Abstract
Objectives:
To evaluate the prevalence of occult hepatitis B viral infections (OBIs) among blood donors considering the clinical and epidemiological importance of identifying OBIs.
Methods:
A cross-sectional study conducted at King Khalid University Hospital, Riyadh, Saudi Arabia between January 2011 and January 2012. Blood donors (n=8501) were screened for Hepatitis B virus surface antigen (HBsAg) and hepatitis B core antibodies (HBcAb). All HBsAg-negative and HBcAb-positive samples were tested further for hepatitis B surface antibodies (HBsAb), hepatitis B virus (HBV)-DNA, and HBV genotyping.
Results:
Of the 8501 serum samples tested, 56 (0.7%) were positive and 8445 (99.3%) were negative for HBsAg. Among the HBsAg-negative samples, 198 (2.3%) were positive for HBcAb and these patients were suspected to have OBIs. Among the HBcAb-positive samples, 119 (60.1%) were positive while 79 (39.9%) were negative for HBsAb. Analysis of HBV-DNA for the suspected OBIs showed that 17 out of 198 samples (8.6%) yielded positive results, and all of them were HBsAb-negative. The viral load was low (<20-186 IU/mL) in all OBIs. Hepatitis B virus genotyping showed that 15 out of 17 samples (88.2%) were genotype D, and the other 2 samples (11.8%) were genotype E.
Conclusion:
The prevalence of OBIs among blood donors in Riyadh was 0.2%. Therefore, it is recommended that HBV molecular testing should be incorporated with serological assays for screening of blood donors.
Hepatitis B infection is a major health problem worldwide.1,2 It is caused by hepatitis B virus (HBV), which primarily targets the liver and causes acute and chronic hepatitis that may progress to cirrhosis, hepatocellular carcinoma, or in rare conditions, fulminant hepatitis.1,2 Hepatitis B virus is an enveloped virus with a double-stranded circular DNA genome. It belongs to the family of Hepadnaviridae and has 8 different genotypes, namely HBV genotypes A-H.1 The HBV genotype is a variable that can potentially influence the outcome of chronic hepatitis B and the success of antiviral therapy. Hepatitis B virus has 3 antigens, surface antigen (HBsAg), e antigen (HBeAg), and core antigen (HBcAg), and therefore, after infection 3 antibodies are generated by the immune system: HBsAb, HBeAb, and HBcAb. These HBV markers along with the viral load are particularly important in defining the stage of HBV infections and in their management.1 Hepatitis B virus is transmitted parentally by different routes including exposure to infectious blood or body fluids, organ transplant, or by sexual contact.2 Evidence has shown that HBV transmission can occur during the serological window period when the HBsAg is absent and the viral DNA is present in the circulation of infected carriers.3 It has been estimated that more than 2 billion people have been infected with HBV worldwide.4 This includes 350 million chronic carriers of the virus. The disease has caused epidemics in different parts of the world including Asia and Africa.4 Moreover, it is endemic in China, Thailand, Vietnam, Brazil, Peru, Venezuela, and southern African countries.5 Despite the introduction of the HBV subunit vaccine in Saudi Arabia in 1989, HBV infection was the predominant type of viral hepatitis identified among Saudi adults for the 2000-2010 period comprising 49.3-53% of all cases.6-8 However, a subgroup of HBV-infected individuals are characterized by the persistent presence of HBV-DNA and absence of HBsAg in their circulation; this condition is known as an occult HBV infection (OBI).9,10 The prevalence of OBIs among HBcAb-positive blood donors has been reported as 12.2% in Iran, 2.8% in Lebanon, and 2.9% in Pakistan.11-13 Previous study revealed that OBIs may persist for years in infected individuals without apparent symptoms of overt HBV infection; however, it can be still transmitted to others.14 Other reports have shown that co-infection, drug abuse, or immunosuppressants can trigger an increase in HBV-DNA levels without an increase in HBsAg.15,16 The advent of highly sensitive polymerase chain reaction (PCR) assays has made it possible to identify HBV carriers who are negative for HBsAg, but who harbour low levels of HBV-DNA in serum or liver tissue, especially among blood and organ donors. Considering the clinical and epidemiological importance of detecting OBIs, we aimed to utilize molecular and the serological testing for the characterization and evaluation of the prevalence of OBIs among blood donors in Riyadh, the central region of Saudi Arabia.
Methods
A cross-sectional study performed at King Khalid University Hospital (KKUH) between January 2011 and January 2012. A total of 8501 volunteer donors were enrolled in this study including 8161 males (96%) and 340 females (4%). The mean age of the participants was 40 years (range, 17-63 years). All participants were eligible to donate blood and were selected based on physical examination and medical history, including a modified version of the general blood bank questionnaire form from the American Association of Blood Banks (AABB). Serum samples were received by the Virology and Molecular Biology laboratories for routine blood screening and molecular testing at KKUH. All samples that were positive for hepatitis C virus antibodies (HCV Ab), human immunodeficiency virus antigen and antibodies (HIV Ag/Ab), and human T lymphocytic leukaemia virus I and II antibodies (HTLV I and II Ab) were excluded. This study was approved by and performed according to the guidelines of KKUH and the college of medicine institutional review board committee. All procedures performed in were in accordance with the ethical standards of the institutional, or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all participants included in the study.
Detection of HBsAg, HBcAb, and HBsAb
Serum samples were tested for HBsAg, HBcAb (total antibodies IgM and IgG), and HBsAb based on chemiluminescent microparticle immunoassays (CMIAs). These tests were performed using CE-approved kits (ARCHITECT® HBsAg, ARCHITECT® anti-HBc, ARCHITECT® anti-HBs) and the Architect i2000 analyzer (Abbott, Lake Forest, IL, USA).
Assessment of HBV viral load and genotyping
Serum from HBsAg-negative and HBcAb-positive volunteer blood donors were processed for HBV-DNA quantification and genotyping. The HBV viral load was measured for individual donors using the COBAS® TaqMan® analyzer and COBAS® AmpliPrep/COBAS® TaqMan® HBV Quantities test, version 2.0 (Roche Diagnostics, Germany) as previously described.17 The limit of detection of the assay was 16.4 IU/mL and the linear range of quantification was 20 IU/mL as stated by the manufacturer kit insert. Serum samples containing HBV-DNA were subjected to HBV genotyping using nested PCR and an INNO-LiPA® HBV Genotyping kit (INNOGENETICS® N.V, Belgium) as described previously.18
Statistical analysis
Data were collected and entered into a Microsoft Office Excel file for statistical and descriptive analysis. The percentage and the mean values were used where applicable.
Results
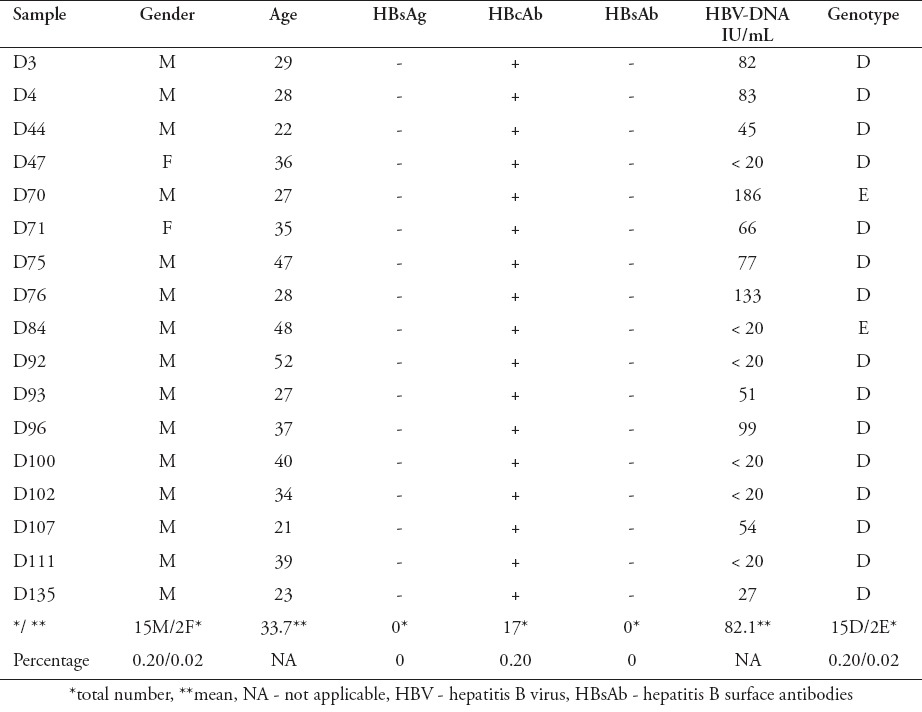
Of the 8501 blood donors samples screened for HBsAg, 56 samples (0.7%) were positive, confirming current HBV infection, whereas 8445 samples (99.3%) were negative (Figure 1). Among the HBsAg-negative samples, 198 (2.3%) were positive for HBcAb and 8247 (97.7%) were negative. All HBcAb-positive samples were subjected to HBsAb and HBV viral load testing. Of the 198 HBcAb-positive samples, 119 (60.1%) were positive for HBsAb, but all of them were negative for HBV-DNA (Figure 1). Moreover, 79 out of 198 samples (39.9%) were negative for HBsAb, but among those, 17 samples (21.5%) were HBV-DNA positive, confirming OBIs, and 62 samples (78.5%) were HBV-DNA negative (Figure 1 and Table 1). The viral load of HBV-DNA-positive samples was low, ranging between 20 and 186 IU/mL (Table 1). Analysis of HBV genotyping for those samples showed that 15 out of 17 samples (88.2%) were genotype D, and the other 2 samples (11.8%) were genotype E (Figure 1 & Table 1).
Figure 1.
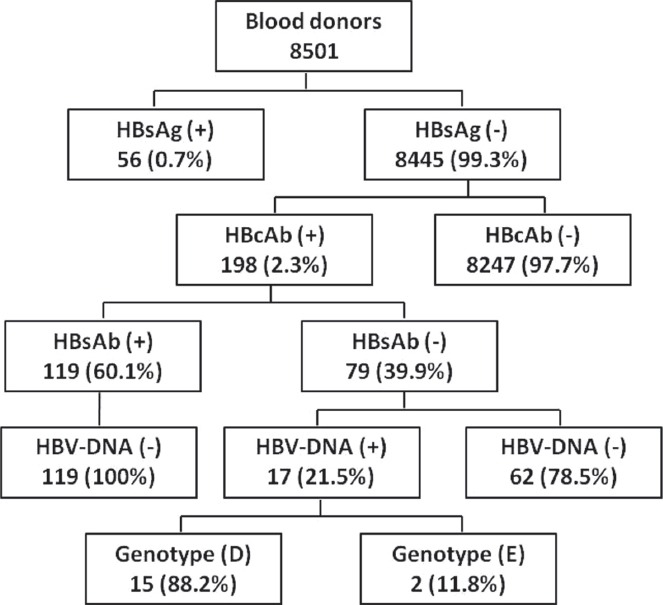
Prevalence of serological and molecular markers of hepatitis B virus (HBV) infection among blood donors in central, Saudi Arabia. HBsAb - hepatitis B surface antibodies
Table 1.
Demographic and serological profile of occult HBV infections.
Discussion
This is the first report that characterizes and defines the frequency of OBIs among blood donors in Riyadh. The prevalence of HBV infection among blood donors was 0.9%. This includes a 0.7% HBsAg-positive and a 0.2% HBV-DNA-positive, but HBsAg-negative population (OBIs). In agreement with these results, a recent report has shown that the prevalence of HBsAg among blood donors was 1.5%.19 Other studies have revealed that the HBsAg positivity rates in blood donors was 4.3% in Egypt and 2.21% in Pakistan in 2006.20 On the other hand, the prevalence of OBI among blood donors was 0.04-0.2% in Italy, 0.02-0.15% in India, 0.016% in Korea, and 1.18% in Egypt.14,21,22
In this study, 198 out of 8501 samples (2.3%) were HBsAg-negative, but HBcAb-positive and these donors were suspected to have an OBI. This result was markedly lower than that of a previous study carried out in 1997, which reported the prevalence of HBcAb among Saudi blood donors as 16.4%.23 However, our data were consistent with other recent reports which have observed an overall reduction in HBV infection over the last ten years because of the introduction of the HBV vaccine.24 Previous published studiesrevealed that the prevalence of HBcAb within HBsAg-negative blood donors was 0.56% in the United Kingdom, 0.8% in the United State of America, 1.4% in Germany, 2.1% in Iran, 3.1% in Brazil, 4.9% in Italy, 13.5 in Korea, 15% in Greece, and 76% in Ghana.21,22,25-30 In the current study, 198 samples were suspected with an OBI and 17 samples were confirmed by using the real-time PCR. However, 181 samples were positive for HBcAb but negative for HBV-DNA, and those were still considered as suspected HBV infection cases. The failure of detecting HBsAg and HBV-DNA could be due to viral mutation, or due to the limitation of the serological and molecular assays used. It has been suggested that such conditions may be observed during infection with mutant HBV, or during the period when the levels of circulating HBsAg in the infected individuals are below the detection threshold.1,10
In our study, all HBV-DNA-positive cases had low viral load (<186 IU/mL) and were negative for HBsAb. Thus, these cases may have recovered from previous infections but had persistently low levels of HBV. In agreement with this result, several studies have reported low HBV-DNA levels (<200 IU/ml) in subjects with OBI.14,22,27,31 Also, a study carried out in Iran has demonstrated that all OBI cases were individuals negative for HBsAb.11 Other studies have shown that there was no significant difference in HBV-DNA positivity between HBsAb-positive and HBsAb-negative samples.31,32 These results suggested that blood obtained from donors positive for HBsAb and HBcAb markers was not necessarily safer than that from donors positive HBcAb alone.
Hepatitis B virus genotype D infection is the most prevalent genotype in Saudi Arabia comprising an approximately 81.4% of HBV infections, followed by genotype E with 5.7%.24 It has been reported that treatment of HBV genotype D infections in the absence of HBeAg with peginterferon alpha-2a for 72 weeks reduced the viral load to <400 copies/mL in only 23% of the patients.33 Compared with this observation therapy with the nucleoside analogues such as entecavir, or tenofovir for 48 weeks has been shown to reduce the viral load to undetectable levels in 58.4-66% of the HBV infected patients with negative HBeAg.34,35 In the current study, the majority of OBIs identified (88.2%) were genotype D. However, the result obtained was not surprising since the vast majority of HBV infections in Saudi Arabia are genotype D.24 Similar to our study, a study conducted on blood donors from Italy and Poland showed that the prevalence of HBV genotype D among OBIs was significantly higher than that of other genotypes.36 Another recent report from Colombia concluded that HBV genotype F is the most prevalent OBI among blood donors.37
In this study, the HBV-DNA detection assay was conducted only on samples from blood donors who tested positive for HBcAb. This is because HBcAb is considered a pathognomonic marker of HBV infection especially in the absence of HBsAg. It has been suggested that HBcAb screening has the advantage of excluding the majority of OBIs, leaving only a few cases of undetectable OBIs.38 However, this approach is not practical in areas where the prevalence of HBcAb is more than 10%, because many donor rejections will negatively affect the blood supply. In the current study, we found that the prevalence of HBcAb in the Riyadh region was low (2.3%). Thus, the positive HBcAb blood units can either be directly discarded to cut costs, or screened for HBV-DNA and discarded only when positive for HBV-DNA, thus saving more blood units. By contrast, in other countries such as Korea, Greece, and Ghana where the prevalence of HBcAb is high (>10%),21,26,28 it would be mandatory to screen all blood units for HBV-DNA and discard only the positive samples in order to maintain adequate blood supply. Maximal prevention of HBV blood transmission can be achieved by developing tests that are more sensitive for HBsAg and HBcAb detection. Moreover, high-sensitivity HBV-DNA testing can be performed on blood donors either in individual samples using real-time PCR, which has a sensitivity of 12-20 IU/mL,16 or in minipool samples using nucleic acid testing (NAT), which has sensitivity of 3-8 IU/mL,39 to exclude all blood units containing HBV-DNA.10,38
Study limitation
One of the limitations of this study was that low numbers of OBIs were identified, and thus we could not correlate the findings with the patients’ demographic data.
In conclusion, all identified OBIs had a low viral load (<186 IU/mL) and mostly were genotype D. Thus, screening of blood and organ donors with more sensitive methods, including nucleic acid testing along with serological assays, especially in endemic areas, can potentially reduce the incidence of HBV blood transmission.
Footnotes
References
- 1.Liang TJ. Hepatitis B: the virus and disease. Hepatology. 2009;49:S13–S21. doi: 10.1002/hep.22881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Franco E, Bagnato B, Marino MG, Meleleo C, Serino L, Zaratti L, Hepatitis B. Epidemiology and prevention in developing countries. World J Hepatol. 2012;4:74–80. doi: 10.4254/wjh.v4.i3.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Thiers V, Nakajima E, Kremsdorf D, Mack D, Schellekens H, Driss F, et al. Transmission of hepatitis B from hepatitis-B-seronegative subjects. Lancet. 1988;2:1273–1276. doi: 10.1016/s0140-6736(88)92891-7. [DOI] [PubMed] [Google Scholar]
- 4.Andre F. Hepatitis B epidemiology in Asia, the Middle East and Africa. Vaccine. 2000;18:S20–S22. doi: 10.1016/s0264-410x(99)00456-9. [DOI] [PubMed] [Google Scholar]
- 5.Williams R. Global challenges in liver disease. Hepatology. 2006;44:521–526. doi: 10.1002/hep.21347. [DOI] [PubMed] [Google Scholar]
- 6.Memish ZA, Knawy BA, El-Saed A. Incidence trends of viral hepatitis A, B, and C seropositivity over eight years of surveillance in Saudi Arabia. Int J Infect Dis. 2010;14:e115–e120. doi: 10.1016/j.ijid.2009.03.027. [DOI] [PubMed] [Google Scholar]
- 7.Al-Tawfiq JA, Anani A. Profile of viral hepatitis A, B, and C in a Saudi Arabian hospital. Med Sci Monit. 2008;14:CR52–CR56. [PubMed] [Google Scholar]
- 8.Alshabanat AA, Albacker RB, Basalama AA, Bin Salamah AA, SalehAlfrayh A. Profile of viral hepatits in Saudi Arabia. Biomed Res. 2013;24:396–399. [Google Scholar]
- 9.Raimondo G, Pollicino T, Romano L, Zanetti AR. A 2010 update on occult hepatitis B infection. Pathol Biol (Paris) 2010;58:254–257. doi: 10.1016/j.patbio.2010.02.003. [DOI] [PubMed] [Google Scholar]
- 10.Kwak MS, Kim YJ. Occult hepatitis B virus infection. World J Hepatol. 2014;6:860–869. doi: 10.4254/wjh.v6.i12.860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Behzad-Behbahani A, Mafi-Nejad A, Tabei SZ, Lankarani KB, Torab A, Moaddeb A. Anti-HBc & HBV-DNA detection in blood donors negative for hepatitis B virus surface antigen in reducing risk of transfusion associated HBV infection. Indian J Med Res. 2006;123:37–42. [PubMed] [Google Scholar]
- 12.Bhatti FA, Ullah Z, Salamat N, Ayub M, Ghani E. Anti-hepatits B core antigen testing, viral markers, and occult hepatitis B virus infection in Pakistani blood donors: implications for transfusion practice. Transfusion. 2007;47:74–79. doi: 10.1111/j.1537-2995.2007.01066.x. [DOI] [PubMed] [Google Scholar]
- 13.Ramia S, Ramlawi F, Kanaan M, Klayme S, Naman R. Frequency and significance of antibodies against hepatitis B core (anti-HBc) antigen as the only serological marker for hepatitis B infection in Lebanese blood donors. Epidemiol Infect. 2005;133:695–699. doi: 10.1017/s0950268805003948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ismail AM, Devakumar S, Anantharam R, Fletcher GJ, Subramani T, John GT, et al. Low frequency of occult hepatitis B infection in anti-HBc seropositive blood donors: experience from a tertiary care centre in South India. Blood Transfus. 2012;10:230–232. doi: 10.2450/2011.0046-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jeantet D, Chemin I, Mandrand B, Tran A, Zoulim F, Merle P, et al. Cloning and expression of surface antigens from occult chronic hepatitis B virus infections and their recognition by commercial detection assays. J Med Virol. 2004;73:508–515. doi: 10.1002/jmv.20119. [DOI] [PubMed] [Google Scholar]
- 16.Garfein RS, Bower WA, Loney CM, Hutin YJ, Xia GL, Jawanda J, et al. Factors associated with fulminant liver failure during an outbreak among injection drug users with acute hepatitis B. Hepatology. 2004;40:865–873. doi: 10.1002/hep.20383. [DOI] [PubMed] [Google Scholar]
- 17.Chevaliez S, Bouvier-Alias M, Laperche S, Hezode C, Pawlotsky JM. Performance of version 2.0 of the Cobas AmpliPrep/Cobas TaqMan real-time PCR assay for hepatitis B virus DNA quantification. J Clin Microbiol. 2010;48:3641–3647. doi: 10.1128/JCM.01306-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Osiowy C, Giles E. Evaluation of the INNO-LiPA HBV genotyping assay for determination of hepatitis B virus genotype. J Clin Microbiol. 2003;41:5473–5477. doi: 10.1128/JCM.41.12.5473-5477.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.El-Hazmi MM. Prevalence of HBV, HCV, HIV-1, 2 and HTLV-I/II infections among blood donors in a teaching hospital in the Central region of Saudi Arabia. Saudi Med J. 2004;25:26–33. [PubMed] [Google Scholar]
- 20.Mujeeb SA, Aamir K, Mehmood K. Seroprevalence of HBV, HCV and HIV infections among college going first time voluntary blood donors. J Pak Med Assoc. 2006;56:S24–S25. [PubMed] [Google Scholar]
- 21.Seo DH, Whang DH, Song EY, Kim HS, Park Q. Prevalence of antibodies to hepatitis B core antigen and occult hepatitis B virus infections in Korean blood donors. Transfusion. 2011;51:1840–1846. doi: 10.1111/j.1537-2995.2010.03056.x. [DOI] [PubMed] [Google Scholar]
- 22.Manzini P, Girotto M, Borsotti R, Giachino O, Guaschino R, Lanteri M, et al. Italian blood donors with anti-HBc and occult hepatitis B virus infection. Haematologica. 2007;92:1664–1670. doi: 10.3324/haematol.11224. [DOI] [PubMed] [Google Scholar]
- 23.Bernvil SS, Andrews V, Kuhns MC, McNamara AL. Hepatitis B core antigen antibody as an indicator of a low grade carrier state for hepatitis B virus in a Saudi Arabian blood donor population. Transfus Sci. 1997;18:49–53. doi: 10.1016/s0955-3886(96)00076-8. [DOI] [PubMed] [Google Scholar]
- 24.Abdo AA, Al-Jarallah BM, Sanai FM, Hersi AS, Al-Swat K, Azzam NA, Al-Dukhayil M, et al. Hepatitis B genotypes: relation to clinical outcome in patients with chronic hepatitis B in Saudi Arabia. World J Gastroenterol. 2006;12:7019–7024. doi: 10.3748/wjg.v12.i43.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kleinman SH, Kuhns MC, Todd DS, Glynn SA, McNamara A, DiMarco A, et al. Frequency of HBV DNA detection in US blood donors testing positive for the presence of anti-HBc: implications for transfusion transmission and donor screening. Transfusion. 2003;43:696–704. doi: 10.1046/j.1537-2995.2003.00391.x. [DOI] [PubMed] [Google Scholar]
- 26.Allain JP, Candotti D, Soldan K, Sarkodie F, Phelps B, Giachetti C, et al. The risk of hepatitis B virus infection by transfusion in Kumasi, Ghana. Blood. 2003;101:2419–2425. doi: 10.1182/blood-2002-04-1084. [DOI] [PubMed] [Google Scholar]
- 27.Hennig H, Puchta I, Luhm J, Schlenke P, Goerg S, Kirchner H. Frequency and load of hepatitis B virus DNA in first-time blood donors with antibodies to hepatitis B core antigen. Blood. 2002;100:2637–2641. doi: 10.1182/blood-2002-03-0798. [DOI] [PubMed] [Google Scholar]
- 28.Zervou EK, Dalekos GN, Boumba DS, Tsianos EV. Value of anti-HBc screening of blood donors for prevention of HBV infection: results of a 3-year prospective study in Northwestern Greece. Transfusion. 2001;41:652–658. doi: 10.1046/j.1537-2995.2001.41050652.x. [DOI] [PubMed] [Google Scholar]
- 29.Wolff FH, Fuchs SC, Brandao AB. Absence of occult hepatitis B among blood donors in southern Brazil. Braz J Infect Dis. 2011;15:159–162. [PubMed] [Google Scholar]
- 30.Sofian M, Aghakhani A, Izadi N, Banifazl M, Kalantar E, Eslamifar A, et al. Lack of occult hepatitis B virus infection among blood donors with isolated hepatitis B core antibody living in an HBV low prevalence region of Iran. Int J Infect Dis. 2010;14:e308–e310. doi: 10.1016/j.ijid.2009.05.011. [DOI] [PubMed] [Google Scholar]
- 31.Silva CM, Costi C, Costa C, Michelon C, Oravec R, Ramos AB, et al. Low rate of occult hepatitis B virus infection among anti-HBc positive blood donors living in a low prevalence region in Brazil. J Infect. 2005;51:24–29. doi: 10.1016/j.jinf.2004.07.007. [DOI] [PubMed] [Google Scholar]
- 32.Fang Y, Shang QL, Liu JY, Li D, Xu WZ, Teng X, et al. Prevalence of occult hepatitis B virus infection among hepatopathy patients and healthy people in China. J Infect. 2009;58:383–388. doi: 10.1016/j.jinf.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 33.Al-Ashgar HI, Khan MQ, Aljumah A, Sanai FM, Abdo AA, Dafalla MM, Fagih MA, Bzeizi KI. Efficacy of peginterferon alpha-2a and predictors of response in HBeAg-negative, genotype D-naive patients. Hepatol Int. 2012;6:718–726. doi: 10.1007/s12072-011-9319-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Al-Ashqar HI, Al-Quaiz M, Dahab ST, Peedikayil MC. Entecavir for the treatment of real-life chronic hepatitis B patients: a study from Saudi Arabia. Ann Saudi Med. 2013;33:119–123. doi: 10.5144/0256-4947.2013.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Alsohaibani F, Alturaif N, Abdulshakour A, Alghamdi S, Alshaibani A, Alashgar H, et al. Tenofovir in the Treatment of Naive and Refractory Chronic Hepatitis B: A Single Center Experience in Saudi Arabia. Saudi J Gastroenterol. 2015 doi: 10.4103/1319-3767.164189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Candotti D, Grabarczyk P, Ghiazza P, Roig R, Casamitjana N, Iudicone P, et al. Characterization of occult hepatitis B virus from blood donors carrying genotype A2 or genotype D strains. J Hepatol. 2008;49:537–547. doi: 10.1016/j.jhep.2008.04.017. [DOI] [PubMed] [Google Scholar]
- 37.Rios-Ocampo WA, Cortes-Mancera F, Olarte JC, Soto A, Navas MC. Occult hepatitis B virus infection among blood donors in Colombia. Virol J. 2014;11:206. doi: 10.1186/s12985-014-0206-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu CJ, Chen DS, Chen PJ. Epidemiology of HBV infection in Asian blood donors: emphasis on occult HBV infection and the role of NAT. J Clin Virol. 2006;36:S33–S44. doi: 10.1016/s1386-6532(06)80007-7. [DOI] [PubMed] [Google Scholar]
- 39.Albertoni G, Castelo Girao MJ, Schor N. Mini review: current molecular methods for the detection and quantification of hepatitis B virus, hepatitis C virus, and human immunodeficiency virus type 1. Int J Infect Dis. 2014;25:145–149. doi: 10.1016/j.ijid.2014.04.007. [DOI] [PubMed] [Google Scholar]


