Abstract
Objectives:
To determine the prevalence of hypertension, obesity, hematuria, and proteinuria among healthy adolescents and to determine the associated risk factors.
Methods:
This is a cross-sectional study of 8 intermediate schools in Jeddah, Saudi Arabia between March 2015 and June 2015. Samples were selected randomly and equal proportions from each school for both genders were ensured. Both blood pressure and body mass index were measured and a brief questionnaire was filled out for the specified studied group. Urine dipstick analysis was carried out for 294 children. A second questionnaire was completed for hypertensive and obese subjects in addition to those with hematuria and proteinuria.
Results:
A total of 401 children (200 males) with a mean (SD) age of 13.87 (1.27) were included. Hypertension was found in 17.2% with a male to female ratio of 1.4:1. Pre-hypertension was found in 4.2% of our sample with a male to female ratio of 2.1:1. Obesity was found in 19.2% with a male to female ratio of 1.5:1. Obesity was found to be the most significant risk factor for hypertension with a related risk: 2.87, 95% and confidence interval: 1.9-4.3. For urine abnormalities, 10.2% of samples were positive for proteinuria, 17% for hematuria, and 3.1% for both.
Conclusion:
It was found that there is a positive correlation between the incidence of obesity and hypertension in adolescents. Hematuria and proteinuria were also found to be high. Screening and prevention programs are therefore recommended.
Cardiovascular morbidity and mortality have been increasing globally.1,2 Hypertension is a major risk factor for cardiovascular disease and is capable of causing damage to both renal and central nervous systems.2 Recently cardiovascular risk factors were more recognizable in children due to the increase in the prevalence of hypertension in the pediatric population.3 Pediatric hypertension is thought to be a prevailing medical concern in the Kingdom of Saudi Arabia (KSA). However, there is a paucity of studies regarding its prevalence. Unfortunate changes in the lifestyle of teenagers involving a decrease in physical activity as well as an increase in the consumption of junk food have been thought to be the primary causes behind widely increased hypertension in pediatric population. A national study was conducted that selected 16,226 children and adolescents in the 13 regions of the country.4 The study evaluated the normal blood pressure values in Saudi children, and as expected, it showed a steady increase in blood pressure levels alongside an increase in age in both genders.4 Hematuria is an abnormality that could indicate glomerular, or non-glomerular kidney disease.5 The prevalence of microscopic hematuria is variable from 0.18 to 16.1% in different populations.6,7 Urine dipstick is usually used for screening, but is found to be relatively inaccurate with a high false-positive rate. Therefore, confirmatory microscopic urinalysis should be performed in patients with positive dipstick.8 The short-term prognosis in the absence of proteinuria, high blood pressure, or renal impairment is almost always excellent.7 Nevertheless, the long-term follow-up of a considerable number of patients demonstrated a significant increase in the life-time risk of end stage kidney disease.5,9 The current recommendation is to follow up the affected subjects on an annual, or biennial basis. On the other hand, immediate attention should be given to children with proteinuria as it usually reflects diseases requiring intervention.10 Positive urine dipsticks for proteinuria should be confirmed by laboratory measurement of urine protein to creatinine ratio.11 There is once again a scarceness in the number of studies carried out in the Kingdom of Saudi Arabia regarding the prevalence of pediatric hematuria as there are only 2.12,13 The first study involved the screening of preschool children attending the out-patient clinics of a children’s hospital and the results revealed a high prevalence of hematuria being 16.9% of which 2.5% was confirmed by urine microscopy.12 The second study was conducted in both primary and preparatory schools in the city of Makkah and Albaha region. It revealed 7% prevalence of hematuria.13 In this study, we investigate the prevalence of obesity, hypertension, hematuria, and proteinuria in the preparatory (intermediate) schools of the city of Jeddah, KSA.
Methods
A cross-sectional study was conducted in 8 intermediate public schools in Jeddah, Saudi Arabia between March 2015 and June 2015. We included healthy children, with the age range between 11-18 years. Children who are known to have any renal diseases, hypertension, or other comorbidities were excluded from the study. To calculate the sample size, we expected a prevalence of 50% (this will provide the largest sample size) and would like to estimate it to within 5% (0.05) of the true value (margin of error) with 95% confidence interval (CI). The calculated sample size was 385 subjects. In order to increase the precision of the estimate, the sample size was increased to 400 subjects. Formula used with 95% CI: n = t2 (1.96)2 x p (1-p) / d2.
The total sample size was 401 children (200 males and 201 females). One public school was randomly selected from each region in Jeddah (for both genders). Fifty children were selected randomly from each school ensuring a fair distribution with regards to age and grade. Ethical approval and consent were obtained from the authorized personnel and the participants themselves. The study was also approved by the Research Ethical Committee of the Medical School of King Abdulaziz University (KAU).
Procedures
A brief questionnaire was filled out for each student during a short face to face interview. It included demographic data (name, age, date of birth, nationality, and gender), ethnic background, parent’s consanguinity, family characteristics and income, parent’s occupation and smoking history.
Weight and height were measured manually using a beam balance scale. The body mass index (BMI) was calculated using the formula BMI = weight (kg)/height (m2). The percentile for weight, height, and BMI was calculated based on the Center for Disease Control and Prevention (CDC) charts. This was carried out specifically for age between 2-20 years for each gender.14
We defined obesity according to the CDC definition of BMI ≥95th percentile, while children with BMI ≥85th < 95th percentile were considered as overweight.15
Blood pressure was obtained using a professional automated blood pressure machine (Dinamap) with the appropriate cuff size covering two thirds of the child’s upper-arm while the child is in a fully rested position and sitting with the arm at the level of the heart. The blood pressure percentile was obtained from the National of Heart, Lung and Blood Institute (NHLBI) charts based on age and height percentile for each gender.16 Prehypertension was defined as systolic blood pressure (SBP) or diastolic blood pressure (DBP) ≥90th <95th percentile. While SBP or DBP ≥95th percentile was considered as hypertension.16 Children with blood pressure (BP) of >90th percentile were reevaluated with 2 additional readings taken after 15 minutes rest. The lowest reading was considered and recorded as the measurement of BP. A mid-stream urine sample was obtained in a sterile container after ensuring a clear explanation of the procedure was given to each child. Urine dipstick analysis was performed in search of protein, bloods, nitrite, leukocyte esterase, glucose and ketone body. Sixteen girls were excluded from the study as they were menstruating at the time of conducting the study. Another 91 children were not tested as some children refused to do the urine test and one of the schools were not cooperative in allowing the children to carry out urine test. An additional questionnaire was conducted for those obese, pre-hypertensive, or hypertensive children. It included questions on the past medical history, family history, and brief evaluation of their diet and activity. All pre-hypertensive and hypertensive children and those with abnormal findings in urine analysis (proteinuria or hematuria) were offered a referral to the pediatric nephrology clinic at KAU Hospital for further evaluation. The related studies, which have been used within and throughout our study discussion were obtained from a variety of reliable online databases such as PubMed and WebMD.
Statistical analysis
The data was entered using the Excel software of Microsoft windows 10. Statistical analysis was carried out using IBM SPSS Statistics software Version 20 (IBMCorp, Armonk, NY, USA). As for the data clearing, it was under supervision of an experienced and professional statistician. Descriptive statistics was calculated as the mean and standard deviation for continuous variables and proportions for categorical variables. Chi-squared test was used for the categorical variables. For all the statistical tests, a p<0.05 was considered statistically significant.
Results
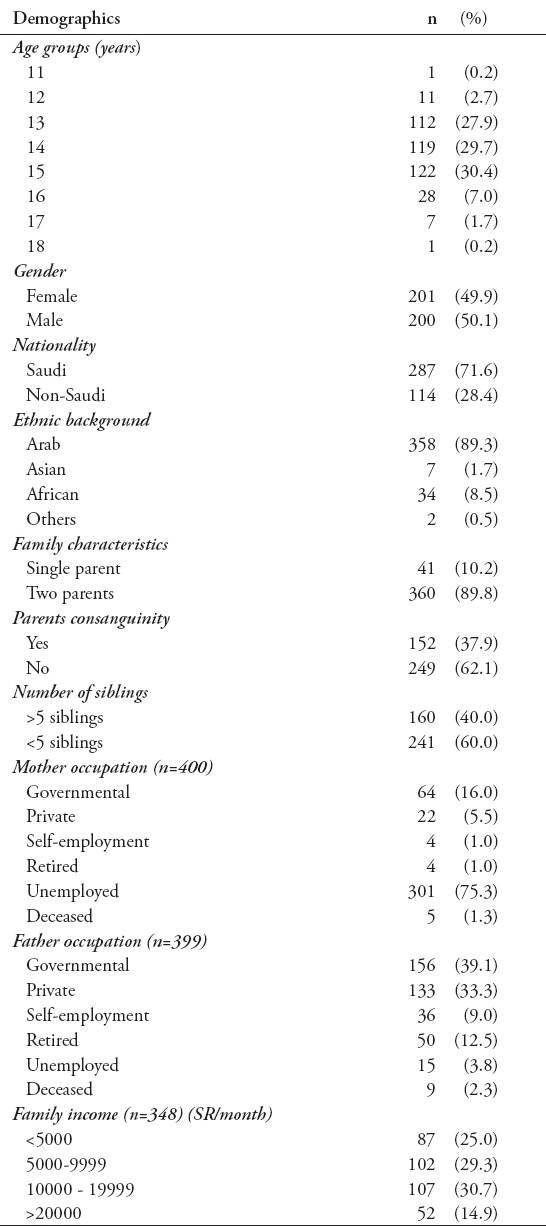
Four hundred and one adolescents (200 males) were included in the study. Their mean + standard deviation (SD) age was 13.87±1.27 years, BMI 21.89 kg/m2, systolic BP 119±13.37 mm Hg, and diastolic BP 72.52±7.85 mm Hg. The demographic and socioeconomic characteristics of the participants are shown in Table 1.
Table 1.
Demographic and socioeconomic status among 401 adolescents included in the study.
Hypertension was found in 69 children (with a prevalence of 17.2% in general (18.5% in Saudis and 14% in non-Saudis). The male to female ratio of the hypertensive group was 1.4:1 and the mean age was 13.99±0.98 years. The mean increase in systolic BP >95th percentile for age and height was 8.24±6.9 mm Hg and the mean increase in diastolic BP >95th percentile for age and height was 4.1±3.5 mm Hg. Prehypertension was found in 17 children (4.2%) of our sample with a male to female ratio of 2.1:1. Obesity was found in 77 children (19.2%) with a male to female ratio of 1.5:1. While 60 children (15%) were classified as overweight and 33 children (8.2%) as underweight. Fifty-six children (14%) confessed that they were smokers with a male to female ratio of 1.3:1
In hypertensive children, the mean (SD) intake of junk food was 1.86±1.4 times/week, soft drinks 5±4.2 times/week, crisps 3.72±3.34 times/week, and chocolates 4.32±4.22 times/week. While the mean (SD) intake of vegetables was 2.5±2.69 times/week and fruits 3.07±2.7. The mean (SD) vigorous activity was carried out on 1.64±1.2 days/week for 38.6±37.1 minutes and for moderate activity 0.83±0.67 days/week for 16.74±14.7 minutes. Walking was executed on 1.94±1.31 days/week for 23.55±17.55 minutes while sitting time was 317.36±189.6 minutes/day. The data of eating and exercise were not available for the normotensive children as they were not offered the second questionnaire.
The independent risk factors for hypertension are listed in Table 2. The same risk factors were found in pre-hypertensive children. Other variables which were non-significant statistically as risk factors for hypertension, or pre-hypertension include Saudi nationality (odds ratio [OR]: 1.387, 95% confidence interval [CI]: 756-2.545, p=0.289), family history of cardiovascular diseases (OR: 0.695, 95% CI: 281-1.723, p=0.431), family history of hypertension (OR: 498, 95% CI: 243-1.020, p=0.055), or consanguinity (OR: 917, 95% CI: 535-1.571, p=0.753). The results of the urine dipstick analysis were available for 294 children. The mean ± standard deviation (SD) age for this group was 13.82±1.12 years. Two hundred and twenty-six children (76.9%) were Saudi and 68 children (23.1%) were from various nationalities. The prevalence of proteinuria is shown in Figure 1. No proteinuria was detected in 264 samples (89.8%). Proteinuria was found in 30 children (10.2%): 1-positive in 24 children (8.2%), 2-positive in 4 (1.4%) and, 3-positive in 2 (0.7%). Proteinuria was observed more frequently in girls and this correlation was significant (p=0.012). It was detected in 12 males and 18 females (OR: 381). No significant correlation was detected in relation to nationality, ethnic background, smoking, or obesity. Prevalence of hematuria is shown in Figure 2. Similar to proteinuria, it was observed more frequently in girls, with a female to male ratio of 1.5:1 and OR: 350 (p=0.001). There was no significant correlation with regards to nationality, ethnic background, or obesity either. Only 2 out of 30 children with proteinuria were obese. Similarly, only 7 out of the 50 children with hematuria were obese. Nine children (3.1%) had both proteinuria and microscopic hematuria. Nitrite was positive in 4 children (1.4%) and leucocyte esterase was positive in 28 children (9.5%).
Table 2.
Independent risk factors for hypertension (n=69) among healthy adolescents in Saudi Arabia.
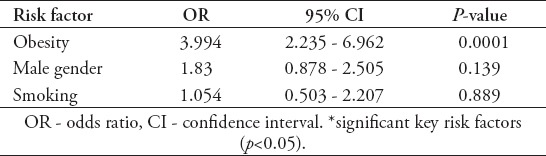
Figure 1.
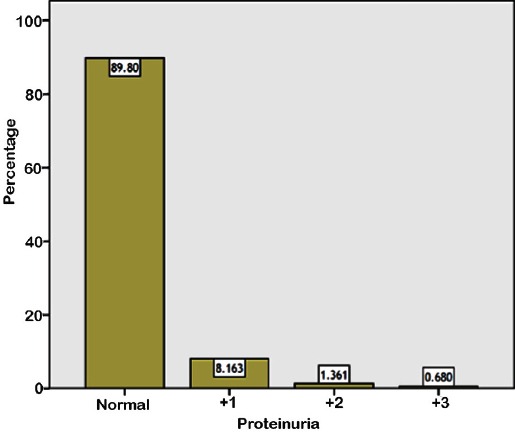
Prevalence of proteinuria among healthy adolescents in Saudi Arabia.
Figure 2.
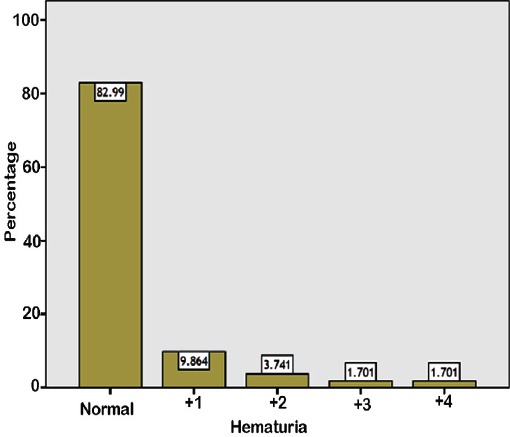
Prevalence of hematuria among healthy adolescents in Saudi Arabia.
Discussion
We found an alarmingly high prevalence of elevated BP levels in adolescents (17.2%), which happens to be much higher than previously reported from other Arab and Asian countries such as Iraq, Pakistan, and India.17-19 A recent study from India from 2009 and 2010 reported the prevalence of hypertension as 5.62% in school adolescents.18 Another study19 from Pakistan reported the prevalence of hypertension to be 3%. Western studies,20,21 also reported a lower prevalence of hypertension in 2-5%. However, the prevalence of prehypertension was lower in our study compared with previous reports.19-21 The true prevalence of hypertension could be lower due to the fact that we did not follow all children, although all were given appointments to our pediatric services. We observed a significant correlation between BMI and high blood pressure levels, which is similar to figures demonstrated in previous studies.22 It is interesting that the Asian population as a whole are subjected to hypertension and other cardiovascular complications at lower BMI kg/m2 as 25 kg/m2 in comparison with the 26 kg/m2 to 31 kg/m2 in Europeans.23 The high prevalence of obesity in our study is similar to the United States of America.24 On the contrary, a huge difference was observed in comparison with other studies from India and Pakistan, which had low rates of 1-2%.18,19 This could be a reflection of the changes in life style that accompany higher socioeconomic statuses and the recent rapid industrialization and modernization in the country. We studied children living in Jeddah and not only Saudi children and therefore we used the CDC charts as an alternative to the Saudi charts.25 The Saudi percentile charts are shifted downwards when compared with CDC charts, which means that using CDC charts would exaggerate the proportion of children with growth deficiency.26 Therefore, using the Saudi charts could reveal a higher percentage of obesity, which is one of the messages of this paper.27,28 It is now widely thought that to achieve an effective functioning cardiorespiratory system, a minimum of moderate and vigorous activity carried out once weekly is required. A previous study29 showed that most Saudi adolescents do not meet these requirements.29 Our results portray that many children live a sedentary lifestyle as they do not achieve their recommended daily activity. The United States Department of Health and Human Services recommendation for adolescents is to have at least 60 minutes of daily activity. Aerobic activity mainly of moderate to vigorous intensity is recommended and should include muscle and bone strengthening activity for a minimum of 3 days a week.30 Prehypertension, which is high in our population was confirmed as a risk factor for the development of hypertension during adolescence.31 Therefore, it should be considered in the screening and prevention programs across the country. We observed a relatively high prevalence of hematuria and proteinuria in our study. Abnormalities in urine tests could be the earliest indicator of chronic kidney disease (CKD). A national screening program for children aged 6-14 was carried out in Japan in 1973.32,33 Taiwan,32 Korea,33 Malaysia,34 and Singapore,35 also developed screening programs. Dipstick urine analysis was used in these studies with the aim of detecting hematuria and/or proteinuria among asymptomatic children as it might indicate an underlying renal disease.32-35
In our study, the prevalence of proteinuria in asymptomatic children in Jeddah was 10.2%, which is much higher than the previously reported 0.5% from Taif in the western region of Saudi Arabia.12 However, preschool children attending hospital clinics were screened unlike our study, which involved the screening of adolescents. Similarly, our observed prevalence was higher than reports from other Arab countries,36 such as Egypt37 reporting only 3%, and Lebanon38 reporting 0.2%. A large study from Korea34 reported annual school urinalysis for 5 million students with 0.2% proteinuria. While the prevalence of proteinuria was 1.2% in a study conducted in 12-year-old school children in Singapore.39 Our finding of 10.2% was the highest in comparison with other Arabic and Asian countries. Our study might possibly be biased, as the sample size is very small compared with the huge number of screened population in Asian studies. In our study, 17% of children had microscopic hematuria which is comparable with the study carried out in Makkah (14.8%),12 but notably higher than the city of Albaha in KSA (7.5%).13 Our observation for the prevalence of hematuria was higher than reports from other Arab countries36 such as Egypt37 (7.3%), and Lebanon38 (2%). It is additionally higher than Asian countries such as Singapore,39 as the reported prevalence of hematuria was 6.8% in 12-year-old school children. Korean annual school urinalysis for 5 million students revealed occult blood in 0.8% of children.34 While in Taiwan,35 0.3% of children were found to have urine abnormalities in the mass urinary screening program for children, which screened approximately 2.7 million children, (34.8% had isolated microscopic hematuria). Combined hematuria and proteinuria was found in 3.1% of our studied children, which is higher than Egypt of only 0.3%.32 Similarly, the reported prevalence from Japan was 0.08% in children aged 12-14 years old, while in Taiwan 14.3% of children with urinary abnormalities (0.3%) had hematuria with mild proteinuria and 34.4% had heavy proteinuria.32,35 Our study demonstrates higher prevalence than that of Egypt, Japan, and Taiwan. Similar to previous study,37 girls were more likely to have urine abnormalities in the present study.
In conclusion, a high prevalence of hypertension and obesity was found in adolescents and there was a strong correlation between them. Therefore, prevention programs are strongly recommended to prevent obesity and to screen for hypertension and urine abnormalities. Adopting such a strategy will lead to early detection of renal diseases and reduce the incidence of CKD. Establishing school heart health curricula is also crucial as they were launched in many countries and proved to be effective in minimizing the risk of cardiovascular diseases as children move into adulthood.
Acknowledgment
We would like to acknowledge the Deanship of Scientific Research, King Abdulaziz University, Jeddah, Saudi Arabia for the technical and financial support. Also thank to Dr. Baker Bin-Sadeq for the statistical analysis.
Footnotes
References
- 1.Harris DE, Aboueissa AM, Hartley D. Myocardial infarction and heart failure hospitalization rates in Maine, USA - variability along the urban-rural continuum. Rural Remote Health. 2008;8:980. [PubMed] [Google Scholar]
- 2.Vishram JK. Prognostic interactions between cardiovascular risk factors. Dan Med J. 2014;61:B4892. [PubMed] [Google Scholar]
- 3.Faienza MF, Wang DQ, Fruhbeck G, Garruti G, Portincasa P. The dangerous link between childhood and adulthood predictors of obesity and metabolic syndrome. Intern Emerg Med. 2016;11:175–182. doi: 10.1007/s11739-015-1382-6. [DOI] [PubMed] [Google Scholar]
- 4.Al Salloum AA, El Mouzan MI, Al Herbish AS, Al Omar AA, Qurashi MM. Blood pressure standards for Saudi children and adolescents. Ann Saudi Med. 2009;29:173–178. doi: 10.4103/0256-4947.51787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Moreno JA, Yuste C, Gutierrez E, Sevillano AM, Rubio-Navarro A, maro-Villalobos JM, et al. Haematuria as a risk factor for chronic kidney disease progression in glomerular diseases: A review. Pediatr Nephrol. 2016;31:523–533. doi: 10.1007/s00467-015-3119-1. [DOI] [PubMed] [Google Scholar]
- 6.Cohen RA, Brown RS. Clinical practice. Microscopic hematuria. N Engl J Med. 2003;348:2330–2338. doi: 10.1056/NEJMcp012694. [DOI] [PubMed] [Google Scholar]
- 7.Gale DP. How benign is hematuria? Using genetics to predict prognosis. Pediatr Nephrol. 2013;28:1183–1193. doi: 10.1007/s00467-012-2399-y. [DOI] [PubMed] [Google Scholar]
- 8.Rao PK, Jones JS. How to evaluate ’dipstick hematuria’: what to do before you refer. Cleve Clin J Med. 2008;75:227–233. doi: 10.3949/ccjm.75.3.227. [DOI] [PubMed] [Google Scholar]
- 9.Vivante A, Afek A, Frenkel-Nir Y, Tzur D, Farfel A, Golan E, et al. Persistent asymptomatic isolated microscopic hematuria in Israeli adolescents and young adults and risk for end-stage renal disease. JAMA. 2011;306:729–736. doi: 10.1001/jama.2011.1141. [DOI] [PubMed] [Google Scholar]
- 10.Yanagihara T, Hamada R, Ishikura K, Uemura O, Matsuyama T, Takahashi S, et al. Urinary screening and urinary abnormalities in 3-year-old children in Japan. Pediatr Int. 2015;57:354–358. doi: 10.1111/ped.12653. [DOI] [PubMed] [Google Scholar]
- 11.Hogg RJ, Furth S, Lemley KV, Portman R, Schwartz GJ, Coresh J, et al. National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative clinical practice guidelines for chronic kidney disease in children and adolescents: evaluation, classification, and stratification. Pediatrics. 2003;111(6 Pt 1):1416–1421. doi: 10.1542/peds.111.6.1416. [DOI] [PubMed] [Google Scholar]
- 12.Alharthi AA, Taha AA, Edrees AE, Elnawawy AN, Abdelrahman AH. Screening for urine abnormalities among preschool children in western Saudi Arabia. Saudi Med J. 2014;35:1477–1481. [PMC free article] [PubMed] [Google Scholar]
- 13.Edress B, Tayeb M, Shandeedi M. Prevalence of hematuria among school children in Makkah and Baha in Saudi Arabia. Jordan Medical Journal. 2013;47:20–25. [Google Scholar]
- 14.Centers for Disease Control and Prevention. Clinical Growth Charts. [Cited 2009 August]. Available from: URL: http://www.cdc.gov/growthcharts/clinical_charts.htm .
- 15.Centers for Disease Control and Prevention. Defining Childhood Obesity. [Cited 2009 August]. Available from: URL: http://www.cdc.gov/obesity/childhood/defining.html .
- 16.US Department of Health and Human Services. A pocket guide to blood pressure measurement in children. [cited 2007 May]. Available from: URL: https://www.nhlbi.nih.gov/files/docs/bp_child_pocket.pdf .
- 17.Subhi MD. Blood pressure profiles and hypertension in Iraqi primary school children. Saudi Med J. 2006;27:482–486. [PubMed] [Google Scholar]
- 18.Kar S, Khandelwal B. Fast foods and physical inactivity are risk factors for obesity and hypertension among adolescent school children in east district of Sikkim, India. J Nat Sci Biol Med. 2015;6:356–359. doi: 10.4103/0976-9668.160004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rahman AJ, Qamar FN, Ashraf S, Khowaja ZA, Tariq SB, Naeem H. Prevalence of hypertension in healthy school children in Pakistan and its relationship with body mass index, proteinuria and hematuria. Saudi J Kidney Dis Transpl. 2013;24:408–412. doi: 10.4103/1319-2442.109619. [DOI] [PubMed] [Google Scholar]
- 20.McNiece KL, Poffenbarger TS, Turner JL, Franco KD, Sorof JM, Portman RJ. Prevalence of hypertension and pre-hypertension among adolescents. J Pediatr. 2007;150:640–644. doi: 10.1016/j.jpeds.2007.01.052. [DOI] [PubMed] [Google Scholar]
- 21.Chiolero A, Paccaud F, Bovet P. Pre-hypertension and hypertension among adolescents of Switzerland. J Pediatr. 2007;151:e24–e25. doi: 10.1016/j.jpeds.2007.08.043. [DOI] [PubMed] [Google Scholar]
- 22.Chiolero A, Madeleine G, Gabriel A, Burnier M, Paccaud F, Bovet P. Prevalence of elevated blood pressure and association with overweight in children of a rapidly developing country. J Hum Hypertens. 2007;21:120–127. doi: 10.1038/sj.jhh.1002125. [DOI] [PubMed] [Google Scholar]
- 23.Appropriate body-mass index for Asian populations and its implications for policy and intervention strategies. Lancet. 2004;363:157–163. doi: 10.1016/S0140-6736(03)15268-3. [DOI] [PubMed] [Google Scholar]
- 24.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents 1999-2010. JAMA. 2012;307:483–490. doi: 10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El-Mouzan MI, Al-Herbish AS, Al-Salloum AA, Qurachi MM, Al-Omar AA. Growth charts for Saudi children and adolescents. Saudi Med J. 2007;28:1555–1568. [PubMed] [Google Scholar]
- 26.El Mouzan MI, Al Herbish AS, Al Salloum AA, Foster PJ, Al Omar AA, Qurachi MM, et al. Comparison of the 2005 growth charts for Saudi children and adolescents to the 2000 CDC growth charts. Ann Saudi Med. 2008;28:334–340. doi: 10.5144/0256-4947.2008.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.El Mouzan M, Foster P, Al Herbish A, Al Salloum A, Al Omer A, Alqurashi M, et al. Regional variations in the growth of Saudi children and adolescents. Ann Saudi Med. 2009;29:348–356. doi: 10.4103/0256-4947.55163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.El Mouzan MI, Al Herbish AS, Al Salloum AA, Al Omer AA, Qurachi MM. Regional prevalence of short stature in Saudi school-age children and adolescents. Scientific World Journal. 2012;2012:505709. doi: 10.1100/2012/505709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Al-Hazzaa HM. Physical activity, fitness and fatness among Saudi children and adolescents: implications for cardiovascular health. Saudi Med J. 2002;23:144–150. [PubMed] [Google Scholar]
- 30.US Department of Health and Human Services. 2008 Physical Activity Guidelines for Americans. [cited 2008]. Available from URL: https://health.gov/paguidelines/pdf/paguide.pdf .
- 31.Redwine KM, Acosta AA, Poffenbarger T, Portman RJ, Samuels J. Development of hypertension in adolescents with pre-hypertension. J Pediatr. 2012;160:98–103. doi: 10.1016/j.jpeds.2011.07.010. [DOI] [PubMed] [Google Scholar]
- 32.Yanagihara T, Kuroda N, Hayakawa M, Yoshida J, Tsuchiya M, Yamauchi K, et al. Epidemiology of school urinary screening over a 30 year period in Tokyo. Pediatr Int. 2007;49:570–576. doi: 10.1111/j.1442-200X.2007.02426.x. [DOI] [PubMed] [Google Scholar]
- 33.Yamagata K, Iseki K, Nitta K, Imai H, Iino Y, Matsuo S, et al. Chronic kidney disease perspectives in Japan and the importance of urinalysis screening. Clin Exp Nephrol. 2008;12:1–8. doi: 10.1007/s10157-007-0010-9. [DOI] [PubMed] [Google Scholar]
- 34.Cho BS, Kim SD. School urinalysis screening in Korea. Nephrology (Carlton) 2007;12(Suppl3):S3–S7. doi: 10.1111/j.1440-1797.2007.00873.x. [DOI] [PubMed] [Google Scholar]
- 35.Lin CY, Hsieh CC, Chen WP, Yang LY, Wang HH. The underlying diseases and follow-up in Taiwanese children screened by urinalysis. Pediatr Nephrol. 2001;16:232–237. doi: 10.1007/s004670000529. [DOI] [PubMed] [Google Scholar]
- 36.El-Tayeb M. ESMESHEY Screening of proteinuria in young adults: Is it worthwhile? Dialysis & Transplantation. 2010;39:522–526. [Google Scholar]
- 37.El-Shafie AM, El-Nemr FM, Bahbah MB, Shokry M, Attia A. The Role of Urine Screening (In School Children of Menoufiya Governorate) In Early Detection of Renal Disorders. Journal of American Science. 2014;10:143–150. [Google Scholar]
- 38.Hajar F, Taleb M, Aoun B, Shatila A. Dipstick urine analysis screening among asymptomatic school children. N Am J Med Sci. 2011;3:179–184. doi: 10.4297/najms.2011.3179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yap HK, Quek CM, Shen Q, Joshi V, Chia KS. Role of urinary screening programmes in children in the prevention of chronic kidney disease. Ann Acad Med Singapore. 2005;34:3–7. [PubMed] [Google Scholar]


