Abstract
Congenital cytomegalovirus (CMV) infection is the most common infectious cause of disability in newborn infants. CMV also causes serious disease in solid organ (SOT) and haematopoietic stem cell transplant (HSCT) recipients. In otherwise healthy children and adults, primary CMV infection rarely causes illness. However, even asymptomatic CMV infections may predispose an individual towards an increased risk of atherosclerosis, cancer and immune senescence over the life course, although such associations remain controversial. Thus, although a vaccine against congenital CMV infection would have the greatest public health impact and cost-effectiveness, arguably all populations could benefit from an effective immunisation against this virus. Currently there are no licensed CMV vaccines, but there is increased interest in developing and testing potential candidates, driven by the demonstration that a recombinant CMV glycoprotein B (gB) vaccine has some efficacy in prevention of infection in young women and adolescents, and in CMV-seronegative SOT recipients. In this review, the recent and current status of candidate CMV vaccines is discussed. Evolving concepts about proposed correlates of protective immunity in different target populations for CMV vaccination, and how these differences impact current clinical trials, are also reviewed.
Keywords: CMV, cytomegalovirus, vaccine, pentameric complex, congenital infection, placenta
Introduction
In this article, the candidate vaccines against CMV infection are described, with an emphasis on recent and current clinical trials. Although the biggest impact from a financial and public health perspective for a CMV vaccine would be the prevention of disabling congenital infection, there are many challenges that make the demonstration of efficacy for this indication a daunting goal. For this reason, the first approved CMV vaccine, which is anticipated to be in the near future – may well end up being licensed for prevention or amelioration of disease in SOT or HSCT patients. Whether the correlates of protective immunity induced by a vaccine for this population would be relevant to the problem of prevention of congenital infection is a provocative question that remains unresolved at this time, but which requires careful scrutiny as trials of various vaccine platforms move forwards in the clinic.
Epidemiology of CMV infection and target populations for vaccination
In a landmark review by Weller published in 1971, CMV was described as a ‘ubiquitous agent with protean clinical manifestations’ [1,2]. Although most primary infections are asymptomatic in otherwise healthy adults, CMV infection can cause a variety of clinical syndromes in both immune competent and immune compromised individuals. CMV, in addition to Epstein-Barr virus, is well recognised to be a cause of mononucleosis in young adults [3]. CMV is a particularly important cause of morbidity and mortality in SOT and HSCT patients, causing viraemia with attendant end-organ diseases such as hepatitis and pneumonitis [4–6]. In addition to these direct effects, CMV is associated with serious indirect effects in transplant patients, such as graft rejection, graft failure, and in HSCT patients, graft-versus-host disease [7,8]. Antiviral therapies, although invaluable in the management of CMV disease in these populations, have limitations, including drug toxicities and the emergence of resistance. Thus, a CMV vaccine administered to the SOT or HSCT recipient prior to transplantation could prove to be of great value in improving transplant outcomes. CMV also produces serious morbidity, including sight-threatening retinitis, in patients with advanced HIV infection [9], although how a CMV vaccine might be of benefit in preventing disease in the HIV-infected population is not immediately intuitive.
In addition to the prospects of a vaccine improving the outcome of CMV in immune compromised patients, an argument can be made for the benefits of universal immunisation of all individuals over the course of life. The rationale for this stems from an emerging body of evidence suggesting that CMV may play a role in the pathogenesis of inflammatory and autoimmune diseases, and of malignancies, in particular glioblastoma multiforme [10]. CMV serostatus may also impact the clinical course of burns, trauma and sepsis [11–13]. Additionally, CMV seropositivity has been associated in some studies with a decreased response to influenza vaccination, suggesting that protection against CMV could provide secondary benefits with respect to an improved response vaccine, with an inferred (but unproven) reduction in risk of acquisition of influenza [14–16]. This phenomenon may be true only in older individuals, since a study of CMV-seropositive young adults demonstrated enhanced antibody responses to influenza vaccination, along with increased CD8+ T cell sensitivity and higher levels of interferon-γ, compared to seronegative individuals [17]. CMV has been proposed to be a co-factor in the pathogenesis of vascular disease, including atherosclerotic coronary artery disease [18-20]. Finally, CMV seropositive status has emerged as a risk factor for all-cause mortality in large population-based cohorts in the United States and Europe [21,22]. Given that the overall seroprevalence of CMV is higher in African-Americans than Caucasians in the United States [23], CMV seropositivity emerges as a contributing factor in the observed socioeconomic disparities identified in all-cause mortality between these two groups [24].
Both from a public health and financial cost perspective, prevention of congenital transmission of CMV is the greatest driving force behind development of a CMV vaccine. CMV transmission to the fetus occurs in between 0.5% and 0.7% of pregnancies in the United States and other developed nations [25]. Estimates are less precise in the developing world, although recent evidence suggests even higher transmission rates – in the range of 4% or greater [26]. Approximately 13.5% of congenitally infected newborns are symptomatic [27]. In the developed world, congenital CMV is the most common infectious cause of brain damage and sensorineural hearing loss, and is an occasional cause of mortality [28]. Infants born to seronegative pregnant women acutely infected in pregnancy are at particularly high risk for infection in the setting of maternal exposure to CMV, with up to ~32% of primary maternal infections leading to congenital CMV transmission in this setting [25,29]. Several lines of evidence suggest that a pre-conception vaccine could modify the risk of congenital infection and its attendant sequelae. At least two studies have compared CMV transmission to the fetus from seronegative and seropositive women, in an attempt to quantify the protective benefit of preconception natural immunity. In one study, protection attributable to seropositive status was 60%, and in another, 91% [30,31]. In addition to protecting against transmission to the fetus, preconception immunity decreases the severity of disease if transmission occurs. It has been reported that 25% of congenitally infected infants born to women with primary CMV infections during pregnancy had at least one sequela, compared with only 8% in infants born to mothers with recurrent infection [32]. The risk for development of adverse long-term neurodevelopmental outcomes appears to be highest in those infants born to mothers with first-trimester maternal CMV infections. In this setting, approximately 20–25% of infants who are congenitally infected will develop sensorineural hearing loss, and up to 35% will have other sequelae involving the central nervous system [33]. Thus, a CMV vaccine that recapitulated the protective features of natural immunity could decrease both the rate and severity of congenital CMV infections if administered to CMV-seronegative women.
The long-term costs to healthcare systems associated with symptomatic congenital CMV infection are substantial. This is not surprising in light of the extensive disability often associated with these infections. Complications include sensorineural hearing loss, cerebral palsy, mental retardation, developmental delay, learning disability and seizure disorders [34], and the most severely affected children may require long-term residential care. The economic impact of congenital CMV was assessed by the Institute of Medicine (IOM) in the 1990s. It was estimated that the costs of medical and educational care for children with congenital infection in the United States amounted to approximately $1.9 billion per year [35–37]. The IOM accordingly ranked the development of a CMV vaccine as having the single highest priority, from a cost-savings perspective, of any potential new vaccine. The IOM model proposed a vaccine that would be universally administered to 12-year-old boys and girls, with the goal of conferring protective immunity prior to entry into child-bearing years. Subsequent work suggested that such an adolescent vaccine would only need to be modestly effective, in the range of ~60%, to provide cost savings to society [38].
A major and unresolved issue complicating the analyses of vaccination strategies against congenital CMV infection is what has been referred to as ‘the paradox of infection’ in infants born to women with immunity prior to pregnancy [39]. Although the risk of congenital CMV transmission in a woman with preconception immunity is lower than in a woman with primary infection during pregnancy, overall as many as three-quarters of congenital CMV infections occur following non-primary maternal infections, because of the high background rates of CMV seroimmunity among women of childbearing age in most populations [29]. It remains incompletely defined whether these infections represent reactivation of latent infection, or re-infection with novel strains of CMV: there is evidence to support both mechanisms [40–42]. Irrespective of the mechanism of re-infection, congenital infections can produce substantial long-term disabilities, including sensorineural hearing loss. Thus, to most effectively prevent congenital CMV infection, a vaccine will be required that has the ability not only to both protect seronegative women from primary infection, but also the capacity to augment the immune response in seropositive individuals in order to prevent reactivation or re-infection.
CMV vaccines currently in clinical trials
Recently completed or currently active clinical trials of candidate CMV vaccines are summarised in Table 1. These include adjuvanted recombinant protein vaccines based on the immunodominant envelope glycoprotein, CMV glycoprotein B (gB); vaccines expressing immunogenic viral gene products (including gB, plus the T cell targets, ppUL83 [pp65] and/or the major immediate early protein 1 [IE1]) using DNA plasmid or peptide-based technologies; vectored vaccine approaches based on the expression of gB and other CMV antigens using live virus and virus-like particle (VLP) systems; and replication-impaired or replication-defective CMV (attenuated vaccines or disabled single-cycle [DISC] vaccines). These individual categories are summarised below.
Table 1. CMV vaccines currently or recently in clinical trials
| Vaccine category | Phase | Vaccine | Antigens used | Adjuvant | Parameters evaluated | Manufacturer | Subjects | Serostatus | Age | Identifier |
|---|---|---|---|---|---|---|---|---|---|---|
| DNA (plasmid) vaccines | 1 | ASP0113 | pp65, gB | CRL1005-BAK | Part 1: pharmacokinetics; Part 2: immunogenicity | Astellas, Vical | Healthy in Part 1, Healthy or dialysis recipients in Part 2 | −/+ if healthy, – if dialysis | 18–70 | NCT02103426 |
| 2 | ASP0113 | pp65, gB | CRL1005-BAK | Viraemia, safety | Astellas | Allogeneic HTC recipients | N/A | 20+ | NCT01903928 | |
| 2 | ASP0113 | pp65, gB | CRL1005-BAK | Viraemia | Astellas, Vical | Seronegative recipient of seropositive kidney | Negative | 18+ | NCT01974206 | |
| 2 | VCL-CB01 | pp65, gB | CRL1005-BAK | Viraemia, T cells | Astellas, Vical | HCT donors/recipients | Positive (HCT recipient) | 18–65 | NCT00285259 | |
| 3 | ASP0113 | pp65, gB | CRL1005-BAK | Viraemia, CMV end-organ disease; overall mortality | Astellas, Vical | Recipients of allogeneic HCT | Positive | 18+ | NCT01877655 | |
| Vectored vaccines | 1 | AVX601 | gB, pp65, IE1 | None | Antibodies, T cells | AlphaVax, Inc (Novartis, GSK) | Healthy | Negative | 18–45 | NCT00439803 |
| 1 | HCMV-MVA Triplex | pp65, UL123/IE1-exon4, UL122/IE2-exon5 | None | Optimal dosage (2-dose), immune response, safety | City of Hope, National Cancer Institute | Healthy | Positive and negative | 18–60 | NCT01941056 | |
| 1 | HB-101 | gB, pp65 | None | Safety, optimal dosage, ELISA antibody, neutralising antibody, T cell, and IFN-γ ELISPOT | Hookipa Biotech | Healthy | Negative | 18–45 | NCT02798692 | |
| 2 | ALVAC-pp65 | pp65 | None | Immunogenicity | NHLBI | SCT donor/recipient | Positive and negative | 18–80 | NCT00353977 | |
| 2 | HCMV-MVA Triplex | pp65, UL123/IE1-exon4, UL122/IE2-exon5 | HCMV reactivation, adverse effects | City of Hope, National Cancer Institute | HCT recipients | Positive | 18–75 | NCT02506933 | ||
| Attenuated and DISC vaccines | 1 | V160-001 | Merck Aluminum Phosphate Adjuvant or none | Antibodies, adverse effects | Merck | Healthy | Positive and negative | 18+ | NCT01986010 | |
| 1 | Towne-Toledo Chimera Vaccines | General safety | CMV Research Foundation, International AIDS Vaccine Initiative | Healthy males with no children <18 yoa in sexual relationship with seropositive individual | Negative | 30–50 | NCT01195571 | |||
| 1 | VCL-CT02, plus Towne CMV | gB, pp65, IE1 | Antibodies, T cells, IFN-γ ELISPOT | UC-SF, Vical | CMV-specific immune response post-Towne vaccine challenge (3000 pfu) in volunteers who received VCL CT02 vaccine in a 3-dose regimen (days 0, 28, 56) administered either ID 100 μg/dose) or IM (1 mg/dose) 9–15 months prior | Negative | 18–45 | NCT00370006 | ||
| 1 | VCL-CT02, plus Towne CMV | gB, pp65, IE1 | Antibodies, T cells, safety | UC-SF, Vical | CMV-specific immune response post-Towne challenge (3000 pfu) in volunteers who received VCL CT02 vaccine (dose of 1 mg weekly × 3 doses) 3 months previously compared to unvaccinated controls | Negative | 18–45 | NCT00373412 | ||
| Recombinant/subunit vaccines | 1 | GSK1492903A | gB | Proprietary | Antibodies, adverse effects | GSK | Healthy males | Negative | 18–40 | NCT00435396 |
| 1 | GSK1492903A | gB | Proprietary | Antibodies | GSK | Healthy male recipients of 3 vaccine doses in NCT00435396 | Negative to be vaccinated, positive as control | 18–45 | NCT01357915 | |
| 2 | gB/MF59 | gB | MF59 | HCMV infection | NIAID | Healthy, female | Negative | 12–17 | NCT00133497 | |
| 2 | gB/MF59 | gB | MF59 | Immunogenicity | NIAID | Healthy, female, vaccinated with gB/MF59 in NCT00133497 study | Negative | 12–17 | NCT00815165 | |
| 2 | gB/MF59 | gB | MF59 | Rate of HCMV infection, antibodies | Robert Pass, NIAID, Sanofi Pasteur | Healthy, postpartum women | Negative | 14–40 | NCT00125502 | |
| 2 | gB subunit | gB | MF59 | Safety, immunogenicity, viral load | University College, London, NIAID | Awaiting kidney/liver transplant | Positive and negative | 18+ | NCT00299260 | |
| 2 | gB subunit | gB | MF59 | Antibodies, viraemia | University College, London | Recipient of vaccine/placebo in NCT00299260 | Positive and negative | 18+ | NCT01883206 | |
| Recombinant/VLP vaccines | 1 | VBI-1501A | gB | ±Alum | Antibody binding titers and avidity measurement; neutralizing antibody | VBI Vaccines and Canadian Center for Vaccinology | Healthy male and female adults | Negative | 18+ | NCT02826798 |
| Peptide vaccines | 1 | CMVpp65-A*0201 peptide; containing either helper T lymphocyte (HTL) PADRE peptide or tetanus toxoid peptide | pp65; T cell epitope fused to either PADRE or CMV tetanus epitope | None | Dose escalation; safety, antibody/T cell response | City of Hope, NCI | Healthy, HLA-A*0201-positive | Positive and negative | 18–65 | NCT00712634 |
| 1 | CMVpp65-A*0201 peptide; containing either helper T lymphocyte (HTL) PADRE peptide or tetanus toxoid peptide | pp65; T cell epitope fused to either PADRE or CMV tetanus epitope | ±CpG 7909 adjuvant | T cells, correct dosage | City of Hope, National Cancer Institute | Healthy, HLA A*0201 positive or positive tetramer-binding using HCMV peptide 495-503 | Positive or negative | 18–55 | NCT00722839 | |
| 1 | Tetanus-HCMVpp65 fusion peptide (CMVpp65-A*0201; CMVPepVax) | pp65; T cell epitope fused to tetanus epitope | PF03512676 (TLR9 agonist) | Safety, GVHD, T cells, PD-1 expression | City of Hope, National Cancer Institute | HLA A*0201 subtype HCT recipients | Positive | 18–75 |
NCT01588015
PMID: 26853648 |
|
| 2 | Tetanus-HCMVpp65 fusion peptide (CMVpp65-A*0201; CMVPepVax) | pp65; T cell fused to tetanus epitope | PF03512676 (TLR9 agonist | Viraemia, GVHD, adverse CMV-related effects post-transplant | City of Hope, National Cancer Institute | Planned HCT recipients, HLA A*0201 | Positive | 18–75 | NCT02396134 |
HTC: haematopoietic cell transplant; GVHD: graft-versus-host disease.
Recombinant gB vaccines
Subunit approaches utilising adjuvanted recombinant formulations of gB have arguably advanced the furthest in clinical trials of CMV vaccines performed to date. These trials have been driven by the observations that antibodies to gB are invariantly present in CMV-seropositive individuals, and are capable of viral neutralisation [43,44]. Several Phase I and Phase II clinical trials using a recombinant CMV gB in microfluidised adjuvant 59 (MF59), a proprietary oil-in-water emulsion from Novartis first used in influenza vaccines, have been completed, [45–50]. Most studies have employed a three-dose series of vaccine. The gB construct has been based on sequence derived from the Towne strain of CMV, modified such that the transmembrane domain and the furin cleavage site have been removed. The carboxy-terminal cytoplasmic component downstream of the transmembrane domain was re-engineered as an in-frame fusion with the truncated gB ORF [51]. As such, the vaccine is expressed as a truncated, secreted polypeptide, and the protein is purified by chromatography from tissue culture supernatants in Chinese hamster ovary (CHO) cells.
The gB/MF59 vaccine has been evaluated in a Phase II study in postpartum women. This study found the gB/MF59 vaccine to have 50% efficacy against primary CMV infection in seronegative women vaccinated within 1 year of giving birth compared to women in the same cohort who received the placebo [47]. Women who enrolled in this study but were found to be CMV-immune were also vaccinated with either the gB/MF59 vaccine or a placebo, in a parallel study aiming to evaluate whether vaccination could augment the antibody response in women who were seropositive [48]. Using ELISA data and neutralising antibody titres, gB specific responses were shown to be boosted in vaccinated seropositive women compared to controls, and antibody titres remained higher in seropositive vaccine recipients at 6 months after the final vaccine dose than at day 0 of the vaccination series. The CD4+ T cell response to gB and levels of IFN-γ producing T cells were also both higher for vaccinees at the majority of time points, including 6 months after the final vaccination. Since the majority of congenital CMV infections occur in the setting of preconception immunity to the virus, these data could have important implications for how a vaccine against congenital infection could be implemented into clinical practice – if the observed augmentation in antibody and cell-mediated immune responses correlated with improved protection of the fetus.
Another clinical trial targeting young women was recently reported in healthy, CMV-seronegative adolescents. The incidence of CMV infection after three vaccinations was reduced in the vaccine group compared to placebo, though this difference was not significant (P=0.2) [49]. CMV viraemia was detected via PCR of urine and blood samples and if viraemia was detected, the subject was placed into a substudy ( http://www.clinicaltrials.gov NCT00815165). This substudy retained blinding and is examining CMV-specific cell mediated responses in greater detail. Data from this substudy are still under analysis and are not currently available.
Towards the goal of testing the gB/MF59 vaccine in a transplant setting, a Phase II double-blinded study ( http://www.clinicaltrials.gov NCT00299260) examined immunogenicity and CMV disease, as assessed by viraemia (DNAemia), in kidney or liver transplant patients [50]. Seronegative organ recipients who received gB/MF59 and had seropositive organ donors demonstrated reduction in viraemia and days of ganciclovir treatment compared to those who received placebo. Additionally, duration of viraemia post-transplantation was inversely correlated to the magnitude of the gB antibody response. The study authors hypothesised that the immune response to gB/MF59 vaccination blocked the ability of the donated organ to transmit CMV to the new host. The mechanism was not defined, but was speculated to be related to vaccine-engendered antibody-dependent cellular cytotoxicity [50].
Another gB subunit vaccine, designated as GSK1492903A, is a recombinant gB vaccine adjuvanted in a proprietary system developed by GlaxoSmithKline (GSK) laboratories ( http://www.clinicaltrials.gov NCT00435396). The gB subunit sequence used in the making of this vaccine was based on the AD169 CMV strain. As with the Sanofi gB construct, the furin cleavage site and transmembrane domains were deleted, and the terminal sequences of the extracellular domains were fused to the cytoplasmic tail. In addition, the carboxy-terminal 394 amino acids of the herpes simplex virus type 1 (HSV-1) glycoprotein D (gD) were fused to the AD169 derived gB sequence to facilitate secretion [52]. In a Phase I study, there were no serious adverse effects noted in any vaccinee. Although these results are not yet published, the data are publicly available ( www.gsk-clinicalstudyregister.com/study/108890#rs, and www.gsk-clinicalstudyregister.com/study/115429#rs).
DNA vaccines for CMV
ASP0113 (previously known as VCL-CB01 and TransVax) is a DNA vaccine against CMV that was developed by Vical Corporation and is currently under license to Astellas for Phase III clinical trials and commercialisation. ASP0113 is a bivalent CMV DNA vaccine consisting of two plasmids, VCL-6368 and VCL-6365 formulated with poloxamer CRL1005 and a cationic surfactant, benzalkonium chloride in PBS [53,54]. VCL-6368 encodes the pp65 protein from AD169 with the putative protein kinase domain removed by deletion of amino acids 435–438. VCL-6365 encodes the extracellular domain (amino acids 1–713) of CMV gB derived from the AD169 sequence. Formulation of the two plasmids with CRL1005 and benzalkonium chloride produces a thermodynamically stable, self-assembled nanoparticle system with a defined particle size, surface charge and stability profile. A Phase I clinical trial evaluating the safety of ASP0113 found no serious adverse events in the 22 CMV seropositive and 22 seronegative individuals immunised [54]. The most common complications included pain and tenderness at the injection site, induration, erythema, malaise and myalgia. Vaccination of seronegative subjects elicited pp65 and/or gB specific T cell responses, as well as gB antibody responses, whereas seropositive vaccinated groups showed increases only in pp65-specific T cell responses.
Subsequent evaluation of ASP0113 efficacy has been completed in a double-blind, placebo-controlled, parallel group, Phase II trial in seropositive patients following HSCT ( http://www.clinicaltrials.gov NCT00285259) [55]. There were no significant differences in adverse events comparing vaccine and placebo, and CMV viraemia (DNAemia) was significantly reduced following vaccination with ASP0113. There was also a non-significant reduction in the rate of initiation of antiviral therapy between the vaccinated and placebo-treated groups (47.5% vs 61.8%). A global, Phase III clinical trial was recently initiated to continue the evaluation of ASP0113 efficacy in HSCT patients ( http://www.clinicaltrials.gov NCT01877655). Similar studies to evaluate the safety and efficacy of this DNA vaccine in solid organ transplant patients (Phase II, http://www.clinicaltrials.gov NCT01974206) and dialysis patients (Phase I, http://www.clinicaltrials.gov NCT02103426) are ongoing.
A non-adjuvanted, trivalent DNA vaccine (VCL-CT02), which includes the T cell target IE1 in addition to the gB and pp65 coding sequences, has also been evaluated in Phase I clinical trials ( http://www.clinicaltrials.gov NCT00370006 and NCT00373412) [56]. These studies were in CMV-seronegative subjects vaccinated intramuscularly or intradermally with the DNA vaccine, followed by administration of Towne vaccine (described below), to examine for immune priming by the DNA vaccine. Vical has proposed further development of the trivalent DNA vaccine as a platform for immunisation against congenital CMV infection, but the current state of this vaccine in clinical development is uncertain. Vical has also recently published results from preclinical evaluation of gB and pp65 plasmids delivered in combination with a different adjuvant system, the cationic lipid-based adjuvant Vaxfectin, which has been observed to increase the immunogenicity of antigens delivered as plasmid DNA [57,58].
Peptide vaccines
Pilot trials suggesting pp65-specific cytotoxic T lymphocyte (CTL) responses can protect HSCT patients from post-transplant CMV disease prompted the development of vaccines focusing on delivery of pp65 epitopes as peptide vaccines [107]. The CTL epitope HLA A*0201 pp65495–503 was identified as a promising peptide sequence due to its limited sequence variation among analysed viral isolates. HLA A*0201 pp65495–503 was fused to either a synthetic pan-DR epitope (PADRE) or to a natural tetanus (Tet) sequence, both of which are known to be universal T helper epitopes. In a Phase I trial evaluating these systems ( http://clinicaltrials.gov NCT00722839), healthy participants were vaccinated with escalating doses of PADRE or Tet pp65495–503 vaccines with and without CpG 7909 adjuvant. CpG 7909, also known as PF03512676, is an immunomodulating synthetic oligonucleotide designed to be a TLR9 antagonist [59,60]. It acts through the TLR9 receptor in B cells and plasmacytoid dendritic cells to stimulate a variety of host immune responses. These include human B-cell proliferation and antigen-specific antibody production, along with IFN-α production, IL-10 secretion, and NK cell activity. The combination of this adjuvant with the PADRE and Tet pp65495–503 vaccines increased the stimulation of vaccine responses in human subjects [60]. It has been estimated that the HLA A*2010 pp65495–503 epitope will cover 30–40% of the at-risk population based on the frequency of the HLA A*2010 allele in the population [60].
This vaccine construct was also studied in seropositive patients undergoing HSCT who were at risk for CMV reactivation post-transplant ( http://clinicaltrials.gov NCT01588015; [61]). This open-label, Phase Ib trial was primarily focused on safety. The trial showed no adverse effects on HSCT, no acute graft-versus-host disease, no development of anti-dsDNA antibodies and no unexpected adverse effects. Additionally, 54 grade 3–4 adverse events were reported in vaccinees, as compared to 91 adverse effects in patients who did not receive the vaccination and were simply under observation. Interestingly, although no virological data was reported and the study was not powered to examine CMV-related disease outcomes, it was noteworthy that, compared with observation, there was better relapse-free overall survival recorded in patients that received the vaccine when compared to those in the observation group [61]. Based on these encouraging preliminary data, Phase II studies of this Tet-pp65 vaccine, designated as CMVpp65-A*0201 or CMVPepVax, are now in progress ( http://clinicaltrials.gov NCT02396134), with enrolment targeting HLA-A*0201-positive, CMV-seropositive HSCT recipients at the City of Hope (Duarte, California) and the University of Minnesota. Study endpoints will include CMV-related events such as viraemia, initiation of anti-CMV antivirals, and CMV end-organ disease, and other HSCT-related events such as disease-free mortality, graft-versus-host disease, and overall time to engraftment.
Enveloped virus-like particle vaccines
Enveloped virus-like particles (eVLPs) are protein structures that mimic wild-type viruses but do not have a viral genome, creating, in principle, safer vaccine candidates. An eVLP CMV vaccine, manufactured by VBI laboratories, is currently in Phase I studies in CMV seronegative subjects. The technology is based on co-transfection of the vaccine immunogen of interest (in this case, CMV gB) with the Moloney murine leukaemia virus (MLV) gag protein in human embryonic kidney (HEK) cells. Expression of MLV gag promotes genesis of eVLPs: the gag protein is cleaved by cellular proteases to yield the viral matrix, capsid and nucleocapsid proteins. Capsid proteins spontaneously assemble into VLPs which then acquire a lipid envelope as they are released from the cell. Inclusion of gB allows this protein to be expressed in the eVLP, with an authentic glycosylation profile derived from post-translational processing in HEK cells. In preclinical studies, eVLPs using two gB variants were examined in mice: a gB-based VLP, with an expression construct encoding the extracellular portion, transmembrane domain (TM), and cytoplasmic portion of gB (906 amino acids); and gB-G, a truncated sequence of gB encoding the extracellular portion only (amino acids 1–752) fused with the TM and cytoplasmic domains of vesicular stomatitis virus (VSV) G protein [62]. Both vaccines were found to induce neutralising antibody titres 10-fold higher than titres induced with the same dose of soluble recombinant gB protein in BALB/c mice, with titre levels comparable to those observed with immunoglobulin (Cytogam) treatment. Notably, however, the gB-G VLP was more immunogenic, which was proposed to be due to the gB-G assuming a ‘post-fusion’ conformation in transfected cells. Improved neutralisation levels were observed when the neutralising capacity of vaccine-induced antibody was evaluated in both fibroblasts and in epithelial cells.
A Phase I study of this vaccine, VBI-1501A, was initiated in early 2016 and is currently enrolling ( https://clinicaltrials.gov/ct2/show/NCT02826798). This study will compare safety and immunogenicity of four dose formulations of vaccine, ranging from 0.5 μg to 2 μg gB content with aluminum phosphate (alum), 1.0 μg gB content without alum, and placebo in approximately 125 healthy CMV-seronegative volunteer participants between 18 and 40 years of age. An additional eVLP CMV vaccine candidate has been described by VBI vaccines, targeting both gB and pp65. This vaccine consists of a Gag plasmid fused in-frame with pp65 along with co-transfected CMV gB plasmid. This vaccine has been proposed for use as a therapeutic vaccine, to be administered in combination with GM-CSF, for CMV-associated glioblastoma multiforme [63]. A pre-IND meeting is anticipated for 2016.
Another candidate eVLP vaccine against CMV was developed by Redvax GmbH, a spin-off from Redbiotec AG, a privately held Swiss biopharmaceutical company. In contrast to the VBI approach, which uses mammalian (HEK) cells, this technology is based on a baculovirus expression system. Although initially the purification of these eVLPs was difficult due to their relatively large size, modifications were introduced into the production system that allowed for easier clarification and concentration of these particles [64]. The Redvax technology has recently been purchased by Pfizer vaccines, which has stated its commitment to developing a congenital CMV vaccine programme.
Vectored vaccines
Another category of CMV vaccine that has reached Phase II clinical trials is based on the approach of expressing viral antigens via a viral vector. Typically such vectors are capable of infecting human cells and expressing one or more viral proteins without establishing a productive infection. Two vaccines have been developed and tested that have utilised an attenuated canary pox vector to deliver either gB (ALVAC-CMV[vCP139]) or pp65 (ALVAC-CMV[vCP260]) [65,66]. The gB-expressing ALVAC vaccine failed to increase neutralising titres among seropositive recipients and did not induce significant neutralising titres in seronegative subjects as compared to baseline titre levels. Therefore, it was studied as a part of a ‘prime-boost’ strategy in which priming with the ALVAC vaccine was followed by subsequent doses of either Towne vaccine (described below) or gB/MF59 subunit vaccine [65,67]. The ALVAC-gB vaccine primed for an improved response to subsequent Towne vaccination. Subjects primed with ALVAC-gB developed neutralising titres and ELISA antibodies to gB sooner upon Towne boosting than did controls immunised with a recombinant ALVAC-rabies glycoprotein control. In contrast, no benefit of priming with ALVAC-gB was noted with respect to subsequent responses to purified, adjuvanted gB subunit vaccine. In addition to the ALVAC-gB vaccine, the pp65-expressing ALVAC (vCP260) has progressed to Phase I clinical trials. Results indicate that the vaccine has a favourable safety profile and is capable of eliciting robust pp65-specific CTL and antibody responses in healthy, CMV-seronegative adults [66]. A follow-up Phase II clinical trial in HSCT patients ( http://www.clinicaltrials.gov NCT00353977) was completed in 2008.
An alphavirus-based vectored vaccine platform has undergone clinical trial evaluation. This vaccine, originally developed by AlphaVax, was designated as AVX601. It was derived from Venezuelan equine encephalitis (VEE) virus. In this vaccine, the VEE structural proteins were replaced with genes expressing the extracellular domain of Towne gB and a pp65-IE1 fusion protein in a double-promoter replicon [68,69]. Seronegative volunteers in a Phase I clinical trial received either three low or high doses of the vaccine over a 24-week period. The vaccine was well tolerated, with only mild local responses following administration, and participants developed CTLs and neutralising antibody responses to all three CMV antigens following vaccination [70]. This platform was acquired by Novartis Corporation in 2008, and is now held by GSK following their purchase of the Novartis vaccine portfolio. Plans for Phase II studies are unknown at this time.
Another vectored vaccine approach that has recently entered Phase I study utilises an attenuated recombinant lymphocytic choriomeningitis virus (LCMV) platform [71]. This vector utilises producer cells that constitutively express the LCMV viral glycoprotein (GP), making it possible to replace the gene encoding LCMV GP with vaccine antigens of interest. These rLCMV vaccines are replication-defective, and elicit not only robust CTL and CD4+ T cell responses, but also high-magnitude neutralising antibody responses. These vectors also do not appear to elicit vector-specific antibody immunity, which would permit re-administration of vector for booster vaccinations, a feature that might be highly desirable for protection of a woman during her reproductive years. Moreover, LCMV has a very low seroprevalence in humans, another useful attribute of using this platform. Hookipa Biotech AG has developed a replication-deficient LCMV-vectored CMV vaccine, designated HB-101, which is a bivalent vaccine containing two vectors, one expressing the CMV pp65 protein and one expressing gB. A Phase I dose escalation study has started to enroll three successive cohorts of 18 healthy volunteers; each cohort will receive either low, middle or high doses of the vaccine (n=14 volunteers), or placebo (n=4; https://clinicaltrials.gov NCT02798692), using a three-dose series of vaccine at 0, 1 and 4 months by intramuscular route. The primary study endpoint is safety, and secondary endpoints will include ELISA and virus-neutralisation titres; IFN-γ ELISPOT specific for gB and pp65; and intracellular cytokine staining assays.
Several other vectored vaccines have been explored in preclinical studies. Recent studies have investigated the immunogenicity of modified vaccinia virus Ankara (MVA) vaccines that express a variety of CMV antigens, including pp65, gB, and the pentameric complex (PC) of proteins gH/gL/UL128/130/131, in rodent or nonhuman primate model systems [72–77]. Of particular note, a recent study demonstrated that when mice or macaques were vaccinated with an MVA vector expressing all five of the protein components of the PC, neutralising antibody responses were engendered that were capable of blocking CMV infection of Hofbauer macrophages, a fetal-derived cell localised within the placenta [78]. This result may have particular relevance to a CMV vaccine designed to elicit immune responses capable of blocking transplacental viral transmission to the developing fetus.
Attenuated and ‘DISC’ vaccines
The earliest attempts to develop a vaccine against CMV infection used live, attenuated viruses. Initial studies focused on the highly tissue culture-passaged attenuated CMV strains AD169 and Towne. A detailed account of the attenuating mutations that accumulated in these strains during the process of tissue culture passage is beyond the scope of this article, but is the subject of several excellent reviews [79–81]. It is of particular interest that both strains of CMV have mutations in open reading frames (ORFs) that disrupt expression of the PC. The Towne strain contains a two base-pair insertion (TT) in UL130 leading to a frameshift mutation in this ORF, and the AD169 strain has a one base-pair insertion (A) similarly leading to a frameshift mutation in UL131. Mutations in the PC components of these passaged isolates are likely to contribute to their attenuation, but also probably impair immunogenicity in the vaccinated host, particularly with respect to the ability to engender antibody responses that could block infectivity of epithelial and endothelial cells, important targets of infection in vivo.
AD169 was the first CMV vaccine studied in humans, when lysate from sonicated AD169-infected cells was administered subcutaneously to healthy students and staff volunteers at St George's Hospital Medical School in London, UK in doses ranging from 100 to 300,000 plaque-forming units (pfu) [82]. While a 96% conversion rate was achieved in the 10,000 pfu group, only half of the volunteers had detectable CMV antibody responses when they were evaluated 8 years post-vaccination [83,84] – an interesting observation in light of similar concerns about duration of immunity that have been raised with respect to the MF59/gB vaccine study reported in 2009 [47].
Immune responses after vaccination with the Towne strain of CMV are well documented in healthy, seronegative adults [31,85]. Towne vaccination also provided some protection against severe CMV disease in transplant recipients, although it did not protect against CMV infection post-transplantation and generated a limited neutralising antibody response, as measured via ELISA, when compared to that of wild-type infection [86]. Towne's diminished ability to induce protective immunity has been attributed both to its attenuation and lower neutralising antibody titres compared to wild-type infection [31].
In an effort to find a vaccine with the safety profile of Towne but without the virus's limiting attenuations, efforts were undertaken to generate ‘chimeric’ viruses containing components of the highly attenuated Towne strain and the less attenuated clinical CMV isolate, Toledo [87]. These Towne/Toledo chimeric viruses containing various genome combinations of both viruses were generated by co-transfection of overlapping cosmid libraries. These vaccines were initially evaluated in a Phase I trial in CMV-seropositive subjects [88]. This approach was intended to identify Towne/Toledo recombinants that were attenuated relative to Toledo but were less attenuated (and therefore more immunogenic and protective) than Towne vaccine. All vaccines contained the Toledo-derived region of the genome referred to as the ULb’ region, a region that rapidly undergoes deletions and mutations when CMV is passaged serially in fibroblasts [79–81]. In the Phase I study, transaminase levels and leukocyte counts, along with CMV culture results post-vaccination, were compared among the Towne/Toledo recombinant vaccine recipients and against historical controls from virus challenge studies in which subjects were experimentally infected with the Toledo strain [88]. This side-by-side analysis suggested that the Towne/Toledo chimeric vaccines were attenuated relative to the parental Toledo strain. These chimeras were next evaluated in CMV-seronegative recipients in a dose-range study (101–103 pfu/dose) for safety and immunogenicity ( http://www.clinicaltrials.gov NCT01195571). No subject had CMV in urine or saliva. Eleven of 36 CMV seronegative men enrolled in the study underwent seroconversion. Seroconversion was more commonly observed with vaccine chimeras 2 and 4. All 11 seroconverters developed low but detectable levels of neutralising antibody, and some subjects demonstrated CD8+ T responses to IE1. All of the chimeras contained a disrupted UL128 sequence derived from the Toledo strain and therefore, like Towne and AD169, are presumed to be incapable of eliciting PC-specific antibody responses [89]. Future work should concentrate on evaluating chimeras 2 and 4 in follow-up clinical trials, and on elucidating the molecular basis for the different immune responses induced by these chimeric vaccines.
In light of concerns about the safety profile of live, attenuated CMV vaccines, generation of transgenic disabled infectious single cycle (DISC) vaccine strains represents an attractive alternative. These viruses are propagated in specific cell types or under certain growth conditions that allow for viral replication [90-92]. In vivo, DISC viruses are replication incompetent and express a limited subset, if any, of the viral proteome. One such virus, V160, is currently undergoing Phase I clinical trials in both seronegative and seropositive subjects in which restoration of expression of UL131 was achieved by bacterial artificial chromosome (BAC) recombineering [90]. The frame-shift mutation in the first exon of UL131 underlying the epithelial tropism deficiency in AD169 was repaired in E. coli by deletion of an adenine in the 7 nucleotide A-stretch in UL131 to rescue the epithelial and endothelial cell tropism and thus allow proper processing and expression of the pentameric gH complex. This clone was further modified by removing the BAC segment by Cre/lox recombination. The modified BAC DNA was transfected into human retinal pigmented epithelial (ARPE-19) cells, to recover infectious virus. The virus has been further modified so that the essential viral proteins IE1/IE2 and UL51 are expressed as individual fusion proteins with an unstable variant of the FKBP12 protein. FKBP12 is a rapamycin-binding protein within the family of FK506, or tacrolimus, binding proteins, called FKPBs [91]. UL51 is essential for viral cleavage–genome packaging, while IE1/IE2 are necessary during both acute infection and reactivation from viral latency [93,94]. V160 is able to propagate in retinal pigment epithelial cells in the presence of a synthetic stabilising ligand, Shield-1. In the absence of this ligand, the fusion protein is rapidly degraded and viral replication is inhibited [93]. Other conditional replication-deficient CMV (rdCMV)/DISC vaccine strains contain different FKBPs fused to the essential CMV proteins UL52, UL79, and UL84. These fusion proteins regulate the transcription of viral gene products, viral DNA synthesis, and processing and packaging of viral genomes [95,96]. Since the Shield-1 ligand does not exist in nature, the V160 DISC virus should be unable, in principle, to revert to replication competence, ensuring an excellent safety profile for this vaccine.
Perspective
In recent years there has been a surge in interest on the part of the pharmaceutical industry in developing a CMV vaccine. This development is gratifying for clinicians who have recognised this unmet need for decades [97], and has been driven both by the success (albeit suboptimal) of the recombinant gB vaccine [47] as well as by an increase in overall knowledge and awareness of the problem of congenital CMV.
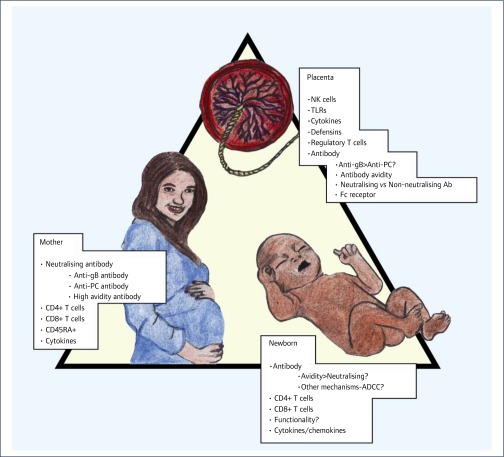
In spite of these advances, many challenges remain. A major unmet need is the lack of knowledge of a correlate of protective immunity for the developing fetus. Indeed, at an even more basic level, clarity is required in addressing the issue of what component of the maternal–placental–fetal triad we are targeting with a CMV vaccine (Figure 1). The immunological correlates of protection in the context of congenital CMV infection are the subject of a recent review [98]. Will a CMV vaccine be successful by limiting maternal DNAemia, thereby limiting haematogenous seeding of the placenta and (therefore) preventing fetal infection? In this setting, a role for neutralising antibody as a correlate of protection against congenital transmission has been proposed, although a recent placebo-controlled trial of CMV-Ig, perhaps surprisingly, failed to demonstrate a benefit against transplacental transmission [99]. CMV-Ig was not shown to reduce the maternal or placental viral load compared to placebo and it did not have an effect on virus-specific T-cell responses [99]. These recent results stand in contrast to a non-randomised study in 2005 that showed that CMV-Ig significantly reduced both the rate of mother-to-fetus transmission and the risk of congenital disease in cases of primary maternal infections [100]. The nature of the antibody response – in particular, whether a woman has high-titre antibody targeting the PC proteins – may emerge as a key predictor of transmission [101], an observation which in turn would be highly informative to vaccine design. A currently active NIH-funded clinical trial of passive antibody therapy, under the leadership of Brenna Anderson at Duke University, will study the role of CMV-Ig for prevention of fetal CMV infection, and may resolve this question ( http://clinicaltrials.gov NCT01376778). Other correlates of protection may emerge as key effectors of CMV control in the context of the maternal immune response. These include maternal CD4+ T cells, which were recently shown to be critical in protection against congenital CMV in a rhesus macaque model [102], and also appear to play a role in protection against transmission in women [103,104]. Indeed, delayed CD4+ and/or CD8+ responses and decreased functionality of T cells (diminished or modified cytokine production, functional exhaustion), as well as decreased percentages of CD45RA+ effector memory T cells, all seem to be important factors in potentiating the risk of congenital CMV transmission [98,103,105–107].
Figure 1.
Schematic representation of the potential targets of a vaccine against congenital CMV infection. Clarity is required regarding the issue of whether a vaccine primarily needs to target the maternal, placental, or fetal compartment. Congenital infection, including attendant sequelae, occurs even in the face of high-titre neutralising antibody in the neonate. The paradox of re-infection with subsequent transmission [39] also needs to be resolved. If sterilising immunity can be achieved in the mother, placental and fetal transmission become moot points. Engendering protective immunity in a vaccine is likely to require robust maternal antibody and T-cell responses, particularly CD4+ responses
Once CMV has reached the placental–fetal interface, immunological correlates of protection may be different from those in the maternal compartment. The placenta is relatively void of T-lymphocytes [98]. Innate effectors of immunity, particularly NK cells, predominate. Antibody is probably still critical in protection, but it is unclear if neutralising antibody that targets the PC [78] or neutralising antibody that targets gB [108] is of greater importance at the level of the trophoblast. Non-neutralising antibody may paradoxically facilitate the translocation of CMV across the neonatal Fc receptor, expressed on placental trophoblasts, actually promoting congenital transmission [109]. In a study of transplacentally acquired antibodies in infants with and without neurological sequelae, higher levels of both anti-gB and neutralising antibody were observed in those infants with sequelae, and no association between neutralisation and hearing loss was noted [110]. These observations must be kept in mind when contemplating CMV vaccine strategies focused on neutralising antibody as a presumed correlate of protection. The presence of high-avidity antibody may be more important than neutralising antibody in protection against fetal transmission [111–113]. Novel strategies that go beyond the current vaccines in clinical trials are needed. Non-neutralising functions of IgG, such as antibody-mediated cellular cytotoxicity, need to be examined in more depth. Examination of the B-cell repertoire in immune individuals may, through analytic vaccinology techniques, provide insights into novel, heretofore unrecognised subunit vaccine candidates [114]. Finally, the study endpoints that would be required for a clinical trial prior to licensure of a vaccine against congenital CMV need to be considered. Although a recent consensus statement suggested that prevention of congenital infection would be an appropriate endpoint for such a trial [115], it should be remembered that most congenital CMV infections produce no sequelae or disability. Therefore, reduction in fetal viral load may be an alternative (and appropriate) endpoint to consider in evaluation of CMV vaccines, both in preclinical ‘proof-of-concept’ models and in clinical trials in women.
Acknowledgements
Grant support from NIH awards HD044864, HD079918, and AI114013 is acknowledged. Support from the Minnesota Vikings CHIP Award, ‘If You Don't Pass, Screen’ is acknowledged. Illustrations provided by Emily Eck designs ( www.emilyeckdesigns.com).
References
- 1. Weller TH. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. I. N Engl J Med 1971; 285: 203– 214. [DOI] [PubMed] [Google Scholar]
- 2. Weller TH. The cytomegaloviruses: ubiquitous agents with protean clinical manifestations. II. N Engl J Med 1971; 285: 267– 274. [DOI] [PubMed] [Google Scholar]
- 3. Cohen JI, Corey GR.. Cytomegalovirus infection in the normal host. Medicine (Baltimore) 1985; 64: 100– 114. [DOI] [PubMed] [Google Scholar]
- 4. Ramanan P, Razonable RR.. Cytomegalovirus infections in solid organ transplantation: a review. Infect Chemother 2013; 45 : 260– 271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. McIntosh M, Hauschild B, Miller V.. Human cytomegalovirus and transplantation: drug development and regulatory issues. J Virus Erad 2016; 2: 143– 148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Reddehase MJ. Mutual interference between cytomegalovirus and reconstitution of protective immunity after hematopoietic cell transplantation. Front Immunol 2016; 7: 294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rubin RH. The indirect effects of cytomegalovirus infection on the outcome of organ transplantation. JAMA 1989; 261: 3607– 3609. [PubMed] [Google Scholar]
- 8. Griffiths PD, Mahungu T.. Why CMV is a candidate for elimination and then eradication. J Virus Erad 2016; 2: 131– 135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Effros RB. The silent war of CMV in aging and HIV infection. Mech Ageing Dev 2016; 158: 46– 52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Söderberg-Nauclér C. Does cytomegalovirus play a causative role in the development of various inflammatory diseases and cancer? J Intern Med 2006; 259: 219– 246. [DOI] [PubMed] [Google Scholar]
- 11. Söderberg-Nauclér C. Treatment of cytomegalovirus infections beyond acute disease to improve human health. Expert Rev Anti Infect Ther 2014; 12 : 211– 222. [DOI] [PubMed] [Google Scholar]
- 12. Limaye AP, Kirby KA, Rubenfeld GD et al. Cytomegalovirus reactivation in critically ill immunocompetent patients. JAMA 2008; 300: 413– 422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Osawa R, Singh N.. Cytomegalovirus infection in critically ill patients: a systematic review. Crit Care 2009; 13: R68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Derhovanessian E, Maier AB, Hähnel K et al. Latent infection with cytomegalovirus is associated with poor memory CD4 responses to influenza A core proteins in the elderly. J Immunol 2014; 193: 3624– 3631. [DOI] [PubMed] [Google Scholar]
- 15. Frasca D, Diaz A, Romero M et al. Cytomegalovirus (CMV) seropositivity decreases B cell responses to the influenza vaccine. Vaccine 2015; 33: 1433– 1439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Frasca D, Blomberg BB.. Aging, cytomegalovirus (CMV) and influenza vaccine responses. Hum Vaccin Immunother 2016; 12: 682– 690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Furman D, Jojic V, Sharma S et al. Cytomegalovirus infection enhances the immune response to influenza. Sci Transl Med 2015; 7: 281ra43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Roberts ET, Haan MN, Dowd JB, Aiello AE.. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol 2010; 172: 363– 371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ji YN, An L, Zhan P, Chen XH.. Cytomegalovirus infection and coronary heart disease risk: a meta-analysis. Mol Biol Rep 2012; 39: 6537– 6546. [DOI] [PubMed] [Google Scholar]
- 20. Nikitskaya E, Lebedeva A, Ivanova O et al. Cytomegalovirus-productive infection is associated with acute coronary syndrome. J Am Heart Assoc 2016; 5: e003759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Simanek AM, Dowd JB, Pawelec G et al. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS One 2011; 6: e16103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gkrania-Klotsas E, Langenberg C, Sharp SJ et al. Seropositivity and higher immunoglobulin G antibody levels against cytomegalovirus are associated with mortality in the population-based European prospective investigation of Cancer-Norfolk cohort. Clin Infect Dis 2013; 56: 1421– 1427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Bate SL, Dollard SC, Cannon MJ.. Cytomegalovirus seroprevalence in the United States: the national health and nutrition examination surveys, 1988–2004. Clin Infect Dis 2010; 50: 1439– 1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Feinstein L, Douglas CE, Stebbins RC et al. Does cytomegalovirus infection contribute to socioeconomic disparities in all-cause mortality? Mech Ageing Dev 2016; 158: 53– 61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kenneson A, Cannon MJ.. Review and meta-analysis of the epidemiology of congenital cytomegalovirus (CMV) infection. Rev Med Virol 2007; 17: 253– 276. [DOI] [PubMed] [Google Scholar]
- 26. Yamamoto AY, Mussi-Pinhata MM, Boppana SB et al. Human cytomegalovirus reinfection is associated with intrauterine transmission in a highly cytomegalovirus-immune maternal population. Am J Obstet Gynecol 2010; 202: 297 e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Dollard SC, Grosse SD, Ross DS.. New estimates of the prevalence of neurological and sensory sequelae and mortality associated with congenital cytomegalovirus infection. Rev Med Virol 2007; 17: 355– 363. [DOI] [PubMed] [Google Scholar]
- 28. Bristow BN, O’Keefe KA, Shafir SC, Sorvillo FJ.. Congenital cytomegalovirus mortality in the United States, 1990–2006. PLoS Negl Trop Dis 2011; 5: e1140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Wang C, Zhang X, Bialek S, Cannon MJ.. Attribution of congenital cytomegalovirus infection to primary versus non-primary maternal infection. Clin Infect Dis 2011; 52: e11– 3. [DOI] [PubMed] [Google Scholar]
- 30. Fowler KB, Stagno S, Pass RF.. Maternal immunity and prevention of congenital cytomegalovirus infection. JAMA 2003; 289: 1008– 1011. [DOI] [PubMed] [Google Scholar]
- 31. Adler SP, Starr SE, Plotkin SA et al. Immunity induced by primary human cytomegalovirus infection protects against secondary infection among women of childbearing age. J Infect Dis 1995; 171: 26– 32. [DOI] [PubMed] [Google Scholar]
- 32. Fowler KB, Stagno S, Pass RF et al. The outcome of congenital cytomegalovirus infection in relation to maternal antibody status. N Engl J Med 1992; 326: 663– 667. [DOI] [PubMed] [Google Scholar]
- 33. Pass RF, Fowler KB, Boppana SB et al. Congenital cytomegalovirus infection following first trimester maternal infection: symptoms at birth and outcome. J Clin Virol 2006; 35: 216– 220. [DOI] [PubMed] [Google Scholar]
- 34. Swanson EC, Schleiss MR.. Congenital cytomegalovirus infection: new prospects for prevention and therapy. Pediatr Clin North Am 2013; 60: 335– 349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Stratton KR, Durch J, Lawrence RS, eds. Vaccines for the 21st century: a tool for decision making. Washington, DC: National Academy Press; 2000. [PubMed] [Google Scholar]
- 36. Manicklal S, Emery VC, Lazzarotto T et al. The “silent” global burden of congenital cytomegalovirus. Clin Microbiol Rev 2013; 26: 86– 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Arvin AM, Fast P, Myers M, Plotkin S, Rabinovich R; National Vaccine Advisory Committee. Vaccine development to prevent cytomegalovirus disease: report from the National Vaccine Advisory Committee. Clin Infect Dis 2004; 39: 233– 239. [DOI] [PubMed] [Google Scholar]
- 38. Dempsey AF, Pangborn HM, Prosser LA.. Cost-effectiveness of routine vaccination of adolescent females against cytomegalovirus. Vaccine 2012; 30: 4060– 4066. [DOI] [PubMed] [Google Scholar]
- 39. Britt W. Controversies in the natural history of congenital human cytomegalovirus infection: the paradox of infection and disease in offspring of women with immunity prior to pregnancy. Med Microbiol Immunol 2015; 204: 263– 271. [DOI] [PubMed] [Google Scholar]
- 40. Boppana SB, Rivera LB, Fowler KB et al. Intrauterine transmission of cytomegalovirus to infants of women with preconceptional immunity. N Engl J Med 2001; 344: 1366– 1371. [DOI] [PubMed] [Google Scholar]
- 41. Ross SA, Arora N, Novak Z et al. Cytomegalovirus reinfections in healthy seroimmune women. J Infect Dis 2010; 201: 386– 389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Nagamori T, Koyano S, Inoue N et al. Single cytomegalovirus strain associated with fetal loss and then congenital infection of a subsequent child born to the same mother. J Clin Virol 2010; 49: 134– 136. [DOI] [PubMed] [Google Scholar]
- 43. Britt WJ, Vugler L, Butfiloski EJ, Stephens EB.. Cell surface expression of human cytomegalovirus (HCMV) gp55-116 (gB): use of HCMV-recombinant vaccinia virus-infected cells in analysis of the human neutralizing antibody response. J Virol 1990; 64 : 1079– 1085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Marshall GS, Rabalais GP, Stout GG, Waldeyer SL.. Antibodies to recombinant-derived glycoprotein B after natural human cytomegalovirus infection correlate with neutralizing activity. J Infect Dis 1992; 165: 381– 384. [DOI] [PubMed] [Google Scholar]
- 45. Pass RF, Duliegè AM, Boppana S et al. A subunit cytomegalovirus vaccine based on recombinant envelope glycoprotein B and a new adjuvant. J Infect Dis 1999; 180: 970– 975. [DOI] [PubMed] [Google Scholar]
- 46. Mitchell DK, Holmes SJ, Burke RL et al. Immunogenicity of a recombinant human cytomegalovirus gB vaccine in seronegative toddlers. Pediatr Infect Dis J 2002; 21: 133– 138. [DOI] [PubMed] [Google Scholar]
- 47. Pass RF, Zhang C, Evans A et al. Vaccine prevention of maternal cytomegalovirus infection. N Engl J Med 2009; 360: 1191– 1199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Sabbaj S, Pass RF, Goepfert PA, Pichon S.. Glycoprotein B vaccine is capable of boosting both antibody and CD4 T-cell responses to cytomegalovirus in chronically infected women. J Infect Dis 2011; 203: 1534– 1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Bernstein DI, Munoz FM, Callahan ST et al. Safety and efficacy of a cytomegalovirus glycoprotein B (gB) vaccine in adolescent girls: A randomized clinical trial. Vaccine 2016; 34: 313– 319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Griffiths PD, Stanton A, McCarrell E et al. Cytomegalovirus glycoprotein-B vaccine with MF59 adjuvant in transplant recipients: a phase 2 randomised placebo-controlled trial. Lancet 2011; 377: 1256– 1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Spaete RR. A recombinant subunit vaccine approach to HCMV vaccine development. Transplant Proc 1991; 23( 3 Suppl 3): 90– 96. [PubMed] [Google Scholar]
- 52. Baudoux GJMFP, Blais N, Marchand M.. 2012; http://www.google.co.ug/patents/WO2012049317A2?cl=tr
- 53. Selinsky C, Luke C, Wloch M et al. A DNA-based vaccine for the prevention of human cytomegalovirus-associated diseases. Hum Vaccin 2005; 1: 16– 23. [DOI] [PubMed] [Google Scholar]
- 54. Wloch MK, Smith LR, Boutsaboualoy S et al. Safety and immunogenicity of a bivalent cytomegalovirus DNA vaccine in healthy adult subjects. J Infect Dis 2008; 197: 1634– 1642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Kharfan-Dabaja MA, Boeckh M, Wilck MB et al. A novel therapeutic cytomegalovirus DNA vaccine in allogeneic haemopoietic stem-cell transplantation: a randomised, double-blind, placebo-controlled, phase 2 trial. Lancet Infect Dis 2012; 12: 290– 299. [DOI] [PubMed] [Google Scholar]
- 56. Jacobson MA, Adler SP, Sinclair E et al. A CMV DNA vaccine primes for memory immune responses to live-attenuated CMV (Towne strain). Vaccine 2009; 27: 1540– 1548. [DOI] [PubMed] [Google Scholar]
- 57. Sullivan SM, Doukas J, Hartikka J et al. Vaxfectin: a versatile adjuvant for plasmid DNA- and protein-based vaccines. Expert Opin Drug Deliv 2010; 7: 1433– 1446. [DOI] [PubMed] [Google Scholar]
- 58. McVoy MA, Lee R, Saccoccio FM et al. A cytomegalovirus DNA vaccine induces antibodies that block viral entry into fibroblasts and epithelial cells. Vaccine 2015; 33: 7328– 7336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. CpG 7909: PF 3512676, PF-3512676. Drugs R D 2006; 7: 312– 316. [DOI] [PubMed] [Google Scholar]
- 60. La Rosa C, Longmate J, Lacey SF et al. Clinical evaluation of safety and immunogenicity of PADRE-cytomegalovirus (CMV) and tetanus-CMV fusion peptide vaccines with or without PF03512676 adjuvant. J Infect Dis 2012; 205: 1294– 1304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Nakamura R, La Rosa CL, Longmate J et al. Viraemia, immunogenicity, and survival outcomes of cytomegalovirus chimeric epitope vaccine supplemented with PF03512676 (CMVPepVax) in allogeneic haemopoietic stem-cell transplantation: randomised phase 1b trial. Lancet Haematol 2016; 3: e87– e98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kirchmeier M, Fluckiger AC, Soare C et al. Enveloped virus-like particle expression of human cytomegalovirus glycoprotein B antigen induces antibodies with potent and broad neutralizing activity. Clin Vaccine Immunol 2014; 21: 174– 180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Soare C, Ahmed T, Fluckiger A et al. CMV gB/pp65 eVLPs formulated with GM-CSF as a therapeutic vaccine against GBM. Abstracts of the Keystone Cancer Vaccine Symposium; 2016. [Google Scholar]
- 64. Vicente T, Burri S, Wellnitz S et al. Fully aseptic single-use cross flow filtration system for clarification and concentration of cytomegalovirus-like particles. Eng Life Sci 2014; 14: 318– 326. [Google Scholar]
- 65. Adler SP, Plotkin SA, Gonczol E et al. A canarypox vector expressing cytomegalovirus (CMV) glycoprotein B primes for antibody responses to a live attenuated CMV vaccine (Towne). J Infect Dis 1999; 180: 843– 846. [DOI] [PubMed] [Google Scholar]
- 66. Berencsi K, Gyulai Z, Gönczöl E et al. A canarypox vector-expressing cytomegalovirus (CMV) phosphoprotein 65 induces long-lasting cytotoxic T cell responses in human CMV-seronegative subjects. J Infect Dis 2001; 183: 1171– 1179. [DOI] [PubMed] [Google Scholar]
- 67. Bernstein DI, Schleiss MR, Berencsi K et al. Effect of previous or simultaneous immunization with canarypox expressing cytomegalovirus (CMV) glycoprotein B (gB) on response to subunit gB vaccine plus MF59 in healthy CMV-seronegative adults. J Infect Dis 2002; 185: 686– 690. [DOI] [PubMed] [Google Scholar]
- 68. Reap EA, Morris J, Dryga SA et al. Development and preclinical evaluation of an alphavirus replicon particle vaccine for cytomegalovirus. Vaccine 2007; 25: 7441– 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Reap EA, Dryga SA, Morris J et al. Cellular and humoral immune responses to alphavirus replicon vaccines expressing cytomegalovirus pp65, IE1, and gB proteins. Clin Vaccine Immunol 2007; 14: 748– 755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Bernstein DI, Reap EA, Katen K et al. Randomized, double-blind, Phase 1 trial of an alphavirus replicon vaccine for cytomegalovirus in CMV seronegative adult volunteers. Vaccine 2009; 28: 484– 93. [DOI] [PubMed] [Google Scholar]
- 71. Flatz L, Hegazy AN, Bergthaler A et al. Development of replication-defective lymphocytic choriomeningitis virus vectors for the induction of potent CD8+ T cell immunity. Nat Med 2010; 16: 339– 345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang D, Shenk T.. Human cytomegalovirus UL131 open reading frame is required for epithelial cell tropism. J Virol 2005; 79: 10330– 10338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Hahn G, Revello MG, Patrone M et al. Human cytomegalovirus UL131-128 genes are indispensable for virus growth in endothelial cells and virus transfer to leukocytes. J Virol 2004; 78: 10023– 10033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Ryckman BJ, Rainish BL, Chase MC et al. Characterization of the human cytomegalovirus gH/gL/UL128-131 complex that mediates entry into epithelial and endothelial cells. J Virol 2008; 82: 60– 70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Wussow F, Yue Y, Martinez J et al. A vaccine based on the rhesus cytomegalovirus UL128 complex induces broadly neutralizing antibodies in rhesus macaques. J Virol 2013; 87: 1322– 1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Gillis PA, Hernandez-Alvarado N, Gnanandarajah JS et al. Development of a novel, guinea pig-specific IFN-γ ELISPOT assay and characterization of guinea pig cytomegalovirus GP83-specific cellular immune responses following immunization with a modified vaccinia virus Ankara (MVA)-vectored GP83 vaccine. Vaccine 2014; 32: 3963– 3970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Wussow F, Chiuppesi F, Martinez J et al. Human cytomegalovirus vaccine based on the envelope gH/gL pentamer complex. PLoS Pathog 2014; 10: e1004524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Chiuppesi F, Wussow F, Johnson E et al. Vaccine-derived neutralizing antibodies to the human cytomegalovirus gH/gL pentamer potently block primary cytotrophoblast infection. J Virol 2015; 89: 11884– 11898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Prichard MN, Penfold ME, Duke GM et al. A review of genetic differences between limited and extensively passaged human cytomegalovirus strains. Rev Med Virol 2001; 11: 191– 200. [DOI] [PubMed] [Google Scholar]
- 80. Murphy E, Yu D, Grimwood J et al. Coding potential of laboratory and clinical strains of human cytomegalovirus. Proc Natl Acad Sci USA 2003; 100: 14976– 14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Murphy E, Shenk T.. Human cytomegalovirus genome. Curr Top Microbiol Immunol 2008; 325: 1– 19. [DOI] [PubMed] [Google Scholar]
- 82. Heineman TC. Human cytomegalovirus vaccines In: Arvin A C-FG, Mocarski E, et al., editor. Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. 2007; Cambridge: Cambridge University Press. [PubMed] [Google Scholar]
- 83. Stern H. Live cytomegalovirus vaccination of healthy volunteers: eight-year follow-up studies. Birth Defects Orig Artic Ser 1984; 20: 263– 269. [PubMed] [Google Scholar]
- 84. Neff BJ, Weibel RE, Buynak EB et al. Clinical and laboratory studies of live cytomegalovirus vaccine Ad-169. Proc Soc Exp Biol Med 1979; 160: 32– 37. [DOI] [PubMed] [Google Scholar]
- 85. Starr SE, Glazer JP, Friedman HM et al. Specific cellular and humoral immunity after immunization with live Towne strain cytomegalovirus vaccine. J Infect Dis 1981; 143: 585– 589. [DOI] [PubMed] [Google Scholar]
- 86. Plotkin SA, Higgins R, Kurtz JB et al. Multicenter trial of Towne strain attenuated virus vaccine in seronegative renal transplant recipients. Transplantation 1994; 58: 1176– 8. [PubMed] [Google Scholar]
- 87. Cha TA, Tom E, Kemble GW et al. Human cytomegalovirus clinical isolates carry at least 19 genes not found in laboratory strains. J Virol 1996; 70: 78– 83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Heineman TC, Schleiss M, Bernstein DI et al. A phase 1 study of 4 live, recombinant human cytomegalovirus Towne/Toledo chimeric vaccines. J Infect Dis 2006; 193: 1350– 1360. [DOI] [PubMed] [Google Scholar]
- 89. Adler SP, Manganello AM, Lee R et al. A phase 1 study of four live, recombinant human cytomegalovirus Towne/Toledo chimera vaccines in CMV seronegative men. J Infect Dis 2016; in press, PMID: 27521362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fu TM, An Z, Wang D.. Progress on pursuit of human cytomegalovirus vaccines for prevention of congenital infection and disease. Vaccine 2014; 32: 2525– 2533. [DOI] [PubMed] [Google Scholar]
- 91. Banaszynski LA, Chen LC, Maynard-Smith LA et al. A rapid, reversible, and tunable method to regulate protein function in living cells using synthetic small molecules. Cell 2006; 126: 995– 1004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Glass M, Busche A, Wagner K et al. Conditional and reversible disruption of essential herpesvirus proteins. Nat Methods 2009; 6: 577– 9. [DOI] [PubMed] [Google Scholar]
- 93. Borst EM, Kleine-Albers J, Gabaev I et al. The human cytomegalovirus UL51 protein is essential for viral genome cleavage-packaging and interacts with the terminase subunits pUL56 and pUL89. J Virol 2013; 87: 1720– 1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Paulus C, Nevels M.. The human cytomegalovirus major immediate-early proteins as antagonists of intrinsic and innate antiviral host responses. Viruses 2009; 1: 760– 779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Borst EM, Wagner K, Binz A et al. The essential human cytomegalovirus gene UL52 is required for cleavage-packaging of the viral genome. J Virol 2008; 82: 2065– 2078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Colletti KS, Xu Y, Yamboliev I, Pari GS.. Human cytomegalovirus UL84 is a phosphoprotein that exhibits UTPase activity and is a putative member of the DExD/H box family of proteins. J Biol Chem 2005; 280: 11955– 11960. [DOI] [PubMed] [Google Scholar]
- 97. Yow MD, Demmler GJ.. Congenital cytomegalovirus disease–20 years is long enough. N Engl J Med 1992; 326: 702– 703. [DOI] [PubMed] [Google Scholar]
- 98. Schleiss MR. Cytomegalovirus in the neonate: immune correlates of infection and protection. Clin Dev Immunol 2013; 2013: 501801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Revello MG, Lazzarotto T, Guerra B et al. A randomized trial of hyperimmune globulin to prevent congenital cytomegalovirus. N Engl J Med 2014; 370: 1316– 26. [DOI] [PubMed] [Google Scholar]
- 100. Nigro G, Adler SP, La Torre R, Best AM; for the Congenital Cytomegalovirus Collaborating Group. Passive immunization during pregnancy for congenital cytomegalovirus infection. N Engl J Med 2005; 353: 1350– 1362. [DOI] [PubMed] [Google Scholar]
- 101. Lilleri D, Kabanova A, Revello MG et al. Fetal human cytomegalovirus transmission correlates with delayed maternal antibodies to gH/gL/pUL128-130-131 complex during primary infection. PLoS One 2013; 8: e59863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Bialas KM, Tanaka T, Tran D et al. Maternal CD4+ T cells protect against severe congenital cytomegalovirus disease in a novel nonhuman primate model of placental cytomegalovirus transmission. Proc Natl Acad Sci USA 2015; 112: 13645– 13650. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Lilleri D, Fornara C, Furione M et al. Development of human cytomegalovirus-specific T cell immunity during primary infection of pregnant women and its correlation with virus transmission to the fetus. J Infect Dis 2007; 195: 1062– 1070. [DOI] [PubMed] [Google Scholar]
- 104. Fornara C, Furione M, Arossa A et al. Comparative magnitude and kinetics of human cytomegalovirus-specific CD4⁺ and CD8⁺ T-cell responses in pregnant women with primary versus remote infection and in transmitting versus non-transmitting mothers: Its utility for dating primary infection in pregnancy. J Med Virol 2016; 88: 1238– 1246. [DOI] [PubMed] [Google Scholar]
- 105. Revello MG, Lilleri D, Zavattoni M et al. Lymphoproliferative response in primary human cytomegalovirus (HCMV) infection is delayed in HCMV transmitter mothers. J Infect Dis 2006; 193: 269– 276. [DOI] [PubMed] [Google Scholar]
- 106. Fornara C, Lilleri D, Revello MG et al. Kinetics of effector functions and phenotype of virus-specific and γδ T lymphocytes in primary human cytomegalovirus infection during pregnancy. J Clin Immunol 2011; 31: 1054– 1064. [DOI] [PubMed] [Google Scholar]
- 107. Eldar-Yedidia Y, Bar-Meir M, Hillel M et al. Low interferon relative-response to cytomegalovirus is associated with low likelihood of intrauterine transmission of the virus. PLoS One 2016; 11: e0147883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Zydek M, Petitt M, Fang-Hoover J, et al. HCMV infection of human trophoblast progenitor cells of the placenta is neutralized by a human monoclonal antibody to glycoprotein B and not by antibodies to the pentamer complex. Viruses 2014; 6: 1346– 1364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Maidji E, McDonagh S, Genbacev O et al. Maternal antibodies enhance or prevent cytomegalovirus infection in the placenta by neonatal Fc receptor-mediated transcytosis. Am J Pathol 2006; 168: 1210– 1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Boppana SB, Miller J, Britt WJ.. Transplacentally acquired antiviral antibodies and outcome in congenital human cytomegalovirus infection. Viral Immunol 1996; 9: 211– 218. [DOI] [PubMed] [Google Scholar]
- 111. Furione M, Rognoni V, Sarasini A et al. Slow increase in IgG avidity correlates with prevention of human cytomegalovirus transmission to the fetus. J Med Virol 2013; 85: 1960– 1967. [DOI] [PubMed] [Google Scholar]
- 112. Saldan A, Forner G, Mengoli C et al. Strong cell-mediated immune response to human cytomegalovirus is associated with increased risk of fetal infection in primarily infected pregnant women. Clin Infect Dis 2015; 61: 1228– 1234. [DOI] [PubMed] [Google Scholar]
- 113. Forner G, Saldan A, Mengoli C et al. Cytomegalovirus (CMV) enzyme-linked immunosorbent spot assay but not CMV QUANTIFERON assay is a novel biomarker to determine risk of congenital CMV infection in pregnant women. J Clin Microbiol 2016; 54: 2149– 2154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114. Kabanova A, Lilleri D. Analytic vaccinology: Antibody-driven design of a human cytomegalovirus subunit vaccine. Methods Mol Biol 2016; 1403: 167– 86. [DOI] [PubMed] [Google Scholar]
- 115. Krause PR, Bialek SR, Boppana SB et al. Priorities for CMV vaccine development. Vaccine 2013; 32: 4– 10. [DOI] [PMC free article] [PubMed] [Google Scholar]