Abstract
Size-independent emissions have been widely observed from ultrasmall thiolated gold nanoparticles (AuNPs) but remain a mystery in fundamental understanding of photoluminescence mechanisms of noble metals on the nanoscale. Herein, we report a correlation between emission wavelengths and local binding geometries of a thiolate ligand (glutathione) on AuNPs with identical size (~2.5 nm) but two distinct emission wavelengths. Using circular dichroism, X-ray absorption and fluorescence spectroscopies, we found that high Au-S coordination number (CN)/high surface coverage resulted in strong Au(I)-ligand charge transfer, chiral conformation and 600 nm emission while low Au-S CN/low surface coverage led to weak charge transfer, achiral conformation and 810 nm emission. By fine tuning of surface coverage, these two size-independent emissions can be integrated into one single 2.5 nm AuNP, where a ratiometric pH response was observed due to strong energy transfer between two emission centers, opening up a new path to design ultrasmall ratiomteric pH nanoindicators.
Keywords: gold, luminescence, nanoparticles, pH sensors, size-independent emission
Graphical abstract
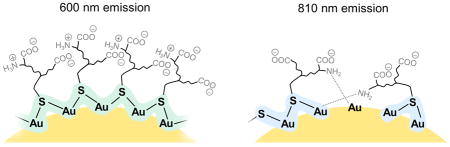
Size-independent emissions of 2.5 nm thiolated gold nanoparticles at 600 nm and 810 nm were found to originate from different surface coverages and local bonding enviroment of ligand on particle surface.
While size-dependent emission as a characteristic phenomenon of noble metals on the nanoscale has been well understood in the past decade,[1] size-independent emission is also widely observed from noble metal nanoparticles (NPs)[2] and found broad applications in cancer detection,[3] kidney functional imaging[4] and chemical sensing.[5] For instance, strong size-dependent luminescence has been observed from Au nanoclusters (NCs) encapsulated by amine-terminated dendrimers: following a free-electron model, their emission maxima were shifted from UV to IR with a size increase from Au5 to Au31.[1a] In contrast, coated with a thiolate ligand glutathione (GSH), the same sized AuNPs (2~3 nm) can exhibit different colored emissions at either 600 nm[6] or 810 nm[3a, 7]. In addition to these few-nm GSH-coated AuNPs (GS-AuNPs), different sized few-atom GSH-protected AuNCs (Au29SG27, Au30SG28, Au36SG32, Au39SG35, Au43SG37) can exhibit the same emission peak at 610 nm.[8] Wang and co-workers reported a series of thiolated AuNCs (Au11, Au38, Au140, Au201) with NIR luminescence spectra ranging from 800 to 1300 nm,[2a] similar to the NIR emission found in ~1.8 nm AuNPs[9]. Moreover, not limited to GS-AuNPs, AuNPs coated by other thiolate ligands also exhibit different colored emissions even though their sizes are comparable. For example, 6-mercapto-1-hexanol-capped AuNPs can fluoresce at different wavelengths from 510 to 600 nm while particle size changes little within 2~3 nm.[10] These results clearly indicate that particle size might not be a critical factor in determining the emission wavelengths of the ultrasmall thiolated AuNPs. However, the origin of size-independent emission remains a long-term mystery in the fundamental understanding of photoluminescence of noble metal NPs.
Herein, we revisited the structure-emission relationships of 600 nm- and 810 nm-emitting GS-AuNPs with identical sizes of 2.5 nm. Using circular dichroism (CD) spectroscopy and elemental analysis, we found a significant difference between these two types of GS-AuNPs: high surface coverage of GSH on 600 nm-emitting NPs resulted in an ordered and chiral conformation, while low surface coverage of GSH on 800 nm-emitting NPs led to little chirality. As further revealed by X-ray absorption spectroscopy, the 600 nm-emitting GS-AuNPs had a higher degree of Au-S bonding than those of the 810 nm-emitting GS-AuNPs, indicating that distinct Au local bonding environments is responsible for 600 nm and 800 nm emission (Figure 1A). Since different colored emissions were independent of AuNP size but depended on surface coverage and local bonding environment, we were able to incorporate the 600 nm and 810 nm emissions into one single AuNP by simply tuning surface coverage of GSH. Interestingly, the dual-emissive AuNPs exhibited unique ratiometric pH-dependent emissions, due to strong energy transfer between the two emission centers on one particle.
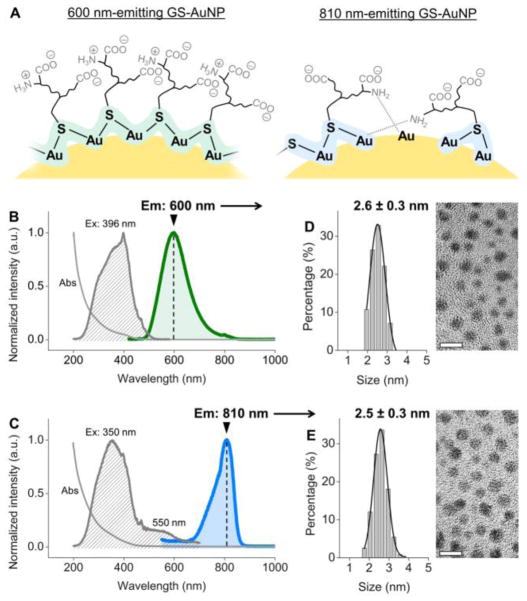
Figure 1.
(A) A scheme showing two different local Au bonding environments of glutathione-coated AuNPs (GS-AuNPs) that correspond to 600 nm and 810 nm emissions. (B, C) Absorption, excitation and emission spectra of 600 nm-emitting (B) and 810 nm-emitting (C) GS-AuNPs. (D, E) TEM images and core size distributions of 600 nm-emitting (D) and 810 nm-emitting (E) GS-AuNPs. Scale bar = 5 nm.
GS-AuNPs with 600 nm or 810 nm emission were synthesized by controlling GSH-to-HAuCl4 ratios during reduction of HAuCl4 in the presence of L-GSH at 95 °C (Supporting Information).[3a, 7a] Using a GSH-to-HAuCl4 ratio of 1.6:1, we synthesized luminescent AuNPs with a single emission peak at 600 nm (quantum yield = 9.3%, by using Nile blue A as standard, Figure 1B). Once the GSH-to-HAuCl4 ratio decreased to 0.8:1, the 600 nm emission disappeared and we obtained AuNPs with a single emission peak at 810 nm (quantum yield = 1.8%, by using Nile blue A as standard, Figure 1C). The excitation spectrum also changed: the excitation maximum of the 600 nm-emitting GS-AuNPs was located at 396 nm, while that of the 810 nm-emitting GS-AuNPs was located at 350 nm with a shoulder peak at 550 nm (Figure 1B&1C). Despite these distinct excitation and emission spectra, the 600 nm-emitting and 810 nm-emitting AuNPs were almost identical in core size (2.6 ± 0.3 nm vs. 2.5 ± 0.3 nm) (Figure 1D&1E). The absorption spectra of these two AuNPs were also very similar and showed strong absorption in the UV region, as well as a shoulder peak at ~400 nm (Figure 1B&1C). Infrared spectroscopy confirmed the two types of AuNPs had the same ligand, GSH. Characteristic peaks of GSH were observed, including νs(COO−) at ~1390 cm−1, νas(COO−) at ~1595 cm−1, and amide II at 1523 cm−1, while the S-H stretching mode observed at ~2522 cm−1 for GSH disappeared due to the formation of Au-S bonds (Figure S1).
The large Stokes shifts (>200 nm, Figure 1B&1C) and microsecond lifetimes[6a] observed from these GS-AuNPs suggested the emission is involved with the charge transfer between the Au and surface ligand (Au (I)→S)[1c] rather than Au-N interaction.[1a] Therefore, we first studied the ligand conformation using CD spectroscopy. As shown in the CD spectra (Figure 2A), the 600 nm-emitting GS-AuNPs exhibited a negative band at 354 nm and a positive band at 400 nm, suggesting a highly ordered and chiral conformation of GSH on the AuNPs, consistent with the previous reports on GSH-protected AuNCs[11] and other thiolated AuNPs.[12] Since L-GSH has weak CD response above 250 nm,[11a] the observation of such CD signals in the range of 300–500 nm implies a chiral arrangement of monomeric SR–Au–SR or dimeric SR–Au–SR–Au–SR staple motifs.[12e, 13] Like free L-GSH, the 810 nm-emitting GS-AuNPs did not show a significant peak within 300–500 nm, indicating a random arrangement of GSH on the Au surface.
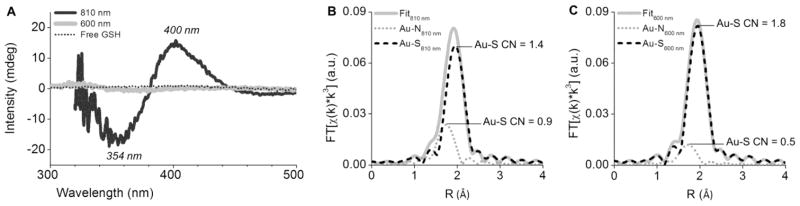
Figure 2.
(A) Circular dichroism spectra of 810 nm- and 600 nm-emitting GS-AuNPs. Data for free GSH was provided as a control. (B, C) EXAFS spectra of 810 nm-emitting (B) and 600 nm-emitting GS-AuNPs at pH 7.0.
The two types of GS-AuNPs were also dramatically different in the local Au bonding environment as revealed by extended X-ray absorption fine structure (EXAFS) spectroscopy, a technique that has been intensively used to study the Authiolate NCs.[14] For 810 nm-emitting GS-AuNPs, the average coordination number (CN) of Au-S was measured to be 1.4 (Figure 2B and Table S1). In the case of 600 nm-emitting GS-AuNPs, the Au-S CN increased to 1.8 (Figure 2C and Table S1). In contrast to the ~29% increase in Au-S CN (1.4 vs. 1.8), the Au-N CN decreased ~44% (0.9 vs. 0.5) once the emission was shifted from 800 nm to 600 nm, further suggesting two distinctly different types of local bonding environments.
These differences in ligand conformation and local bonding environment fundamentally originated from the different surface coverage of GSH on AuNPs. Consistent with a higher GSH-to-HAuCl4 ratio used for making 600 nm-emitting AuNPs, the surface coverage of GSH on the 600 nm-emitting AuNPs was nearly 2-fold of that on the 810 nm-emitting AuNPs (quantified by elementary analysis, Supporting Information). The high surface coverage of GSH on the 600 nm-emitting NPs was also correlated with the large Au-S CN of these NPs (1.8), where densely packed GSH showed a chiral conformation. On the other hand, due to relatively low surface coverage of GSH on the 810 nm-emitting AuNPs, there were no enough S atoms to stabilize the NPs; thus N atoms started interacting with Au, leading to an increase in Au-N CN, which, however, is not involved in the luminescence.[1a] As a result, the distinct surface coverage of GSH resulted in two types of emission centers: higher degree of Au-S bonding resulted in strong Au→S charger transfer and served as the “600 nm emission center”, whereas lower degree of Au-S bonding led to relatively weak Au →S charger transfer and formed the “810 nm emission center” (Figure 1A).
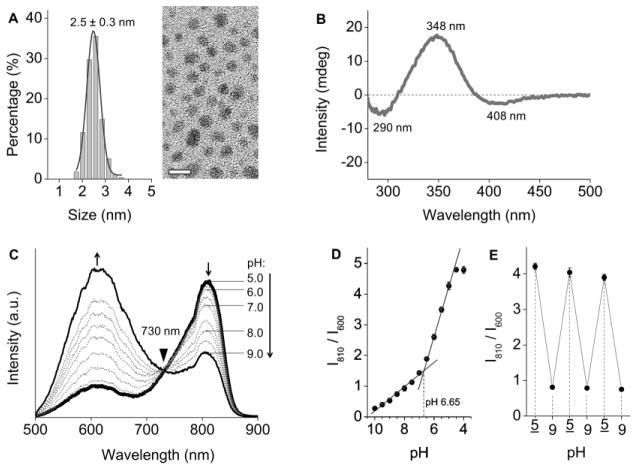
Inspired by the finding that the size-independent emissions from GS-AuNPs were governed by surface coverage, we controlled the surface coverage by tuning the GSH-to-HAuCl4 ratio and were able to synthesize AuNPs with dual emissions at 600 nm and 810 nm (Supporting Information). The dual-emissive AuNPs had core size identical to those of single-emissive GS-AuNPs (2.5 ± 0.3 nm, Figure 3A). Infrared spectra confirmed that the ligand was GSH (Figure S2). The surface coverage of GSH on the dual-emissive NPs was 55% less than that on the 600 nm-emitting GS-AuNPs but 14% higher than that on the 810 nm-emitting GS-AuNPs (Supporting Information). The CD spectrum showed a positive peak at 348 nm and two negative peaks at 290 and 408 nm (Figure 3B), implying existence of Au-S motifs but slightly different binding geometry of GSH on the AuNPs.
Figure 3.
(A) TEM image and core size distribution of the dual-emissive GS-AuNPs. Scale bar = 5 nm. (B) Circular dichroism spectrum of the dual-emissive GS-AuNPs; (C) Luminescence spectra of dual-emissive GS-AuNPs in PBS buffer at different pHs. (D) Ratiometric plots of luminescence intensities at 600 nm and 810 nm at different pHs. (E) Reversibility of the pH dependent ratiomentric emissions between pH 5.0 and pH 9.0.
Integration of the 600 nm and 810 nm emission centers on one single NP resulted in a synergistic effect: the dual-emissive GS-AuNPs can response to the pH changes in a ratiometric way. As pH was increased from 5.0 to 9.0, the 600 nm emission intensity increased 4 fold, while the 810 nm emission intensity decreased 2 fold, giving an isosbestic point at 730 nm (Figure 3C). Neither of these two single-emissive GS-AuNPs, nor a mixture of them possess this property. Single-emissive 810 nm emission was insensitive to pH changes, whereas single-emissive 600 nm emission only increased 1.8 fold when pH changed from 5.0 to 9.0 (Figures S3&S4). The two single-emissive GS-AuNPs independently responded to the pH changes in a mixture, generating 2.4-fold intensity increase at 600 nm and negligible intensity change at 810 nm (no isosbestic point was detected, Figure S5). These differences confirmed that the dual emissions showing a ratiometric pH response indeed originated from a single particle containing two coupled emission centers. Furthermore, the solution pH can be read by taking the ratio of intensities of 810 nm and 600 nm emissions (I810/I600). The value of I810/I600 changed nearly 4-fold within a physiological pH range from 7.4 to 5.0. The pH threshold for the observed pH-dependent emission was pH 6.65 (Figure 3D). The reversible pH responses suggested a high stability of GS-AuNPs in this pH range (Figure 3E). The emission spectrum of the dual-emissive GS-AuNPs was also independent of ionic strength (Figure S6).
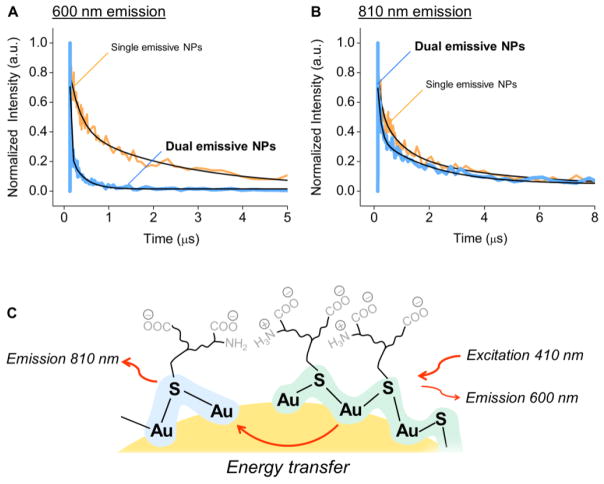
The origin of such a ratiometric response from dual-emissive GS-AuNPs was attributed to energy transfer between the 600 nm and 810 nm emission centers on the same particle. We used time-resolved fluorescence spectroscopy to study the luminescence lifetimes of the different types of GS-AuNPs at pH 7.5 (at this pH, the dual-emissive NPs had equal intensities at 600 nm and 810 nm). Under 410 nm excitation, both 600 nm-emitting and 810 nm-emitting GS-AuNPs showed average luminescence lifetimes on the microsecond scale: 1.54 μs for 600 nm emission, and 1.93 μs for 810 nm emission (Figures 4A&4B). However, in the case of dual-emissive GS-AuNPs, we detected a nearly one-order decrease in the average lifetime of 600 nm emission (0.14 μs), and a slight decrease in the average lifetime of 810 nm emission to 1.20 μs. These results unambiguously indicated a strong energy transfer from 600 nm emission center to 810 nm emission center within the same dual-emissive ultrasmall AuNPs (Figure 4C).
Figure 4.
Luminescence lifetimes of (A) 600 nm emission and (B) 810 nm emission in single-emissive GS-AuNPs (orange) and dual-emissive GS-AuNPs (blue) at pH 7.5. Excitation = 410 nm for all measurements. (C) A scheme showing energy transfer from 600 nm emission center to 810 nm emission center on one single AuNP.
The energy transfer between these two emission centers is fundamentally due to the overlap between emission spectrum of 600 nm emission center (donor) and broad excitation spectrum of 810 nm emission center (acceptor) (Figures 1B&1C). Interestingly, the energy transfer was sensitive to pH. As shown in Figure 3C, the donor emission (600 nm) was dominant at pH 9, whereas the acceptor emission (810 nm) reached the highest value at pH 5. Not only the intensities but also lifetimes of dual emissions were dependent of pH. Lowering pH from 9 to 5 resulted in a reduction of the average lifetime of 600 nm emission from 0.17 μs to 0.064 μs and an increase of average lifetime of 800 nm emission from 1.17 μs to 1.67 μs, respectively (Table S2). These results clearly indicate a significant enhancement of energy transfer efficiency when pH decreased from 9 to 5. Since energy transfer efficiency is known to depend on the distance between emission centers,[15] the observed increase in energy transfer efficiency suggests the formation of more 600 nm emission centers on the particle surface, which was confirmed by EXAFS studies on Au-S CNs of dual-emissive AuNPs. As shown in Table S3, the Au-S CNs of dual-emissive NPs increased from 1.3, 1.6 to 2.3 when pH decreased from 9, 7 to 5. On the other hand, CNs of single-emissive AuNPs have little response to pH changes (Table S4&S5). Thus, these changes on CNs of Au-S and Au-N in dual-emissive AuNPs are likely due to the protonation of amine group in the acidic environment. At pH 5, the high degree of Au-S bonding suggests formation of more 600 nm emission centers (donor), which might make energy transfer between 600 nm and 800 nm emission centers more efficient. Thus, even though 810 nm emission is pH insensitive in single-emissive GS-AuNPs,[5b] it became highly pH sensitive due to stronger coupling with more 600 nm emission centers on the same NP.
In summary, with the assistance of CD, X-ray absorption and optical spectroscopies, we unraveled the origin of size-independent emissions from ultrasmall GS-AuNPs with the similar sizes but distinct emissions. Our results showed strong correlation of emission wavelengths with surface coverage and local binding structure of GSH on AuNPs: high surface coverage resulted in high degree of Au-S bonding and strong Au(I)-S charge transfer as well as 600 nm emission, whereas low surface coverage led to low degree of Au-S bonding, relatively weak Au(I)-ligand charge transfer and 810 nm emission. The observation of different CD responses from 600 nm- and 810 nm-emitting GS-AuNPs with the same sizes suggested that CD signal is strongly dependent of surface ligand coverage. While size-independent emissions suggested that surface coverage and local bonding environment played more important roles in the emissions from thiolated AuNPs, it should be noted that gold core is important to stabilizing surface gold atoms and ligands. Moreover, based on the previous studies on long-lived NIR luminescence of Au25, d band of Au13 cores in Au25 is involved in the relaxation of excited electrons from Au-S charge transfer states to the ground states.[2b, 16] Thus, d band of Au cores in these luminescent AuNPs might also be involved in the electron relaxation rather than excitation; however, more detailed ultrafast spectroscopic studies on these luminescent AuNPs need to be done for complete understanding of their photoluminescence mechanism. Nevertheless, because the different colored emissions were independent of particle size but depended on the surface coverage, two different colored emissions can be integrated into one single AuNP by tuning surface coverage of the ligands. The obtained dual-emissive AuNPs exhibited unique pH-dependent ratiometric emissions owing to energy transfer between the two emission centers. Such synergy not only offers us an opportunity to modulate photoluminescence from AuNPs but also provides a new path to apply luminescent AuNPs as ratiometric indicators for quantitative bioimaging.
Supplementary Material
Acknowledgments
This study was supported by the NIH (1R01DK103363), CPRIT (RP140544) and the start-up fund from the University of Texas at Dallas. PND thanks the support of an NSERC CGS scholarship and PZ acknowledges the NSERC DG grant.
Contributor Information
Dr. Jinbin Liu, Department of Chemistry and Biochemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX 75080 (USA).
Paul N. Duchesne, Department of Chemistry, Dalhousie University, 6274 Coburg Rd., Halifax, NS, B3H 4J3 (Canada).
Dr. Mengxiao Yu, Department of Chemistry and Biochemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX 75080 (USA).
Xingya Jiang, Department of Chemistry and Biochemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX 75080 (USA).
Xuhui Ning, Department of Chemistry and Biochemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX 75080 (USA).
Prof. Dr. Peng Zhang, Department of Chemistry, Dalhousie University, 6274 Coburg Rd., Halifax, NS, B3H 4J3 (Canada)
Prof. Dr. Jie Zheng, Email: jiezheng@utdallas.edu, Department of Chemistry and Biochemistry, The University of Texas at Dallas, 800 W. Campbell Rd., Richardson, TX 75080 (USA). Department of Urology, The University of Texas Southwestern Medical Center, 5323 Harry Hines Blvd., Dallas, TX 75390 (USA)
References
- 1.a) Zheng J, Zhang CW, Dickson RM. Phys Rev Lett. 2004;93 doi: 10.1103/PhysRevLett.93.077402. [DOI] [PubMed] [Google Scholar]; b) Zheng J, Nicovich PR, Dickson RM. Annu Rev Phys Chem. 2007;58:409–431. doi: 10.1146/annurev.physchem.58.032806.104546. [DOI] [PMC free article] [PubMed] [Google Scholar]; c) Zheng J, Zhou C, Yu M, Liu J. Nanoscale. 2012;4:4073–4083. doi: 10.1039/c2nr31192e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.a) Wang G, Huang T, Murray RW, Menard L, Nuzzo RG. J Am Chem Soc. 2005;127:812–813. doi: 10.1021/ja0452471. [DOI] [PubMed] [Google Scholar]; b) Devadas MS, Kim J, Sinn E, Lee D, Goodson T, Ramakrishna G. J Phys Chem C. 2010;114:22417–22423. [Google Scholar]
- 3.a) Liu J, Yu M, Zhou C, Yang S, Ning X, Zheng J. J Am Chem Soc. 2013;135:4978–4981. doi: 10.1021/ja401612x. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Liu J, Yu M, Ning X, Zhou C, Yang S, Zheng J. Angew Chem Int Edit. 2013;52:12572–12576. doi: 10.1002/anie.201304465. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2013;125:12804–12808. [Google Scholar]; c) Liu J, Yu M, Zhou C, Zheng J. Mater Today. 2013;16:477–486. [Google Scholar]; d) Zhang J, Fu Y, Conroy CV, Tang Z, Li G, Zhao RY, Wang G. J Phys Chem C. 2012;116:26561–26569. doi: 10.1021/jp306036y. [DOI] [PMC free article] [PubMed] [Google Scholar]; e) Yu M, Zheng J. ACS Nano. 2015;9:6655–6674. doi: 10.1021/acsnano.5b01320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.a) Yu M, Liu J, Ning X, Zheng J. Angew Chem Int Ed. 2015;54:15434–15438. doi: 10.1002/anie.201507868. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2015;127:15654–15658. [Google Scholar]; b) Yu M, Zhou J, Du B, Ning X, Authement C, Gandee L, Kapur P, Hsieh JT, Zheng J. Angew Chem Int Ed. 2016;55:2787–2791. doi: 10.1002/anie.201511148. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2016;128:2837–2841. [Google Scholar]
- 5.a) Huang CC, Yang Z, Lee KH, Chang HT. Angew Chem Int Ed. 2007;46:6824–6828. doi: 10.1002/anie.200700803. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2007;119:6948–6952. [Google Scholar]; b) Sun S, Ning X, Zhang G, Wang YC, Peng C, Zheng J. Angew Chem Int Ed. 2016;55:2421–2424. doi: 10.1002/anie.201509515. [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew Chem. 2016;128:2467–2470. [Google Scholar]
- 6.a) Zhou C, Sun C, Yu M, Qin Y, Wang J, Kim M, Zheng J. J Phys Chem C. 2010;114:7727–7732. doi: 10.1021/jp9122584. [DOI] [PMC free article] [PubMed] [Google Scholar]; b) Yu M, Zhou C, Liu J, Hankins JD, Zheng J. J Am Chem Soc. 2011;133:11014–11017. doi: 10.1021/ja201930p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.a) Tu XJ, Chen WB, Guo XQ. Nanotechnology. 2011;22:7. [Google Scholar]; b) Zhou C, Hao GY, Thomas P, Liu JB, Yu MX, Sun SS, Oz OK, Sun XK, Zheng J. Angew Chem Int Edit. 2012;51:10118–10122. doi: 10.1002/anie.201203031. [DOI] [PubMed] [Google Scholar]; Angew Chem. 2012;124:10265–10269. [Google Scholar]
- 8.Luo Z, Yuan X, Yu Y, Zhang Q, Leong DT, Lee JY, Xie J. J Am Chem Soc. 2012;134:16662–16670. doi: 10.1021/ja306199p. [DOI] [PubMed] [Google Scholar]
- 9.a) Huang T, Murray RW. J Phys Chem B. 2001;105:12498–12502. [Google Scholar]; b) Crawford SE, Andolina CM, Smith AM, Marbella LE, Johnston KA, Straney PJ, Hartmann MJ, Millstone JE. J Am Chem Soc. 2015;137:14423–14429. doi: 10.1021/jacs.5b09408. [DOI] [PubMed] [Google Scholar]
- 10.Chen P-C, Yeh T-Y, Ou C-M, Shih C-C, Chang H-T. Nanoscale. 2013;5:4691–4695. doi: 10.1039/c3nr00713h. [DOI] [PubMed] [Google Scholar]
- 11.a) Schaaff TG, Whetten RL. J Phys Chem B. 2000;104:2630–2641. [Google Scholar]; b) Wu Z, Gayathri C, Gil RR, Jin R. J Am Chem Soc. 2009;131:6535–6542. doi: 10.1021/ja900386s. [DOI] [PubMed] [Google Scholar]
- 12.a) Yao H, Fukui T, Kimura K. J Phys Chem C. 2008;112:16281–16285. [Google Scholar]; b) Gautier C, Bürgi T. J Am Chem Soc. 2008;130:7077–7084. doi: 10.1021/ja800256r. [DOI] [PubMed] [Google Scholar]; c) Zeng C, Li T, Das A, Rosi NL, Jin R. J Am Chem Soc. 2013;135:10011–10013. doi: 10.1021/ja404058q. [DOI] [PubMed] [Google Scholar]; d) Dolamic I, Varnholt B, Burgi T. Nat Commun. 2015;6:7117. doi: 10.1038/ncomms8117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.a) Qian H, Eckenhoff WT, Zhu Y, Pintauer T, Jin R. J Am Chem Soc. 2010;132:8280–8281. doi: 10.1021/ja103592z. [DOI] [PubMed] [Google Scholar]; b) Jadzinsky PD, Calero G, Ackerson CJ, Bushnell DA, Kornberg RD. Science. 2007;318:430–433. doi: 10.1126/science.1148624. [DOI] [PubMed] [Google Scholar]
- 14.Zhang P. J Phys Chem C. 2014;118:25291–25299. [Google Scholar]
- 15.Lakowicz JR. Principles of Fluorescence Spectroscopy, Third Edition. Springer; New York: 2006. p. 13. [Google Scholar]
- 16.Zhu M, Aikens CM, Hollander FJ, Schatz GC, Jin R. J Am Chem Soc. 2008;130:5883–5885. doi: 10.1021/ja801173r. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.