Abstract
Context
Lung cancer is one of the most common cancers affecting both men and women and is associated with high symptom burden and psychological distress. Lung cancer patients’ family caregivers also show high rates of distress. However, few interventions have been tested to alleviate significant problems of this population.
Objectives
This study examined the preliminary efficacy of telephone-based symptom management (TSM) for symptomatic lung cancer patients and their family caregivers.
Methods
Symptomatic lung cancer patients and caregivers (N=106 dyads) were randomly assigned to 4 sessions of TSM consisting of cognitive-behavioral and emotion-focused therapy or an education/support condition. Patients completed measures of physical and psychological symptoms, self-efficacy for managing symptoms, and perceived social constraints from the caregiver; caregivers completed measures of psychological symptoms, self-efficacy for helping the patient manage symptoms and managing their own emotions, perceived social constraints from the patient, and caregiving burden.
Results
No significant group differences were found for all patient outcomes and caregiver self-efficacy for helping the patient manage symptoms and caregiving burden at 2 and 6-weeks post-intervention. Small effects in favor of TSM were found regarding caregiver self-efficacy for managing their own emotions and perceived social constraints from the patient. Study outcomes did not significantly change over time in either group.
Conclusion
Findings suggest that our brief telephone-based psychosocial intervention is not efficacious for symptomatic lung cancer patients and their family caregivers. Next steps include examining specific intervention components in relation to study outcomes, mechanisms of change, and differing intervention doses and modalities.
Keywords: lung cancer, family caregivers, psychosocial interventions, cognitive-behavioral, symptom management, distress
Introduction
Lung cancer is one of the most frequently diagnosed cancers and the leading cause of cancer deaths in both men and women.1 Most lung cancer patients (85%) have regional or distant metastases at diagnosis, contributing to their high rate (80%) of multiple physical and psychological symptoms.2–4 Lung cancer patients experience higher rates of anxiety and depressive symptoms and breathlessness than other cancer patients.5–7 The most frequent and severe symptoms in lung cancer patients include depression, anxiety, pain, fatigue, and breathlessness, which contribute to impaired quality of life (QoL).2, 8–11 Greater distress and reduced QoL in lung cancer patients have been associated with lower self-efficacy or confidence in their ability to manage symptoms and greater social constraints (e.g., avoidance, criticism) on cancer-related disclosure.12, 13
Family caregivers’ QoL is also affected.14–18 Up to 50% of family caregivers of lung cancer patients experience significant anxiety or depressive symptoms.14, 19–22 Greater caregiver distress has been related to greater caregiving burden and lower self-efficacy in assisting the patient with symptom management.12, 23 Caregivers of lung cancer patients have reported difficulty with caregiving tasks such as providing emotional support and monitoring symptoms.24, 25
Clinical practice guidelines for lung cancer have changed to emphasize the early integration of standard oncologic and palliative care based on evidence that this may improve QoL and possibly survival in advanced lung cancer patients.26–28 Although palliative care services are available in many hospitals, patients with lung and other cancers and their caregivers have reported high rates of unmet needs for symptom management and psychosocial support.29–31 In addition, non-pharmacologic aspects of palliative care have a limited evidence base for use with lung cancer patients and caregivers.32–34 To date, cognitive-behavioral interventions have reduced physical symptom severity in patients with various cancers and chronic illnesses35–41 and reduced distress in primarily early-stage cancer patients and caregivers.32, 38 Two trials have tested emotion-focused interventions for couples coping with cancer and both showed improved relational outcomes.42, 43 Regarding trials specific to lung cancer patients and caregivers, one pilot study found that a telephone-based dyadic intervention reduced advanced lung cancer patient and caregiver anxiety and depressive symptoms and caregiver burden compared to usual care.44 A telephone-based trial for early-stage lung cancer patients and caregivers found that both caregiver-assisted coping skills training and education/support led to improvement in patient depression, lung cancer symptoms, and self-efficacy for symptom control as well as caregiver anxiety and self-efficacy for helping the patient manage symptoms.45
The present study tested a novel telephone-based symptom management (TSM) intervention with lung cancer patients and caregivers jointly participating. Telephone delivery reduces barriers to participation for people with physical impairments. While most prior symptom management trials with cancer patient-caregiver dyads have emphasized patient care,32, 45–49 TSM has a dual focus on patient and caregiver concerns. The intervention involves a blend of evidence-based cognitive-behavioral and emotion-focused strategies42, 45, 50–53 to address patient and caregiver anxiety and depressive symptoms and patient pain, fatigue, and breathlessness. These primary outcomes were chosen because they are amenable to non-pharmacologic intervention and prevalent in this population.2, 8–11, 14, 22 The intervention was framed by Social Cognitive Theory, which hypothesizes that self-efficacy to implement symptom management strategies will result in improved health outcomes.54, 55 The TSM intervention was designed to influence self-efficacy by encouraging practice of new skills, emphasizing the benefits of practicing the skills, and changing maladaptive thoughts. Self-efficacy also may be enhanced as patients and caregivers reinforce each other’s practice of the skills.
We enrolled lung cancer patients who met clinical criteria for at least one of five symptoms targeted in the intervention (i.e., depressive symptoms, anxiety, pain, fatigue, or breathlessness) and their family caregivers. We hypothesized that TSM would lead to improved primary outcomes for patients (i.e., reduced depressive symptoms, anxiety, pain, fatigue, and breathlessness) and caregivers (i.e., reduced depressive symptoms and anxiety) as compared to an education/support condition that controlled for time and attention provided to participants. We also hypothesized that TSM would lead to improved secondary outcomes for patients (i.e., self-efficacy for symptom management and perceived social constraints from the caregiver) and caregivers (i.e., self-efficacy for helping the patient manage symptoms and managing their own emotions, perceived social constraints from the patient, and caregiving burden) as compared to the education/support condition.
Methods
Participants and Setting
Participants were recruited from the Indiana University Simon Cancer Center, the Roudebush VA Medical Center, and Eskenazi Hospital in Indianapolis, IN between March 2013 and April 2015. All study procedures received institutional review board approval (Clinicaltrials.gov number NCT01993550). Patient inclusion requirements were: 1) a diagnosis of small-cell or non-small-cell lung cancer, 2) English fluency, 3) at least one symptom of moderate severity, defined by validated cutpoints for depressive symptoms (Patient Health Questionnaire-2 [PHQ-2] score ≥ 3 on this 0–6 scale);56 anxiety (Generalized Anxiety Disorder two-item scale [GAD-2] score ≥ 3 on this 0–6 scale);57 pain (PEG score ≥ 5 on this 0–10 scale);58 fatigue (SF-36 Vitality score ≤ 45 on this 0–100 scale);59, 60 or breathlessness (Memorial Symptom Assessment Scale [MSAS] shortness-of-breath severity score ≥ 2 on this 1–4 scale),61 and 4) a consenting family caregiver. Patients were excluded from study participation if they: 1) had severe cognitive impairment defined as four or more errors on a six-item cognitive screener,62 or 2) were receiving hospice care at the time of enrollment.
An authorized study team member reviewed medical records and consulted with oncologists to confirm initial patient eligibility. A research assistant approached the patient during an oncology clinic visit to describe the study. Interested patients identified their primary family caregiver (i.e., the person who provided most of their unpaid, informal care) and completed the symptom eligibility screening. With the patient’s written consent, a research assistant approached caregivers in clinic or via telephone to obtain informed consent. Eligible caregivers were adults (18+ years of age) who were fluent in English and lived with the patient or had visited the patient at least twice a week for the past month. At the time of enrollment, all participants received a brochure outlining psychosocial services at the study site.
Adequate sample size was determined on the basis of group comparisons of anxiety and depressive symptoms. An a priori power analysis suggested that a mixed linear model would have 80% power to detect a Cohen’s d of 0.63 (P=0.05, two-sided) in a sample of 42 patient-caregiver dyads, assuming an intraclass correlation coefficient of 0.05.63 This effect size is smaller than that found for anxiety and depressive symptoms in a trial comparing a dyadic telephone-based psychosocial intervention to usual care for advanced lung cancer patients and caregivers.44
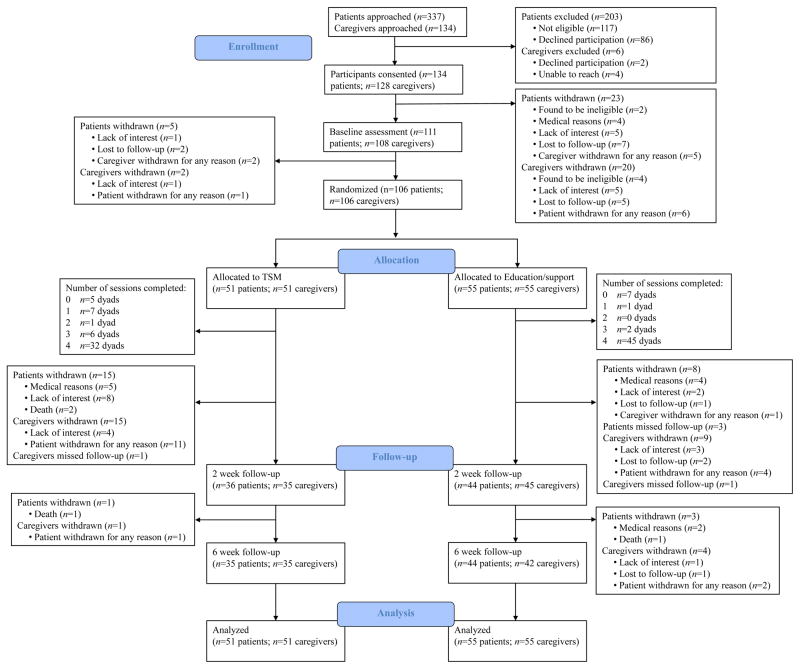
Of the 337 potentially eligible lung cancer patients who were approached regarding this study, 86 declined to participate, 117 were deemed ineligible, and 134 consented. The most common reasons for study refusal were lack of interest, time constraints, and personal stress. Most ineligible patients did not meet the symptom criterion for study entry. Of the 134 patients and 128 caregivers who consented, 50 withdrew before randomization primarily because of medical reasons, loss of interest, or an inability to reach them via phone. Thus, 106 patient-caregiver dyads were included in the current analyses (Fig. 1).
Figure 1.
Study Flow Chart
Procedures
All participants completed an individual baseline assessment and then patient-caregiver dyads were randomly assigned to one of two conditions: 1) telephone symptom management (TSM; n=51 dyads) or 2) an education/support condition (n=55 dyads). Randomization assignments were generated by a person who was not a study interviewer or therapist using a SAS procedure. Randomization was stratified by patient gender and performance status (self-reported Eastern Cooperative Oncology Group scores 0 or 1 vs. ≥2).64, 65 Patients and caregivers completed an individual follow-up assessment at 2 weeks and 6 weeks post-intervention because we were interested in short-term effects of the intervention and two weeks matched the time frame of certain measures. Research assistants who were blind to study condition conducted all assessments via telephone.
General Aspects of Treatment Procedures and Therapist Training
Participants in both study conditions (TSM and education/support) participated in four weekly 45-minute telephone sessions. Both dyad members participated simultaneously via speakerphone, and all sessions were audiorecorded. Both study conditions were delivered by licensed clinical social workers who were trained by a PhD-level psychologist. Training included didactic instruction and role-plays of treatment sessions detailed in manuals. Following the initial training, social workers received weekly supervision from the psychologist who reviewed 53% of audiorecordings for adherence to the study protocol and provided feedback on treatment adherence and quality. The average fidelity rating was 97.5%.
Telephone Symptom Management
Participants in this condition received instruction in symptom management strategies. Each person was mailed identical handouts detailing major points discussed during the sessions and home practice assignments as well as a CD with instructions for relaxation exercises. The primary goal of the intervention was to teach patients and caregivers various evidence-based cognitive-behavioral and emotion-focused strategies for managing anxiety and depressive symptoms, pain, fatigue, and breathlessness. All sessions had a dual focus on patient and caregiver concerns. A summary of the intervention components appears in Table 1.
Table 1.
Summary of Topics Covered in Each Intervention Condition
| Telephone Symptom Management | Education/Support |
|---|---|
|
| |
|
|
During the first session, the social worker introduced the sessions as providing information and skills for coping with lung cancer and discussed cancer-related changes that the patient and caregiver had experienced. During the four sessions, the patient and caregiver received instruction in symptom management strategies, including relaxation exercises, problem-solving, cognitive restructuring, emotion-focused/self-soothing approaches, communication skills, pleasant activity scheduling, and activity pacing. The symptoms endorsed by the patient or caregiver were emphasized when presenting the strategies, but all dyads received training in the same strategies. Skill practice comprised the majority of each session, and dyad members practiced the skills simultaneously (e.g., relaxation) or consecutively (e.g., communication). Each session began with a review of the patient’s and caregiver’s practice of the skills and ended with a discussion of a home practice assignment. Dyad members were encouraged to practice the skills together at home.
Education/Support Condition
The primary goal of this intervention was to direct participants to resources for practical and health information and psychosocial services. A similar comparison group was used in a prior psychosocial intervention trial with gastrointestinal cancer patients and caregivers.43 The therapists were the same as those for the TSM condition. Table 1 provides a summary of the intervention components. The sessions included the following topics: orientation to the medical center and treatment team, the impact of cancer on QoL, resources for health information, psychosocial support, and financial concerns, and evaluating health information on the Internet. Each person was mailed handouts summarizing the topics for each session and was asked to review them at home. Instruction in symptom management strategies did not occur in the education/support condition.
Measures
The primary and secondary outcomes were assessed with validated self-report measures used with cancer patients and caregivers.
Primary Outcomes
The Patient Health Questionnaire-8 (PHQ-8)66, 67 and Generalized Anxiety Disorder seven-item scale (GAD-7)57 were used to assess patient and caregiver depressive and anxiety symptoms, respectively. In addition, the following measures assessed patient physical symptoms: 1) the Brief Pain Inventory Short Form consisting of pain severity and pain interference subscales;68, 69 2) the Fatigue Symptom Inventory consisting of fatigue frequency, severity, and interference subscales;70, 71 and 3) four items from the MSAS assessing the frequency and severity of breathlessness as well as distress related to breathlessness.61
Secondary Outcomes
A 16-item standard self-efficacy scale modified from the arthritis literature was used to assess patients’ perceived ability to manage pain, other symptoms, and function.12, 72 A parallel version of this scale was administered to caregivers to assess their confidence in their ability to help the patient manage symptoms.12, 72 In addition, eight items developed by Kilbourn et al.73 were used to assess caregivers’ self-efficacy for managing their own emotions. Patients and caregivers also completed the 5-item social constraints scale assessing perceived constraints on cancer-related disclosure from the other dyad member.74 Finally, the Caregiver Reaction Assessment was used to evaluate caregiver burden and included the following subscales: impact on schedule, caregiver’s esteem, lack of family support, impact on health, and impact on finances.75
Sociodemographic and Medical Variables
Patients and caregivers reported their demographic information and use of mental health services at baseline. Patient medical information was obtained via chart review.
Statistical Analyses
Baseline comparisons (Fisher’s exact tests and t-tests) assessed differences between the TSM and education/support groups for patients and caregivers separately. Possible gender differences were examined. Linear mixed-model repeated measures analyses in SPSS were used to examine the preliminary efficacy of TSM. Because these analyses use all available data, an intent-to-treat framework was implemented. For outcomes reported by patients and caregivers, multilevel modeling for dyadic data was used to account for the non-independence of data from two members of the same dyad.76, 77 Models included the main effects of time, study group, and social role (patient or caregiver) as well as time x group and time x group x role interactions. Both time and study group were treated as categorical variables in these models, which focuses the analyses on mean differences between groups and across time. A significant treatment effect is indicated either by a significant study group main effect or a significant time x group interaction. A significant time x group x role interaction indicates that the treatment effect differs for patients and caregivers. For outcome measures that only patients or caregivers completed, models included main effects of time and study group (TSM or education/support) and the time x group interaction. Again, all variables were treated as categorical. Gender was not included in the models because only two significant gender differences in outcomes were found (data not shown).
Results
Participant Characteristics
Table 2 presents participant characteristics by study group and group comparisons at baseline. About half of the patients (53%) were women, and most caregivers (73%) were women. Patients and caregivers were primarily White and had completed an average of 13 years of education. The median annual household income was over $30,000. Sixty-three percent of caregivers were spouses or partners of the patient. No significant baseline differences on demographic, medical, or outcome variables were found for patients and caregivers randomized to TSM and education/support conditions, with the exception of caregiver income.
Table 2.
Patient and Caregiver Characteristics and Group Comparisons at Baseline
| Characteristic | Patients (n = 106)
|
t-test/Fisher’s exact test p | Caregivers (n = 106)
|
t-test/Fisher’s exact test p | ||
|---|---|---|---|---|---|---|
| TSM (n = 51) | Education/support (n = 55) | TSM (n = 51) | Education/support (n = 55) | |||
| Sex, n (%) | 0.70 | 0.99 | ||||
| Male | 23 (45.10) | 27 (49.09) | 14 (27.45) | 15 (27.27) | ||
| Female | 28 (54.90) | 28 (50.91) | 37 (72.55) | 40 (72.73) | ||
| Age | 0.33 | 0.88 | ||||
| Mean | 63.47 | 61.96 | 56.33 | 56.75 | ||
| SD | 7.68 | 8.20 | 14.09 | 13.81 | ||
| Range | 45–85 | 42–82 | 20–76 | 20–80 | ||
| Race, n (%) | ||||||
| non-Hispanic White | 45 (88.24) | 51 (92.73) | 0.52 | 44 (86.27) | 51 (92.73) | 0.51 |
| Missing | 0 (0.00) | 0 (0.00) | 1 (1.96) | 0 (0.00) | ||
| Household income, n (%) | ||||||
| $0 – $20,999 | 10 (19.61) | 10 (18.18) | 0.80 | 8 (15.69) | 8 (14.55) | 0.79 |
| $21,000 – $50,999 | 12 (23.53) | 21 (38.18) | 0.20 | 11 (21.57) | 26 (47.27) | 0.01 |
| $51,000 – $99,999 | 13 (25.49) | 11 (20.00) | 0.35 | 17 (33.33) | 9 (16.36) | 0.02 |
| $100,000 or more | 7 (13.73) | 8 (14.55) | 0.99 | 9 (17.65) | 10 (18.18) | 0.99 |
| Missing | 9 (17.65) | 5 (9.09) | 6 (11.76) | 2 (3.64) | ||
| Employment status, n (%) | ||||||
| Employed full or part-time | 9 (17.65) | 13 (23.64) | 0.48 | 23 (45.10) | 30 (54.55) | 0.44 |
| Retired | 25 (49.02) | 20 (36.36) | 0.24 | 16 (31.37) | 15 (27.27) | 0.67 |
| Unemployed/other (e.g., sick leave, homemaker) | 17 (33.33) | 22 (40.00) | 0.55 | 11 (21.57) | 10 (18.18) | 0.64 |
| Missing | 0 (0.00) | 0 (0.00) | 1 (1.96) | 0 (0.00) | ||
| Years of education | 0.57 | 0.35 | ||||
| Mean | 12.92 | 13.16 | 13.94 | 13.45 | ||
| SD | 2.22 | 2.11 | 2.85 | 2.54 | ||
| Range | 9–19 | 9–19 | 8–20 | 9–19 | ||
| Caregiver relationship to the patient, n (%) | ||||||
| Spouse/partner | 32 (62.75) | 34 (61.82) | 0.99 | |||
| Son/daughter | 9 (17.65) | 12 (21.82) | 0.63 | |||
| Other family member or friend | 10 (19.61) | 9 (16.37) | 0.80 | |||
| Caregiver lives with the patient, n (%) | 37 (72.55) | 41 (74.55) | 0.83 | |||
| Married/living with partner, n (%) | 35 (68.63) | 37 (67.27) | 0.99 | 41 (80.39) | 41 (74.55) | 0.50 |
| Psychiatric medication, n (%)1 | 22 (43.14) | 34 (61.82) | 0.08 | 13 (25.49) | 20 (36.36) | 0.29 |
| Psychotherapy/counseling, n (%)1 | 1 (1.96) | 5 (9.09) | 0.21 | 2 (3.92) | 1 (1.82) | 0.61 |
| Study site, n (%) | ||||||
| Indiana University Simon Cancer Center | 39 (76.47) | 42 (76.36) | 0.99 | |||
| Roudebush VA Medical Center | 10 (19.61) | 10 (18.18) | 0.99 | |||
| Eskenazi Hospital in Indianapolis | 2 (3.92) | 3 (5.45) | 0.99 | |||
| Type of lung cancer, n (%) | 0.77 | |||||
| NSCLC | 44 (86.27) | 49 (89.09) | ||||
| SCLC | 7 (13.73) | 6 (10.91) | ||||
| Stage of NSCLC, n (%) | ||||||
| Stage I | 12 (23.53) | 6 (10.91) | 0.11 | |||
| Stage II | 4 (7.84) | 10 (18.18) | 0.15 | |||
| Stage III | 9 (17.65) | 9 (16.36) | 0.99 | |||
| Stage IV | 19 (37.25) | 24 (43.64) | 0.68 | |||
| Stage of SCLC, n (%) | 0.56 | |||||
| Limited-stage | 3 (5.88) | 1 (1.82) | ||||
| Extensive-stage | 4 (7.84) | 5 (9.09) | ||||
| Time since diagnosis in years | 0.65 | |||||
| Mean | 1.26 | 1.09 | ||||
| SD | 2.12 | 1.46 | ||||
| Range | 0.07–11.99 | 0.10–8.52 | ||||
| Missing, n (%) | 1 (1.96) | 2 (3.64) | ||||
| Lung cancer treatments received, n (%) | ||||||
| Chemotherapy | 27 (52.94) | 34 (61.82) | 0.43 | |||
| Radiation | 13 (25.49) | 15 (27.27) | 0.99 | |||
| Chemoradiation | 12 (23.53) | 11 (20.00) | 0.81 | |||
| Surgery | 24 (47.06) | 23 (41.82) | 0.70 | |||
| Patient self-reported ECOG score | 0.65 | |||||
| Mean | 1.43 | 1.51 | ||||
| SD | 0.92 | 0.86 | ||||
| Range | 0–3 | 0–4 | ||||
| Depressive symptoms, n (%)2 | 19 (37.25) | 24 (43.64) | 0.56 | |||
| Anxiety, n (%)2 | 20 (39.22) | 18 (32.73) | 0.55 | |||
| Pain, n (%)2 | 17 (33.33) | 19 (34.55) | 0.99 | |||
| Fatigue, n (%)2 | 27 (52.94) | 26 (47.27) | 0.70 | |||
| Breathlessness, n (%)2 | 34 (66.67) | 38 (69.09) | 0.84 | |||
| Number of symptoms2 | 0.93 | |||||
| Mean | 2.29 | 2.27 | ||||
| SD | 1.17 | 1.28 | ||||
| Range | 1–5 | 1–5 | ||||
Note. TSM = Telephone-based symptom management intervention; SD = standard deviation; NSCLC = non-small cell lung cancer; SCLC = small cell lung cancer; ECOG = Eastern Cooperative Oncology Group.
Treatment received in the past month at baseline.
Symptoms assessed at screening.
Primary Outcomes
Results from the mixed model dyadic analyses revealed no main effect of study group or time x group effect for anxiety and depressive symptoms (Table 3). However, there was a main effect of role on depressive symptoms such that patients, on average, had higher levels of depressive symptoms than caregivers. In addition, mixed model analyses showed no main effects of study group or time x group effect for patient pain, fatigue, or breathlessness (Table 4). There were also no significant main effects of time, indicating that in general primary outcome variables did not change on average over the study period.
Table 3.
Intent-to-Treat Results for Multilevel Linear Models Predicting Dyadic Outcomes (n = 106 dyads)
| Outcome Fixed effect |
TSM
|
Education/support
|
df | F | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 2 weeks post-intervention | 6 weeks post-intervention | Baseline | 2 weeks post-intervention | 2 weeks post-intervention | ||||
|
|
|
||||||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||
| Patient Depressive Symptoms | 7.33 (4.78) | 6.36 (3.75) | 6.71 (4.77) | 8.27 (5.48) | 8.36 (5.67) | 7.18 (5.25) | |||
| Caregiver Depressive Symptoms | 5.67 (5.59) | 5.09 (4.88) | 4.83 (4.90) | 5.33 (5.02) | 5.89 (5.22) | 5.64 (5.67) | |||
| Group | 103 | 0.56 | 0.45 | ||||||
| Time | 161 | 0.32 | 0.72 | ||||||
| Role | 106 | 11.49 | 0.00 | ||||||
| Time X Role | 165 | 0.33 | 0.72 | ||||||
| Group X Time | 161 | 0.69 | 0.50 | ||||||
| Group X Role | 106 | 0.21 | 0.65 | ||||||
| Group X Time X Role | 165 | 1.15 | 0.32 | ||||||
| Patient Anxiety Symptoms | 5.12 (4.89) | 3.72 (3.45) | 4.06 (3.82) | 6.31 (5.91) | 6.68 (6.48) | 5.45 (5.93) | |||
| Caregiver Anxiety Symptoms | 6.10 (5.19) | 5.06 (4.28) | 5.00 (4.77) | 6.02 (5.74) | 6.51 (6.04) | 5.86 (6.25) | |||
| Group | 106 | 2.76 | 0.10 | ||||||
| Time | 164 | 1.78 | 0.17 | ||||||
| Role | 104 | 0.30 | 0.59 | ||||||
| Time X Role | 162 | 0.09 | 0.92 | ||||||
| Group X Time | 164 | 2.58 | 0.08 | ||||||
| Group X Role | 104 | 0.77 | 0.38 | ||||||
| Group X Time X Role | 162 | 0.68 | 0.51 | ||||||
Note. TSM = Telephone-delivered symptom management intervention; SD = standard deviation.
Table 4.
Intent-to-Treat Results for Multilevel Linear Models Predicting Patient Outcomes (n = 106)
| Outcome Fixed effect |
TSM
|
Education/support
|
df | F | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 2 weeks post-intervention | 6 weeks post-intervention | Baseline | 2 weeks post-intervention | 6 weeks post-intervention | ||||
|
|
|
||||||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||
| Pain Severity | 2.61 (2.47) | 2.24 (2.16) | 2.64 (2.49) | 2.82 (2.42) | 2.62 (2.34) | 2.77 (2.48) | |||
| Group | 107 | 0.26 | 0.61 | ||||||
| Time | 162 | 1.34 | 0.27 | ||||||
| Group X Time | 162 | 0.14 | 0.87 | ||||||
| Pain Interference | 2.27 (2.75) | 1.81 (2.15) | 2.66 (2.77) | 2.72 (2.79) | 2.75 (2.69) | 2.61 (2.42) | |||
| Group | 102 | 0.73 | 0.40 | ||||||
| Time | 163 | 1.00 | 0.37 | ||||||
| Group X Time | 163 | 1.69 | 0.19 | ||||||
| Fatigue Frequency | 5.37 (2.17) | 5.17 (2.17) | 5.27 (2.23) | 5.69 (2.41) | 5.00 (2.57) | 4.64 (2.71) | |||
| Group | 108 | 0.16 | 0.69 | ||||||
| Time | 170 | 2.66 | 0.07 | ||||||
| Group X Time | 170 | 1.81 | 0.17 | ||||||
| Fatigue Severity | 4.25 (2.04) | 3.84 (2.03) | 4.28 (2.47) | 4.47 (2.09) | 3.81 (2.31) | 3.63 (2.86) | |||
| Group | 109 | 0.01 | 0.92 | ||||||
| Time | 169 | 2.65 | 0.07 | ||||||
| Group X Time | 169 | 0.93 | 0.40 | ||||||
| Fatigue Interference | 3.22 (2.44) | 2.53 (2.04) | 2.87 (2.44) | 3.65 (2.53) | 3.42 (2.86) | 3.20 (2.89) | |||
| Group | 106 | 1.36 | 0.25 | ||||||
| Time | 165 | 1.72 | 0.18 | ||||||
| Group X Time | 165 | 0.49 | 0.62 | ||||||
| Breathlessness Frequency | 1.73 (1.37) | 1.75 (1.44) | 1.71 (1.41) | 1.73 (1.35) | 1.86 (1.34) | 1.98 (1.21) | |||
| Group | 107 | 0.30 | 0.58 | ||||||
| Time | 168 | 1.20 | 0.30 | ||||||
| Group X Time | 168 | 0.47 | 0.62 | ||||||
| Breathlessness Severity | 1.35 (1.00) | 1.19 (0.95) | 1.23 (0.97) | 1.45 (1.09) | 1.48 (1.09) | 1.43 (0.82) | |||
| Group | 106 | 0.96 | 0.33 | ||||||
| Time | 168 | 0.02 | 0.98 | ||||||
| Group X Time | 168 | 0.22 | 0.80 | ||||||
| Breathlessness Distress | 1.22 (1.12) | 1.33 (1.35) | 1.20 (1.23) | 1.25 (1.19) | 1.43 (1.37) | 1.39 (1.22) | |||
| Group | 106 | 0.34 | 0.56 | ||||||
| Time | 167 | 1.74 | 0.18 | ||||||
| Group X Time | 167 | 0.23 | 0.80 | ||||||
| Self-efficacy for Symptom Management | 63.51 (16.80) | 62.69 (17.41) | 61.78 (19.59) | 59.82 (17.33) | 60.55 (19.75) | 62.09 (20.87) | |||
| Group | 106 | 0.23 | 0.63 | ||||||
| Time | 167 | 0.08 | 0.92 | ||||||
| Group X Time | 167 | 0.85 | 0.43 | ||||||
| Social Constraints | 1.54 (0.69) | 1.58 (0.82) | 1.36 (0.56) | 1.61 (0.71) | 1.58 (0.68) | 1.70 (0.92) | |||
| Group | 106 | 1.13 | 0.29 | ||||||
| Time | 170 | 0.28 | 0.76 | ||||||
| Group X Time | 170 | 2.35 | 0.10 | ||||||
Note. TSM = Telephone-delivered symptom management intervention; SD = standard deviation.
Secondary Outcomes
Mixed model analyses revealed no main effects of study group or time x group effect for patient self-efficacy for symptom management or perceived social constraints from the caregiver (Table 4). Regarding secondary outcomes for caregivers, there was a significant time x group effect for self-efficacy for managing their own emotions. Means found in Table 5 showed a small increase in self-efficacy for managing emotions in the TSM group, whereas the mean scores for the education/support group showed a slight decline. In addition, there was a main effect of study group on caregiver reports of perceived social constraints from the patient in favor of TSM (Table 5). Thus, caregivers assigned to TSM felt less constrained in discussing the illness with the patient than those assigned to education/support. In addition, there were no main effects of study group or time x group effect for caregivers’ self-efficacy for managing the patient’s symptoms and all aspects of caregiving burden (Table 5).
Table 5.
Intent-to-Treat Results for Multilevel Linear Models Predicting Caregiver Outcomes (n = 106)
| Outcome Fixed effect |
TSM
|
Education/support
|
df | F | p | ||||
|---|---|---|---|---|---|---|---|---|---|
| Baseline | 2 weeks post-intervention | 6 weeks post-intervention | Baseline | 2 weeks post-intervention | 6 weeks post-intervention | ||||
|
|
|
||||||||
| Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||||
| Self-efficacy for Managing the Patient’s Symptoms | 60.01 (22.96) | 52.89 (22.12) | 59.16 (23.67) | 58.22 (20.77) | 58.48 (21.65) | 56.79 (21.65) | |||
| Group | 104 | 0.20 | 0.66 | ||||||
| Time | 157 | 1.16 | 0.31 | ||||||
| Group X Time | 157 | 1.61 | 0.20 | ||||||
| Self-efficacy for Managing Their Own Emotions | 7.27 (1.82) | 7.38 (1.92) | 7.59 (1.66) | 7.19 (1.64) | 6.74 (2.01) | 6.92 (2.18) | |||
| Group | 106 | 2.41 | 0.12 | ||||||
| Time | 161 | 0.73 | 0.48 | ||||||
| Group X Time | 161 | 3.21 | 0.04 | ||||||
| Caregiving Burden: Impact on Schedule | 13.69 (3.70) | 13.74 (4.45) | 13.89 (4.37) | 14.64 (4.62) | 13.89 (4.86) | 14.10 (5.24) | |||
| Group | 107 | 0.62 | 0.43 | ||||||
| Time | 162 | 0.04 | 0.96 | ||||||
| Group X Time | 162 | 0.28 | 0.75 | ||||||
| Caregiving Burden: Caregiver’s Esteem | 32.10 (2.76) | 32.49 (2.47) | 32.54 (2.65) | 32.04 (2.87) | 32.13 (2.68) | 32.31 (2.97) | |||
| Group | 103 | 0.20 | 0.65 | ||||||
| Time | 156 | 0.03 | 0.97 | ||||||
| Group X Time | 156 | 0.42 | 0.66 | ||||||
| Caregiving Burden: Lack of Family Support | 10.14 (4.58) | 9.57 (4.67) | 9.29 (5.17) | 9.67 (4.38) | 9.91 (4.94) | 9.86 (4.78) | |||
| Group | 103 | 0.08 | 0.78 | ||||||
| Time | 156 | 0.39 | 0.68 | ||||||
| Group X Time | 156 | 0.14 | 0.87 | ||||||
| Caregiving Burden: Impact on Health | 7.82 (3.22) | 8.23 (3.38) | 8.11 (3.64) | 8.04 (2.75) | 7.93 (2.96) | 8.17 (2.75) | |||
| Group | 106 | 0.02 | 0.88 | ||||||
| Time | 162 | 1.24 | 0.29 | ||||||
| Group X Time | 162 | 0.32 | 0.73 | ||||||
| Caregiving Burden: Impact on Finances | 6.48 (2.71) | 6.66 (3.16) | 6.34 (2.72) | 6.96 (3.07) | 6.31 (2.96) | 6.60 (3.22) | |||
| Group | 105 | 0.35 | 0.56 | ||||||
| Time | 161 | 0.02 | 0.98 | ||||||
| Group X Time | 161 | 1.02 | 0.36 | ||||||
| Social Constraints | 1.86 (0.85) | 1.60 (0.71) | 1.61 (0.80) | 2.06 (0.79) | 2.07 (0.92) | 2.05 (0.90) | |||
| Group | 103 | 7.09 | 0.01 | ||||||
| Time | 160 | 0.39 | 0.68 | ||||||
| Group X Time | 160 | 2.65 | 0.07 | ||||||
Note. TSM = Telephone-delivered symptom management intervention; SD = standard deviation.
Discussion
This study is one of the first to examine a dyadic psychosocial intervention for lung cancer patients and caregivers and to focus on lung cancer patients with clinically meaningful symptoms. Lung cancer patient-caregiver dyads were randomized to four sessions of TSM consisting of evidence-based cognitive-behavioral and emotion-focused therapy or four sessions of an education/support condition. Compared to the education/support condition, TSM did not result in improved patient and caregiver depressive symptoms or anxiety or improved patient pain, fatigue, or breathlessness. In addition, compared to education/support, TSM did not improve patient self-efficacy for managing their symptoms or perceived social constraints from the caregiver. TSM also did not improve caregiver self-efficacy for assisting the patient with symptom management and caregiving burden. In contrast, caregivers assigned to TSM showed better self-efficacy for managing their emotions and decreases in perceived social constraints from the patient across follow-ups; however, the effect sizes were small. Study outcomes did not significantly change over time for either group. Thus, findings do not support the efficacy of our brief telephone-based dyadic psychosocial intervention for symptomatic lung cancer patients and caregivers.
The current results are partially consistent with those of prior intervention studies with this population.33, 44, 45 For example, Porter and colleagues45 tested 14 telephone sessions of caregiver-assisted coping skills training or education/support for early-stage lung cancer patients and caregivers and found no differences in psychological distress or symptoms between study conditions. Both groups showed improved outcomes following the intervention. Conversely, another study found large effects of a 6-session telephone dyadic psychosocial intervention on advanced lung cancer patient and caregiver anxiety and depressive symptoms and caregiver burden compared to usual care.44 Differences in findings across studies may be related to characteristics of the sample, intervention and control groups, and assessments.
Several potential explanations for the current findings warrant consideration. First, brief cognitive-behavioral and emotion-focused therapy may not be sufficient for addressing the high symptom burden and unique challenges of lung cancer patients and caregivers. Similar to the general population of lung cancer patients,78 participants were, on average, socioeconomically disadvantaged, which may have contributed to suboptimal outcomes and barriers to participation. A meta-analysis found limited and inconclusive evidence that non-pharmacologic interventions impact lung cancer patient outcomes.33 In addition, meta-analyses have found small effects of couple-based and caregiver-focused interventions on psychological outcomes for cancer patients’ caregivers.32, 34 Thus, this study contributes to a limited but growing literature suggesting that novel approaches are needed to address the significant problems faced by lung cancer patients and caregivers.
Another possible explanation for the current findings is that the brief intervention length and telephone delivery lessened the impact of the intervention. As noted earlier, Porter and colleagues45 found that lung cancer patients and caregivers assigned to 14 sessions of telephone-based coping skills training or education/support showed improved symptom outcomes over time. Meta-analytic evidence regarding the effect of intervention dose on cancer patient and caregiver outcomes has been mixed.32, 34 One meta-analysis of individual and dyadic interventions for cancer patients’ caregivers found that fewer intervention sessions were associated with lower levels of depression and caregiving burden.32 Another meta-analysis of couple-based interventions did not find an association between the number of sessions and cancer patient and caregiver outcomes.34 Further work is needed to determine the optimal intervention dose. With respect to intervention modality, there is no evidence that telephone delivery is inferior to in-person treatment with respect to cancer patient and caregiver psychological outcomes, but few comparisons of different modalities have been conducted.32
Finally, this study did not include a usual care group. This group may have shown worsening outcomes over time relative to the TSM and education/support conditions. Three-arm trials are needed which compare new interventions to attention control conditions and usual care. Such trials would allow for more definitive conclusions regarding the impact of interventions.
Results of this study suggest several potential directions for future research. First, examining associations between specific intervention components and outcomes will allow researchers to develop more efficacious interventions. In addition, understanding the mechanisms underlying the effects of interventions will advance the science of symptom management and translation of findings to clinical care. A focus on participants with clinically meaningful symptoms will enhance the generalizability of findings to those who warrant clinical attention. Finally, comparing a dyadic to an individual intervention approach and different intervention modalities will clarify the most feasible and effective approach for this population.
Limitations of this study should be noted. The sample was primarily Caucasian and was recruited from three medical centers in the Midwestern U.S. Thus, the findings may not generalize to ethnic minorities and those in other geographic regions. Additionally, caregivers were eligible for this study regardless of their distress, which may have reduced intervention effects. Furthermore, the attrition rate was 40% at 6 weeks post-intervention, which is comparable to attrition rates in other studies with this population.45, 79 Finally, the study was underpowered for detecting moderators of the intervention’s effects. Further work with larger sample sizes is needed to determine for whom psychosocial interventions are most efficacious.
Our findings suggest that symptomatic lung cancer patients and caregivers may require more intensive intervention to produce symptom reduction. Next steps include examining the effects of specific components of our intervention on outcomes. Identifying the most efficacious approaches for symptom reduction and mechanisms underlying their effects is a critical issue in palliative care for this large, underserved population.
Acknowledgments
This work was supported by grant PEP-13-236-01-PCSM from the American Cancer Society, an American Cancer Society Institutional Research grant, and K07CA168883 and K05CA175048 from the National Cancer Institute. The authors thank Susan Daily, BS, RT(T), Barbara A. Given, PhD, study therapists, the physicians and staff at the Indiana University Simon Cancer Center, Roudebush VA Medical Center, and Eskenazi Hospital for their assistance with recruitment, and the study participants for their time and effort. This material is the result of work supported with resources and the use of facilities at the Roudebush VA Medical Center in Indianapolis, IN.
Footnotes
Disclosures
The contents do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. The study sponsors were not involved in the study design, the collection, analysis and interpretation of data, the writing of this report, or the decision to submit the article for publication.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer facts and figures 2015. Atlanta, GA: American Cancer Society; 2015. [Google Scholar]
- 2.Hopwood P, Stephens RJ. Symptoms at presentation for treatment in patients with lung cancer: implications for the evaluation of palliative treatment. The Medical Research Council (MRC) Lung Cancer Working Party. Br J Cancer. 1995;71:633–636. doi: 10.1038/bjc.1995.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kurtz ME, Kurtz JC, Stommel M, Given CW, Given B. Predictors of depressive symptomatology of geriatric patients with lung cancer-a longitudinal analysis. Psychooncology. 2002;11:12–22. doi: 10.1002/pon.545. [DOI] [PubMed] [Google Scholar]
- 4.Rolke HB, Bakke PS, Gallefoss F. Health related quality of life, mood disorders and coping abilities in an unselected sample of patients with primary lung cancer. Respir Med. 2008;102:1460–1467. doi: 10.1016/j.rmed.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 5.Dudgeon DJ, Kristjanson L, Sloan JA, Lertzman M, Clement K. Dyspnea in cancer patients: prevalence and associated factors. J Pain Symptom Manage. 2001;21:95–102. doi: 10.1016/s0885-3924(00)00258-x. [DOI] [PubMed] [Google Scholar]
- 6.Zabora J, BrintzenhofeSzoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psychooncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 7.Linden W, Vodermaier A, Mackenzie R, Greig D. Anxiety and depression after cancer diagnosis: prevalence rates by cancer type, gender, and age. J Affect Disord. 2012;141:343–351. doi: 10.1016/j.jad.2012.03.025. [DOI] [PubMed] [Google Scholar]
- 8.Cooley ME, Short TH, Moriarty HJ. Symptom prevalence, distress, and change over time in adults receiving treatment for lung cancer. Psychooncology. 2003;12:694–708. doi: 10.1002/pon.694. [DOI] [PubMed] [Google Scholar]
- 9.Hopwood P, Stephens RJ. Depression in patients with lung cancer: prevalence and risk factors derived from quality-of-life data. J Clin Oncol. 2000;18:893–903. doi: 10.1200/JCO.2000.18.4.893. [DOI] [PubMed] [Google Scholar]
- 10.Shin JA, Kosiba JD, Traeger L, et al. Dyspnea and panic among patients with newly diagnosed non-small cell lung cancer. J Pain Symptom Manage. 2014;48:465–470. doi: 10.1016/j.jpainsymman.2013.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Iyer S, Roughley A, Rider A, Taylor-Stokes G. The symptom burden of non-small cell lung cancer in the USA: a real-world cross-sectional study. Support Care Cancer. 2014;22:181–187. doi: 10.1007/s00520-013-1959-4. [DOI] [PubMed] [Google Scholar]
- 12.Porter LS, Keefe FJ, Garst J, McBride CM, Baucom D. Self-efficacy for managing pain, symptoms, and function in patients with lung cancer and their informal caregivers: associations with symptoms and distress. Pain. 2008;137:306–315. doi: 10.1016/j.pain.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chambers SK, Baade P, Youl P, et al. Psychological distress and quality of life in lung cancer: the role of health-related stigma, illness appraisals and social constraints. Psychooncology. 2015;24:1569–1577. doi: 10.1002/pon.3829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carmack Taylor CL, Badr H, Lee JH, et al. Lung cancer patients and their spouses: psychological and relationship functioning within 1 month of treatment initiation. Ann Behav Med. 2008;36:129–140. doi: 10.1007/s12160-008-9062-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Persson C, Östlund U, Wennman-Larsen A, Wengström Y, Gustavsson P. Health-related quality of life in significant others of patients dying from lung cancer. Palliat Med. 2008;22:239–247. doi: 10.1177/0269216307085339. [DOI] [PubMed] [Google Scholar]
- 16.Ellis J. The impact of lung cancer on patients and carers. Chron Respir Dis. 2012;9:39–47. doi: 10.1177/1479972311433577. [DOI] [PubMed] [Google Scholar]
- 17.Fujinami R, Sun V, Zachariah F, et al. Family caregivers’ distress levels related to quality of life, burden, and preparedness. Psychooncology. 2015;24:54–62. doi: 10.1002/pon.3562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Grant M, Sun V, Fujinami R, et al. Family caregiver burden, skills preparedness, and quality of life in non-small cell lung cancer. Oncol Nurs Forum. 2013;40:337–346. doi: 10.1188/13.ONF.337-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim Y, Duberstein PR, Sörensen S, Larson MR. Levels of depressive symptoms in spouses of people with lung cancer: Effects of personality, social support, and caregiving burden. Psychosomatics. 2005;46:123–130. doi: 10.1176/appi.psy.46.2.123. [DOI] [PubMed] [Google Scholar]
- 20.Östlund U, Wennman-Larsen A, Persson C, Gustavsson P, Wengström Y. Mental health in significant others of patients dying from lung cancer. Psychooncology. 2010;19:29–37. doi: 10.1002/pon.1433. [DOI] [PubMed] [Google Scholar]
- 21.Haun MW, Sklenarova H, Brechtel A, Herzog W, Hartmann M. Distress in cancer patients and their caregivers and association with the caregivers’ perception of dyadic communication. Oncol Res Treat. 2014;37:384–388. doi: 10.1159/000364885. [DOI] [PubMed] [Google Scholar]
- 22.Mosher CE, Champion VL, Hanna N, et al. Support service use and interest in support services among distressed family caregivers of lung cancer patients. Psychooncology. 2013;22:1549–1556. doi: 10.1002/pon.3168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Braun M, Mikulincer M, Rydall A, Walsh A, Rodin G. Hidden morbidity in cancer: Spouse caregivers. J Clin Oncol. 2007;25:4829–4834. doi: 10.1200/JCO.2006.10.0909. [DOI] [PubMed] [Google Scholar]
- 24.Bakas T, Lewis RR, Parsons JE. Caregiving tasks among family caregivers of patients with lung cancer. Oncol Nurs Forum. 2001;28:847–854. [PubMed] [Google Scholar]
- 25.Mosher CE, Jaynes HA, Hanna N, Ostroff JS. Distressed family caregivers of lung cancer patients: An examination of psychosocial and practical challenges. Support Care Cancer. 2013;21:431–437. doi: 10.1007/s00520-012-1532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Temel JS, Greer JA, Muzikansky A, et al. Early palliative care for patients with metastatic non–small-cell lung cancer. N Engl J Med. 2010;363:733–742. doi: 10.1056/NEJMoa1000678. [DOI] [PubMed] [Google Scholar]
- 27.Smith TJ, Temin S, Alesi ER, et al. American Society of Clinical Oncology provisional clinical opinion: The integration of palliative care into standard oncology care. J Clin Oncol. 2012;30:880–887. doi: 10.1200/JCO.2011.38.5161. [DOI] [PubMed] [Google Scholar]
- 28.Ford DW, Koch KA, Ray DE, Selecky PA. Palliative and end-of-life care in lung cancer: Diagnosis and management of lung cancer, 3rd ed: American College of Chest Physicians evidence-based clinical practice guidelines. Chest. 2013;143:e498S–e512S. doi: 10.1378/chest.12-2367. [DOI] [PubMed] [Google Scholar]
- 29.Sanders SL, Bantum EO, Owen JE, Thornton AA, Stanton AL. Supportive care needs in patients with lung cancer. Psychooncology. 2010;19:480–489. doi: 10.1002/pon.1577. [DOI] [PubMed] [Google Scholar]
- 30.Osse BH, Vernooij-Dassen MJ, Schadé E, Grol RP. Problems experienced by the informal caregivers of cancer patients and their needs for support. Cancer Nurs. 2006;29:378–388. doi: 10.1097/00002820-200609000-00005. [DOI] [PubMed] [Google Scholar]
- 31.Northouse LL, Williams AL, Given B, McCorkle R. Psychosocial care for family caregivers of patients with cancer. J Clin Oncol. 2012;30:1227–1234. doi: 10.1200/JCO.2011.39.5798. [DOI] [PubMed] [Google Scholar]
- 32.Northouse LL, Katapodi MC, Song L, Zhang L, Mood DW. Interventions with family caregivers of cancer patients: meta-analysis of randomized trials. CA Cancer J Clin. 2010;60:317–339. doi: 10.3322/caac.20081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rueda JR, Solà I, Pascual A, Subirana Casacuberta M. Non-invasive interventions for improving well-being and quality of life in patients with lung cancer. Cochrane Database Syst Rev. 2011;9:CD004282. doi: 10.1002/14651858.CD004282.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Badr H, Krebs P. A systematic review and meta-analysis of psychosocial interventions for couples coping with cancer. Psychooncology. 2013;22:1688–1704. doi: 10.1002/pon.3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dixon KE, Keefe FJ, Scipio CD, Perri LM, Abernethy AP. Psychological interventions for arthritis pain management in adults: a meta-analysis. Health Psychol. 2007;26:241–250. doi: 10.1037/0278-6133.26.3.241. [DOI] [PubMed] [Google Scholar]
- 36.Malouff JM, Thorsteinsson EB, Rooke SE, Bhullar N, Schutte NS. Efficacy of cognitive behavioral therapy for chronic fatigue syndrome: a meta-analysis. Clin Psychol Rev. 2008;28:736–745. doi: 10.1016/j.cpr.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 37.Tatrow K, Montgomery GH. Cognitive behavioral therapy techniques for distress and pain in breast cancer patients: a meta-analysis. J Behav Med. 2006;29:17–27. doi: 10.1007/s10865-005-9036-1. [DOI] [PubMed] [Google Scholar]
- 38.Redd WH, Montgomery GH, DuHamel KN. Behavioral intervention for cancer treatment side effects. J Natl Cancer Inst. 2001;93:810–823. doi: 10.1093/jnci/93.11.810. [DOI] [PubMed] [Google Scholar]
- 39.Kangas M, Bovbjerg DH, Montgomery GH. Cancer-related fatigue: a systematic and meta-analytic review of non-pharmacological therapies for cancer patients. Psychol Bull. 2008;134:700–741. doi: 10.1037/a0012825. [DOI] [PubMed] [Google Scholar]
- 40.Morley S, Eccleston C, Williams A. Systematic review and meta-analysis of randomized controlled trials of cognitive behaviour therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain. 1999;80:1–13. doi: 10.1016/s0304-3959(98)00255-3. [DOI] [PubMed] [Google Scholar]
- 41.Luebbert K, Dahme B, Hasenbring M. The effectiveness of relaxation training in reducing treatment-related symptoms and improving emotional adjustment in acute non-surgical cancer treatment: a meta-analytical review. Psychooncology. 2001;10:490–502. doi: 10.1002/pon.537. [DOI] [PubMed] [Google Scholar]
- 42.McLean LM, Walton T, Rodin G, Esplen MJ, Jones JM. A couple-based intervention for patients and caregivers facing end-stage cancer: outcomes of a randomized controlled trial. Psychooncology. 2013;22:28–38. doi: 10.1002/pon.2046. [DOI] [PubMed] [Google Scholar]
- 43.Porter LS, Keefe FJ, Baucom DH, et al. Partner-assisted emotional disclosure for patients with gastrointestinal cancer: results from a randomized controlled trial. Cancer. 2009;115:4326–4338. doi: 10.1002/cncr.24578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Badr H, Smith CB, Goldstein NE, Gomez JE, Redd WH. Dyadic psychosocial intervention for advanced lung cancer patients and their family caregivers: Results of a randomized pilot trial. Cancer. 2015;121:150–158. doi: 10.1002/cncr.29009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Porter LS, Keefe FJ, Garst J, et al. Caregiver-assisted coping skills training for lung cancer: Results of a randomized clinical trial. J Pain Symptom Manage. 2011;41:1–13. doi: 10.1016/j.jpainsymman.2010.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Given C, Given B, Rahbar M, et al. Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. J Clin Oncol. 2004;22:507–516. doi: 10.1200/JCO.2004.01.241. [DOI] [PubMed] [Google Scholar]
- 47.Given B, Given CW, Sikorskii A, et al. The impact of providing symptom management assistance on caregiver reaction: results of a randomized trial. J Pain Symptom Manage. 2006;32:433–443. doi: 10.1016/j.jpainsymman.2006.05.019. [DOI] [PubMed] [Google Scholar]
- 48.Kurtz ME, Kurtz JC, Given CW, Given B. A randomized, controlled trial of a patient/caregiver symptom control intervention: Effects on depressive symptomatology of caregivers of cancer patients. J Pain Symptom Manage. 2005;30:112–122. doi: 10.1016/j.jpainsymman.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.O’Hara RE, Hull JG, Lyons KD, et al. Impact on caregiver burden of a patient-focused palliative care intervention for patients with advanced cancer. Palliat Support Care. 2010;8:395–404. doi: 10.1017/S1478951510000258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greer JA, Park ER, Prigerson HG, Safren SA. Tailoring cognitive-behavioral therapy to treat anxiety comorbid with advanced cancer. J Cogn Psychother. 2010;24:294–313. doi: 10.1891/0889-8391.24.4.294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Moorey S, Greer S. Cognitive-behavioral therapy for people with cancer. New York: Oxford University Press; 2002. [Google Scholar]
- 52.Keefe FJ, Abernethy AP, Campbell LC. Psychological approaches to understanding and treating disease-related pain. Annu Rev Psychol. 2005;56:601–630. doi: 10.1146/annurev.psych.56.091103.070302. [DOI] [PubMed] [Google Scholar]
- 53.Barlow J, Wright C, Sheasby J, Turner A, Hainsworth J. Self-management approaches for people with chronic conditions: a review. Patient Educ Couns. 2002;48:177–187. doi: 10.1016/s0738-3991(02)00032-0. [DOI] [PubMed] [Google Scholar]
- 54.Bandura A. Social learning theory. Englewood Cliffs, NJ: Prentice-Hall; 1977. [Google Scholar]
- 55.Bandura A. Health promotion by social cognitive means. Health Educ Behav. 2004;31:143–164. doi: 10.1177/1090198104263660. [DOI] [PubMed] [Google Scholar]
- 56.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41:1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 57.Kroenke K, Spitzer RL, Williams JB, Monahan PO, Lowe B. Anxiety disorders in primary care: prevalence, impairment, comorbidity, and detection. Ann Intern Med. 2007;146:317–325. doi: 10.7326/0003-4819-146-5-200703060-00004. [DOI] [PubMed] [Google Scholar]
- 58.Krebs EE, Lorenz KA, Bair MJ, et al. Development and initial validation of the PEG, a three-item scale assessing pain intensity and interference. J Gen Intern Med. 2009;24:733–738. doi: 10.1007/s11606-009-0981-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.McHorney CA, Ware JE, Jr, Raczek AE. The MOS 36-Item Short-Form Health Survey (SF-36): II. Psychometric and clinical tests of validity in measuring physical and mental health constructs. Med Care. 1993;31:247–263. doi: 10.1097/00005650-199303000-00006. [DOI] [PubMed] [Google Scholar]
- 60.O’Connor PJ. Evaluation of four highly cited energy and fatigue mood measures. J Psychosom Res. 2004;57:435–441. doi: 10.1016/j.jpsychores.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 61.Portenoy RK, Thaler HT, Kornblith AB, et al. The Memorial Symptom Assessment Scale: an instrument for the evaluation of symptom prevalence, characteristics and distress. Eur J Cancer. 1994;30A:1326–1336. doi: 10.1016/0959-8049(94)90182-1. [DOI] [PubMed] [Google Scholar]
- 62.Callahan CM, Unverzagt FW, Hui SL, Perkins AJ, Hendrie HC. Six-item screener to identify cognitive impairment among potential subjects for clinical research. Med Care. 2002;40:771–781. doi: 10.1097/00005650-200209000-00007. [DOI] [PubMed] [Google Scholar]
- 63.Kraemer HC, Thiemann S. How many subjects? Statistical power analysis in research. Thousand Oaks, CA: Sage Publications; 1987. [Google Scholar]
- 64.Bauer J, Capra S, Ferguson M. Use of the scored Patient-Generated Subjective Global Assessment (PG-SGA) as a nutrition assessment tool in patients with cancer. Eur J Clin Nutr. 2002;56:779. doi: 10.1038/sj.ejcn.1601412. [DOI] [PubMed] [Google Scholar]
- 65.Dajczman E, Kasymjanova G, Kreisman H, et al. Should patient-rated performance status affect treatment decisions in advanced lung cancer? J Thorac Oncol. 2008;3:1133–1136. doi: 10.1097/JTO.0b013e318186a272. [DOI] [PubMed] [Google Scholar]
- 66.Kroenke K, Spitzer RLMD. The PHQ-9: A new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509–515. [Google Scholar]
- 67.Kroenke K, Strine TW, Spitzer RL, et al. The PHQ-8 as a measure of current depression in the general population. J Affect Disord. 2009;114:163–173. doi: 10.1016/j.jad.2008.06.026. [DOI] [PubMed] [Google Scholar]
- 68.Cleeland CS, Gonin R, Hatfield AK, et al. Pain and its treatment in outpatients with metastatic cancer. N Engl J Med. 1994;330:592–596. doi: 10.1056/NEJM199403033300902. [DOI] [PubMed] [Google Scholar]
- 69.Kroenke K, Theobald D, Norton K, et al. The Indiana Cancer Pain and Depression (INCPAD) trial: Design of a telecare management intervention for cancer-related symptoms and baseline characteristics of study participants. Gen Hosp Psychiatry. 2009;31:240–253. doi: 10.1016/j.genhosppsych.2009.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Donovan KA, Jacobsen PB, Small BJ, Munster PN, Andrykowski MA. Identifying clinically meaningful fatigue with the Fatigue Symptom Inventory. J Pain Symptom Manage. 2008;36:480–487. doi: 10.1016/j.jpainsymman.2007.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hann DM, Denniston MM, Baker F. Measurement of fatigue in cancer patients: further validation of the Fatigue Symptom Inventory. Qual Life Res. 2000;9:847–854. doi: 10.1023/a:1008900413113. [DOI] [PubMed] [Google Scholar]
- 72.Anderson KO, Dowds BN, Pelletz RE, Edwards WT, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain. 1995;63:77–84. doi: 10.1016/0304-3959(95)00021-J. [DOI] [PubMed] [Google Scholar]
- 73.Kilbourn KM, Costenaro A, Madore S, et al. Feasibility of a telephone-based counseling program for informal caregivers of hospice patients. J Palliat Med. 2011;14:1200–1205. doi: 10.1089/jpm.2011.0186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lepore SJ, Silver RC, Wortman CB, Wayment HA. Social constraints, intrusive thoughts, and depressive symptoms among bereaved mothers. J Pers Soc Psychol. 1996;70:271–282. doi: 10.1037//0022-3514.70.2.271. [DOI] [PubMed] [Google Scholar]
- 75.Given CW, Given B, Stommel M, et al. The caregiver reaction assessment (CRA) for caregivers to persons with chronic physical and mental impairments. Res Nurs Health. 1992;15:271–283. doi: 10.1002/nur.4770150406. [DOI] [PubMed] [Google Scholar]
- 76.Kenny D, Kashy D, Cook W. Dyadic data analysis. New York: Guilford Press; 2006. [Google Scholar]
- 77.Atkins DC. Using multilevel models to analyze couple and family treatment data: basic and advanced issues. J Fam Psychol. 2005;19:98–110. doi: 10.1037/0893-3200.19.1.98. [DOI] [PubMed] [Google Scholar]
- 78.Hastert TA, Beresford SA, Sheppard L, White E. Disparities in cancer incidence and mortality by area-level socioeconomic status: a multilevel analysis. J Epidemiol Community Health. 2015;69:168–176. doi: 10.1136/jech-2014-204417. [DOI] [PubMed] [Google Scholar]
- 79.Northouse LL, Mood DW, Schafenacker A, et al. Randomized clinical trial of a brief and extensive dyadic intervention for advanced cancer patients and their family caregivers. Psychooncology. 2013;22:555–563. doi: 10.1002/pon.3036. [DOI] [PMC free article] [PubMed] [Google Scholar]