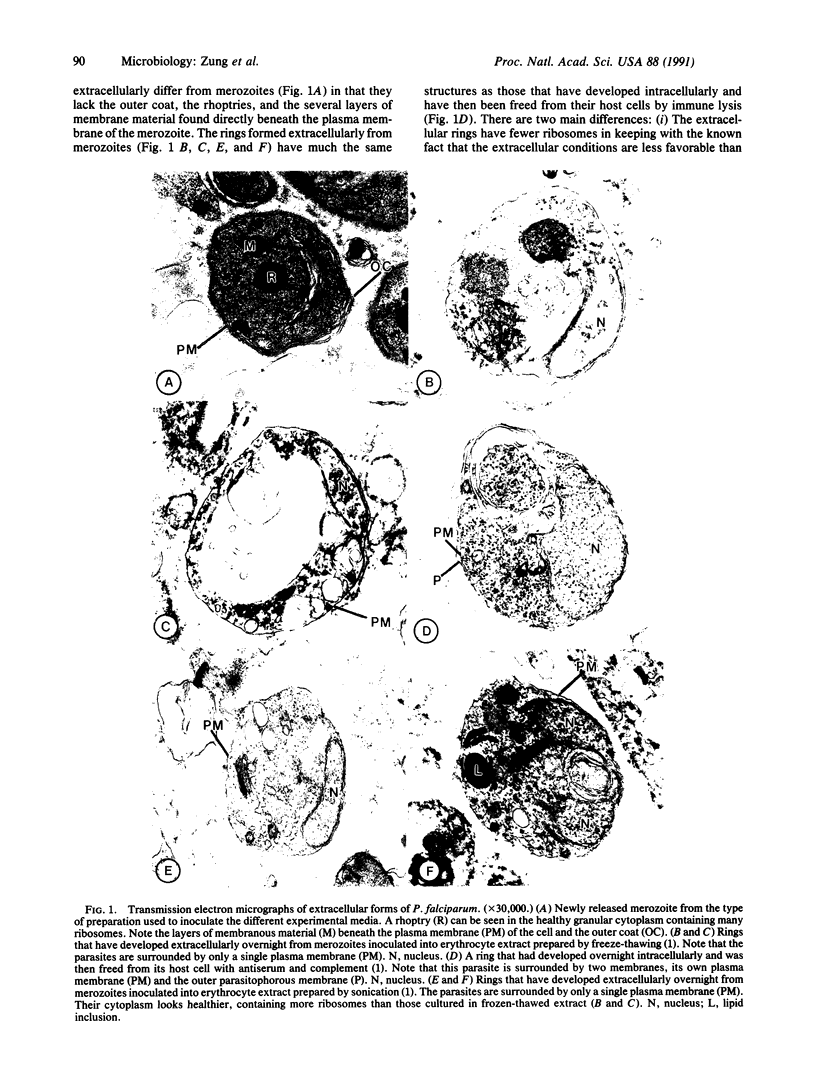
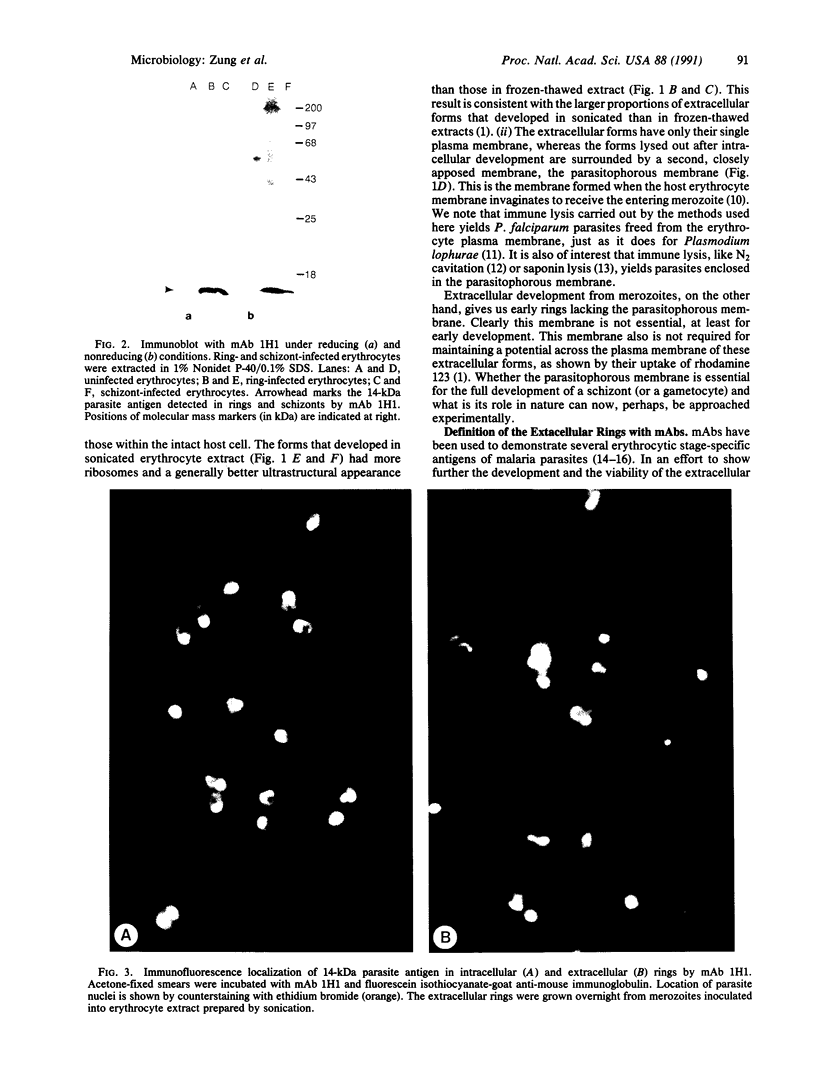
Abstract
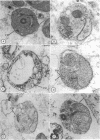
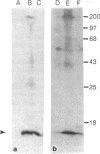
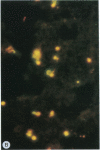
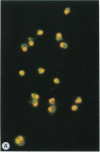
Merozoites of the erythrocytic stage of the human malaria parasite Plasmodium falciparum, when placed under appropriate conditions in a culture medium with erythrocyte extract, differentiate into early trophic forms. These forms have much the same ultrastructure as rings of the same age that have developed intracellularly and have then been freed from their host cells by immune lysis. However, these forms differ in two respects: the extracellular forms have only their single plasma membrane, whereas the forms freed from host cells have, in addition, a surrounding parasitophorous vacuole membrane; the forms that develop extracellularly have fewer ribosomes. Five monoclonal antibodies against the ring stage have been prepared and characterized. Their pattern of immunofluorescence localization differs in merozoites as compared with rings, but their pattern is identical in rings developed extracellularly and those developed intracellularly. These results and the observations on fine structure demonstrate biochemical and morphological differentiation in the extracellular forms.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Banyal H. S., Inselburg J. Isolation and characterization of parasite-inhibitory Plasmodium falciparum monoclonal antibodies. Am J Trop Med Hyg. 1985 Nov;34(6):1055–1064. doi: 10.4269/ajtmh.1985.34.1055. [DOI] [PubMed] [Google Scholar]
- Campbell G. H., Miller L. H., Hudson D., Franco E. L., Andrysiak P. M. Monoclonal antibody characterization of Plasmodium falciparum antigens. Am J Trop Med Hyg. 1984 Nov;33(6):1051–1054. doi: 10.4269/ajtmh.1984.33.1051. [DOI] [PubMed] [Google Scholar]
- Clark J. T., Anand R., Akoglu T., McBride J. S. Identification and characterisation of proteins associated with the rhoptry organelles of Plasmodium falciparum merozoites. Parasitol Res. 1987;73(5):425–434. doi: 10.1007/BF00538200. [DOI] [PubMed] [Google Scholar]
- Giloh H., Sedat J. W. Fluorescence microscopy: reduced photobleaching of rhodamine and fluorescein protein conjugates by n-propyl gallate. Science. 1982 Sep 24;217(4566):1252–1255. doi: 10.1126/science.7112126. [DOI] [PubMed] [Google Scholar]
- Hadley T. J. Invasion of erythrocytes by malaria parasites: a cellular and molecular overview. Annu Rev Microbiol. 1986;40:451–477. doi: 10.1146/annurev.mi.40.100186.002315. [DOI] [PubMed] [Google Scholar]
- Hall R., McBride J., Morgan G., Tait A., Zolg J. W., Walliker D., Scaife J. Antigens of the erythrocytes stages of the human malaria parasite Plasmodium falciparum detected by monoclonal antibodies. Mol Biochem Parasitol. 1983 Mar;7(3):247–265. doi: 10.1016/0166-6851(83)90025-7. [DOI] [PubMed] [Google Scholar]
- Holder A. A., Freeman R. R., Uni S., Aikawa M. Isolation of a Plasmodium falciparum rhoptry protein. Mol Biochem Parasitol. 1985 Mar;14(3):293–303. doi: 10.1016/0166-6851(85)90057-x. [DOI] [PubMed] [Google Scholar]
- Howard R. F., Stanley H. A., Campbell G. H., Reese R. T. Proteins responsible for a punctate fluorescence pattern in Plasmodium falciparum merozoites. Am J Trop Med Hyg. 1984 Nov;33(6):1055–1059. doi: 10.4269/ajtmh.1984.33.1055. [DOI] [PubMed] [Google Scholar]
- Izumo A., Tanabe K., Kato M. A method for monitoring the viability of malaria parasites (Plasmodium yoelii) freed from the host erythrocytes. Trans R Soc Trop Med Hyg. 1987;81(2):264–267. doi: 10.1016/0035-9203(87)90235-5. [DOI] [PubMed] [Google Scholar]
- Kearney J. F., Radbruch A., Liesegang B., Rajewsky K. A new mouse myeloma cell line that has lost immunoglobulin expression but permits the construction of antibody-secreting hybrid cell lines. J Immunol. 1979 Oct;123(4):1548–1550. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langreth S. G., Trager W. Fine structure of the malaria parasite Plasmodium lophurae developing extracellularly in vitro. J Protozool. 1973 Nov;20(5):606–613. doi: 10.1111/j.1550-7408.1973.tb03584.x. [DOI] [PubMed] [Google Scholar]
- Mikkelsen R. B., Wallach D. F., Van Doren E., Nillni E. A. Membrane potential of erythrocytic stages of Plasmodium chabaudi free of the host cell membrane. Mol Biochem Parasitol. 1986 Oct;21(1):83–92. doi: 10.1016/0166-6851(86)90082-4. [DOI] [PubMed] [Google Scholar]
- Murakami K., Tanabe K. An antigen of Plasmodium yoelii that translocates into the mouse erythrocyte membrane upon entry into the host cell. J Cell Sci. 1985 Feb;73:311–320. doi: 10.1242/jcs.73.1.311. [DOI] [PubMed] [Google Scholar]
- OUCHTERLONY O. Diffusion-in-gel methods for immunological analysis. Prog Allergy. 1958;5:1–78. [PubMed] [Google Scholar]
- Roger N., Dubremetz J. F., Delplace P., Fortier B., Tronchin G., Vernes A. Characterization of a 225 kilodalton rhoptry protein of Plasmodium falciparum. Mol Biochem Parasitol. 1988 Jan 15;27(2-3):135–141. doi: 10.1016/0166-6851(88)90033-3. [DOI] [PubMed] [Google Scholar]
- Sam-Yellowe T. Y., Shio H., Perkins M. E. Secretion of Plasmodium falciparum rhoptry protein into the plasma membrane of host erythrocytes. J Cell Biol. 1988 May;106(5):1507–1513. doi: 10.1083/jcb.106.5.1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samal B. B. Drying and storage of polyacrylamide slab gels: a simple procedure. Anal Biochem. 1987 May 15;163(1):42–44. doi: 10.1016/0003-2697(87)90090-x. [DOI] [PubMed] [Google Scholar]
- Schofield L., Bushell G. R., Cooper J. A., Saul A. J., Upcroft J. A., Kidson C. A rhoptry antigen of Plasmodium falciparum contains conserved and variable epitopes recognized by inhibitory monoclonal antibodies. Mol Biochem Parasitol. 1986 Feb;18(2):183–195. doi: 10.1016/0166-6851(86)90037-x. [DOI] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trager W., Lanners H. N. Initial extracellular development in vitro of merozoites of Plasmodium falciparum. J Protozool. 1984 Nov;31(4):562–567. doi: 10.1111/j.1550-7408.1984.tb05503.x. [DOI] [PubMed] [Google Scholar]
- Trager W., Zung J., Tershakovec M. Initial extracellular development in vitro of erythrocytic stages of malaria parasites (Plasmodium falciparum). Proc Natl Acad Sci U S A. 1990 Aug;87(15):5618–5622. doi: 10.1073/pnas.87.15.5618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiser M. F. Characterization of monoclonal antibodies directed against erythrocytic stage antigens of Plasmodium berghei. Eur J Cell Biol. 1986 Oct;42(1):45–51. [PubMed] [Google Scholar]