Abstract
Microglia are immune cells of the brain that display a range of functions. Most of our knowledge about microglia biology and function is based on cells from the rodent brain. Species variation in the complexity of the brain and differences in microglia response in the primate when compared with the rodent, require use of adult human microglia in studies of microglia biology. While methods exist for isolation of microglia from postmortem human brains, none allow culturing cells to high passage. Thus cells from the same case could not be used in parallel studies and multiple conditions. Here we report a method, which includes use of growth factors such as granulocyte macrophage colony stimulating factor, for successful culturing of adult human microglia from postmortem human brains up to 28 passages without significant loss of proliferation. Such cultures maintained their phenotype, including uptake of the scavenger receptor ligand acetylated low density lipoprotein and response to the amyloid-β peptide, and were used to extend in vivo studies in the primate brain demonstrating that inhibition of microglia activation protects neurons from amyloid-β toxicity. Significantly, microglia cultured from brains with pathologically confirmed Alzheimer’s disease displayed the same characteristics as microglia cultured from normal aged brains. The method described here provides the scientific community with a new and reliable tool for mechanistic studies of human microglia function in health from childhood to old age, and in disease, enhancing the relevance of the findings to the human brain and neurodegenerative conditions.
Keywords: Phenotype, primary human microglia cultures, reactive oxygen species, scavenger receptor ligand
INTRODUCTION
Microglia are macrophage lineage resident immune cells of the CNS that express pattern recognition receptors, which mediate their innate interactions with foreign or modified host proteins [1–5]. In response to various stimuli, microglia produce and secrete chemokines, such as monocyte chemoattractant protein-1 which stimulates recruitment of monocytes/microglia, cytokines such as interleukin-1β and tumor necrosis factor-α, and reactive oxygen species (ROS) [5, 6].
Pro-inflammatory and potentially neurotoxic activation of microglia, particularly in relation to neurodegenerative disorders and brain trauma, has been the subject of extensive experimental attention [6–9]. Similar to peripheral macrophages, microglia also display an anti-inflammatory and protective activation state [10, 11]. In fact, it is now clear that in addition to their inflammatory reaction in response to damaged or foreign proteins, microglia play important roles in normal brain function [12], including synaptic remodeling and pruning during development [13], and circuit plasticity [14, 15] and regulation of neuronal activity in the adult brain [12].
The evolutionary pressures that caused increased complexity of the CNS and culminated in the human brain, resulted in significant differences in nervous system structure and function. In addition to dramatic differences in anatomy and connectivity, there are differences in molecular signatures of cells in the human and non-human primate brains when compared with the rodent [16–18], with profound functional implications. Primate microglia also differ from their counterparts in the rodent. For example, we have shown that injections of small quantities of the fibrillar conformation of the amyloid-β peptide (Aβ) in the cerebral cortex of aged rhesus and marmoset monkeys result in significant recruitment and activation of microglia and neuronal loss [19]. Inhibition of microglia activation resulted in preservation of neurons [20]. In contrast, identical injections in the aged rat cortex failed to activate microglia [19]. Furthermore, it has been shown that rodent microglia produce and secrete nitric oxide in response to Aβ, while human microglia do not, in vitro or in vivo [21–24].
The overwhelming majority of studies available in the literature on microglia biology and function are based on cells from the rodent brain. Species differences in the structural and functional complexity of the brain suggest the use of human microglia in such studies to ensure results are applicable to humans. This suggestion is reinforced by the species differences observed in microglia response. Furthermore, since changes in the gene expression profiles of microglia have been described during development, it is critical to use human microglia from the mature adult brain in which many inflammatory and protective functions of microglia are detected.
A number of methods have been described for isolation of microglia from embryonic or adult postmortem human brains [25]. Microglia isolated from embryonic brains show a relatively high rate of proliferation [25, 26]. Despite this proliferative capacity, only one group of investigators attempted to culture human embryonic microglia to high passage (50 passages), with good yield [27]. A few groups have isolated and cultured microglia from adult human brains [25, 28]. The majority have specifically noted very low or absent basal rates of proliferation of isolated microglia in the resting state [25, 29, 30]. Two studies reported higher proliferation rates when microglia were exposed to granulocyte macrophage colony stimulating factor (GM-CSF) [31, 32]. However, even these did not attempt to passage microglia, presumably due to inadequate yield. Nearly all in vitro studies of adult human microglia have either isolated cells and used them directly in experiments (passage [P] 1), or cultured the isolated cells to the desired level of confluence, dissociated the cells, and used them in experiments (i.e., P2) [25]. This has meant that cells from the same case could not be used in parallel studies and multiple conditions.
The goal of the present experiments was to generate adult human microglia cultures of high passage. Here we report a method for successfully culturing adult human microglia isolated from postmortem human cerebral cortex up to 28 passages without significant loss of proliferative capacity. We found that high passage adult human microglia cultures maintain their phenotype, including response to Aβ, and can be used to extend results obtained in the primate brain in vivo.
MATERIALS AND METHODS
Human brain tissue
Fresh cortical tissue from frontal poles (15–20 grams per case) of 25 postmortem human brains were harvested at autopsy and used for preparation of cultures.
Preparation of adult human microglia cultures
Immediately after harvest, brain tissue was placed in a 50 ml culture tube containing cold RPMI 1640 medium (1X) on ice. After a rinse in RPMI 1640, the meninges and visible blood vessels were removed and primary microglia were prepared from mixed cortical cell preparations. Blocks of cortex (dissected gray matter only) were cut into small pieces and incubated in a 0.25% trypsin EDTA solution in PBS at room temperature for 1 h. An equal volume of trypsin neutralizing solution (ScienCell, Carlsbad, CA) was added, and the tissue was triturated by vigorous pipetting to break up cell clumps (30–40 times each via a 10 ml graduated pipette, followed by a 9 inch Pasteur pipette). The suspension was passed sequentially through 100 μm and 40 μm Nitex mesh filters and centrifuged in 15 ml culture tubes at 1500 RPM for 5 min. The pellets were pooled and resuspended in microglia medium (ScienCell, Carlsbad, CA), supplemented with 5% fetal bovine serum, 100 U/ml penicillin, 100 μg/ml streptomycin, 1 ml/500ml primocin, 1% microglia growth supplement (ScienCell), and 10 ng/ml GM-CSF (Sigma-Aldrich). Cells were seeded in 10 poly-D-lysine coated T75 flasks, incubated in 5% CO2 at 37°C and allowed to adhere to the bottom. Culture medium was changed twice a week. In select cases, microglia were isolated from trypsinized tissue using magnetic cell sorting. For this purpose, following filtration, the cell suspension was incubated in culture media containing the microglia marker CD11b (1/800 dilution) conjugated to magnetic beads and then passed through a column in the presence of a strong magnet. Cells collected in the column were cultured as described above.
Passaging of microglia cultures
When initial cultured cells (passage 1 [P1]) were approximately 30% confluent, they were trypsinized (5 min), diluted by addition of an equal volume of RPMI, centrifuged (1000 RPM for 3 min), and the pellets resuspended in 3 ml of the same supplemented microglia medium. The second passage of cells was divided into 2–3 aliquots and each was seeded in a T25 flask. Splitting into third and higher passages was carried out when cells were 70–80% confluent.
Cryopreservation of microglia cultures
Cultured cells of various passages were washed 2 X in PBS and trypsinized (5 min) when they were 70–80% confluent. An equal volume of RPMI was added, and the mixture was centrifuged (1000 RPM, for 3 min). The pellet was resuspended in supplemented microglia medium containing 10% (v/v) high purity, sterile dimethyl sulfoxide (DMSO), at a final density of 0.5–1.5 × 104/ml. The cell suspension was placed in cryovials in 1 ml aliquots, frozen, and stored at −80°C.
Microglia cultures were re-established by thawing cryopreserved cells in a water bath at 37°C. The cell suspension was centrifuged at 1000 RPM for 3 min, and the pellet was resuspended in the supplemented microglia medium and cultured as described above.
Determination of microglia phenotype
Uptake of DiI-AcLDL
As a measure of microglia phenotype in cultures and as an indication of the status of scavenger receptors, uptake of DiI conjugated acetylated low density lipoprotein (DiI-AcLDL, 10 mg/ml diluted in media), a microglia scavenger receptor ligand, by cultured human microglia was monitored. The number of cells with DAPI stained nuclei that took up DiI-LDL in each culture was determined and expressed as the percentage of total number of cells in the culture. Alternatively, numbers of DiI containing cells and cells devoid of DiI were determined using fluorescent activated cell sorting (FACS) analysis. The percentage of cells that displayed DiI fluorescence were determined in early as compared with late passage cultures.
Expression of CD68
As a second measure of microglia phenotype, presence of the microglia marker cluster of differentiations 68 (CD68) and the specific astrocyte marker glial fibrillary acidic protein (GFAP) were determined in cultured cells of different passages using immunohistochemistry and western blot analysis.
ROS production
ROS production was measured by the nitro blue tetrazolium (NBT) reduction assay [5]. Briefly, microglia were cultured in multi-chamber slides at a density of 2,500 cells with 500 μl media per chamber, allowed to adhere overnight, and were stimulated in HBSS containing 1 mg/ml BSA alone or HBSS/BSA containing 5 μg of fibrillar Aβ (fAβ) or 5 μg of zymosan at 37°C for 15 min. Then 50 μl of 1 mg/ml of NBT was added to each chamber and cells were incubated at 37°C for 1 h. ROS production correlates with formation of a dark blue colored insoluble formazan deposit and was quantified by solubilizing the precipitated formazan in a mixture of DMSO and KOH and measuring the optical density of the solution at 650 nm.
Extension of in vivo findings
We have shown that injections of macrophage/ microglia inhibiting factor (MIF, fragment of tuftsin, the endogenous macrophage/microglia stimulating factor [33, 34]) in the cerebral cortex of aged rhesus monkeys inhibits microglia recruitment and neuronal toxicity following fAβ injections. To determine the effects of MIF on ROS production by cultured human microglia, we exposed cultures of passage 5–8 in multi-chamber slides to fAβ in the presence or absence of 500 μM MIF and measured production of ROS as above.
Statistical analysis
All data were subjected to tests of normality. When data were normally distributed, ANOVA followed by Tukey’s pairwise comparisons, or t-tests (when only two groups were being compared) were used. When data were not normally distributed, non-parametric equivalents of these tests were used. The probability for considering the results significant was set at p < 0.05.
RESULTS
Proliferation and passaging of human microglia in culture
Microglia cultures were established from mixed cortical cell preparations obtained following dissociation of cortical cells from fresh postmortem tissue. After washing and centrifugation, the resultant cell mixture was passed sequentially through 100 μm and 40 μm Nitex mesh filters and the cells passing through the filters (P1) were cultured in microglia medium (ScienCell, Carlsbad, CA), containing 1% microglia growth supplement (ScienCell) and 10 ng/ml GM-CSF (Sigma-Aldrich). Once the attached cells in the initial culture were approximately 30% confluent, they were split into P2 cultures. Thereafter, cells were split into the next passage when they were 70–80% confluent. Microglia of all passages were cultured in the media described above containing growth supplement and GM-CSF.
The time to initial adherence of cells in culture and reaching 30% confluence was variable. At P2 and thereafter, it took approximately 7–14 days for cells to reach 70–80% confluence. The variability in time to reach confluence was due to variations in microglia isolated from different brains. The initial cell mixture was divided equally and cultured in 7–10 T75 flasks, and each was split into 2–3 T25 flasks to obtain P2 cultures. After P2, we consistently split cells from one T25 flask of a lower passage into three T25 flasks of the next passage with excellent proliferation and yield. Each T25 flask contained 1.0–1.5 × 104 cells when cultures were 70–80% confluent.
Using this method, we successfully cultured microglia from 25 brains (Fig. 1A, B). Seven brains came from normal young individuals (36–63 years), 9 from normal aged individuals (70–95 years), 7 from patients who suffered from Alzheimer’s disease, 1 from a patient with frontotemporal dementia, and 1 from a patient with Lewy body dementia. Pathological diagnoses were rendered by an expert neuropathologist at our center using published criteria. Microglia from all cases were cultured up to P10. In 14 cases, microglia were cultured between P11–P20. To determine the highest passage to which human microglia can be successfully subjected, we have continued passaging one case, with the highest passage currently achieved being P28. Microglia in high passage (>10) cultures maintained their proliferative capacity as evidenced by the length of time to reach confluence (7–14 days). Age, gender, disease status, and postmortem interval (range 4–24 h, mean 14.7 ± 1.5 h) did not influence success in culturing or microglia proliferation.
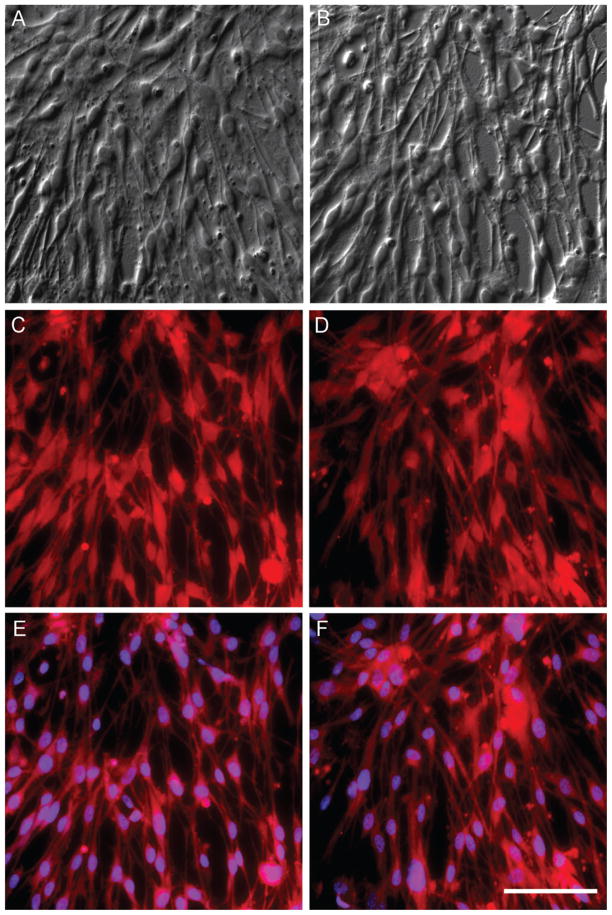
Fig. 1.
Cultured adult human microglia of low and high passage (P) display robust uptake of DiI conjugated scavenger receptor ligand Ac-LDL, demonstrating purity of cultures and preserved microglia phenotype. Differential interference contrast (DIC) imaging of cultured microglia from a 95-year-old normal male at P3 (A) and P13 (B). Note the high density of cells at both passages. Uptake of DiI-Ac-LDL by microglia in the same P3 (C) and P13 (D) cultures and the same fields as above. All cells appear to emit DiI fluorescence at 575 nm, indicating uptake of DiI-Ac-LDL. E) DAPI staining in the same P3 and P13 cultures demonstrates that 96–100% of nucleated cells display prominent uptake of DiI-Ac-LDL as evidenced by emission of DiI fluorescence, an indication of preserved microglia phenotype. Scale bar in F is 25 μm and also applies to A–E.
P1 cultures contained small-size debris likely resulting from degeneration of other cell components in the mixed culture. This debris mostly disappeared by P2–P3. To prepare initial cultures without debris, in select cases, microglia were isolated from a portion of the original mixed cell preparation using magnetic cell sorting. An antibody to the microglia marker CD11b conjugated to magnetic beads was used for this purpose, and the isolated cells were cultured as described. Such cultures were completely free of debris at P1 and thereafter, and showed the same rate of proliferation (i.e., time to reach confluence) as microglia cultured using the filtration method.
To allow use of microglia from different passages in future experiments, cells from T25 flasks of various passages were cryopreserved in supplemented microglia medium containing 10% (v/v) high purity, sterile DMSO, and stored in cryovials in 1 ml aliquots at −80°C. To reestablish cultures, microglia were thawed in a water bath at 37°C, and cultured as described. Cryopreserved and reestablished microglia cultures displayed no changes in proliferation (time to reach confluence) at various passages when compared with regular cultures.
Contribution of GM-CSF to microglia proliferation in culture
To determine the contribution of GM-CSF to proliferation of cultured cells, microglia of various passages (P4–P10, n = 6) were cultured in media with growth factor supplement (ScieCell) in the presence or absence of GM-CSF. Regardless of passage, microglia cultured in the presence of GM-CSF proliferated as described, reaching 70–80% confluence within 7–12 days, allowing continuous splitting to higher passages. Microglia cultured in the absence of growth factors took progressively longer to reach 70–80% confluence, and stopped proliferating after an average of four passages.
Preservation of microglia phenotype and response in high-passage cultures
Expression of CD68
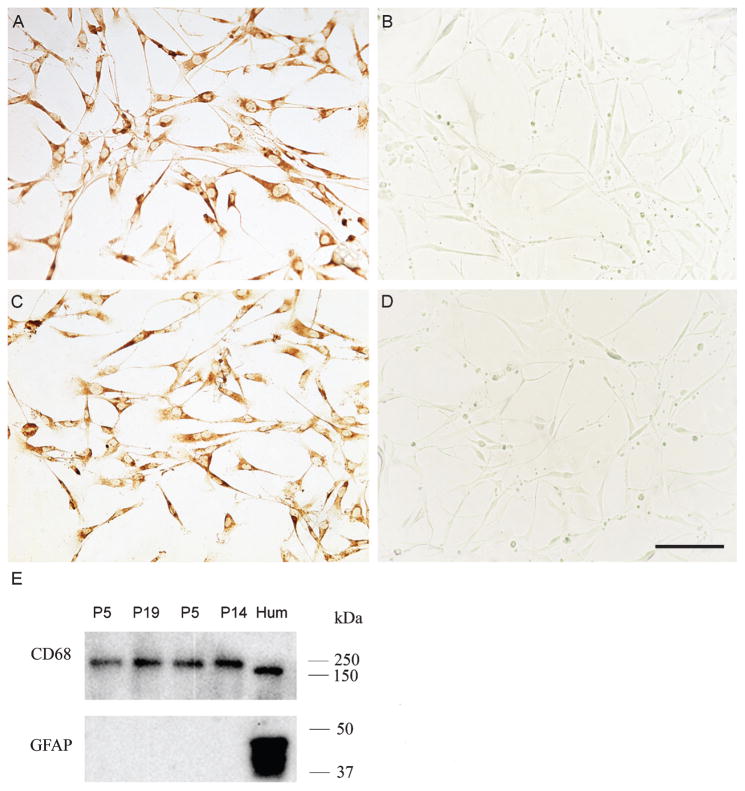
Immunoreactivity for the microglia marker CD68 in high and low passage cultures was visualized and compared with immunoreactivity for the specific astrocyte marker GFAP (n = 6). Immunohistochemical staining revealed robust CD68 immunoreactivity in cultured microglia in both low and high passage cultures while GFAP immunoreactivity was completely absent from both (Fig. 2A–D). Western blot analysis confirmed expression of CD68 and absence of GFAP immunoreactivity in high and low passage cultures (Fig. 2E). As a positive control, homogenate of human cortical tissue was rich in both CD68 and GFAP immunoreactivities.
Fig. 2.
Expression of the microglia marker CD68 in low and high passage (P) human microglia cultures. A) Prominent CD68 immunoreactivity is present in microglia from an 85-year-old participant with Alzheimer’s disease at P7. Immunoreactivity for the astrocyte marker GFAP is completely absent from cultures of the same case at P7. The exact same pattern of CD68 expression (C) and absence of GFAP immunoreactivity (D) was detected in microglia from an 88-year-old normal brain at P13. E) Western blot analysis confirmed expression of CD68 in high and low passage human microglia. Homogenates of microglia from a 95-year-old normal participant (lanes 1 and 2) and a 60-year-old normal individual (lanes 3 and 4) at low (P5, lanes 1 and 3) and high (P19 and P14, respectively, lanes 2 and 4) passages show robust immunoreactivity for CD68, but display no GFAP immunoreactivity. Homogenate of frontal cortex from a normal human brain used as a positive control (Hum) displays strong immunoreactivity for both CD68 and GFAP. Scale bar in D is 25 μm and also applies to A–C.
Uptake of DiI conjugated acetylated low density lipoprotein (DiI-AcLDL)
As a measure of the purity of cultured cells and phenotype of microglia, and as an indication of the status of scavenger receptors, uptake of DiI-AcLDL, a microglia scavenger receptor ligand, by cultured human microglia was monitored. Microscopic examination revealed that, on average, 99% of cells with DAPI-positive nuclei displayed DiI-AcLDL uptake (n = 16, range 95–100%, Fig. 1C–F). Passage number (P3–P15), and age or disease status of the brains from which cultures were derived did not affect the percentage of cells displaying DiI-AcLDL uptake. As a second measure of DiI-AcLDL uptake, select cultures of high and low passage were subjected to FACS analysis. This analysis confirmed that nearly all (96–99%) cultured cells with DAPI-positive nuclei display DiI-AcLDL uptake. The percentage of total nucleated cells with DiI fluorescence were identical when early passage cultures were compared with late passages (Fig. 3).
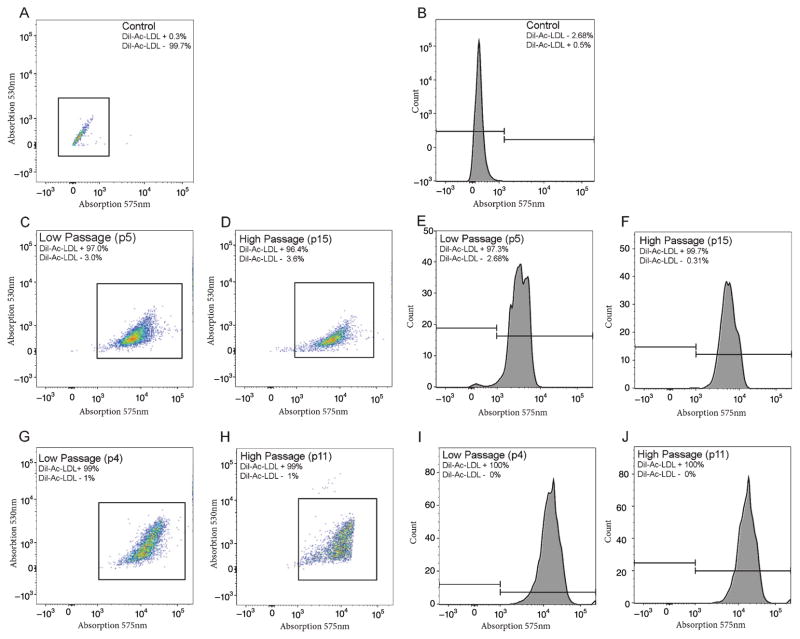
Fig. 3.
Fluorescence activated cell sorting (FACS) analysis confirms uptake of DiI-Ac-LDL by human microglia cultures of high and low passage. Control microglia stained with DAPI in the absence of DiI-Ac-LDL show virtually no fluorescence at 575 nm as indicated by two dimensional color density plot (A) or one dimensional histogram (B), both plotted on logarithmic axes. C–F) Microglia from a 60-year-old normal female participant show robust uptake of DiI-Ac-LDL as indicated by DiI fluorescence in color density plots (C and D) and histograms (E and F), in both low (P4, C and E) and high (P15, D and F) passage cultures. Microglia from a 65-year-old female with Alzheimer’s disease show an identical pattern of DiI-Ac-LDL uptake to the above at low (P4, G and I) and high (P11, H and J) passages. Regardless of passage, virtually all cultured human microglia (96–99%) displayed strong DiI-Ac-LDL uptake.
ROS production
As a measure of the response of human microglia, ROS production was monitored separately in cultures of low, intermediate, and high passage following stimulation. The nitro blue tetrazolium reduction assay was used for this purpose and formation of the blue formazan salt was quantified as a measure of ROS (Fig. 4A, B). Microglia were stimulated with 5 μg/ml of fAβ for 10 min. The optical density of solubilized formazan remained unchanged in cultures of low, intermediate, and high passage (n = 12, p > 0.05, Fig. 4C). Furthermore, ROS production in the presence of Aβ was not different in cultures from normal elderly when compared with AD (optical density, normal = 0.05 ± 0.02; AD = 0.04 ± 0.02; p > 0.05).
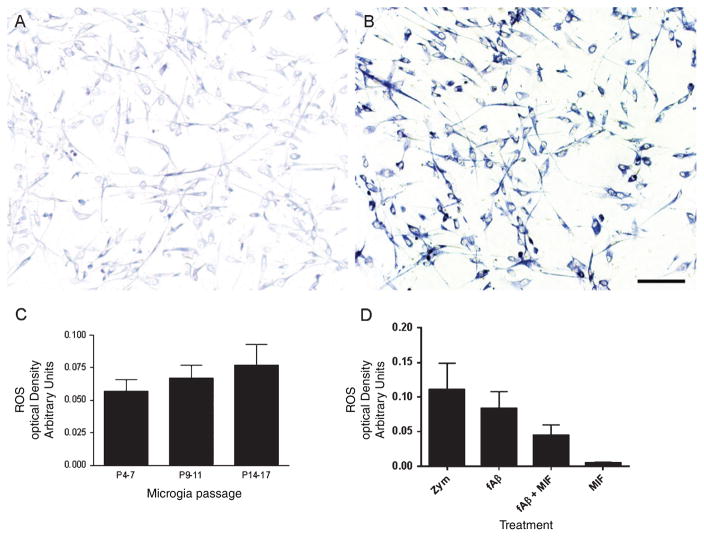
Fig. 4.
Cultured human microglia maintain their response to the fibrillar form of the amyloid-β peptide (fAβ) regardless of passage, as evidenced by production of reactive oxygen species (ROS). A) The nitro blue tetrazolium assay indicated minimal production of ROS when cultures were exposed to vehicle, while cultures exposed to 5 μg of fAβ (B) displayed significant production of ROS, as indicated by deposition of the dark blue formazan salt. C) Spectrophotometric measurement of solubilized formazan at 650 nm indicated no significant differences in ROS production by microglia at low, intermediate and high passages following exposure to fAβ (p > 0.05). D) Use of cultured human microglia from normal and Alzheimer brains allowed extension of previous in vivo findings. Cultures of passage 5–8 displayed robust production of ROS when exposed to 5 μg of the fungal ligand zymosan (positive control) or fAβ. The presence of macrophage/microglia inhibitory factor (MIF) resulted in significant reduction of ROS production following exposure to fAβ(p < 0.05). Exposure of human microglia to MIF alone did not result in appreciable ROS production. Values in C and D represent experimental condition minus vehicle ± standard error of the mean (SEM). Scale bar in B is 25 μm and also applies to A.
To determine whether human microglia maintain their response to other stimulants in culture, we exposed cultures to 5 μg/ml of the fungal ligand zymosan. Zymosan exposure resulted in robust production of ROS in microglia that was significantly greater than cultures exposed to vehicle (p < 0.001) or Aβ (p < 0.05) (Fig. 4D).
Extension of in vivo findings
We have demonstrated abundant recruitment and activation of microglia and neuronal loss at sites of fAβ injection in the aged rhesus monkey cerebral cortex [19]. Tuftsin is an endogenous microglia stimulating factor, and a fragment of tuftsin (macrophage/MIF), has been shown to interfere with the normal functions of tuftsin and to inhibit microglia activation and inflammation [33–35]. We observed significant reduction in microglia recruitment/activation and neuronal loss when fAβ was co-injected with MIF in the aged rhesus cortex [20]. To compare the response of cultured human microglia to in vivo conditions and to determine the potential role of ROS production by microglia in our in vivo experiments, we exposed P5–P8 cultures from normal aged and AD brains (n = 5 each) to fAβ in the presence or absence of MIF. We confirmed the nearly identical levels of ROS production by microglia from normal aged brains when compared with AD brains in the presence of fAβ (p > 0.05). When data from the aged and AD were pooled, significant production of ROS was detected in the presence of fAβ when compared with vehicle (p < 0.001, Fig. 4D). The presence of MIF resulted in significant reduction of ROS production by microglia from aged and AD brains exposed to fAβ (p < 0.05). Exposure of microglia to MIF alone did not result in changes in ROS production when compared with vehicle (p > 0.05).
DISCUSSION
We have developed a method that allows isolation and culturing of adult human microglia from postmortem brains to high passage. Using this method, we have successfully cultured microglia from 25 brains to high passage (up to passage 28). Two factors, among others, appear to have contributed to the success of our method, when compared with previous attempts at isolation and culturing of adult human microglia [25, 28–32]. First, we used a relatively large amount of tissue from each case (15–20 g), which resulted in high initial yield of cells. Second, the microglia media we used contained growth factors, including GM-CSF. We found that microglia cultured without GM-CSF show progressively slower proliferation and stop proliferating after approximately four passages. The potential reason previous attempts at culturing human microglia in the presence of GM-CSF were not carried to high passage [31, 32] may be that they did not use other growth factors. The media used in this study contained a microglia growth supplement (ScienCell). The presence of this supplement is most likely the reason we were able to passage microglia up to P4 without GM-CSF. Our results suggest that the presence of this growth supplement in addition to GM-CSF is necessary to successfully produce microglia cultures of high passage.
The method described here resulted in relatively high yield of microglia from each case. On average, at P2 we obtained 20–30 T25 flasks, each containing 1.0–1.5 × 104 microglia when 70–80% confluent. Thereafter, each T25 flask of a lower passage was split into three T25 flasks of a higher passage with the same yield of microglia. Thus, our method would allow microglia from the same cases and various passages to be used in parallel conditions and multiple experiments, permitting proper comparisons between various experimental manipulations.
Cultured human microglia maintained their rate of proliferation, phenotype, and response to stimulants to high passage. Regardless of the passage tested, human microglia displayed strong CD68 immunoreactivity, robust uptake of DiI-AcLDL, and high levels of ROS production in response to the endogenous ligand fAβ and the exogenous fungal lig-and zymosan. While a complete characterization of microglia phenotype is beyond the scope of any single study, we found maintained microglia phenotype and response in high passage cultures in all measures obtained.
Microglia are known to undergo inflammatory activation in a number of neurodegenerative disorders and their animal models [6–9]. At present, it is not known whether the response of microglia in brains with neurodegenerative disorders is due to the abnormal environment within which microglia must function or due to permanent alterations in microglia characteristics [36]. Our findings are supportive of the former possibility. We observed nearly identical proliferation, phenotype and response of microglia cultured from brains of patients with Alzheimer’s disease, Lewy body dementia, and frontotemporal lobar degeneration, when compared with microglia isolated from normal brains. This observation has important therapeutic implications and indicates that microglia in brains with neurodegenerative disorders may be amenable to manipulation so that they display responses known to occur in microglia under normal conditions.
To demonstrate feasibility of the use of passaged human microglia cultures in experimental work, we extended our in vivo findings using P5–P8 microglia cultures from normal and Alzheimer’s disease patients. We had shown extensive microglia activation and neuronal loss at sites of fAβ injection in the aged rhesus cortex, and reversal of neuronal loss by co-injection of fAβ with tuftsin fragment (MIF) [19, 20]. Our present results demonstrate inhibition of ROS production by cultured adult human microglia exposed to fAβ in the presence of MIF. Thus, the in vivo protective effects of MIF against fAβ neurotoxicity are due, at least in part, to inhibition of ROS production, and perhaps also inhibition of production of potentially cytotoxic cytokines, by microglia.
The method described here for culturing adult human microglia to high passage provides the scientific community with a new and reliable tool for mechanistic studies of human microglia function in health from childhood to old age, and in disease, enhancing the relevance of the findings to the human brain and neurodegenerative conditions. It will allow testing of drugs that can regulate microglial functions from normal and diseased brains. It will also provide high quantities of microglial RNA and protein for transcriptomic and proteomic studies. It must be noted that the majority of studies on microglia function have not only used cells from the rodent brain, but have used microglia cultured from embryonic brains. The method described here may also allow culturing of microglia from adult rodent brain to high passage so that studies concentrating on rodent microglia function are rendered relevant to processes in operation in the adult brain.
Acknowledgments
This work was supported by the National Institute of Neurological Disorders and Stroke (NS084210), the Dana Foundation Neuroimmunology Program, the Alzheimer’s Disease Fund of the Illinois Department of Public Health, and Scientific Research Funding, Science and Technology Agency (2013FB045), and Provincial Education Department (2012Z093), Yunnan Province, China. A portion of the human brain tissue used in these experiments was obtained from the Northwestern University Alzheimer’s Disease Center Brain Bank (AG13854). We are grateful to Girgis Girgis for expert technical assistance.
Authors’ disclosures available online (http://j-alz.com/manuscript-disclosures/16-0394r1).
References
- 1.El Khoury J, Hickman SE, Thomas CA, Cao L, Silverstein SC, Loike JD. Scavenger receptor-mediated adhesion of microglia to beta-amyloid fibrils. Nature. 1996;382:716–719. doi: 10.1038/382716a0. [DOI] [PubMed] [Google Scholar]
- 2.Paresce DM, Ghosh RN, Maxfield FR. Microglial cells internalize aggregates of the Alzheimer’s disease amyloid beta-protein via a scavenger receptor. Neuron. 1996;17:553–565. doi: 10.1016/s0896-6273(00)80187-7. [DOI] [PubMed] [Google Scholar]
- 3.Christie RH, Freeman M, Hyman BT. Expression of the macrophage scavenger receptor, a multifunctional lipoprotein receptor, in microglia associated with senile plaques in Alzheimer’s disease. Am J Pathol. 1996;148:399–403. [PMC free article] [PubMed] [Google Scholar]
- 4.Honda M, Akiyama H, Yamada Y, Kondo H, Kawabe Y, Takeya M, Takahashi K, Suzuki H, Doi T, Sakamoto A, Ookawara S, Mato M, Gough PJ, Greaves DR, Gordon S, Kodama T, Matsushita M. Immunohistochemical evidence for a macrophage scavenger receptor in Mato cells and reactive microglia of ischemia and Alzheimer’s disease. Biochem Biophys Res Commun. 1998;245:734–740. doi: 10.1006/bbrc.1998.8120. [DOI] [PubMed] [Google Scholar]
- 5.Coraci IS, Husemann J, Berman JW, Hulette C, Dufour JH, Campanella GK, Luster AD, Silverstein SC, El Khoury JB. CD36, a class B scavenger receptor, is expressed on microglia in Alzheimer’s disease brains and can mediate production of reactive oxygen species in response to beta-amyloid fibrils. Am J Pathol. 2002;160:101–112. doi: 10.1016/s0002-9440(10)64354-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.El Khoury J, Hickman SE, Thomas CA, Loike JD, Silver-stein SC. Microglia, scavenger receptors, and the pathogenesis of Alzheimer’s disease. Neurobiol Aging. 1998;19(1 Suppl):S81–S84. doi: 10.1016/s0197-4580(98)00036-0. [DOI] [PubMed] [Google Scholar]
- 7.Lee YJ, Han SB, Nam SY, Oh KW, Hong JT. Inflammation and Alzheimer’s disease. Arch Pharm Res. 2010;33:1539–1556. doi: 10.1007/s12272-010-1006-7. [DOI] [PubMed] [Google Scholar]
- 8.Loane DJ, Byrnes KR. Role of microglia in neurotrauma. Neurotherapeutics. 2010;7:366–377. doi: 10.1016/j.nurt.2010.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Qian L, Flood PM, Hong JS. Neuroinflammation is a key player in Parkinson’s disease and a prime target for therapy. J Neural Transm. 2010;117:971–979. doi: 10.1007/s00702-010-0428-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Duffield JS. The inflammatory macrophage: A story of Jekyll and Hyde. Clin Sci. 2003;104:27–38. doi: 10.1042/. [DOI] [PubMed] [Google Scholar]
- 11.Colton CA, Wilcock DM. Assessing activation states in microglia. CNS Neurol Disord Drug Targets. 2010;9:174–191. doi: 10.2174/187152710791012053. [DOI] [PubMed] [Google Scholar]
- 12.Tremblay ME, Stevens B, Sierra A, Wake H, Bessis A, Nimmerjahn A. The role of microglia in the healthy brain. J Neurosci. 2011;31:16064–16069. doi: 10.1523/JNEUROSCI.4158-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schafer DP, Stevens B. Synapse elimination during development and disease: Immune molecules take centre stage. Biochem Soc Trans. 2010;38:476–481. doi: 10.1042/BST0380476. [DOI] [PubMed] [Google Scholar]
- 14.Tremblay ME, Lowery RL, Majewska AK. Microglial interactions with synapses are modulated by visual experience. PLoS Biol. 2010;8:e1000527. doi: 10.1371/journal.pbio.1000527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wake H, Moorhouse AJ, Jinno S, Kohsaka S, Nabekura J. Resting microglia directly monitor the functional state of synapses in vivo and determine the fate of ischemic terminals. J Neurosci. 2009;29:3974–3980. doi: 10.1523/JNEUROSCI.4363-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Geula C, Mesulam MM, Kuo CC, Tokuno H. Postnatal development of cortical acetylcholinesterase-rich neurons in the rat brain: Permanent and transient patterns. Exp Neurol. 1995;134:157–178. doi: 10.1006/exnr.1995.1046. [DOI] [PubMed] [Google Scholar]
- 17.Geula C, Schatz CR, Mesulam MM. Differential localization of NADPH-diaphorase and calbindin-D28k within the cholinergic neurons of the basal forebrain, striatum and brainstem in the rat, monkey, baboon and human. Neuroscience. 1993;54:461–476. doi: 10.1016/0306-4522(93)90266-i. [DOI] [PubMed] [Google Scholar]
- 18.Mesulam MM, Geula C. Acetylcholinesterase-rich neurons of the human cerebral cortex: Cytoarchitectonic and ontogenetic patterns of distribution. J Comp Neurol. 1991;306:193–220. doi: 10.1002/cne.903060202. [DOI] [PubMed] [Google Scholar]
- 19.Geula C, Wu CK, Saroff D, Lorenzo A, Yuan M, Yankner BA. Aging renders the brain vulnerable to amyloid á-protein neurotoxicity. Nat Med. 1998;4:827–831. doi: 10.1038/nm0798-827. [DOI] [PubMed] [Google Scholar]
- 20.Leung E, Guo L, Bu J, Maloof M, El Khoury J, Geula C. Microglia activation mediates fibrillar amyloid-beta toxicity in the aged primate cortex. Neurobiol Aging. 2011;32:387–397. doi: 10.1016/j.neurobiolaging.2009.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vitek MP, Snell J, Dawson H, Colton CA. Modulation of nitric oxide production in human macrophages by apolipoprotein-E and amyloid-beta peptide. Biochem Biophys Res Commun. 1997;240:391–394. doi: 10.1006/bbrc.1997.7408. [DOI] [PubMed] [Google Scholar]
- 22.Wallace MN, Geddes JG, Farquhar DA, Masson MR. Nitric oxide synthase in reactive astrocytes adjacent to beta-amyloid plaques. Exp Neurol. 1997;144:266–272. doi: 10.1006/exnr.1996.6373. [DOI] [PubMed] [Google Scholar]
- 23.Colton CA, Chernyshev ON, Gilbert DL, Vitek MP. Microglial contribution to oxidative stress in Alzheimer’s disease. Ann N Y Acad Sci. 2000;899:292–307. doi: 10.1111/j.1749-6632.2000.tb06195.x. [DOI] [PubMed] [Google Scholar]
- 24.Wong A, Luth HJ, Deuther-Conrad W, Dukic-Stefanovic S, Gasic-Milenkovic J, Arendt T, Munch G. Advanced glycation endproducts co-localize with inducible nitric oxide synthase in Alzheimer’s disease. Brain Res. 2001;920:32–40. doi: 10.1016/s0006-8993(01)02872-4. [DOI] [PubMed] [Google Scholar]
- 25.Gibbons HM, Dragunow M. Adult human brain cell culture for neuroscience research. Int J Biochem Cell Biol. 2010;42:844–856. doi: 10.1016/j.biocel.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 26.Hassan NF, Campbell DE, Rifat S, Douglas SD. Isolation and characterization of human fetal brain-derived microglia in in vitro culture. Neurosci. 1991;41:149–158. doi: 10.1016/0306-4522(91)90205-3. [DOI] [PubMed] [Google Scholar]
- 27.Jordan CA, Watkins BA, Kufta C, Dubois-Dalcq M. Infection of brain microglial cells by human immunodeficiency virus type 1 is CD4 dependent. J Virol. 1991;65:736–742. doi: 10.1128/jvi.65.2.736-742.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rustenhoven J, Park TI, Schweder P, Scotter J, Correia J, Smith AM, Gibbons HM, Oldfield RL, Bergin PS, Mee EW, Faull RL, Curtis MA, Scott Graham E, Dragunow M. Isolation of highly enriched primary human microglia for functional studies. Sci Rep. 2016;6:19371. doi: 10.1038/srep19371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gibbons HM, Hughes SM, Van Roon-Mom W, Greenwood JM, Narayan PJ, Teoh HH, Bergin PM, Mee EW, Wood PC, Faull RL, Dragunow M. Cellular composition of human glial cultures from adult biopsy brain tissue. J Neurosci Methods. 2007;166:89–98. doi: 10.1016/j.jneumeth.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Williams K, Bar-Or A, Ulvestad E, Olivier A, Antel JP, Yong VW. Biology of adult human microglia in culture: Comparisons with peripheral blood monocytes and astrocytes. J Neuropathol Exp Neurol. 1992;51:538–549. doi: 10.1097/00005072-199209000-00009. [DOI] [PubMed] [Google Scholar]
- 31.De Groot CJ, Montagne L, Janssen I, Ravid R, Van Der Valk P, Veerhuis R. Isolation and characterization of adult microglial cells and oligodendrocytes derived from postmortem human brain tissue. Brain Res Protoc. 2000;5:85–94. doi: 10.1016/s1385-299x(99)00059-8. [DOI] [PubMed] [Google Scholar]
- 32.Lee SC, Liu W, Brosnan CF, Dickson DW. GM-CSF promotes proliferation of human fetal and adult microglia in primary cultures. Glia. 1994;12:309–318. doi: 10.1002/glia.440120407. [DOI] [PubMed] [Google Scholar]
- 33.Najjar VA. Tuftsin, a natural activator of phagocyte cells: An overview. Ann NY Acad Sci. 1983;419:1–11. doi: 10.1111/j.1749-6632.1983.tb37086.x. [DOI] [PubMed] [Google Scholar]
- 34.Siemion IZ, Kluczyk A, Slon JJ, Zimecki M, Wieczorek Z. Immunosuppressive activity of analogs of tripeptide Lys-Arg-Pro with D-amino acid residues. Arch Immunol Ther Exp (Warsz) 1994;42:205–207. [PubMed] [Google Scholar]
- 35.Cillari E, Arcoleo F, Dieli M, D’Agostino R, Gromo G, Leoni F, Milano S. The macrophage-activating tetrapeptide tuftsin induces nitric oxide synthesis and stimulates murine macrophages to kill Leishmania parasites in vitro. Infect Immun. 1994;62:2649–2652. doi: 10.1128/iai.62.6.2649-2652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Streit WJ. Microglia and Alzheimer’s disease pathogenesis. J Neurosci Res. 2004;77:1–8. doi: 10.1002/jnr.20093. [DOI] [PubMed] [Google Scholar]