Abstract
Introduction
No comprehensive reports have been published on epidemiological status of Rhinocerebral zygomycosis infections and its outcome in our population, Hence, the current study came to address epidemiological characteristics as well as clinical outcome of patients with Rhinocerebral zygomycosis infection referred to a referral hospital in Iran.
Methods
This retrospective study was performed at the Rasoul-e-Akram hospital, an 800-bed tertiary care teaching hospital in Tehran, Iran. The pathology recorded charts were reviewed to identify all cases of Rhinocerebral zygomycosis from patients admitted between April 2007 and March 2014. A diagnosis of Rhinocerebral zygomycosis was based on histopathological assessments.
Results
Sixty four patients with Rhinocerebral zygomycosis were assessed. The mean age of the patients was 46.07 ± 22.59 years and 51.6% were female. Among those, 67.2% were diabetic, 26.6% were hypertensive and 29.7% had history of cancer. Different sinuses were infected in 73.4% of the patients. Out of all the patients 26.6% underwent surgical procedures and 17.2% were controlled medically. Extensive debridement was carried out in 40.6%. Neutropenia (<1500 cell/ µl) was revealed in 12.5%. In-hospital mortality rate was 35.9% and prolonged hospital stay (> 14 days) was found in 60.9%. According to the Multivariable logistic regression analysis, the main predictors of in-hospital mortality included female gender, advanced age, the presence of sinus infection, and neutropenia, while higher dosages of amphotericin administered had a protective role in preventing early mortality. In a similar Multivariate model, history of cancer could predict prolonged hospital stay, whereas using higher dose of amphotericin could lead to shortening length of hospital stay.
Conclusion
There is no difference in demographic characteristics between our patients with Rhinocerebral zygomycosis and other nations. The presence of diabetes mellitus is closely associated with the presence of this infection. Sinus involvement is very common in those with Rhinocerebral zygomycosis leading to high mortality and morbidity. Besides female gender, advanced age, and presence of neutropenia was a major risk factor for increasing early mortality. The use of higher doses of antifungal treatment such as amphotericin can prevent both mortality and prolonged hospital stay. The cancer patients may need longer hospital stay because of needing comprehensive in-hospital treatment.
Keywords: Rhinocerebral zygomycosis, in-hospital mortality, morbidity
Introduction
Zygomycosis refers to infectious disease variants caused by Mucorales fungi species [1]. It may occur in progressive underlying risk profile patients such as immunosuppression, diabetic ketoacidosis, malignancies, extreme malnutrition, any conditions led to iron overload, burns, and trauma. Zygomycosis has a life-threatening nature with multi-organ involvements such as cutaneous, pulmonary, and even neurologic invasions [2]. Thus, proper management and treatment of this multi-infection needs early detection of underlying risk factors along with medical and even surgical aggressive therapies. However, misdiagnosing identifiable risk factors is now a major problematic issue to improve disease progression and outcome [3, 4]. Moreover, because of identified different routes of infection such as inhalation of conidia, gastrointestinal ingestion (among malnourished patients), non-sterile tape and contaminated wooden splints, traumatic inoculation, and even via natural disasters [5–9], the problem is compounded for disease control and treatment. Descriptions of zygomycosis appear to be increasing, perhaps given higher numbers of persons at risk [10]. Epidemiologically, the infection by zygomycosis has a worldwide distribution with no known gender, age, or interracial preferences; however some institutional studies have shown a gender tendency with a male-to-female ratio of 3:1 [11]. Regarding disease prognosis, the presence of some serious comorbidities and needing aggressive coordinated treatment strategies may potentially affect prognosis, however despite all efforts to identify disease risk factors as well as to control disease progress, zygomycosis may carry a mortality rate of 50-85% mostly due to its serious consequences including rhinocerebral and pulmonary events [12–14]. Unfortunately, no comprehensive reports have been published on epidemiological status of mucormycosis infections and its outcome in our population from 2007, Hence, the current study came to address epidemiological characteristics as well as clinical outcome of patients with mucormycosis infection referred to a referral hospital in Iran.
Methods
This retrospective study was performed at the Rasoul-e-Akram hospital, an 800-bed tertiary care teaching hospital in Tehran, Iran. The pathology recorded charts were reviewed to identify all cases of zygomycosis from patients admitted between April 2007 and March 2014. Patients were included in the study if they met the criteria for proven invasive Rhinocerebral zygomycosis based on the revised definitions of invasive fungal disease of the European Organization for Research and Treatment of Cancer/Mycosis Study Group (EORTC/MSG) [15]. In total, 91 patients were identified that among them, 16 were excluded because of discharge against medical advice. The cases that were diagnosed on an outpatient basis or on the day of surgery and were not admitted to either hospital were also excluded. Data was collected on patients´ demographics, underlying conditions, concomitant immunosuppressive medications, laboratory data, radiologic findings, clinical features, antifungal treatment, surgical procedures, and outcomes. A diagnosis of Rhinocerebral zygomycosis was based on histopathological demonstration of broad, ribbon-like, wide-angled branching, non-septate hyphae even in the absence of positive cultures, and accompanying tissue invasion by fungal hyphae [16, 17]. The zygomycosis genus was determined by morphological examination of conidia, hyphae, and whole colonies. The study endpoint was first to assess in-hospital mortality and length of hospital stay and second was to determine main predictors of mortality and prolonged hospital stay in the patients. Results were presented as mean ± standard deviation (SD) for quantitative variables and were summarized by frequency (percentage) for categorical variables. Continuous variables were compared using t test or Mann-Whitney U test whenever the data did not appear to have normal distribution or when the assumption of equal variances was violated across the study groups. Categorical variables were, on the other hand, compared using chi-square test. The multivariable regression models were applied to determine indicators of disease outcome. For the statistical analysis, the statistical software SPSS version 16.0 for windows (SPSS Inc., Chicago, IL) was used. P values of 0.05 or less were considered statistically significant.
Results
In total, 64 patients with Rhinocerebral zygomycosis were assessed. The mean age of the patients was 46.07 ± 22.59 years (ranged 4 to 87 years) from which 51.6% were female. The mean age of affected men was similar to diseased women (45.89 ± 26.36 years versus 46.24 ± 18.80 years, p = 0.950). The overall prevalence of malignant conditions was 29.7%, 67.2% were diabetic, and 26.6% were hypertensive. The average dose of amphotericin in administered patients was 2135.64 ± 1870.91 mg. Different sinuses were infected in 73.4%, 26.6% underwent surgical procedures once and 56.2% underwent these procedures more than once, and 17.2% were controlled medically (Figure 1). Functional endoscopic sinus surgery (FESS) was also done on 53.1% of patients. Eye enucleating was considered for 18.8% of cases. Regarding diagnostic approaches, pathological assessment was planned for 85.9% and 25.0% were also assessed by endoscopy. Extensive debridement was carried out in 40.6% as a surgical procedure. The mean WBC count of the patients was 7947.60 ± 5446.86 cells per microliter (µl) of blood that neutropenia (<1500 cell/ µl) was revealed in 12.5%. In total, in-hospital mortality rate was 35.9%. The mean length of hospital stay was 26.94 ± 24.70 days that prolonged hospital stay (> 14 days) was found in 60.9% of the patients. According to the Multivariable logistic regression analysis (Table 1), the main predictors of in-hospital mortality included female gender (OR = 5.263, P = 0.002), advanced age (OR = 1.063, P = 0.001), the presence of sinus infection (OR = 4.836, P = 0.018), and neutropenia (OR = 31.250, P = 0.003), while higher dosages of amphotericin deoxycholate administered had a protective role in preventing early mortality (OR = 0.825, P = 0.025). In a similar Multivariate model (Table 2), history of cancer could predict prolonged hospital stay (OR = 8.413, P = 0.043), whereas using higher dose of amphotericin could lead to shortening length of hospital stay (OR = 0.998, P < 0.001).
Figure 1.
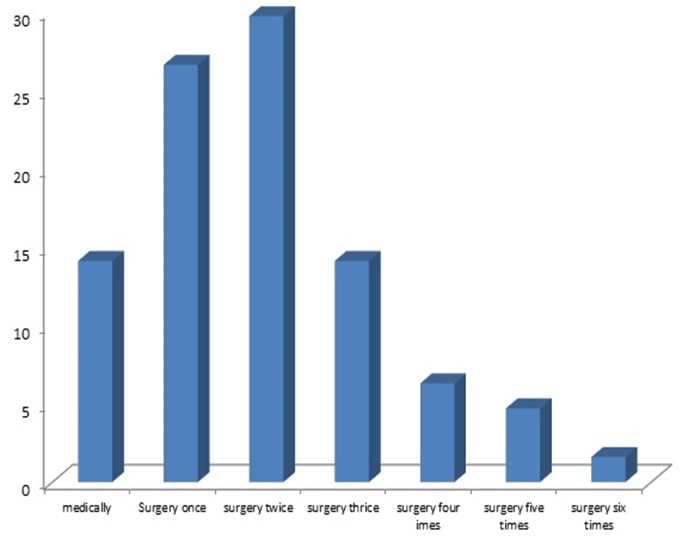
Treatment approach in patients with sinus involvement related to mucormycosis
Table 1.
Main predictors of in-hospital mortality
| Variable | B | S.E. | Wald | p-value | OR | 95.0% CI for OR | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Female gender | 1.662 | .544 | 9.338 | 0.002 | 5.263 | 1.815 | 15.385 |
| Age | 0.060 | .018 | 11.099 | 0.001 | 1.063 | 1.026 | 1.101 |
| Diabetes | -0.083 | .980 | 0.007 | 0.932 | 0.920 | 0.135 | 6.283 |
| Hypertension | -0.107 | .484 | 0.049 | 0.825 | 0.898 | 0.348 | 2.319 |
| Amphotericin use | 0.226 | 0.568 | 5.021 | 0.025 | 0.825 | 0.295 | 0.979 |
| Sinus infection | 1.576 | 1.665 | 5.610 | 0.018 | 4.836 | 1.312 | 17.822 |
| Neutropenia | -3.451 | 1.157 | 8.898 | 0.003 | 31.250 | 3.268 | 333.33 |
| Cancer | 0.036 | 0.662 | 0.003 | 0.956 | 1.037 | 0.284 | 3.794 |
| Constant | 7.912 | 2.392 | 10.944 | 0.001 | 2.730 | ||
Table 2.
Main predictors of prolonged hospital stay (LOS > 14 days)
| Variable | B | S.E. | Wald | p-value | OR | 95.0% CI for OR | |
|---|---|---|---|---|---|---|---|
| Lower | Upper | ||||||
| Female gender | -1.116 | 0.656 | 2.888 | .089 | 0.328 | 0.091 | 1.186 |
| Age | 0.034 | 0.020 | 2.846 | .092 | 1.035 | 0.995 | 1.076 |
| Diabetes | 0.324 | 1.138 | 0.081 | .776 | 1.383 | 0.149 | 12.880 |
| Hypertension | 0.778 | 0.734 | 1.123 | .289 | 2.178 | 0.516 | 9.184 |
| Amphotericin use | -0.002 | 0.000 | 22.696 | .000 | 0.998 | 0.998 | 0.999 |
| Sinus infection | 1.239 | 0.800 | 2.399 | .121 | 3.453 | 0.720 | 16.571 |
| Neutropenia | 0.258 | 1.056 | 0.060 | .807 | 1.294 | 0.163 | 10.250 |
| Cancer | 2.130 | 1.052 | 4.096 | .043 | 8.413 | 1.070 | 66.169 |
| Constant | -1.782 | 2.418 | 0.543 | .461 | 0.168 | ||
Discussion
As the first results and regarding demographic characteristics of our patients who suffered Rhinocerebral zygomycosis, we showed the prevalence of disease in an age wide spectrum from childhood to old adulthood. Also, men and women were similarly affected by this infection. Among underlying risk profiles, high prevalence of diabetes mellitus was revealed among patients that was shown in about two-third of them. Epidemiological studies showed high prevalence of diabetes mellitus in the patients in both developed and developing nations, however some clear differences were also revealed in the epidemiological aspects of disease between the nations that in developed countries, the disease remains uncommon and mostly occur in patients with diabetes mellitus and hematological malignancies, while in developing countries, zygormycosis has a sporadic pattern closely related to uncontrolled diabetes or trauma [18–20]. It seems that the relationship between high incidence rate of zygomycosis and diabetes is mainly influenced by uncontrolled situation of diabetes or occurring ketoacidosis as a serious predisposing factor for this infection. In total, reviewing the literature demonstrated the risky role of diabetes mellitus in 36% to 88% of patients [20–25]. In contrast, controlling diabetes, proper management of ketoacidosis and the use of statins as a main treatment protocols for metabolic syndrome could effectively reduce the incidence of zygomycosis [26–28]. Sinuses involvement is a common clinical manifestations affecting disease poor prognosis. This complication commonly appears as rhinocerebral mucormycosis, that is manifested by either sinusitis or periorbital cellulitis [29]. If untreated, sinusitis can spread from the sinuses and extend into the neighboring tissues such as orbit or orbital muscular complex leading to potential visual complications or may extend into the mouth and produce painful, necrotic ulcerations of the hard palate [30]. Another serious complication can be the spreading infection from sinus posteriorly to central nervous system and leading to cerebral vascular invasion and cerebral infarction, a serious cause for increasing disease-related mortality [31]. In our observation, 73.4% of patients suffered sinuses infections that mostly underwent invasive surgical procedure to prevent its progression. More importantly, the presence of sinusitis has been identified as an important predicting factor for early mortality in these patients leading to4.8 times more risk for mortality.
We saw an early mortality of 35% for affected patients with Rhinocerebral zygomycosis that seems to be considerably high despite performing curative management in our center. Naturally, the early and long-term mortality following Rhinocerebral zygomycosis is strongly associated with the presence of underlying comorbidities. It has been shown that the overall mortality of pulmonary mucormycosis is approximately 50 to 70% [32, 33]. Cutaneous and subcutaneous disease may lead to necrotizing fasciitis, which has a mortality approaching 80% [34, 35]. The mortality associated with dissemination to the brain approaches 100% [36]. In this regard, managing disease by proper antifungal agents leads to considerably decrease in mortality. It has been indicated that in cases with rhinocerebral mucormycosis, the mortality is about 70% in cases treated with antifungal agents alone versus 14% in cases treated with antifungal agents plus surgery [37, 38]. Similarly, in a combined series of rhinocerebral, cutaneous, and pulmonary mucormycosis, 65% of patients treated with surgery plus antifungal agents survived the infection, compared to none of the patients treated with antifungal agents alone [39]. Thus, the death rate is directly associated with whether the patients were managed medically or surgically. In our study, regardless of the type of comorbidity or treatment approach, high early mortality was reported following Rhinocerebral zygomycosis infection. However, as shown in our multivariable analysis, administrating antifungal treatment by high dose amphotericin, not only reduced in-hospital mortality, but also shortened hospital stay. However, it seems that the rate obtained for early mortality is notably high requiring deep assessment of its causes among our population.
Two underlying conditions including history of cancer and presence of neutropenia can adversely affect outcome of Rhinocerebral zygomycosis as shown in our analysis. It has been clearly shown that the overall mortality rate for Rhinocerebral zygomycosis remains higher than 50%, and it approaches 100% among patients with persistent neutropenia [32, 40]. In fact, the neutrophils are critical for inhibiting fungal spore proliferation and thus decrease in neutrophil count can predispose the patients to progressive Rhinocerebral zygomycosis. The risky role of neutropenia is more prominent in patients with malignancies especially hematological cancers with immunosuppressive patterns.
Conclusion
In summary, comparing our results with data previously reported in the literature shows similarities in demographic distribution of Rhinocerebral zygomycosis in our population. Among common comorbidities, the presence of diabetes mellitus is closely associated with the presence of this infection because more than two-third of affected patients with this infection are diabetics. Sinus involvement is very common in those with Rhinocerebral zygomycosis even in early stages of disease leading to high mortality and morbidity in the patients needing invasive surgical treatments. Besides female gender, advanced age, and presence of neutropenia (an indicator for immunosuppression) act as a major risk factor for increasing early mortality, the use of antifungal treatment such as high dose amphotericin can prevent both mortality and prolonged hospital stay. The cancer patients may need longer hospital stay because of needing comprehensive in-hospital treatments.
What is known about this topic
Presence of diabetes mellitus is closely associated with rhinocerebral zygomycosis;
Neutropenia is a risk factor for early mortality in patients with rhinocerebral zygomycosis
What this study adds
High dose amphotericin can reduce both mortality and prolonged hospital stay in patients with rhinocerebral zygomycosis;
Cancer patients need longer hospital stay for treatment of rhinocerebral zygomycosis
Competing interests
Authors declare no competing interests.
Authors’ contributions
All authors read and approved the final version of this manuscript and equally contributed to its content.
References
- 1.Kontoyiannis DP, Lewis RE. Agents of mucormycosis and Entomophthoramycosis. In: Mandell GL, Bennett GE, Dolin R, editors. Mandell, Douglas and Bennett's Principles and Practice of Infectious Diseases. 7th ed. Philadelphia, Pa: Churchill Livingstone; 2010. pp. 3257–69. [Google Scholar]
- 2.Kwon-Chung KJ. Taxonomy of fungi causing mucormycosis and entomophthoramycosis (zygomycosis) and nomenclature of the disease: molecular mycologic perspectives. Clinical Infectious Diseases. 2012 Feb 1;54(suppl 1):S8–15. doi: 10.1093/cid/cir864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mohindra S, Mohindra S, Gupta R, Bakshi J, Gupta SK. Rhinocerebral mucormycosis: the disease spectrum in 27 patients. Mycoses. 2007 Jul 1;50(4):290–6. doi: 10.1111/j.1439-0507.2007.01364.x. [DOI] [PubMed] [Google Scholar]
- 4.Rahman A, Akter K, Hossain S, Rashid HU. Rhino-orbital mucourmycosis in a non-immunocompromised patient. BMJ case reports. 2013 Feb 7;2013 doi: 10.1136/bcr-2012-007863. bcr2012007863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lalayanni C, Baliakas P, Xochelli A, Apostolou C, Arabatzis M, Velegraki A. Outbreak of cutaneous zygomycosis associated with the use of adhesive tape in haematology patients. J Hosp Infect. 2012 Jul;81(3):213. doi: 10.1016/j.jhin.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 6.Rammaert B, Lanternier F, Zahar JR, Dannaoui E, Bougnoux ME, Lecuit M, Lortholary O. Healthcare-associated mucormycosis. Clinical Infectious Diseases. 2012 Feb 1;54(suppl 1):S44–54. doi: 10.1093/cid/cir867. [DOI] [PubMed] [Google Scholar]
- 7.Andresen D, Donaldson A, Choo L, Knox A, Klaassen M, Ursic C, Vonthethoff L, Krilis S, Konecny P. Multifocal cutaneous mucormycosis complicating polymicrobial wound infections in a tsunami survivor from Sri Lanka. The Lancet. 2005 Mar 11;365(9462):876–8. doi: 10.1016/S0140-6736(05)71046-1. [DOI] [PubMed] [Google Scholar]
- 8.NeblettFanfair R, Benedict K, Bos J, Bennett SD, Lo YC, Adebanjo T. Necrotizing cutaneous mucormycosis after a tornado in Joplin, Missouri, in 2011. N Engl J Med. 2012 Dec 6;367(23):2214–25. doi: 10.1056/NEJMoa1204781. [DOI] [PubMed] [Google Scholar]
- 9.Kouadio IK, Aljunid S, Kamigaki T, Hammad K, Oshitani H. Infectious diseases following natural disasters: prevention and control measures. Expert Rev Anti Infect Ther. 2012 Jan;10(1):95–104. doi: 10.1586/eri.11.155. [DOI] [PubMed] [Google Scholar]
- 10.Pagano L, Ricci P, Tonso A, et al. Mucormycosis in patients with haematological malignancies: a retrospective clinical study of 37 cases, GIMEMA Infection Program (Gruppo Italiano Malattie Ematologiche Malignedel l'Adulto) Br J Haematol. 1997 Nov;99(2):331–6. doi: 10.1046/j.1365-2141.1997.3983214.x. [DOI] [PubMed] [Google Scholar]
- 11.Lee FY, Mossad SB, Adal KA. Pulmonary mucormycosis: the last 30 years. Arch Intern Med. 1999 Jun 28;159(12):1301–9. doi: 10.1001/archinte.159.12.1301. [DOI] [PubMed] [Google Scholar]
- 12.Hong HL, Lee YM, Kim T, Lee JY, Chung YS, Kim MN, Kim SH, Choi SH, Kim YS, Woo JH, Lee SO. Risk factors for mortality in patients with invasive mucormycosis. Infection & chemotherapy. 2013 Sep 1;45(3):292–8. doi: 10.3947/ic.2013.45.3.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Riera F, Marangoni LD, Allende BL, Allende C, Minoldo E, Martinatto C, Paoletti OA, Bergallo C. Mucormycosis clinical cases and update. Rev FacCien Med UnivNac Cordoba. 2014;71(4):192–8. [PubMed] [Google Scholar]
- 14.Ibrahim AS, Kontoyiannis DP. Update on mucormycosis pathogenesis. CurrOpin Infect Dis. 2013 Dec;26(6):508–15. doi: 10.1097/QCO.0000000000000008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Pauw B, Walsh TJ, Donnelly JP, Stevens DA, Edwards JE, Calandra T, Pappas PG, Maertens J, Lortholary O, Kauffman CA, Denning DW. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin Infect Dis. 2008 Jun 15;46(12):1813–21. doi: 10.1086/588660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Frater JL, Hall GS, Procop GW. Histologic features of zygomycosis: emphasis on perineural invasion and fungal morphology. Archives of pathology & laboratory medicine. 2001 Mar;125(3):375–8. doi: 10.5858/2001-125-0375-HFOZ. [DOI] [PubMed] [Google Scholar]
- 17.Parfrey NA. Improved Diagnosis and Prognosis of Mucormyucosis: A CLINICOPATHOLOGIC STUDY OF 33 CASES. Medicine. 1986 Mar 1;65(2):113–23. doi: 10.1097/00005792-198603000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clinical Microbiology Reviews. 2005 Jul 1;18(3):556–69. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prabhu RM, Patel R. Mucormycosis and entomophthoramycosis: a review of the clinical manifestations, diagnosis and treatment. Clinical Microbiology and Infection. 2004 Mar 1;10(s1):31–47. doi: 10.1111/j.1470-9465.2004.00843.x. [DOI] [PubMed] [Google Scholar]
- 20.Chakrabarti A, Das A, Mandal J, Shivaprakash MR, George VK, Tarai B, Rao P, Panda N, Verma SC, Sakhuja V. The rising trend of invasive zygomycosis in patients with uncontrolled diabetes mellitus. Medical mycology. 2006 Jan 1;44(4):335–42. doi: 10.1080/13693780500464930. [DOI] [PubMed] [Google Scholar]
- 21.Ludvigsson J. Why diabetes incidence increases—a unifying theory. Annals of the New York Academy of Sciences. 2006 Oct 1;1079(1):374–82. doi: 10.1196/annals.1375.058. [DOI] [PubMed] [Google Scholar]
- 22.Nithyanandam S, Jacob MS, Battu RR, Thomas RK, Correa MA, D'Souza O. Rhino-orbito-cerebral mucormycosis: a retrospective analysis of clinical features and treatment outcomes. Indian journal of ophthalmology. 2003 Sep 1;51(3):231. [PubMed] [Google Scholar]
- 23.Joshi N, Caputo GM, Weitekamp MR, Karchmer AW. Infections in patients with diabetes mellitus. New England Journal of Medicine. 1999 Dec 16;341(25):1906–12. doi: 10.1056/NEJM199912163412507. [DOI] [PubMed] [Google Scholar]
- 24.Chayakulkeeree M, Ghannoum MA, Perfect JR. Zygomycosis: the re-emerging fungal infection. European Journal of Clinical Microbiology and Infectious Diseases. 2006 Apr 1;25(4):215–29. doi: 10.1007/s10096-006-0107-1. [DOI] [PubMed] [Google Scholar]
- 25.Sugar AM, Mandell GL, Douglas RG, Jr, Bennett JE. Agents of mucormycosis and related species. Principles and practice of infectious diseases. (Ed. 3) 1990:1962–72. [Google Scholar]
- 26.Greenberg RN, Scott LJ, Vaughn HH, Ribes JA. Zygomycosis (mucormycosis): emerging clinical importance and new treatments. Current opinion in infectious diseases. 2004 Dec 1;17(6):517–25. doi: 10.1097/00001432-200412000-00003. [DOI] [PubMed] [Google Scholar]
- 27.Helderman JH, Cooper HS, Mann JO. Letter: Chronic phycomycosis in a controlled diabetic. Annals of internal medicine. 1974 Mar;80(3):419. doi: 10.7326/0003-4819-80-3-419. [DOI] [PubMed] [Google Scholar]
- 28.Bhansali A, Bhadada S, Sharma A, Suresh V, Gupta A, Singh P, Chakarbarti A, Dash RJ. Presentation and outcome of rhino-orbital-cerebral mucormycosis in patients with diabetes. Postgraduate medical journal. 2004 Nov 1;80(949):670–4. doi: 10.1136/pgmj.2003.016030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fatterpekar G, Mukherji S, Arbealez A, Maheshwari S, Castillo M. Fungal diseases of the paranasal sinuses. Semin Ultrasound CT MR. 1999;20:391–401. doi: 10.1016/s0887-2171(99)90023-9. [DOI] [PubMed] [Google Scholar]
- 30.Talmi YP, Goldschmied-Reouven A, Bakon M, Barshack I, Wolf M, Horowitz Z, Berkowicz M, Keller N, Kronenberg J. Rhino-orbital and rhino-orbito-cerebral mucormycosis. Otolaryngology--Head and Neck Surgery. 2002 Jul 1;127(1):22–31. doi: 10.1067/mhn.2002.126587. [DOI] [PubMed] [Google Scholar]
- 31.Thajeb P, Thajeb T, Dai D. Fatal strokes in patients with rhino-orbito-cerebral mucormycosis and associated vasculopathy. Scandinavian journal of infectious diseases. 2004 Sep 1;36(9):643–8. doi: 10.1080/00365540410020794. [DOI] [PubMed] [Google Scholar]
- 32.Gleissner B, Schilling A, Anagnostopolous I, Siehl I, Thiel E. Improved outcome of zygomycosis in patients with hematological diseases? Leukemia & lymphoma. 2004 Jul 1;45(7):1351–60. doi: 10.1080/10428190310001653691. [DOI] [PubMed] [Google Scholar]
- 33.Tedder M, Spratt JA, Anstadt MP, Hegde SS, Tedder SD, Lowe JE. Pulmonary mucormycosis: results of medical and surgical therapy. The Annals of thoracic surgery. 1994 Apr 30;57(4):1044–50. doi: 10.1016/0003-4975(94)90243-7. [DOI] [PubMed] [Google Scholar]
- 34.Patiño JF, Castro D. Necrotizing lesions of soft tissues: a review. World journal of surgery. 1991 Mar 1;15(2):235–9. doi: 10.1007/BF01659058. [DOI] [PubMed] [Google Scholar]
- 35.Prasad RM, Bose SM, Vaiphei K, Verma GR. Post operative abdominal wall mucormycosis mimicking as bacterial necrotising fasciitis. Journal of postgraduate medicine. 2003 Apr 1;49(2):187. [PubMed] [Google Scholar]
- 36.Straatsma BR, Zimmerman LE, Gass JDM. Phycomycosis: a clinicopathologic study of fifty-one cases. Lab. Investig. 1962;11:963–985. [PubMed] [Google Scholar]
- 37.Khor BS, Lee MH, Leu HS, Liu JW. Rhinocerebral mucormycosis in Taiwan. Journal of microbiology, immunology, and infection= Wei mian yu gan ran za zhi. 2003 Dec;36(4):266–9. [PubMed] [Google Scholar]
- 38.Peterson KL, Wang M, Canalis RF, Abemayor E. Rhinocerebralmucormycosis: evolution of the disease and treatment options. Laryngoscope. 1997;107:855–862. doi: 10.1097/00005537-199707000-00004. [DOI] [PubMed] [Google Scholar]
- 39.Petrikkos G, Skiada A, Sambatakou H, Toskas A, Vaiopoulos G, Giannopoulou M, Katsilambros N. Mucormycosis: ten-year experience at a tertiary-care center in Greece. European Journal of Clinical Microbiology and Infectious Diseases. 2003 Dec 1;22(12):753–6. doi: 10.1007/s10096-003-1035-y. [DOI] [PubMed] [Google Scholar]
- 40.Spellberg B, Edwards J, Ibrahim A. Novel perspectives on mucormycosis: pathophysiology, presentation, and management. Clinical Microbiology Reviews. 2005 Jul 1;18(3):556–69. doi: 10.1128/CMR.18.3.556-569.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]


