsening of fatigue from T1 to T3.
Conclusions
Immunological changes prior to chronic fatigue development may reflect the long term response to radiation therapy-induced damage.
Implications for Practice
Early biomarkers for chronic fatigue related to cancer therapy will help advance our understanding of the etiology of this distressing symptom and will help nurses identify patients at risk for developing chronic fatigue after cancer treatment. This information will also aide in patient education, as well as symptom management.
Keywords: cancer-related fatigue, radiation therapy, prostate cancer, immune dysfunction, cytokines
Introduction
Cancer-related fatigue (CRF) is defined as an unusual, persistent, subjective sense of tiredness related to cancer or cancer treatment that interferes with normal functioning and is not relieved by rest or sleep1. CRF, which has a prevalence ranging from 59–100%, has been ranked by cancer patients as the longest lasting and most disruptive symptom2, 3. A portion of cancer patients who completed therapy continue to experience fatigue more than one year after treatment, suggesting a long-lasting effect of cancer and/or cancer treatment4, 5.
Although the causes of fatigue in cancer patients remain unknown, efforts have been made to understand the underlying mechanisms. It is now widely accepted that infection and inflammation can result in a variety of symptoms including fatigue, lethargy, weakness, anorexia, and anhedonia, collectively termed “sickness behavior”6. Cytokines are important mediators of sickness behavior; they represent a large family of secreted glycoproteins that act as key mediators in cell signaling and immune response7. Upon binding to cytokine receptors, cytokines cause dimerization of the receptors leading to Janus kinase family of tyrosine kinases (JAK) activation and signal transducers and activators of transcription (STAT) phosphorylation. Phosphorylated STATs dimerize and translocate into the nucleus where they induce expression of numerous genes including those that are important in immune regulation8.
There is ample evidence in the existing literature that suggests inflammation plays a role in CRF development7, 9. For example, single nucleotide polymorphisms in the promoter regions of tumor necrosis factor (TNF) and interleukin-6 (IL6) were correlated with CRF in breast cancer patients10. In another study using plasma samples collected from ovarian cancer patients, IL6 was correlated with fatigue induced by poor sleep11. C-reactive protein was found to be correlated with fatigue in breast cancer survivors12. Higher concentrations of IL6, IL10, and interferon-gamma (IFNγ) were observed in patients with testicular cancer13. Interestingly, activation of the pro-inflammatory NF-kB transcription pathway appears to be associated with cancer-related fatigue14.
Despite this abundance of literature showing a possible link between cytokines and fatigue symptoms15, the biologic mechanism of persistent fatigue from cancer treatment remains underexplored. This study will specifically focus on the persistent effects of radiation therapy (RT). RT is one of the most common therapies for prostate cancer, but there are a number of side effects associated with RT including fatigue as well as urinary, bowel, and sexual dysfunctions16. Prostate cancer is a leading cause of death among men in the United States, representing 27% of new cancer cases and 10% of cancer-related deaths17. Despite the high rate of deaths, the five-year survival rate is 99.8% in men with prostate cancer17. Current clinical guidelines for treating prostate cancer include active surveillance, radical prostatectomy, and external-beam radiation therapy (EBRT)18.
Ionizing radiation damages tumors by inducing nuclear DNA breakage and is commonly used in cancer therapy due to its effectiveness. At the same time, radiation is a double-edged sword that gives rise to reactive oxygen species (ROS), which in turn induce lipid peroxidation as well as oxidation of proteins and DNA19. ROS generated during radiation therapy can trigger inflammatory events, and when unchecked, induce long term damage that may result in the symptom of fatigue20. It is unclear how peripheral pro-inflammatory markers triggered by localized radiation induce the intensification or even the persistence of a centrally-regulated symptom, such as fatigue. It is worthwhile to investigate if a compromise in the blood-brain-barrier (BBB) is associated with the persistence of fatigue following EBRT.
We hypothesize that immunological changes that occur during RT can have long-lasting effects on fatigue. In this study, our objective was to identify early biomarkers that can be detected during RT for persistent fatigue in prostate cancer patients one year after completing RT. We want to examine correlations of levels of cytokines during EBRT with the change of fatigue symptom before EBRT to one year following treatment completion. The development of peripheral biomarkers detectable during cancer treatment could help clinicians understand the underlying mechanisms of fatigue, evaluate the risk for developing chronic fatigue after treatment, and ultimately help with symptom management and improving patients’ quality of life.
Methods
Participants
The current study (NCT00852111) was approved by the Institutional Review Board of the National Institutes of Health (NIH), Bethesda, Maryland. All participants enrolled in this study were men, 18 years of age or older, diagnosed with non-metastatic prostate cancer with or without prior prostatectomy, and scheduled to receive EBRT with or without concurrent androgen deprivation therapy (ADT). The entire EBRT treatment lasted 38–42 days. Potential participants were excluded if they had progressive diseases causing significant fatigue, psychiatric disease within the past five years, uncorrected hypothyroidism or anemia, or a second malignancy. Individuals who used sedatives, steroids, or non-steroidal anti-inflammatory agents were also excluded. Participants were recruited from September 2009 to November 2013 at the Magnuson Clinical Research Center at the NIH, and signed written informed consents were obtained prior to study participation.
Instruments
Clinical and demographic data (e.g., age, race, stage of prostate cancer, EBRT dose, EBRT technique used, and laboratory tests) were obtained from chart review at baseline (prior to EBRT initiation; T1). Blood cell counts were measured using standard procedures adapted by the Department of Laboratory Medicine, NIH at baseline (T1), midpoint of EBRT (day 19–21 after EBRT initiation; T2), and one year after EBRT (T3).
Fatigue was assessed using the 13-item Functional Assessment of Cancer Therapy–Fatigue (FACT-F) questionnaire, which is a frequently used, validated, reliable, stand-alone measure of fatigue in cancer therapy (coefficient alpha = 0.95–0.96)21. The FACT-F questionnaires were administered by investigators experienced with FACT-F administration in an outpatient setting, before clinical procedures began, in order to avoid extraneous influences on the responses. Each item response was rated on a 0–4 scale, where a 0 represents “Not at all” and a 4 indicates that the respondent relates to the corresponding statement “very much.” Total scores ranged from 16–53 with lower scores reflecting high fatigue intensity. A FACT-F score change of ≥3 is considered clinically significant22. High fatigue was defined as a decrease in FACT-F score ≥3 points from T1 to T3, while low fatigue subjects had a <3-point decrease in FACT-F scores between the time points mentioned.
Participants were screened for depression using the validated Hamilton Depression Rating Scale (HAM-D), a 21-item, clinician-rated paper questionnaire with good internal reliability (α=0.81 to 0.98).23. A score between 0 and 7 indicates normal, whereas scores higher than 8 reflect mild to severe depression. Questionnaires were administered by investigators experienced with HAM-D administration. Both FACT-F and HAM-D were administered at T1, T2, and T3.
Serum sample preparation
A total volume of 4 ml of whole blood was collected from each subject in a serum separator tube (Becton, Dickinson, and Company, Franklin Lakes, NJ) at T1, T2, and T3. Blood samples were centrifuged at 3000 rpm for 10 minutes at 4 °C, and collected serum was stored at −80 °C prior to analysis.
Cytokine Measurement
To address the purpose of the study to identify early biomarkers of persistent fatigue, a panel of 48 cytokines was measured in 50 μl of non-diluted serum samples from each participant at T1 and T2 using the Bio-Plex Pro Human Cytokine Kits (Bio-Rad Laboratories, Hercules, CA) according to manufacturer’s instructions. Briefly, anti-cytokine conjugated beads were added to each well in a 96-well plate. Following washing with the wash buffer, 50 μl of standard or undiluted serum sample was added to each well and the plate was incubated at room temperature for 30 minutes. The plates were washed, and 25 μl of prediluted Bio-Plex detection antibody was added; the plates were then incubated for an additional 30 minutes at room temperature. After three washes, 50 μl of prediluted streptavidin-phycoerythrin was added for 10 minutes. Following an additional wash, 125 μl of Bio-Plex assay buffer was added to each well. Concentrations of the cytokines were quantified using the Luminex® 100 instrument.
CGRP ELISA
To ensure that the changes in serum concentrations of pro-inflammatory cytokines and worsening of fatigue symptoms were not associated with radiation-related disruption in gastrointestinal integrity24, serum calcitonin gene related peptide (CGRP) concentrations were measured in all study time points (T1, T2, T3) using the Human CGRP ELISA Kit (MyBioSource, San Diego, CA) based on the standard protocol provided by the manufacturer. Briefly, 100 μl of standard or undiluted serum was added in duplicates to each well. Following incubation at 37 °C for 2 hours, the plates were incubated for 1.5 hours with 100 μl of biotin antibody added to each well. Subsequently, the plates were incubated in the presence of 100 μl of HRP-avidin solution per well for 1 hour at 37 °C. The plates were washed 5 times and incubated with 90 μl of TMB substrate solution added to each well. Absorbance was measured at 450 nm on a SpectraMax M3 (Molecular Devices, Sunnyvale, CA) plate reader and CGRP concentrations were calculated based on a standard curve.
S100B ELISA
To determine whether a BBB compromise can explain the association of peripheral expression of cytokines and persistent fatigue, serum samples collected at T1 and T2 were used to measure S100 calcium-binding protein B (S100B) levels, a validated serum marker for BBB disruption,25, 26 using the Human S100B ELISA Kit (Millipore, Billerica, MA) based on the standard protocol provided by the manufacturer. Briefly, 50 μl of S100B standards (reconstituted from lyophilized S100B) and undiluted serum was added in duplicates to each standard and sample wells in a 96-well plate coated with pre-titered Human S100B antibodies. Following incubation at room temperature for 2 hours, the wells were washed five times with TBS/Tween-20. The plates were then incubated for 1.5 hours with 100 μl of detection antibody added to each well, and followed by addition of 100 μl of enzyme solution and substrate solution to each well. Absorbance of the reaction product in each well was measured at 450 nm and 590 nm on a SpectraMax M3 (Molecular Devices, Sunnyvale, CA) plate reader, and S100B concentrations were calculated based on a standard curve generated using a 4-parameter logistic function. The intra-assay variations (CVs) were below 10%.
Statistical Analysis
Descriptive analyses were used to describe demographic characteristics of the sample. All data are expressed as mean ± SEM. Repeated measures one-way analysis of variance (ANOVA) with post hoc paired t-test comparisons were conducted to compare fatigue scores, PSA, CGRP, and S100B levels between time points. Correlation plots and coefficients for the analysis of associations between cytokines and changes in fatigue scores were obtained using MATLAB (MathWorks, Natick, MA). For each cytokine, Pearson’s correlation coefficients and p values were calculated by comparing all study participants’ T2 cytokine concentrations with all changes in FACT-F scores from T1 to T3. Plots were generated for all significant correlations, omitting single measurements that fell more than 3 standard deviations from the mean across all participants. The cytokines measured are involved in overlapping signaling pathways; because of this, their measurements should not be treated as independent variables. Hence, the resulting correlations were evaluated one biomarker at a time and considered statistically significant at p < .05 without correction for multiple comparison. A more in-depth assessment of statistical significance is addressed in the discussion section. Cytokines that were found to correlate significantly with changes in fatigue scores were further analyzed using a combination of pathway analysis using Ingenuity Pathway Analysis (Qiagen, Redwood City, CA) and literature search using Pubmed in order for us to examine the relationships between these cytokines.
Results
Clinical Characteristics
The study enrolled a total of 52 participants. One participant withdrew from the study because of conflict in schedule, and 18 participants have not completed the T3 study time point. This analysis included a cohort of 34 participants (mean age 64.3 ± 8.3 years) who completed all study time points and received an 8-week regimen of EBRT for localized prostate cancer; 27 participants received a total dosage of 75.6 Gray and 7 participants with prior prostatectomy received 66.0–68.4 Gray (Table 1). Prior to EBRT, clinical T2 stage prostate cancer, which indicates that the cancer is localized to the prostate and has not metastasized, was the most prominent diagnosis (61.7%). The average Gleason score of 7.6 ± 0.99 out of 10 suggests that cancer in the majority of participants was moderately aggressive. None of the participants included in the study experienced clinically significant depression symptoms based on the low scores on the HAM-D. Most participants (76.5%) received ADT prior to and during EBRT.
Table 1.
Demographics and Clinical Characteristics of Sample Population
| Variable | Mean | SD | Range | N | Variable | Mean | SD | Range | N |
|---|---|---|---|---|---|---|---|---|---|
| Age in Years | 64.3 | 8.3 | 49–81 | 34 | Total Dosage EBRT (Gray) | ||||
| Ethnicity | 75.6 | 27 | |||||||
| Caucasian | 20 | 68.4 | 6 | ||||||
| African-American | 9 | 66.0 | 1 | ||||||
| Asian | 3 | HAM-D Questionnaire | 34 | ||||||
| Hispanic | 2 | Baseline | 1.15 | 1.89 | 0–8 | ||||
| Clinical T Stage | Midpoint | 2.71 | 3.57 | 0–13 | |||||
| T1 (a-c) | 8 | Endpoint | 1.65 | 1.94 | 0–8 | ||||
| T2 (a-c) | 21 | Albumin Levels (g/dL) | 30 | ||||||
| T3 (a-c) | 5 | Baseline | 3.99 | 0.38 | 2.7–4.6 | ||||
| Gleason Score | 7.6 | 0.99 | 6–9 | Testosterone (ng/dL) | 24 | ||||
| BMI | 29.5 | 4.1 | 22.9–40.2 | Baseline | 279 | 174 | 20–707 | ||
| ADT | 26 | TSH (mclU/mL) | 18 | ||||||
| No ADT | 8 | Baseline | 2.26 | 0.98 | 0.76–4.15 | ||||
| Prostatectomy plus EBRT | 7 | Hemoglobin (g/dL) | 33 | ||||||
| Irradiated Pelvis | 30 | Baseline | 13.5 | 1.07 | 10.9–15.2 | ||||
| Absolute Monocytes (1000/uL) | 34 | ||||||||
| Baseline | 0.50 | 0.15 | 0.22–0.81 | ||||||
Abbreviations: BMI, body mass index; EBRT, external beam radiation therapy; PSA, prostate specific antigen; TSH, thyroid stimulating hormone; HAM-D, Hamilton Depression; g, gram; dL, deciliter; ng, nanogram; uL, microliter
A significant portion of the study cohort (41%) continued to experience high fatigue at T3, which is the focus of this study; whereas FACT-F scores for the rest of the participants went back to baseline levels or higher (Figure 1). To rule out common causes of inflammation and fatigue, we compared clinical characteristics of subjects with high fatigue change versus those with low fatigue. The participants with high fatigue at T3 did not differ from the low fatigue subjects in age (high fatigue: 65.6 ± 1.9; low fatigue: 66.0 ± 1.9, p = 0.79), BMI (high fatigue: 31.5 ± 1.6; low fatigue: 29.0 ± 1.2, p = 0.13), and concomitant ADT use (high fatigue: 77% of participants; low fatigue: 78% of participants). Further, the prostate-specific antigen (PSA) levels of all participants remained similar (below 1) at T2 and T3 (Figure 2A); hence, inflammation and fatigue may not be related to recurrence of prostate cancer. In addition, chronic fatigue or inflammation also did not appear to be a result of gastrointestinal involvement from radiation because there was an absence of changes in the concentrations of CGRP, a marker of gastrointestinal integrity24, from baseline to midpoint or one year post EBRT (Figure 2B).
Figure 1.
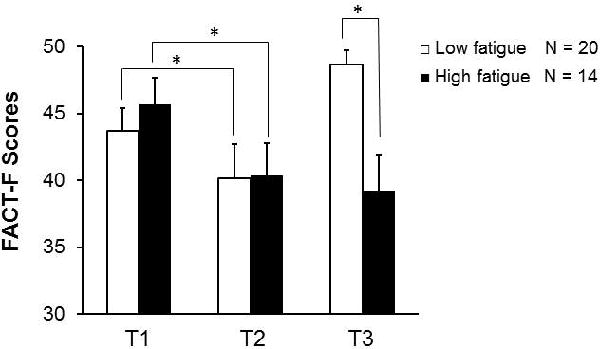
Fatigue levels at baseline (T1), at midpoint (T2), and after radiation therapy (T3). Fatigue persisted in 41% (14 out of 34) of participants at T3. The Functional Assessment of Cancer Therapy – Fatigue (FACT-F) scores of all subjects were >40 at T1, indicating that all subjects were not fatigued prior to radiation therapy. FACT-F scores of study participants in the high fatigue group and low fatigue group were comparable at T1 (p = .88) and T2 (p = .67); however, the difference between the two fatigue groups became significant at T3 (p = 3×10−6). Compared to T1, FACT-F scores in the high fatigue participants decreased at T2 (p = .03) and remained low at T3 (p = .01), indicating both treatment-related fatigue as well as chronic fatigue. Values are expressed as mean ± SEM. * indicates p < .05
Figure 2.
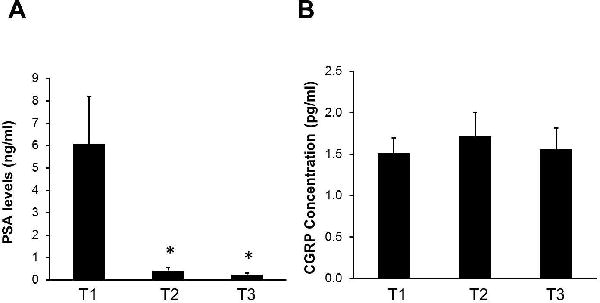
Fatigue during and after treatment was not related to the health status of study participants. (A) Prostate Specific Antigen (PSA) levels significantly decreased at midpoint (T2) of EBRT and remained low one year post-EBRT (T3). (B) EBRT did not alter calcitonin gene-related peptide (CGRP) concentrations at midpoint (T2) or one year post-EBRT (T3). Values are expressed as mean ± SEM. * indicates p < .05.
Significant Relationships of T2 Data
We hypothesize that inflammatory response during EBRT contributes to chronic fatigue experience months after treatment completion. In order to identify early biomarkers during treatment that can predict chronic fatigue, we measured a panel of 48 cytokines (listed in Table 2) at T2 and the following cytokines were positively correlated with changes in FACT-F score from T1 to T3: 1) pro-inflammatory cytokines IL3, IL8, IL16, IFNα2, IFNγ; 2) anti-inflammatory cytokine IL10; 3) hematopoietic cytokines IL3, IL9; 4) chemokines: interferon gamma-induced protein 10 (IP10), stromal cell-derived factor 1α (SDF1α) (Figure 3).
Table 2.
Correlation between T1 – T3 FACT-F Score Change and Cytokine Measurements at T1 or T2.
| T1 | T2 | |||
|---|---|---|---|---|
| Correlation | p-Value | Correlation | p-Value | |
| IL2Ra | 0.42 | .018 | 0.06 | .771 |
| IL3 | 0.12 | .531 | 0.44 | .023 |
| IL12p40ad | – | – | 0.22 | .280 |
| IL16 | 0.33 | .062 | 0.49 | .008 |
| IL18 | −0.16 | .386 | 0.02 | .935 |
| CTACK | 0.30 | .098 | 0.13 | .502 |
| GROa | −0.22 | .229 | 0.21 | .291 |
| HGF | 0.14 | .429 | −0.10 | .630 |
| IFNa2 | 0.20 | .280 | 0.58 | .002 |
| LIF’ | 0.16 | .393 | 0.27 | .171 |
| MCP3 | 0.18 | .335 | 0.27 | .159 |
| MCSF | 0.13 | .478 | – | – |
| MIF | 0.30 | .101 | 0.26 | .176 |
| MIG | 0.06 | .751 | 0.22 | .256 |
| bNGF | 0.25 | .178 | 0.35 | .076 |
| SCF | 0.16 | .386 | −0.23 | .243 |
| SCGFb | 0.31 | .088 | 0.11 | .583 |
| SDF1a | 0.23 | .236 | 0.49 | .008 |
| TRAIL | −0.02 | .901 | 0.34 | .077 |
| IL1b | −0.16 | .378 | 0.29 | .129 |
| IL1ra | 0.04 | .830 | 0.22 | .268 |
| IL2 | −0.25 | .181 | 0.28 | .160 |
| IL4 | −0.13 | .470 | 0.20 | .312 |
| IL5 | −0.20 | .264 | 0.12 | .529 |
| IL6 | −0.01 | .937 | 0.33 | .089 |
| IL7 | −0.09 | .638 | 0.27 | .160 |
| IL8 | −0.05 | .772 | 0.42 | .028 |
| IL9 | −0.11 | .550 | 0.44 | .021 |
| IL10 | 0.10 | .590 | 0.44 | .021 |
| IL12p70 | 0.02 | .918 | 0.24 | .229 |
| IL13 | 0.14 | .434 | 0.07 | .736 |
| IL17 | −0.32 | .073 | 0.02 | .910 |
| Eotaxin | −0.07 | .685 | 0.18 | .349 |
| FGFbasic | −0.05 | .796 | 0.02 | .910 |
| GCSF | 0.05 | .807 | 0.22 | .277 |
| GMCSF | 0.04 | .836 | – | – |
| IFNg | −0.08 | .654 | 0.40 | .033 |
| IP10 | −0.02 | .930 | 0.43 | .022 |
| MCP1 | −0.17 | .356 | 0.11 | .589 |
| MIP1a | 0.10 | .592 | 0.24 | .230 |
| PDGFbb | −0.35 | .051 | 0.01 | .947 |
| MIP1b | −0.20 | .275 | 0.19 | .351 |
| RANTES | −0.12 | .513 | 0.32 | .102 |
| TNFa | −0.02 | .905 | 0.14 | .500 |
| VEGF | −0.19 | .293 | 0.15 | .445 |
Figure 3.
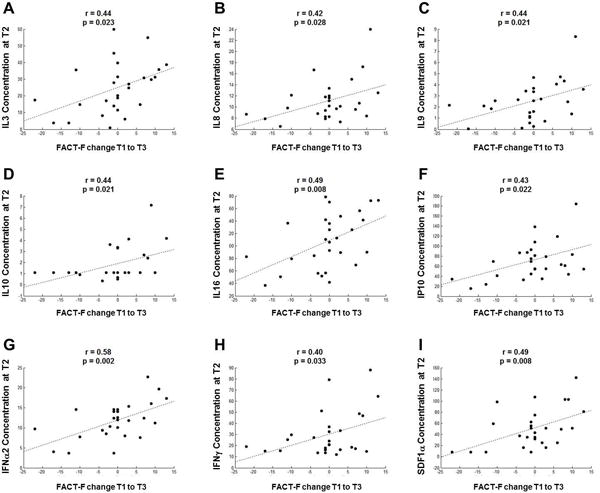
Cytokine levels during EBRT correlated with fatigue changes from baseline to one year post-EBRT. Among the 48 cytokines tested, concentrations of 9 cytokines (pg/ml) measured at midpoint of EBRT showed significant correlations with FACT-F score changes from T1 to T3: 1) proinflammatory cytokines IL3 (A; r = 0.44, p = .023), IL8 (B; r = 0.42, p = .028), IL16 (E; r = 0.49, p = .008), IFNα2 (G; r = 0.58, p = .002), IFNγ (H; r = 0.40, p = .033); 2) anti-inflammatory cytokine IL10 (D; r = 0.44, p = .021); 3) hematopoietic cytokines IL3 (A; r = 0.44, p = .023), IL9 (C; r = 0.44, p = .021); 4) IP10 (F; r = 0.43, p = .022), SDF1α (I; r = 0.49, p = .008).
Peripheral cytokines can contribute to fatigue either indirectly possibly by activating microglia, or directly affecting neural network activity by entering the CNS through the BBB27, 28. To test whether there is a disruption of BBB integrity in prostate cancer patients receiving EBRT that might explain the influence of this peripheral observation with a central behavior such as fatigue, we measured serum concentrations of S100B, which is an established marker for BBB disruption25, 26. Serum S100B concentrations did not significantly change over the course of EBRT (Figure 4). We also did not find a statistically significant correlation between S100B concentrations and FACT-F scores (p > .05).
Figure 4.
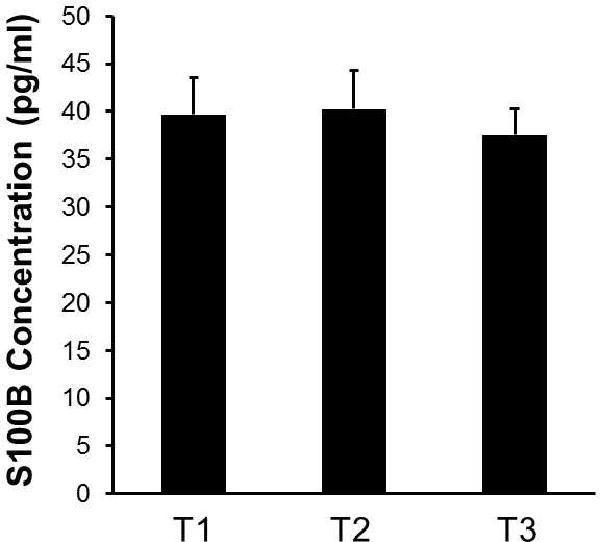
EBRT did not alter the integrity of blood-brain-barrier. Compared to the baseline (T1) S100B concentrations did not change at midpoint of EBRT (T2) or at one year after treatment completion (T3). Results suggest that EBRT dosage received by study participants did not result in disturbances in the blood-brain-barrier. Values are mean ± SEM.
We also examined the correlation between fatigue and different blood cell types in order to identify potential contributors to changes in the cytokines that we observed. T2 absolute eosinophil counts and FACT-F scores were negatively correlated (r = −0.42, p = .027, Figure 5A). Macrophage migration inhibitory factor (MIF) concentrations, one of the 48 cytokines measured, also negatively correlated with FACT-F scores (Figure 5B).
Figure 5.
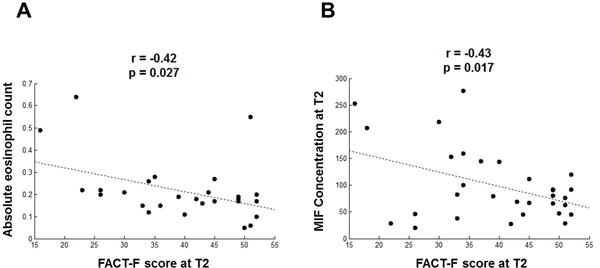
Correlations between eosinophils/MIF and FACT-F scores. (A) Eosinophil numbers (absolute eosinophil count) correlate negatively with FACT-F scores at midpoint (r = −0.42, p = 0.027). (B) Serum macrophage migration inhibitory factor (MIF) levels negatively correlated with FACT-F scores indicating a positive correlation with fatigue intensity (r = −0.43, p = 0.017).
Discussion
In the current study, we demonstrated that 41% of subjects experience chronic fatigue one year post-EBRT suggesting that chronic fatigue is affecting a large number of men following RT. We observed that immunological changes occurring during EBRT may explain the chronic fatigue experience one year after treatment completion. Specifically, we found serum concentrations of nine cytokines (IL3, IL8, IL9, IL10, IL16, IFNα2, IFNγ, IP10, and SDF1α) measured during EBRT (T2) correlating significantly with worsening fatigue from baseline to one year post RT. In contrast, none of these 9 cytokines measured at T1 showed significant correlation with changes in FACT-F score from baseline to one year post RT (Table 2), suggesting that the associations of cytokine concentrations and chronic fatigue may be treatment-induced and are not pre-existing.
The importance of these findings from this pilot study is three-fold: 1) changes in cytokines during radiation therapy may allow us to identify patients at risk for developing chronic fatigue after cancer treatment; 2) immunological dysfunction as evidenced by changes in cytokines during treatment precedes development of chronic fatigue and may help us understand the upstream mechanistic pathways of chronic fatigue; 3) interventions that modulate cytokine changes during RT may help alleviate chronic fatigue.
The pathway analysis explains the relationships between pro-inflammatory (IL3, IL8, IL16, IFNα2, IFNγ) and anti-inflammatory (IL10) cytokines, as well as hematopoietic cytokines (IL3, IL9) and chemokines (IP10 and SDF1α) with chronic fatigue, which are illustrated in Figure 6. These inflammatory markers are part of a complex interconnected network involving both type-1 and type-2 immunity (Figure 6). IP10 is secreted by multiple cell types in response to IFNγ and acts to attract monocytes and macrophages29. Similarly, SDF1α is known to recruit macrophages as well as contribute to tumor progression30. Upon antigen detection, macrophages release IL8 to attract granulocytes31. Secreted by basophils and activated T cells, IL3 stimulates proliferation of cells of the myeloid lineage including eosinophils32. Also known as lymphocyte chemoattractant factor, IL16 has been shown to attract activated T cells as well as other CD4+ cells including monocytes, dendritic cells, and eosinophils33. Our observation of an association between IFNα2 and fatigue is consistent with previous studies that demonstrated elevated levels of IFNα in patients with chronic fatigue syndrome34. Occupying the central hub of the cytokine network found in our study, IFNγ in the CNS induces indoleamine 2,3-dioxygenase (IDO) production by monocytes and/or microglia. IDO is the rate-limiting enzyme in the kynurenine pathway, which converts tryptophan into quinolinic acid and/or kynurenine. Because the kynurenine pathway and 5-HT production share the same precursor tryptophan, overexpression of IDO is associated with 5-HT depletion, which has both physiological and psychological consequences35. Interestingly, quinolinic acid produced in this pathway is an NMDA receptor agonist, while kynurenine is an NMDA receptor antagonist. As NMDA receptors play an important role in maintaining the balance of CNS excitation and inhibition, it is very likely that disturbances in NMDA activity may have a global effect on the depressive state of the CNS and, consequently, fatigue.
Figure 6.
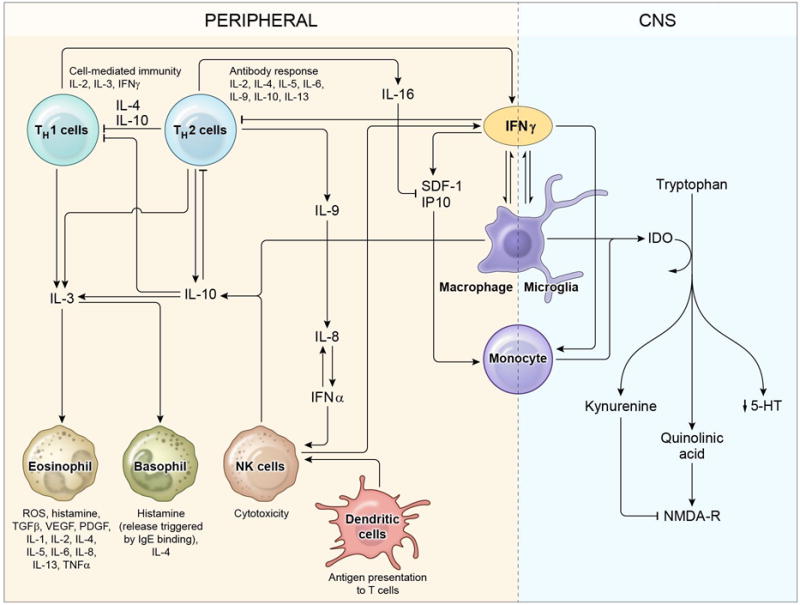
Proposed mechanism of fatigue as a result of immunological dysfunction. Radiation-induced activation of the cytokine network results in a positive-feedback network of immune cells and cytokines. Increased levels of cytokines in the periphery result in activation of multiple immune cells including eosinophils. Activation of eosinophils further contributes to inflammation by producing more cytokines. Peripheral inflammation may result in abnormal muscle function leading to muscle fatigue. Cytokines may either directly enter the CNS or activate neuro-immune interactions resulting in decreased 5-HT production and abnormal NMDA receptor activity leading to central fatigue.
We hypothesize that fatigue is a result of multiple pathogenic processes involving both the peripheral immune system and the CNS. Peripheral immune cells such as eosinophils release a variety of cytokines contributing to peripheral inflammation and leading to peripheral fatigue. Additionally, cytokines in the CNS can result in decreased serotonin (5-HT) production, disruption in long-term potentiation (LTP), and NMDA receptor-induced excitotoxicity35–39.
Our study did not observe any changes in S100B levels over time suggesting that the radiation received by the study participants did not result in any detectable BBB leakage and that fatigue symptoms were not attributable to cytokines entering the CNS through a compromised BBB. So, how do peripheral cytokines influence symptoms such as chronic fatigue, which is known to be centrally regulated? We propose that changes in cytokines during RT influence CNS physiology via: 1) passive diffusion through a BBB via the destruction of tight junctions of endothelial cells lining the BBB microvasculature; 2) entering the brain through existing BBB openings—circumventricular organs; 3) retrograde axonal transport; and 4) activating immune-to-brain pathways in the absence of BBB disturbances (i.e., peripheral cytokines can induce cytokine production in CNS macrophages and microglia)40. Indeed, direct effects of cytokines have been observed in the CNS. For example, TNFα has been shown to inhibit LTP in rat hippocampal slices41. Similarly, IL18 had an inhibitory effect on LTP in the rat dentate gyrus, which was attenuated by application of mGluR antagonists36. Cytokines can induce long term effects on neuronal networks by affecting synaptic scaling (i.e. scaling up of excitatory synapses in response to inactivity and scaling down in response to over-excitation)42. CNS response to peripheral inflammation results in compensatory changes including alterations in neurotransmitter metabolism and changes in the hypothalamic-pituitary-adrenal (HPA) axis, both of which can cause changes in behavior including fatigue43, 44. Furthermore, chronic peripheral administration of IFNα reduced striatal dopamine release and anhedonia-like behavior in non-human primates45. These findings are particularly important because fatigue is associated with impaired motivation and reward-based decision making, which is indicative of a dysfunctional frontostriatal network7. It is also worth mentioning that systemic infection as measured by increased serum IL6 concentrations was correlated with increased cognitive demand on the Stroop task46, which is similar to our own observation in fatigued patients (data not shown).
The novel correlation between fatigue intensification and eosinophil counts found in this study may be attributable to the increased concentrations of MIF, which has been shown to induce eosinophil infiltration and activation47, 48. Interestingly, eosinophils are a major source of cytokines and have been shown to release up to 35 different cytokines49. Consistent with our finding, other groups have shown evidence to suggest a correlation between eosinophils and fatigue symptoms in chronic fatigue syndrome, cancer-related fatigue, and eosinophilia-myalgia50–52. However, the mechanism and the exact role of eosinophils in fatigue development remain elusive. It is likely that inflammation induced by radiation results in abnormal immune activation including increased cytokine release from eosinophils. Cytokines can then exert their effects either peripherally through causing muscle function impairment or centrally by affecting NMDA receptor activity and the frontostriatal pathway, which lead to a combination of peripheral and central fatigue.
This study was an initial exploration of the relationship between immune signaling and cancer-related fatigue; as a preliminary study, our sample size was small, limiting our statistical power. We have forgone a multiple-comparisons correction, because the levels of many of the cytokines are regulated by each other or by common upstream signaling molecules. Thus, we would expect to see many rise and fall in tandem, and their measurements should not be treated as independent variables. In fact, this interdependence may be what leads to chronic fatigue correlating with many cytokine measurements taken during treatment (T2, 9 of 43 with p < .05) but only one taken before treatment (T1, one of 44 with p < .05) (Table 2). For comparison, if the measurements were independent and normally distributed, we would expect to see roughly two false positives for each of T1 and T2. Because there is only a 0.025% chance of seeing this many positive results from a single time point by chance alone, we conclude that our results cross the threshold for considering this a “statistically significant” effect. Future study should be conducted with a larger sample size and a restricted list of cytokine measurements to thoroughly address concerns about multiple comparisons.
It is worth noting that while using peripheral biomarkers has the advantage over central markers, since blood samples are easily obtainable, it certainly has the downside that external factors unrelated to the disease state such as sample processing and storage condition can affect cytokine measurements53. Further, peripheral cytokine measurements may not reflect levels in the central nervous system. Future studies should include alternate measures such as cerebrospinal fluid samples to better understand peripheral versus central fatigue.
Using early biomarkers to identify patients who are high risk of developing chronic fatigue a year after EBRT completion has important clinical implications. In the context of individualizing care, this knowledge empowers nurses with the advantage of planning ahead through focused education related to energy conservation or balance in rest and activity. Encouraging physical activity for at risk individuals, a well-known anti-inflammatory strategy54, during EBRT that is recommended by national organizations like the National Comprehensive Cancer Network55, can improve treatment outcomes and patients’ quality of life. Furthermore, biomarkers found in this study can be easily measured in serum samples using routine, commercially-available immunoassay methods including ELISA. Therefore, further validation of these markers using a larger sample would be important to pursue.
In conclusion, prostate cancer patients receiving radiation therapy in this study exhibited signs of immunological changes that occurred during EBRT and these changes correlated significantly with chronic fatigue one year after treatment completion. These findings suggest that immunological changes may be upstream in the fatigue pathogenic pathway, and that changes in these cytokines may advance our understanding of the biological underpinnings of chronic fatigue related to cancer therapy, so optimal management to improve the lives of cancer patients can be developed.
Acknowledgments
This study is fully supported by the Division of Intramural Research, National Institute of Nursing Research, NIH. The authors thank Dr. Sungyoung Auh (Statistician), National Institute of Neurological Disorders and Stroke, for her statistical assistance. The authors also thank Cindy Clark, NIH Library Editing Service, for reviewing the manuscript.
Abbreviations
- ADT
androgen deprivation therapy
- BBB
blood-brain-barrier
- BMI
body mass index
- CGRP
calcitonin gene related peptide
- CNS
central nervous system
- CRF
Cancer-related fatigue
- dL
deciliter
- EBRT
external-beam radiation therapy
- FACT-F
Functional Assessment of Cancer Therapy–Fatigue
- g
gram
- HAM-D
Hamilton Depression Rating Scale
- HPA
hypothalamic-pituitary-adrenal axis
- 5-HT
serotonin
- IDO
indoleamine 2,3-dioxygenase
- IFN
interferon
- IL
interleukin
- JAK
Janus kinase family of tyrosine kinases
- mGluR
metabotropic glutamate receptor
- MIF
macrophage migration inhibitory factor
- ng
nanogram
- NM-PC
non-metastatic prostate cancer
- PSA
prostate specific antigen
- ROS
reactive oxygen species
- RT
radiation therapy
- SEM
standard error of mean
- STAT
signal transducers and activators of transcription
- T1
baseline
- T2
midpoint of EBRT
- T3
one year following EBRT
- TNF
tumor necrosis factor
- TSH
thyroid stimulating hormone
- uL
microliter
Footnotes
Conflicts of Interest: The authors have no funding or conflicts of interest to disclose.
Financial/nonfinancial disclosures: The authors have reported no potential conflicts of interest that exist with any companies/organizations whose products or services may be discussed in this article.
References
- 1.Berger AM, Abernethy AP, Atkinson A, et al. Cancer-Related Fatigue. Journal of the National Comprehensive Cancer Network. 2010 Aug 1;8(8):904–931. doi: 10.6004/jnccn.2010.0067. 2010. [DOI] [PubMed] [Google Scholar]
- 2.Weis J. Cancer-related fatigue: prevalence, assessment and treatment strategies. Expert Review of Pharmacoeconomics & Outcomes Research. 2011;11(4):441–446. doi: 10.1586/erp.11.44. 2011/08/01. [DOI] [PubMed] [Google Scholar]
- 3.Husson O, Mols F, van de Poll-Franse L, de Vries J, Schep G, Thong MY. Variation in fatigue among 6011 (long-term) cancer survivors and a normative population: a study from the population-based PROFILES registry. Supportive Care in Cancer. 2015:1–10. doi: 10.1007/s00520-014-2577-5. [DOI] [PubMed] [Google Scholar]
- 4.Goldstein D, Bennett BK, Webber K, et al. Cancer-Related Fatigue in Women With Breast Cancer: Outcomes of a 5-Year Prospective Cohort Study. Journal of Clinical Oncology. 2012 May 20;30(15):1805–1812. doi: 10.1200/JCO.2011.34.6148. 2012. [DOI] [PubMed] [Google Scholar]
- 5.Berger AM, Gerber LH, Mayer DK. Cancer-related fatigue. Cancer. 2012;118(S8):2261–2269. doi: 10.1002/cncr.27475. [DOI] [PubMed] [Google Scholar]
- 6.Gaykema RPA, Goehler LE. Ascending caudal medullary catecholamine pathways drive sickness-induced deficits in exploratory behavior: Brain substrates for fatigue? Brain Behavior and Immunity. 2011 Mar;25(3):443–460. doi: 10.1016/j.bbi.2010.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dantzer R, Heijnen CJ, Kavelaars A, Laye S, Capuron L. The neuroimmune basis of fatigue. Trends in Neurosciences. 2014;37(1):39–46. doi: 10.1016/j.tins.2013.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghoreschi K, Jesson MI, Li X, et al. Modulation of Innate and Adaptive Immune Responses by Tofacitinib (CP-690,550) The Journal of Immunology. 2011;186(7):4234–4243. doi: 10.4049/jimmunol.1003668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Arnett SV, Clark IA. Inflammatory fatigue and sickness behaviour — Lessons for the diagnosis and management of chronic fatigue syndrome. Journal of Affective Disorders. 2012;141(2–3):130–142. doi: 10.1016/j.jad.2012.04.004. [DOI] [PubMed] [Google Scholar]
- 10.Bower JE, Ganz PA, Irwin MR, Castellon S, Arevalo J, Cole SW. Cytokine Genetic Variations and Fatigue Among Patients With Breast Cancer. Journal of Clinical Oncology. 2013 May 1;31(13):1656–1661. doi: 10.1200/JCO.2012.46.2143. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Clevenger L, Schrepf A, Christensen D, et al. Sleep disturbance, cytokines, and fatigue in women with ovarian cancer. Brain Behavior and Immunity. 2012 Oct;26(7):1037–1044. doi: 10.1016/j.bbi.2012.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alfano CM, Imayama I, Neuhouser ML, et al. Fatigue, Inflammation, and ω-3 and ω-6 Fatty Acid Intake Among Breast Cancer Survivors. Journal of Clinical Oncology. 2012 Apr 20;30(12):1280–1287. doi: 10.1200/JCO.2011.36.4109. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Christensen JF, Tolver A, Andersen JL, Rørth M, Daugaard G, Hojman P. Resistance Training Does Not Protect Against Increases in Plasma Cytokine Levels Among Germ Cell Cancer Patients During and After Chemotherapy. The Journal of Clinical Endocrinology & Metabolism. 2014;99(8):2967–2976. doi: 10.1210/jc.2013-4495. [DOI] [PubMed] [Google Scholar]
- 14.Bower JE, Ganz PA, Irwin MR, Arevalo JMG, Cole SW. Fatigue and gene expression in human leukocytes: Increased NF-κB and decreased glucocorticoid signaling in breast cancer survivors with persistent fatigue. Brain Behavior and Immunity. 2011 Jan;25(1):147–150. doi: 10.1016/j.bbi.2010.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Miaskowski C, Aouizerat BE. Biomarkers: Symptoms, Survivorship, and Quality of Life. Seminars in Oncology Nursing. 2012;28(2):129–138. doi: 10.1016/j.soncn.2012.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Budäus L, Bolla M, Bossi A, et al. Functional Outcomes and Complications Following Radiation Therapy for Prostate Cancer: A Critical Analysis of the Literature. European Urology. 2012 Jan;61(1):112–127. doi: 10.1016/j.eururo.2011.09.027. [DOI] [PubMed] [Google Scholar]
- 17.Woolen S, Holzmeyer C, Nesbitt E, Siami PF. Long-Term Efficacy and Tolerability of Abdominal Once-Yearly Histrelin Acetate Subcutaneous Implants in Patients with Advanced Prostate Cancer. 2014 doi: 10.1155/2014/490315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Keyes M, Crook J, Morton G, Vigneault E, Usmani N, Morris WJ. Treatment options for localized prostate cancer. Canadian Family Physician. 2013;59(12):1269–1274. [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao W, Robbins ME. Inflammation and chronic oxidative stress in radiation-induced late normal tissue injury: therapeutic implications. Curr Med Chem. 2009;16(2):130–143. doi: 10.2174/092986709787002790. [DOI] [PubMed] [Google Scholar]
- 20.Multhoff G, Radons J. Radiation, inflammation and immune responses in cancer. Frontiers in Oncology. 2012;2 doi: 10.3389/fonc.2012.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yellen SB, Cella DF, Webster K, Blendowski C, Kaplan E. Measuring fatigue and other anemia-related symptoms with the Functional Assessment of Cancer Therapy (FACT) measurement system. Journal of Pain and Symptom Management. 1997;13(2):63–74. doi: 10.1016/s0885-3924(96)00274-6. [DOI] [PubMed] [Google Scholar]
- 22.Cella D, Eton DT, Lai J-S, Peterman AH, Merkel DE. Combining Anchor and Distribution-Based Methods to Derive Minimal Clinically Important Differences on the Functional Assessment of Cancer Therapy (FACT) Anemia and Fatigue Scales. Journal of Pain and Symptom Management. 2002;24(6):547–561. doi: 10.1016/s0885-3924(02)00529-8. [DOI] [PubMed] [Google Scholar]
- 23.Lydiatt WM, Denman D, McNeilly DP, Puumula SE, Burke WJ. A randomized, placebo-controlled trial of citalopram for the prevention of major depression during treatment for head and neck cancer. Arch Otolaryngol Head Neck Surg. 2008;134(5):528–535. doi: 10.1001/archotol.134.5.528. [DOI] [PubMed] [Google Scholar]
- 24.Esfandyari T, Macnaughton WK, Quirion R, St Pierre S, Junien J-L, Sharkey KA. A novel receptor for calcitonin gene-related peptide (CGRP) mediates secretion in the rat colon: implications for secretory function in colitis. The FASEB Journal. 2000;14(10):1439–1446. doi: 10.1096/fj.14.10.1439. [DOI] [PubMed] [Google Scholar]
- 25.Blyth BJ, Farhavar A, Gee C, et al. Validation of Serum Markers for Blood-Brain Barrier Disruption in Traumatic Brain Injury. Journal of Neurotrauma. 2009;26(9):1497–1507. doi: 10.1089/neu.2008.0738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Griffin WS, Stanley LC, Ling C, et al. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proceedings of the National Academy of Sciences. 1989;86(19):7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Besedovsky H, del Rey A. Central and Peripheral Cytokines Mediate Immune-Brain Connectivity. Neurochemical Research. 2011;36(1):1–6. doi: 10.1007/s11064-010-0252-x. 2011/01/01. [DOI] [PubMed] [Google Scholar]
- 28.Bower JE, Lamkin DM. Inflammation and cancer-related fatigue: Mechanisms, contributing factors, and treatment implications. Brain Behavior and Immunity. 2013;30(Supplement(0)):S48–S57. doi: 10.1016/j.bbi.2012.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu W, Joo H, Clayton S, et al. Macrophages induce differentiation of plasma cells through CXCL10/IP-10. The Journal of Experimental Medicine. 2012;209(10):1813–1823. doi: 10.1084/jem.20112142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ma W, Liu Y, Ellison N, Shen J. Induction of C-X-C Chemokine Receptor Type 7 (CXCR7) Switches Stromal Cell-derived Factor-1 (SDF-1) Signaling and Phagocytic Activity in Macrophages Linked to Atherosclerosis. Journal of Biological Chemistry. 2013;288(22):15481–15494. doi: 10.1074/jbc.M112.445510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolf M, Belen Delgado M, Jones SA, Dewald B, Clark-Lewis I, Baggiolini M. Granulocyte chemotactic protein 2 acts via both IL-8 receptors, CXCR1 and CXCR2. European Journal of Immunology. 1998;28(1):164–170. doi: 10.1002/(SICI)1521-4141(199801)28:01<164::AID-IMMU164>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 32.Gregory B, Kirchem A, Phipps S, et al. Differential Regulation of Human Eosinophil IL-3, IL-5, and GM-CSF Receptor α-Chain Expression by Cytokines: IL-3, IL-5, and GM-CSF Down-Regulate IL-5 Receptor α Expression with Loss of IL-5 Responsiveness, but Up-Regulate IL-3 Receptor α Expression. The Journal of Immunology. 2003;170(11):5359–5366. doi: 10.4049/jimmunol.170.11.5359. [DOI] [PubMed] [Google Scholar]
- 33.Little F, Cruikshank W. lnterleukin-16: The Ins and Outs of Regulating T-Cell Activation. Critical Review in Immunology. 2008;28(6):467–483. doi: 10.1615/critrevimmunol.v28.i6.10. [DOI] [PubMed] [Google Scholar]
- 34.Vojdani A, Ghoneum M, Choppa PC, Magtoto L, Lapp CW. Elevated apoptotic cell population in patients with chronic fatigue syndrome: the pivotal role of protein Kinase RNA. Journal of Internal Medicine. 1997;242(6):465–478. doi: 10.1111/j.1365-2796.1997.tb00019.x. [DOI] [PubMed] [Google Scholar]
- 35.Yadav MC, Burudi EME, Alirezaei M, et al. IFN-γ-induced IDO and WRS expression in microglia is differentially regulated by IL-4. Glia. 2007;55(13):1385–1396. doi: 10.1002/glia.20544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cumiskey D, Pickering M, O’Connor JJ. Interleukin-18 mediated inhibition of LTP in the rat dentate gyrus is attenuated in the presence of mGluR antagonists. Neurosci Lett. 2007;412(3):206–210. doi: 10.1016/j.neulet.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 37.Harris RE, Sundgren PC, Craig AD, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis & Rheumatism. 2009;60(10):3146–3152. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Morimoto Y, Zhang Q, Adachi K. Effects of Memantine, an N-Methyl-D-aspartate Receptor Antagonist, on Fatigue and Neuronal Brain Damage in a Rat Model of Combined (Physical and Mental) Fatigue. Biological and Pharmaceutical Bulletin. 2012;35(4):481–486. doi: 10.1248/bpb.35.481. [DOI] [PubMed] [Google Scholar]
- 39.Stellwagen D, Malenka RC. Synaptic scaling mediated by glial TNF-[alpha] Nature. 2006;440(7087):1054–1059. doi: 10.1038/nature04671. [DOI] [PubMed] [Google Scholar]
- 40.Yarlagadda A, Alfson E, Clayton AH. The Blood Brain Barrier and the Role of Cytokines in Neuropsychiatry. Psychiatry (Edgmont) 2009;6(11):18–22. [PMC free article] [PubMed] [Google Scholar]
- 41.Butler MP, O’Connor JJ, Moynagh PN. Dissection of tumor-necrosis factor-α inhibition of long-term potentiation (LTP) reveals a p38 mitogen-activated protein kinase-dependent mechanism which maps to early—but not late—phase LTP. Neuroscience. 2004;124(2):319–326. doi: 10.1016/j.neuroscience.2003.11.040. [DOI] [PubMed] [Google Scholar]
- 42.Santello M, Bezzi P, Volterra A. TNFα Controls Glutamatergic Gliotransmission in the Hippocampal Dentate Gyrus. Neuron. 2011;69(5):988–1001. doi: 10.1016/j.neuron.2011.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Felger JC, Miller AH. Cytokine effects on the basal ganglia and dopamine function: The subcortical source of inflammatory malaise. Frontiers in Neuroendocrinology. 2012;33(3):315–327. doi: 10.1016/j.yfrne.2012.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dantzer R. Cytokine, Sickness Behavior, and Depression. Immunology and Allergy Clinics of North America. 2009;29(2):247–264. doi: 10.1016/j.iac.2009.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Felger JC, Mun J, Kimmel HL, et al. Chronic Interferon-[alpha] Decreases Dopamine 2 Receptor Binding and Striatal Dopamine Release in Association with Anhedonia-Like Behavior in Nonhuman Primates. Neuropsychopharmacology. 2013;38(11):2179–2187. doi: 10.1038/npp.2013.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Harrison NA, Brydon L, Walker C, et al. Neural Origins of Human Sickness in Interoceptive Responses to Inflammation. Biol Psychiatry. 2009;66(5):415–422. doi: 10.1016/j.biopsych.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Vieira-de-Abreu A, Calheiros AS, Mesquita-Santos FP, et al. Cross-Talk between Macrophage Migration Inhibitory Factor and Eotaxin in Allergic Eosinophil Activation Forms Leukotriene C4–Synthesizing Lipid Bodies. American Journal of Respiratory Cell and Molecular Biology. 2011;44(4):509–516. doi: 10.1165/rcmb.2010-0004OC. [DOI] [PubMed] [Google Scholar]
- 48.Yoshihisa Y, Makino T, Matsunaga K, et al. Macrophage Migration Inhibitory Factor Is Essential for Eosinophil Recruitment in Allergen-Induced Skin Inflammation. J Invest Dermatol. 2011;131(4):925–931. doi: 10.1038/jid.2010.418. [DOI] [PubMed] [Google Scholar]
- 49.Lacy P, Stow JL. Cytokine release from innate immune cells: association with diverse membrane trafficking pathways. Blood. 2011;118(1):9–18. doi: 10.1182/blood-2010-08-265892. [DOI] [PubMed] [Google Scholar]
- 50.Schacterle RS, Conti F, Magrini L, Komaroff AL, Valesini G. Increased Eosinophil Protein X Levels in Chronic Fatigue Syndrome. Journal of Chronic Fatigue Syndrome. 2001;9(1–2):21–30. [Google Scholar]
- 51.Steel JL, Kim KH, Dew MA, et al. Cancer-Related Symptom Clusters, Eosinophils, and Survival in Hepatobiliary Cancer: An Exploratory Study. Journal of Pain and Symptom Management. 2010;39(5):859–871. doi: 10.1016/j.jpainsymman.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lotfi R, Lee JJ, Lotze MT. Eosinophilic Granulocytes and Damage-associated Molecular Pattern Molecules (DAMPs): Role in the Inflammatory Response Within Tumors. Journal of Immunotherapy. 2007;30(1):16–28. doi: 10.1097/01.cji.0000211324.53396.f6. [DOI] [PubMed] [Google Scholar]
- 53.Parkitny L, McAuley JH, Kelly PJ, Di Pietro F, Cameron B, Moseley GL. Multiplex Cytokine Concentration Measurement: How Much Do the Medium and Handling Matter? 2013:2013. doi: 10.1155/2013/890706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Hojan K, Kwiatkowska-Borowczyk E, Leporowska E, Gorecki M, Ozga-Majchrzak O, Milecki T, Milecki P. Physical exercise for functional capacity, blood immune function, fatigue and wuality of life in high-risk prostate cancer patients during radiotherapy. A prospective, randomised clinical study. European Journal of Physical and Rehabilitation Medicine. 2016 [PubMed] [Google Scholar]
- 55.Berger AM, Mooney K, Alvarez-Perez A, Breitbart WS, Carpenter KM, Cella D, Cleeland C, Dotan E, Eisenberger MA, Escalante CP, Jacobsen PB, Jankowski C, LeBlanc T, Ligibel JA, Loggers ET, Mandrell B, Murphy BA, Palesh O, Pirl WF, Plaxe SC, Riba MB, Rugo HS, Salvador C, Wagner Ll, Wagner-Johnston ND, Zachariah FJ, Bergman MA, Smith C. Cancer-related fatigue, version 2.2015. Journal of the National Comprehensive Care Network. 2015;13(8):1012–1039. doi: 10.6004/jnccn.2015.0122. [DOI] [PMC free article] [PubMed] [Google Scholar]


