Abstract
Gamma radiation is known to induce cell death in several organs. This damage is associated with endonuclease-mediated DNA fragmentation; however, the enzyme that produces the latter and is likely to cause cell death is unknown. To determine whether the most abundant cytotoxic endonuclease DNase I mediates γ-radiation-induced tissue injury, we used DNase I knockout mice and zinc chelate of 3,5-diisopropylsalicylic acid (Zn-DIPS), which, as we show, has DNase I inhibiting activity in vitro. The study demonstrated for the first time that inactivation or inhibition of DNase I ameliorates radiation injury to the white pulp of spleen, intestine villi and bone marrow as measured using a quantitative TUNEL assay. The spleen and intestine of DNase I knockout mice were additionally protected from radiation by Zn-DIPS, perhaps due to the broad radioprotective effect of the zinc ions. Surprisingly, the main DNase I-producing tissues such as the salivary glands, pancreas and kidney showed no effect of DNase I inactivation. Another unexpected observation was that even without irradiation, DNA fragmentation and cell death were significantly lower in the intestine of DNase I knockout mice than in wild-type mice. This points to the physiological role of DNase I in normal cell death in the intestinal epithelium. In conclusion, our results suggested that DNase I-mediated mechanism of DNA damage and subsequent tissue injury are essential in γ-radiation-induced cell death in radiosensitive organs.
INTRODUCTION
Ionizing radiation induces DNA strand breaks through the generation of reactive oxygen species (ROS) that hydrolyze deoxyribose (1) or by internucleosomal endonuclease cleavage of phosphodiether bonds (2). The majority of radiation-induced DNA breaks and cell death appear to be a result of endonuclease-mediated cleavage, which occurs several hours after the initial impact (2). The exact endonuclease that mediates radiation-induced DNA fragmentation is unknown. Several cytotoxic (apoptotic) endonucleases have been shown to be induced in response to γ or β-particle irradiation, including deoxyribonuclease (DNase) I (3, 4), DNase II (5), DNase γ (6) and caspase-activated DNase (CAD) (7). Recent study showed that inhibition of DNase γ in rat splenocytes protected them from γ-radiation injury (8). The inhibition of other endonucleases in vivo had not been used previously for radioprotection because inhibitors of these enzymes that can be used in vivo are virtually nonexistent and the knockout animals were not available.
Our studies using recently developed DNase I knockout mice demonstrated that DNase I is the key enzyme for DNA fragmentation that mediates organ toxicity (9, 10). These studies also identified DNase I as the most active DNA degrading enzyme, at least in some tissues.
DNase I is universally expressed in all cell types and tissues (11). In digestive organs such as the pancreas and salivary glands, it is a secreted enzyme that hydrolyzes DNA consumed with food. In non-digestive tissues (for example, kidney or prostate), the role of DNase I is unknown. In the cells, the enzyme is located in the endoplasmic reticulum or cytoplasm (12). It has also been found inside nuclei, but the mechanism of its introduction into nuclei has not been studied. No known nuclear localization signal was identified in DNase I, and passive leakage through nuclear pores was suggested (13). DNase I degrades DNA by a “double-hit” mechanism, independently cleaving opposite DNA strands (11). The enzyme from all sources endonucleolytically cleaves double- or single-stranded DNA to 39′H and 5′P end oligonucleotides, requires bivalent cations, particularly Ca2+ and Mg2+, and is inhibited by Zn2+z (11, 14).
The ability of DNase I and some other apoptotic endonucleases to generate 3′OH DNA termini during apoptosis is used to identify injured or dead cells by the terminal deoxynucleotidyl transferase (TdT)-mediated deoxyuridine-triphosphate (dUTP)-biotin nick-end labeling (TUNEL) assay. TUNEL is reliable and is the most commonly used assay for identifying apoptotic cells in tissues. Recent studies showed its usefulness for measuring radiation-induced injury (15-17). This assay was applied as our main end point to assess the radiation injury. Cell number, which is affected by the intensity of cell death and delay in cell proliferation, was used as a secondary end point.
The goal of the present study was to determine whether inactivation of DNase I can cause amelioration of DNA fragmentation and thus cell injury caused by γ radiation in the spleen, intestine and bone marrow in mice. The radiation-induced acute injury of these organs is an important component of the acute radiation syndrome that determines survival after acute exposure to radiation. To study the role of DNase I, we used DNase I knockout mice. In addition, we applied a stable zinc chelate of 3,5-diisopropylsalicylic acid, Zn-DIPS, which, as we show, is a DNase I inhibitor in vitro.
METHODS
Plasmid Incision Assay
Recombinant DNase I (Genentech, South San Francisco, CA) and rat blood serum, which is known to contain highly active DNase I (18), were used in the pBR322 plasmid incision assay as described previously (19). Digested plasmid DNA was subjected to 1% agarose gel electrophoresis at 7 V/cm for 1 h at room temperature. The gel was stained in 0.5 μg/ml ethidium bromide solution for 20 min and photographed under UV light. An EagleEye scanning densitometer (Stratagene, La Jolla, CA) was used to quantify the relative amount of endonuclease-treated plasmid DNA present in covalently closed circular DNA (form 1), open circular DNA (form 2), or linear DNA (form 3). One DNase/endonuclease unit was the amount of the enzyme required to convert 1 μg DNA from form 1 to forms 2 and 3.
Total-Body Irradiation of Mice
DNase I knockout mice (CD-1 background) were generated by T. Moroy's group at the University of Essen, Germany (20). The mice were bred as heterozygotes and genotyped by PCR. At the age of 10 to 12 weeks, female DNase I knockout and wild-type mice were subjected to a single whole-body irradiation. Sixteen or 36 h postirradiation, the mice were killed humanely, and organs (jejunum, ileum, spleen, femur with bone marrow, kidney, pancreas and salivary gland) were collected and fixed in 10% buffered formalin (Sigma, St. Louis, MO). All protocols involving animal were approved by the Animal Care and Use Committee of the Central Arkansas Veterans Healthcare System.
Radioprotection using Zn-DIPS
Zinc chelate of 3,5-diisopropylsalicylic acid (DIPS), Zn-DIPS, was provided by Dr. John R. J. Sorenson, College of Pharmacy, University of Arkansas for Medical Sciences. It was tested for radioprotection in mice exposed to γ radiation as described above. The compound was administered subcutaneously (10 mg/kg per dose, 5.6 mg/ml in saline) before and after (two doses at 24 h and 4 h before irradiation and two doses at 4 h and 8 h after irradiation) or only after irradiation (two doses at 4 h and 8 h postirradiation). The time for Zn-DIPS administration was selected based on the knowledge that radiation causes detectable pro-apoptotic changes in cells in several hours (21-23).
TUNEL Assay, Histochemistry and Image Analyses
After fixation for 24 h, tissue samples were dehydrated and embedded in paraffin using methyl benzoate (Sigma) as an intermediate solution. For cutting, the jejunum, ileum and kidney were oriented cross-sectionally; the rest of the organs were oriented longitudinally. For staining, 4-μm-thick sections were cut, dewaxed, rehydrated in phosphate-buffered saline (PBS) (10 mM sodium phosphate buffer, pH 7.4, 140 mM NaCl), and processed for the TUNEL assay using the In Situ Cell Death Detection Kit (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's protocol. Each section was probed with a reaction mixture of TdT and fluorescein (FITC)-labeled precursor in cacodylate-based buffer for 1 h at 37°C, rinsed with 0.05% Tween-20 in PBS three times, and then mounted under a ProLong® Antifade medium containing 4′,6-diamidino-2-phenylindole dihydrochloride (DAPI) (Invitrogen). Control of TUNEL specificity was performed by substitution of the mixture of TdT and probe with the cacodylate buffer. A set of short-band filters with respective emission and excitation wavelengths were used for the green spectrum (FITC) and blue spectrum (DAPI) for TUNEL-positive objects and nuclei, respectively. Images were taken using an Olympus IX-81 microscope (Olympus America Inc., Center Valley, PA) equipped with a Hamamatsu ORCA-ER digital camera (Hamamatsu Photonics K.K., Hamamatsu City, Japan). Slidebook 4.2 software (SciTech Pty Ltd., Australia) was used for the image capturing and analysis. Major tissue compartments (villi, epithelium, stroma, crypt area, bone marrow, spleen white pulp) were marked manually. The areas of the total nuclear DNA (DAPI-positive) and fragmented nuclear DNA (TUNEL-positive) were masked and measured within each tissue compartment under investigation. The results were presented as the percentage of TUNEL-positive nuclear DNA area in the total nuclear DNA area calculated for individual cells.
Statistical Analysis
All parametric results were expressed as means ± SEM. The significance of difference in mean values between groups was examined with an analysis of variance (ANOVA) followed by a parametric Student's t test and non-parametric Mann-Whitney U test (SPSS 13.0 for Windows, SPSS Inc., Chicago, IL). A P value <0.05 was considered statistically significant.
RESULTS
Inhibition of DNase I by Zn-DIPS In Vitro
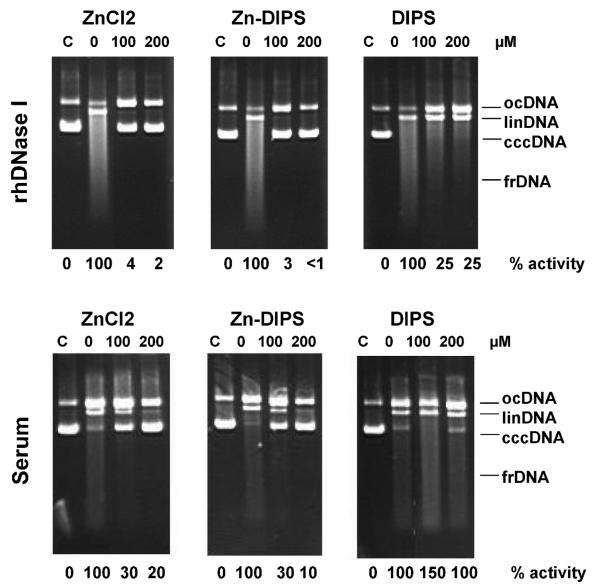
There are no inhibitors that suppress the activity of DNase I (or any other endonuclease) activity in vivo. To have a complementary approach for studying the role of DNase I in radiation injury, we tested Zn-DIPS. In this chelate, DIPS is a hydrophobic ligand that was expected to deliver zinc, an in vitro pan-endonuclease inhibitor, through plasma and intracellular membranes. In this experiment, recombinant DNase I and blood serum DNase [also mainly DNase I (20)] were tested in vitro using a plasmid incision assay (Fig. 1). ZnCl2 was used as positive control and DIPS (ligand) as a negative control. Our experiments showed that both Zn-DIPS and ZnCl2 inhibited recombinant DNase I and serum DNase activities, while the ligand had no effect. In the studies discussed below, we used Zn-DIPS in vivo. Because endonucleases are usually activated for an extended period after the impact, we tested whether Zn-DIPS would act if injected both before and after or only after irradiation.
FIG. 1.
Inhibition of human recombinant DNase I or rat serum DNase (Serum) in the presence of Znchloride, Zn-DIPS or DIPS (0, 100 or 200 μM). C, control non-digested pBR322 plasmid DNA. Circular covalently closed DNA (cccDNA) is converted first to open circular DNA (ocDNA), then to linear DNA (linDNA) and finally is fragmented (frDNA). Endonuclease activity is shown as a percentage of 0 μM samples.
DNA Fragmentation Induced by Total-Body Irradiation
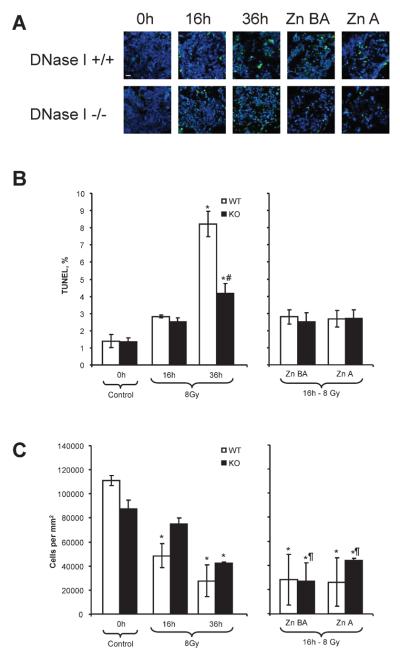
Mice were irradiated with 8 Gy and endonuclease-mediated DNA fragmentation was measured using the TUNEL assay in the organs 16 or 36 h later. The dose was chosen because it is close to the LD50/30 dose in mice (24); the choice of the times was based on previous studies demonstrating that DNA fragmentation detectable by TUNEL develops 14–18 h after irradiation and sometimes extends up to 40 h (21, 25). Radiation induced marked DNA fragmentation in the different organs. As expected, the organs with the most prominent DNA fragmentation, indicating the strongest apoptosis, were the intestine, bone marrow and spleen (white pulp). In the majority of organs, the DNA fragmentation was higher at 16 h and went down at 36 h.
Effects of DNase I Knockout or Zn-DIPS in the Radiation-Induced Injury of Jejunum
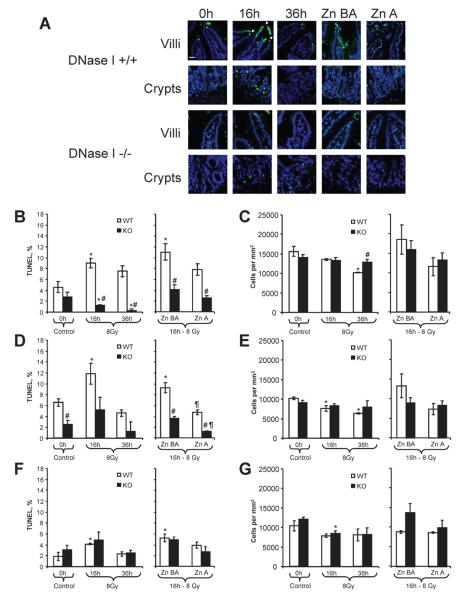
We analyzed jejunum and ileum separately because they often show different radiation responses (26, 27). In the intestine, villi and crypts were analyzed separately because they often differ in their radiation responses (28). In general, the radiation injury in the jejunum villi was greater than in ileum, while the injury of ileum crypts was greater than in the jejunum. The injury was assessed by quantification of TUNEL-type DNA fragmentation and cell numbers determined by nuclear DAPI staining (Fig. 2A). The effect of radiation on jejunal compartments was unevenly distributed. We commonly observed that villi were affected by radiation to a much higher degree than crypts. Frequently, jejunal villi in irradiated mice had a characteristic “gap” look at the tips of the villi. In all epithelial and stromal compartments of villi and in the crypts of the wild-type mouse jejunum, the percentage of TUNEL-positive cells increased significantly by 133%, 96% and 130%, respectively, at 16 h after irradiation (Fig. 2B, D, F). The tissue injury in knockout mice was significantly reduced by 86% and 60% in the epithelial and stromal compartments, respectively, compared to wild-type mice. Between the three studied compartments, the portion of TUNEL-positive cells was significantly less in stromal part of villi in knockout mice than in wild-type mice even before irradiation (Fig. 2D), indicating that DNase I may have a role in the normal physiological cell death of this intestinal compartment. Zn-DIPS injected both before and after irradiation or only after irradiation reduced DNA fragmentation in villi and sometimes below the control (Fig. 2B, D). In this experiment, injury in the stromal compartment was not affected by Zn-DIPS. The evaluation of the cell numbers by nuclear counting (DAPI staining) supported the TUNEL data; however, the changes were less prominent (Fig. 2C, E, F). Irradiation decreased the cell numbers in epithelial and stromal parts of villi in wild-type mice, while the numbers remained unaffected in DNase I knockout mice at 36 h (Fig. 2C, E). Zn-DIPS provided some radioprotection, in terms of cell numbers; however, it did not reach significance in these cases (Fig. 2C, E). Taken together, these data showed that genetic inactivation of DNase I or the use of Zn-DIPS ameliorated radiation-induced injury of jejunum.
FIG. 2.
Amelioration of radiation-induced injury of jejunum in wild-type (WT) and DNase I knockout (KO) mice treated with or without Zn-DIPS. Panel A: Representative images of the radiation-induced DNA fragmentation (TUNEL, green staining) and reduced cell numbers (DAPI, blue color) in villi and crypts. TUNEL-positive cells are present in the epithelium and stroma. Villi of wild-type animals often had interruptions of epithelial line (gaps) at 16 h after irradiation. Scale bar 20 mm. Quantification of TUNEL (panels B, D, F) and cell numbers (panels C, E, G) was performed in the epithelial (panels B, C) and stromal (panels D, E) parts of villi and in the crypt part of the intestine (panels F, G). *P < 0.05 compared to control mice of same genotype, #P < 0.05 compared to irradiated wild-type mice for the same time/treatment, ¶P < 0.05 compared to irradiated mouse of the same genotype at 16 h. n = 6–12 per group.
Effects of DNase I Knockout or Zn-DIPS in the Radiation-Induced Injury of the Ileum
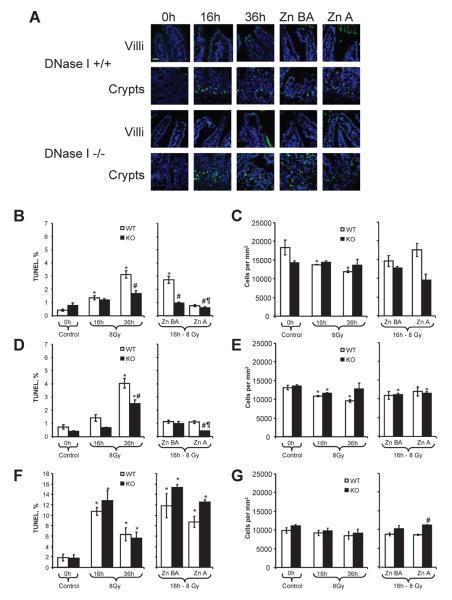
Similarly to the jejunum, our data showed that DNase I knockout or the use of Zn-DIPS ameliorated radiation-induced injury of ileum. Both visually and numerically, the injury of the ileum was very different from that of the jejunum. In the ileum, crypts were affected by radiation approximately seven to eight times more than villi, and thus the injury at 36 h was not less but rather was greater in villi than at 16 h after irradiation (Fig. 3A, B, D). The ileal villi of knockout mice were protected at 36 h (Fig. 3B, D). Zn-DIPS was very effective in decreasing DNA fragmentation to the normal levels in the villi of wild-type mice (Fig. 3B, D). Notably, Zn-DIPS administration caused some additional protection in knockout mice. In the ileal crypts, the injury as measured by TUNEL again peaked at 16 h after irradiation, and similar to the jejunal crypts, no protection was observed in knockout mice (Fig. 3F). Zn-DIPS also had no radioprotective effects in the ileal crypts. In this experiment, radiation decreased the cell numbers moderately in both compartments of villi, while knockout of the DNase I gene and treatment with Zn-DIPS ameliorated the effect (Fig. 3C, E). There was a slight but not significant decrease in cell numbers in the ileal crypts (Figure 3G). Administration of Zn-DIPS did not cause significant changes in this part of the experiment; however, similar to the jejunal crypts, knockout mice had higher cell numbers than wild-type mice. This may be explained by a higher rate of proliferation and possibility migration of other cells to the crypt zone.
FIG. 3.
Amelioration of radiation-induced injury to ileum in wild-type (WT) and DNase I knockout (KO) mice treated with or without Zn-DIPS. Panel A: Representative images of the radiation-induced DNA fragmentation (TUNEL, green staining) and reduced cell numbers (DAPI, blue color) in villi and crypts. Scale bar 20 μm. Quantification of TUNEL (panels B, D, F) and cell numbers (panels C, E, G) was performed in the epithelial (panels B, C) and stromal (panels D, E) villi and in the crypt part of the intestine (panels F, G). *P < 0.05 compared to control mice of the same genotype, #P < 0.05 compared to irradiated wild-type mice for the same time/treatment, ¶P < 0.05 compared to irradiated mouse of the same genotype at 16 h. n = 6–12 per group.
Effects of DNase I Knockout or Zn-DIPS in the Radiation-Induced Injury of Spleen
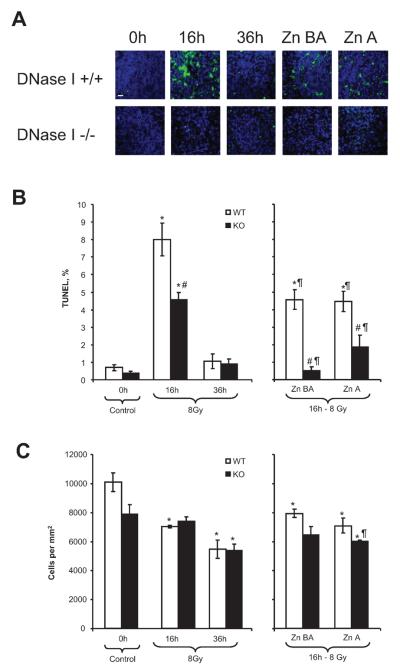
Total-body irradiation induced DNA fragmentation in the white pulp of the spleen and was the highest (864% over control) at 16 h (Fig. 4A, B). DNase I knockout mice were partially protected against radiation-induced DNA fragmentation at this time (Fig. 4A, B). In both schemes of administration, Zn-DIPS ameliorated the cytotoxic effect of radiation not only in wild-type but also in knockout mice, suggesting that in addition to DNase I, another zinc-inhibiting mechanism may be important for the death of splenocytes. Cell numbers decreased steadily at 16 and 36 h after irradiation, respectively (Fig. 4C). Administration of Zn-DIPS did not protect the cell numbers in wild-type mice and provided only partial protection in knockout mice when administered before and after irradiation. The loss of cell numbers while DNA fragmentation was diminished may be explained by the continuous washout of the peripheral cells due to irradiation. Therefore, the data showed that DNase I knockout or Zn-DIPS ameliorated radiation-induced injury of the white pulp of the spleen.
FIG. 4.
Amelioration of radiation-induced injury of the spleen (white pulp) in wild-type (WT) and DNase I knockout (KO) mice with or without Zn-DIPS. Panel A: Representative images of the radiation-induced DNA fragmentation (TUNEL, green staining) and reduced cell number (DAPI, blue color) in white pulp. Scale bar 20 mm. Quantification of TUNEL (panel B) and cell numbers (panel C) in white pulp. *P < 0.05 compared to control mice of same genotype, #P < 0.05 compared to irradiated wild-type mice for the same time/treatment, ¶P < 0.05 compared to irradiated mouse of the same genotype at 16 h. n = 6–12 per group.
Effects of DNase I Knockout or Zn-DIPS in the Radiation-Induced Injury of Bone Marrow
Unlike in the spleen, the maximum DNA fragmentation in bone marrow was at 36 h (Fig. 5A, B). Cell numbers decreased progressively at 16 and 36 h after irradiation, and Zn-DIPS did not significantly affect either DNA fragmentation or cell numbers (Fig. 5C). DNase I knockout mice demonstrated partial protection. Although we did not specifically examine the nature of the TUNEL-positive cells in bone marrow, judging by the morphological features, all hematopoietic cells were injured, with some predominance of myeloid cells and slightly less in lymphoid cells. Therefore, these data also showed that DNase I knockout ameliorated the delayed radiation-induced injury of bone marrow.
FIG. 5.
Amelioration of radiation-induced injury in bone marrow from femurs in wild-type (WT) and DNase I knockout (KO) mice treated with or without Zn-DIPS. Panel A: Representative images of the radiation-induced DNA fragmentation (TUNEL, green staining) and reduced cell numbers (DAPI, blue color) in bone marrow. Scale bar 20 μm. Quantification of the TUNEL (panel B) and cell numbers (panel C) in bone marrow. *P < 0.05 compared to control mice of same genotype, #P < 0.05 compared to irradiated wild-type mice for the same time/treatment, ¶P < 0.05 compared to irradiated mouse of the same genotype at 16 h. n = 6–12 per group.
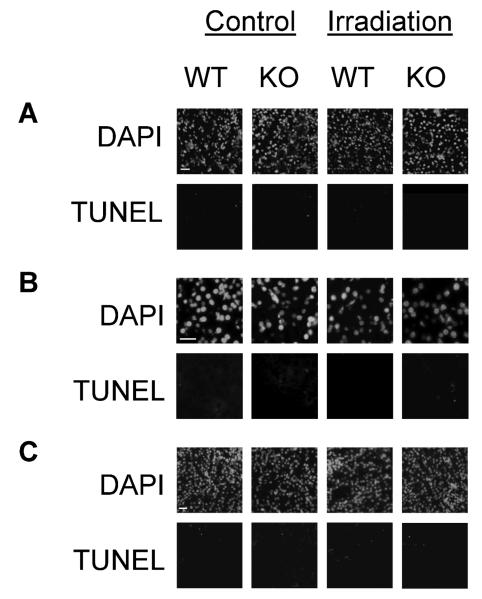
Effects of DNase I Knockout or Zn-DIPS in the Radiation-Induced Injury of Other Organs
Surprisingly, DNase I knockout had no effect in other DNase I-positive organs, some of which are known to be major producers of this enzyme (Fig. 6). The pancreas and salivary glands, the source of the DNase I delivered to gut, did not have any substantial tissue injury after irradiation in either wild-type or DNase I knockout mice. The area of the TUNEL-positive nuclear DNA in the kidneys was less than 0.5% in control and irradiated animals with no significant difference between the two groups (data not shown) and was located mainly in endothelial cells and cellular debris in the tubular lumen.
FIG. 6.
DNase I has no effect on radiation-induced injury of major DNase I-producing organs: salivary glands (panel A), pancreas (panel B) and kidney (panel C). Scale bars 20 μm.
DISCUSSION
In the current study, we hypothesized that DNase I inhibition could be applied as a countermeasure for γ-radiation injury in vivo. This approach to radioprotection challenges the existing paradigm by identifying an apoptotic (cytotoxic) endonuclease as the key modulator of tissue radiosensitivity. Using DNase I knockout mice as the primary model, we determined the essential role of DNase I in radiosensitivity of the lymphoid compartment of the spleen (white pulp), bone marrow and small intestine, injuries to which are known to cause radiation syndrome in mammals. Techniques used in previous studies demonstrating a potential role of DNase I in radiation are not sensitive enough to be sure that DNase I, as opposed to another DNase I-like endonuclease, is actually the enzyme that has been measured (3, 4).
Our secondary approach was using Zn-DIPS as a likely pan-endonuclease inhibitor, which, as we showed for the first time, has strong anti-DNase I activity in vitro. Zn-DIPS has been described previously as a radioprotector (29); however, its activity toward DNase I had not been studied. Unlike zinc salts, zinc chelates are preferable for use in vivo because they are the native form of zinc in vivo and can deliver zinc through cellular membranes (29). Our main concern while using Zn-DIPS was that zinc would not dissociate from the ligand, and thus the Zn-chelate would not be active in vitro. The data suggested that it does dissociate because its inhibiting activity toward DNase I was as powerful as that of zinc chloride. Injection of Zn-DIPS provided protection of the spleen and epithelial and stromal compartments of the intestine to the levels observed in DNase I knockout mice. Importantly, this protection was almost independent of whether the Zn-chelate was injected both before and after irradiation or only after irradiation. This indicates that the Zn-chelate is a powerful inhibitor of DNA fragmentation and subsequent tissue injury induced by endonucleases even after the direct impact of radiation.
In the intestine, the DNA fragmentation indicative of cell death and the desquamation of epithelial cells were especially profound on the tips of villi in jejunum. The jejunal crypts as well as the ileal villi and crypts appeared to be less affected. This supports previous observations that TUNEL-positive cells can be visualized in nonirradiated and irradiated animals in the crypts and on the tips of villi (28, 30). DNase I knockout mice were completely protected against radiation in the jejunal villi. While both epithelial and stromal cells in the villi were protected from radiation in DNase I knockout mice, the crypts were protected slightly or not at all. These data indicate the presence of different cell death mechanisms in those compartments. Previously, Marsh-man et al. observed increased TUNEL staining in mouse intestine after irradiation, but caspase 3 was activated in crypts but not in villi (28). This suggested that at least in villi, apoptosis is likely to be independent of caspase. In support of this, Paris et al. (21) demonstrated that radiation-induced damage of epithelial stem cells in crypts is due to primary endothelial cell injury followed by hypoxia of crypts. This indirectly indicates that the origin of cell death in crypts and villi epithelium may differ.
Our unexpected observation was that even DNA fragmentation and cell death under physiological conditions (without γ irradiation) were significantly lower in the intestine of DNase I knockout mice than in wild-type mice. This is the evidence of the physiological role of DNase I in the death of intestinal epithelial cells and desquamation. Apparently, DNase I activity and/or nuclear DNA accessibility to DNase I are increased after irradiation and increase cell death. It is not clear, though, whether the DNase I that causes intestinal epithelial apoptosis originated from the same cells (endogenous DNase I) or was internalized from the lumen (exogenous DNase I). Some studies show that DNase I and DNase I-like proteins can be consumed by cells and act as toxins (31, 32). It is likely that internalization of exogenous DNase I was important, because the tips of villi were damaged the most, and the ileum, which is located downstream of the jejunum, was damaged less. Consequently, if DNase I is consumed from the lumen, the pancreas should probably be the major source. However, neither the pancreas nor the salivary glands, the two major producers of DNase I in the gut, were affected by radiation and thus showed no difference in DNase I knockout and wild-type mice. It is possible that these DNase I-producing organs have an unknown defense mechanism against endogenous DNase I.
In our experiments, the DNA fragmentation in the white pulp of the spleen was the highest among the radiation-sensitive organs. The white pulp in DNase I knockout mice was partially protected from radiation-induced injury. Our data do not rule out a potential role of other endonucleases, because administration of Zn-DIPS in DNase I knockout mice provided additional radioprotection effect as measured by TUNEL-type DNA fragmentation and cell numbers. It is also possible that DNase I may activate other endonucleases. For example, we have recently shown that EndoG is regulated by DNase I, and thus EndoG may also be involved (33).
Partial inactivation of TUNEL in DNase I knockout mice also indicates that other endonucleases may be involved in the DNA fragmentation induced by radiation. In fact, some other studies suggested the possible involvement of other endonucleases in radiation-induced lymphoid and hematopoietic cell injury. For example, Nakagami et al. showed that DNase II and acid phosphatase translocated to the nuclei after irradiation of HL60 human myeloid leukemia cells and generated TUNEL-positive DNA breaks by the combined action of the enzymes (5). McIlroy et al. reported that exposing Jurkat cells to γ radiation induced caspase-activated DNase (7). Exposure of transgenic mice that expressed a caspase-resistant form of the CAD inhibitor (ICAD) to γ radiation did not increase TUNEL-positive cells compared to wild-type mice, suggesting that CAD (DFF40) may be important for radiosensitivity (7). Shiokawa et al. isolated DNase γ from rat thymocytes that was induced by radiation (34). A recent study from the same group demonstrated that a novel inhibitor of DNase γ, DR396, inhibited apoptosis in rat splenocytes exposed to γ radiation in a dose-dependent manner (8). This appears to be the only study describing radioprotection by inhibiting an endonuclease.
Our proposal that zinc, a pan-endonuclease inhibitor, can be delivered in vivo by a Zn-chelate to suppress DNA fragmentation appears to be correct. Radioprotection by Zn-DIPS in almost all the tissues and organ compartments we examined was independent of whether it is administered both before and after or only after irradiation. These data suggest that endonuclease activity is harmful only after irradiation and that Zn-DIPS can be used as a powerful postirradiation radioprotector. Administration of the Zn-DIPS also enhanced the radioprotection achieved in the DNase I knockout. This may have resulted from Zn-DIPS affecting other cell death mechanisms induced by radiation (for example, other endonucleases or caspases).
Radioprotection by zinc compounds has been shown in numerous studies. Floersheim and Floersheim were the first to report a remarkable radioprotective effect of Zn-aspartate in increasing the survival of irradiated mice (35). Subcutaneous injection of ZnCl2 before irradiation was shown to increase the survival of mice (36). Zinc salts protect cells from γ radiation (37, 38). Znmetallothionein provided protection from radiation-induced DNA damage that was greater that from Cumetallothionein or Cd-metallothionein (39). Promotion of recovery after irradiation has been suggested as a potential mechanism of zinc protection (29). We realize that Zn-DIPS may have broader effects than just inhibition of DNase I. However, the fact that Zn-DIPS did not have a preventive effect in DNase I knockout mice suggested that Zn-DIPS action is mediated by DNase I. Zinc is not specific, but it is the only known universal inhibitor of cytotoxic endonucleases. With the exception of cation-independent DNase II, all known cell death endonucleases, which represent about 90–95% of endonuclease activity in the cell, are inhibited by Zn2+ ions. The expression of the endonucleases varies in different tissues. However, DNase I is the most active endonuclease in the majority of cells.
In conclusion, our study demonstrated for the first time that DNase I is a mediator of cell death induced by γ radiation in the intestine, spleen and bone marrow. Inactivation of DNase I was radioprotective to these organs, while other organs known to produce more DNase I showed no response to radiation and no radioprotective effect of DNase I inactivation. Future studies will need to determine whether DNase I excreted by the pancreas can be internalized by the intestinal epithelium or whether this DNase originates from the intestinal epithelium itself. The search for specific DNase I inhibitors may lead to the discovery of powerful radioprotectors that act by inactivating this endonuclease. One such class of radioprotectors may be the Zn-chelates.
ACKNOWLEDGMENTS
This research was supported by the pilot grant from Duke University Radiation Countermeasures Center of Research Excellence (RadCCORE), the VA Merit Review grant and the National Institutes of Health /NIDDK grant to AGB.
REFERENCES
- 1.Bertoncini CR, Meneghini R. DNA strand breaks produced by oxidative stress in mammalian cells exhibit 3′-phosphoglycolate termini. Nucleic Acids Res. 1995;23:2995–3002. doi: 10.1093/nar/23.15.2995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Skalka M, Matyasova J. The effect of radiation on deoxyribonucleoproteins in animal tissue. 3. The character of the polydeoxyribonucleotides released from irradiated tissues. Fol. Biol. (Praha) 1967;13:457–464. [PubMed] [Google Scholar]
- 3.Swingle KF, Cole LJ. Radiation-induced free polydeoxyribonucleotides in lymphoid tissues: A product of the action of neutral deoxyribonuclease (DNase 1) Radiat. Res. 1967;30:81–95. [PubMed] [Google Scholar]
- 4.Tabachnick J, LaBadie JH. Increased activity of skin surface DNase I after beta-irradiation injury or clipping of guinea pig hair. J. Invest. Dermatol. 1970;55:89–93. doi: 10.1111/1523-1747.ep12291515. [DOI] [PubMed] [Google Scholar]
- 5.Nakagami Y, Ito M, Hara T, Inoue T, Matsubara S. Nuclear translocation of DNase II and acid phosphatase during radiation-induced apoptosis in HL60 cells. Acta Oncol. 2003;42:227–236. doi: 10.1080/02841860310010745. [DOI] [PubMed] [Google Scholar]
- 6.Tanuma S, Shiokawa D. Multiple forms of nuclear deoxyribonuclease in rat thymocytes. Biochem. Biophys. Res. Commun. 1994;203:789–797. doi: 10.1006/bbrc.1994.2252. [DOI] [PubMed] [Google Scholar]
- 7.McIlroy D, Tanaka M, Sakahira H, Fukuyama H, Suzuki M, Yamamura K, Ohsawa Y, Uchiyama Y, Nagata S. An auxiliary mode of apoptotic DNA fragmentation provided by phagocytes. Genes Dev. 2000;14:549–558. [PMC free article] [PubMed] [Google Scholar]
- 8.Nakajima T, Matsunaga T, Kawai S, Hokari S, Inoue I, Katayama S, Nagata A, Komoda T. Characterization of the epitopes specific for the monoclonal antibody 9F5-3a and quantification of oxidized HDL in human plasma. Ann. Clin. Biochem. 2004;41:309–315. doi: 10.1258/0004563041201491. [DOI] [PubMed] [Google Scholar]
- 9.Basnakian AG, Apostolov EO, Yin X, Napirei M, Mannherz HG, Shah SV. Cisplatin nephrotoxicity is mediated by deoxyribonuclease I. J. Am. Soc. Nephrol. 2005;16:697–702. doi: 10.1681/ASN.2004060494. [DOI] [PubMed] [Google Scholar]
- 10.Napirei M, Basnakian AG, Apostolov EO, Mannherz HG. Deoxyribonuclease 1 aggravates acetaminophen-induced liver necrosis in male CD-1 mice. Hepatology. 2006;43:297–305. doi: 10.1002/hep.21034. [DOI] [PubMed] [Google Scholar]
- 11.Lacks SA. Deoxyribonuclease I in mammalian tissues. Specificity of inhibition by actin. J. Biol. Chem. 1981;256:2644–2648. [PubMed] [Google Scholar]
- 12.Peitsch MC, Polzar B, Stephan H, Crompton T, McDonald HR, Mannherz HG, Tschopp T. Characterization of the endogenous deoxyribonuclease involved in nuclear DNA degradation during apoptosis (programmed cell death) EMBO J. 1993;12:371–377. doi: 10.1002/j.1460-2075.1993.tb05666.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Polzar B, Peitsch MC, Loos R, Tschopp J, Mannherz HG. Overexpression of deoxyribonuclease I (DNase I) transfected into COS cells: its distribution during apoptotic cell death. Eur. J. Cell Biol. 1993;62:397–405. [PubMed] [Google Scholar]
- 14.Basnakian AG, Ueda N, Kaushal GP, Mikhailova MV, Shah SV. DNase I-like endonuclease in rat kidney cortex that is activated during ischemia/reperfusion injury. J. Am. Soc. Nephrol. 2002;13:1000–1007. doi: 10.1681/ASN.V1341000. [DOI] [PubMed] [Google Scholar]
- 15.Saito A, Yamauchi H, Ishida Y, Ohmachi Y, Nakayama H. Defect of the cerebellar vermis induced by prenatal gamma-ray irradiation in radiosensitive BALB/c mice. Histol. Histopathol. 2008;23:953–964. doi: 10.14670/HH-23.953. [DOI] [PubMed] [Google Scholar]
- 16.Laposa RR, Huang EJ, Cleaver JE. Increased apoptosis, p53 up-regulation, and cerebellar neuronal degeneration in repair-deficient Cockayne syndrome mice. Proc. Natl. Acad. Sci. USA. 2007;104:1389–1394. doi: 10.1073/pnas.0610619104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vyas D, Robertson CM, Stromberg PE, Martin JR, Dunne WM, Houchen CW, Barrett TA, Ayala A, Perl M, Coopersmith CM. Epithelial apoptosis in mechanistically distinct methods of injury in the murine small intestine. Histol. Histopathol. 2007;22:623–630. doi: 10.14670/hh-22.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yasuda T, Takeshita H, Ueki M, Iida R, Nakajima T, Mori S, Mogi K, Kaneko Y, Kishi K. Tissue-specific in vivo inhibition of DNase I gene expression by somatostatin. Biochem. Biophys. Res. Commun. 2001;283:287–291. doi: 10.1006/bbrc.2001.4770. [DOI] [PubMed] [Google Scholar]
- 19.Wang X, Tryndyak V, Apostolov EO, Yin X, Shah SV, Pogribny IP, Basnakian AG. Sensitivity of human prostate cancer cells to chemotherapeutic drugs depends on EndoG expression regulated by promoter methylation. Cancer Lett. 2008;270:132–143. doi: 10.1016/j.canlet.2008.04.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Napirei M, Karsunky H, Zevnik B, Stephan H, Mannherz HG, Moroy T. Features of systemic lupus erythematosus in DNase1-deficient mice. Nat. Genet. 2000;25:177–181. doi: 10.1038/76032. [DOI] [PubMed] [Google Scholar]
- 21.Paris F, Fuks Z, Kang A, Capodieci P, Juan G, Ehleiter D, Haimovitz-Friedman A, Cordon-Cardo C, Kolesnick R. Endothelial apoptosis as the primary lesion initiating intestinal radiation damage in mice. Science. 2001;293:293–297. doi: 10.1126/science.1060191. [DOI] [PubMed] [Google Scholar]
- 22.Borges HL, Linden R. Gamma irradiation leads to two waves of apoptosis in distinct cell populations of the retina of newborn rats. J. Cell Sci. 1999;112:4315–4324. doi: 10.1242/jcs.112.23.4315. [DOI] [PubMed] [Google Scholar]
- 23.Pena LA, Fuks Z, Kolesnick RN. Radiation-induced apoptosis of endothelial cells in the murine central nervous system: protection by fibroblast growth factor and sphingomyelinase deficiency. Cancer Res. 2000;60:321–327. [PubMed] [Google Scholar]
- 24.Jagetia GC, Venkatesh P, Baliga MS. Fruit extract of Aegle marmelos protects mice against radiation-induced lethality. Integr. Cancer Ther. 2004;3:323–332. doi: 10.1177/1534735404270641. [DOI] [PubMed] [Google Scholar]
- 25.Tu Y, Jerry DJ, Pazik B, Smith Schneider S. Sensitivity to DNA damage is a common component of hormone-based strategies for protection of the mammary gland. Mol. Cancer Res. 2005;3:435–442. doi: 10.1158/1541-7786.MCR-05-0038. [DOI] [PubMed] [Google Scholar]
- 26.Kunjathoor VV, Febbraio M, Podrez EA, Moore KJ, Andersson L, Koehn S, Rhee JS, Silverstein R, Hoff HF, Freeman MW. Scavenger receptors class A-I/II and CD36 are the principal receptors responsible for the uptake of modified low density lipoprotein leading to lipid loading in macrophages. J. Biol. Chem. 2002;277:49982–49988. doi: 10.1074/jbc.M209649200. [DOI] [PubMed] [Google Scholar]
- 27.Sedgwick JD, Czerkinsky C. Detection of cell-surface molecules, secreted products of single cells and cellular proliferation by enzyme immunoassay. J. Immunol. Methods. 1992;150:159–175. doi: 10.1016/0022-1759(92)90075-5. [DOI] [PubMed] [Google Scholar]
- 28.Marshman E, Ottewell PD, Potten CS, Watson AJ. Caspase activation during spontaneous and radiation-induced apoptosis in the murine intestine. J. Pathol. 2001;195:285–292. doi: 10.1002/path.967. [DOI] [PubMed] [Google Scholar]
- 29.Sorenson JR. Cu, Fe, Mn, and Zn chelates offer a medicinal chemistry approach to overcoming radiation injury. Curr. Med. Chem. 2002;9:639–662. doi: 10.2174/0929867023370725. [DOI] [PubMed] [Google Scholar]
- 30.Mylonas PG, Matsouka PT, Papandoniou EV, Vagianos C, Kalfarentzos F, Alexandrides TK. Growth hormone and insulin-like growth factor I protect intestinal cells from radiation induced apoptosis. Mol. Cell. Endocrinol. 2000;160:115–122. doi: 10.1016/s0303-7207(99)00215-4. [DOI] [PubMed] [Google Scholar]
- 31.Oliveri M, Daga A, Lunardi C, Navone R, Millo R, Puccetti A. DNase I behaves as a transcription factor which modulates Fas expression in human cells. Eur. J. Immunol. 2004;34:273–279. doi: 10.1002/eji.200223817. [DOI] [PubMed] [Google Scholar]
- 32.Cortes-Bratti X, Frisan T, Thelestam M. The cytolethal distending toxins induce DNA damage and cell cycle arrest. Toxicon. 2001;39:1729–1736. doi: 10.1016/s0041-0101(01)00159-3. [DOI] [PubMed] [Google Scholar]
- 33.Yin X, Apostolov EO, Shah SV, Wang X, Bogdanov KV, Buzder T, Stewart AG, Basnakian AG. Induction of renal endonuclease G by cisplatin is reduced in DNase I-deficient mice. J. Am. Soc. Nephrol. 2007;18:2544–2553. doi: 10.1681/ASN.2006080896. [DOI] [PubMed] [Google Scholar]
- 34.Shiokawa D, Ohyama H, Yamada T, Tanuma S. Purification and properties of DNase gamma from apoptotic rat thymocytes. Biochem. J. 1997;326:675–681. doi: 10.1042/bj3260675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Floersheim GL, Floersheim P. Protection against ionising radiation and synergism with thiols by zinc aspartate. Br. J. Radiol. 1986;59:597–602. doi: 10.1259/0007-1285-59-702-597. [DOI] [PubMed] [Google Scholar]
- 36.Matsubara J, Shida T, Ishioka K, Egawa S, Inada T, Machida K. Protective effect of zinc against lethality in irradiated mice. Environ. Res. 1986;41:558–567. doi: 10.1016/s0013-9351(86)80150-5. [DOI] [PubMed] [Google Scholar]
- 37.Emonet-Piccardi N, Richard MJ, Ravanat JL, Signorini N, Cadet J, Beani JC. Protective effects of antioxidants against UVA-induced DNA damage in human skin fibroblasts in culture. Free Radic. Res. 1998;29:307–313. doi: 10.1080/10715769800300341. [DOI] [PubMed] [Google Scholar]
- 38.Mathieu J, Ferlat S, Ballester B, Platel S, Herodin F, Chancerelle Y, Mestries JC, Kergonou JF. Radiation-induced apoptosis in thymocytes: inhibition by diethyldithiocarbamate and zinc. Radiat. Res. 1996;146:652–659. [PubMed] [Google Scholar]
- 39.Uribarri J, Peppa M, Cai W, Goldberg T, Lu M, He C, Vlassara H. Restriction of dietary glycotoxins reduces excessive advanced glycation end products in renal failure patients. J. Am. Soc. Nephrol. 2003;14:728–731. doi: 10.1097/01.asn.0000051593.41395.b9. [DOI] [PubMed] [Google Scholar]