Abstract
Context
Episodic breathlessness is common and debilitating in cancer patients.
Objectives
In this pilot study, we examined the effect of prophylactic fentanyl pectin nasal spray (FPNS) on exercise-induced dyspnea, physiologic function and adverse events.
Methods
In this parallel, double-blind randomized placebo-controlled trial, opioid-tolerant patients performed three six-minute walk tests (6MWT) to induce dyspnea. They were randomized to receive either FPNS (15−25% of total daily opioid dose each time) or placebo 20 minutes before the second and third 6MWTs. We compared dyspnea numeric rating scale (NRS, 0−10, primary outcome), walk distance, vital signs, neurocognitive function and adverse events between the first and second 6MWTs (T2-T1) and between the first and third 6MWTs (T3-T1).
Results
Twenty-four patients enrolled, with 96% completion. FPNS was associated with significant within-arm reduction in dyspnea NRS at rest (T2-T1: −0.9 [95% confidence interval [CI] −1.7,−0.1]; T3-T1: −1.3 [95% CI −2.0,−0.5]) and after six minutes (T2-T1: −2.0 [95% CI −3.5,−0.6]; T3-T1: −2.3 [95% CI −4.0,−0.7]), and longer walk distance (T2-T1 +23.8m [95% CI +1.3,+46.2m]; T3-T1: +23.3m [95% CI −1.7,+48.2]). In the placebo arm, we observed no significant change in walk distance nor dyspnea NRS at rest, but significant reduction in dyspnea NRS at 6 minutes (T2-T1: −1.7 [95% CI −3.3,−0.1]; T3-T1: −2.5 [95% CI −4.2,−0.9]). Vital signs, neurocognitive function and adverse effects did not differ significantly.
Conclusion
FPNS was safe, reduced dyspnea at rest and increased walk distance in before-after comparison. The placebo effect was substantial, which needs to be factored in future study designs.(clinicaltrials.gov registration: NCT01832402)
Keywords: dyspnea, exercise, nasal sprays, neoplasms, opioids, randomized controlled trial
Introduction
Episodic breathlessness is characterized by “a severe worsening of breathless intensity or unpleasantness beyond usual fluctuation in the patient’s perception” (1). These episodes occur in 70–90% of patients with dyspnea, and have a significant impact on daily function.(2–4) Patients experience multiple intensive episodes of breathlessness on a daily basis, often triggered by exertion and each lasting for 10–20 minutes.(5, 6)
Because episodes of breathlessness are often short-lived and occur rapidly,(5, 6) opioids with longer onset of action may have limited benefits.(7) Rapid onset opioids that are used for breakthrough cancer pain may be particularly useful in the management of episodic dyspnea.(8, 9) Several small case series suggested that oral transmucosal fentanyl citrate and intranasal fentanyl may be efficacious.(10–12) A small prospective randomized trial also provided preliminary evidence that subcutaneous fentanyl given prophylactically may improve dyspnea while increasing walk distance.(13) Moreover, nebulized fentanyl was found to be associated with a decrease in exertional dyspnea in patients with chronic obstructive pulmonary disease but not in healthy men.(14, 15) A recent systematic review on the effect of fentanyl for refractory breathlessness included 13 studies and only 2 randomized controlled trials.(16) The investigators concluded that fentanyl showed some promise but adequately powered randomized trials are needed. They also emphasized the need for pilot studies “to evaluate effective size, study procedures, and outcome measures”.
Fentanyl pectin nasal spray (FPNS) is approved by the US Food and Drugs Administration (FDA) in 2011 for breakthrough pain in opioid-tolerant cancer patients. It has a bioavailability of 80% and a time to maximal effect (Tmax) of 15–20 minutes, making it a potential appealing option for breathlessness.(17, 18) FPNS has been found in clinical trials to provide greater and more rapid breakthrough pain relief than placebo (19, 20) and oral morphine,(21, 22) and was generally well tolerated.(23) The efficacy of FPNS has never been examined for dyspnea. A better understanding of FPNS’s effect on dyspnea both at rest and with exertion may open up novel therapeutic options for this distress symptom. In this pilot placebo-control randomized controlled trial, we estimated the within-arm effects of prophylactic FPNS and placebo on the intensity of exercise-induced episodic breathlessness. We also examined their effects on dyspnea at rest, 6 minute walk distance, neurocognitive function and adverse effects. Data gathered from this preliminary study is expected to inform future study designs regarding feasibility of patient recruitment, study outcomes and placebo effect.
Methods
Patients
Inclusion criteria were diagnosis of cancer, outpatients at the Supportive Care Center at MD Anderson Cancer Center, age 18 or older, an average intensity of episodic dyspnea ³3/10 on a numeric rating scale (NRS), ambulatory with or without walking aid, Karnofsky performance status ≥50%, and a stable dose of strong opioids with an morphine equivalent daily dose (MEDD) of between 80 mg/day and 500 mg/day. Because our study was based on proportional dosing, the lower limit of MEDD for study entry (80 mg) was calculated based on the lowest dosage form of FPNS (100 mcg) without exceeding the upper limit of proportional dosing (25%). Patients with dyspnea numeric rating scale (NRS) at rest ≥7/10 (i.e. these individuals with poor baseline function and high baseline expression of dyspnea so difficult to worsen further with exertion), supplemental oxygen >6 L/minute, delirium (i.e. Memorial Delirium Assessment Scale >13/30), allergic reaction to fentanyl, history of opioid abuse, or contraindications to completing the 6 minute walk test (6MWT) were excluded. The Institutional Review Board at MD Anderson Cancer Center approved this study. All patients provided written informed consent.
Study Design
In this double-blind, parallel, placebo-controlled randomized trial, patients were asked to perform a baseline 6MWT without any medications. This was followed by a rest period during which they were asked about their level of dyspnea every 5 minutes for up to 1 hour. When their dyspnea level was less than or equal to baseline dyspnea +1, they were given a single dose of FPNS or placebo followed by a second 6MWT 20 minutes later (corresponding to the Tmax of FPNS).(17, 18) The rest period, study intervention and 6MWT were repeated once in an identical fashion to assess the effect of repeated dosing (and hence a higher maximum serum fentanyl concentration).(24)
After enrollment by our study coordinator, the study pharmacist assigned patients in a 1:1 ratio to receive either FPNS or matching placebo based on a computer-generated randomization sequence with permuted blocks. We stratified patients according to the baseline level of dyspnea NRS (0–3 vs. 4–6) because baseline symptom intensity is often associated with response.(25)
Study Interventions
The supply of study medication (both FPNS and placebo) were provided by Depomed, Inc (Newark, CA). Allocation was concealed by using a secured website that was only accessible to the study pharmacist after patient enrollment. The patient, research coordinator conducting the study assessments and principal investigator were blinded to the study intervention and the randomization sequence.
For patients randomized to FPNS arm, the same dose was given prior to the 2nd and the 3rd 6MWT using the following sliding scale: 100 mcg (1 spray), 200 mcg (2 sprays), 300 mcg (3 sprays) and 400 mcg (4 sprays) of FPNS for MEDD of 80–159 mg/day, 160–239 mg/day, 240–319 mg/day and 320–540 mg/day, respectively. Based on our prior study with subcutaneous fentanyl,(13) each dose was designed to be equivalent to 15–25% of MEDD, assuming 80% bioavailability.(17) For instance, 100 mcg of FPNS is equivalent to 80 mcg of intravenous fentanyl, which is in turn equivalent to 8 mg of intravenous morphine or 20 mg of oral morphine. Thus, a 100 mcg dose of FPNS represents 25% of the daily dose for a patient with MEDD of 80 mg/day. A proportional approach was used instead of titration approach to determine the prophylactic dose of FPNS because previous studies supported the use of proportional dosing with rapid onset opioids and this approach was more feasible for study logistical reasons.(13, 26, 27) Specifically, this is a single day study to minimize attrition, and the process of dose titration would significantly prolong this study and require a washout period. FPNS was administered intranasally by a research nurse. Patients randomized to the placebo arm received identically looking placebo and the same number of sprays as they would otherwise receive with FPNS.
Study Assessments and Endpoints
At baseline, we collected information on patient characteristics. We examined dyspnea with the Cancer Dyspnoea Scale (CDS), a validated 12 item questionnaire that assesses the quality of dyspnea over the past few days.(28, 29) Each item has a score between 1 and 5, with a total score of up to 60, and sub-scores for sense of effort, anxiety, and discomfort.
Our primary outcome was dyspnea intensity “now” using a numeric rating scale that ranges from 0 (“no shortness of breath”) to 10 (“worst possible shortness of breath”).(28, 30, 31) This was assessed at 0, 1, 2, 3, 4, 5 and 6 minute during each 6MWT, and every 5 minutes during the rest period. 6MWT were carried out following guidelines from the American Thoracic Society and have been described in detail.(13, 32) The dyspnea NRS score immediately before starting each 6MWT (i.e. at 0 minutes) allowed us to examine the effect of FPNS/placebo on dyspnea at rest because they received the medication 20 minutes prior to the second and third 6MWTs. We also assessed both dyspnea and fatigue using the 0–10 modified Borg scale before and after each 6MWT, and measured the distance walked every minute.(32) The minimal clinically significant difference was 1 point for both the NRS and modified Borg scale.(33, 34)
Other outcomes included vital signs, adverse effects and neurocognitive testing. We assessed heart rate, respiratory rate, blood pressure, and oxygen saturation immediately before and after each 6MWT. Adverse effects such as dizziness, drowsiness, nausea, and nasal symptoms were assessed prior to medication administration and after each 6MWT using an 11-point NRS, with 0 being absent and 10 denoting worst possible. Neurocognitive testing including finger tapping (3 timed trials of 10 sec each, with a 15 sec interval between trials; score represents average number of taps), arithmetic (20 simple arithmetic questions; total score equal to time in seconds plus 10% of the time score for each error), reverse memory of digits (recall series of numbers in reverse order starting with 3 digits; progressively increasing the length by 1 digit for up to 8 digits; if the patient failed with one sequence, they will be given one more opportunity to recall the same number of digits with another sequence before the test is terminated; at each level, 2 points were assigned for a successful first attempt, 1 point for a successful second attempt, and 0 points for failure to recall after two attempts), and visual memory (recall of 3 visual objects in reverse order; 2 points for each object recalled in correct order, range 0–6 points) were assessed within 5 minutes after each 6MWT according to published procedures.(35)
After completion of each intervention, we asked patients about their change in dyspnea (better/same/worse) using the Global Symptom Evaluation.(36, 37)
Statistical Analysis
This study was designed to estimate the effect of FPNS on breathlessness in a before-after comparison. We also included a placebo arm to examine the magnitude of placebo effect. Ten evaluable patients in the fentanyl arm provided 80% power to detect an effect size as small as 1.0 using a two-sided paired t-test with a significance level of 5% to compare the change of dyspnea between the first and second walk tests. This study is not powered for a direct comparison between fentanyl and placebo.
Five patients with MEDD >160 mg/day inadvertently received a lower study medication dose than planned (1 spray instead of multiple sprays; 2 FPNS arm, 3 placebo arm). To ensure there was adequate representation of patients in the high dose range, we enrolled 4 more patients with MEDD >160 mg/day at the end of the study until we exhausted the placebo supply. As a result, we conducted both intention-to-treat analysis that included all 24 patients who started the study, and per protocol analysis that included only the 19 patients who received the correct dose. Unless otherwise specified, our reporting focused on intention-to-treat analysis because the findings were highly similar with the per protocol analysis.
We summarized the baseline demographics using descriptive statistics, including means, standard deviations (SD), ranges, 95% confidence intervals and frequencies. We calculated the mean difference between the first and second 6MWTs and also the mean difference between the first and third 6MWTs, along with 95% confidence interval for study outcomes (Supplemental Fig. 1, available at jpsmjournal.com). We used all available data for analysis and did not conduct imputation for missing data.
The Statistical Analysis System (SAS version 9.2, SAS Institute, Cary, North Carolina) was used for statistical analysis.
Results
Patient Characteristics
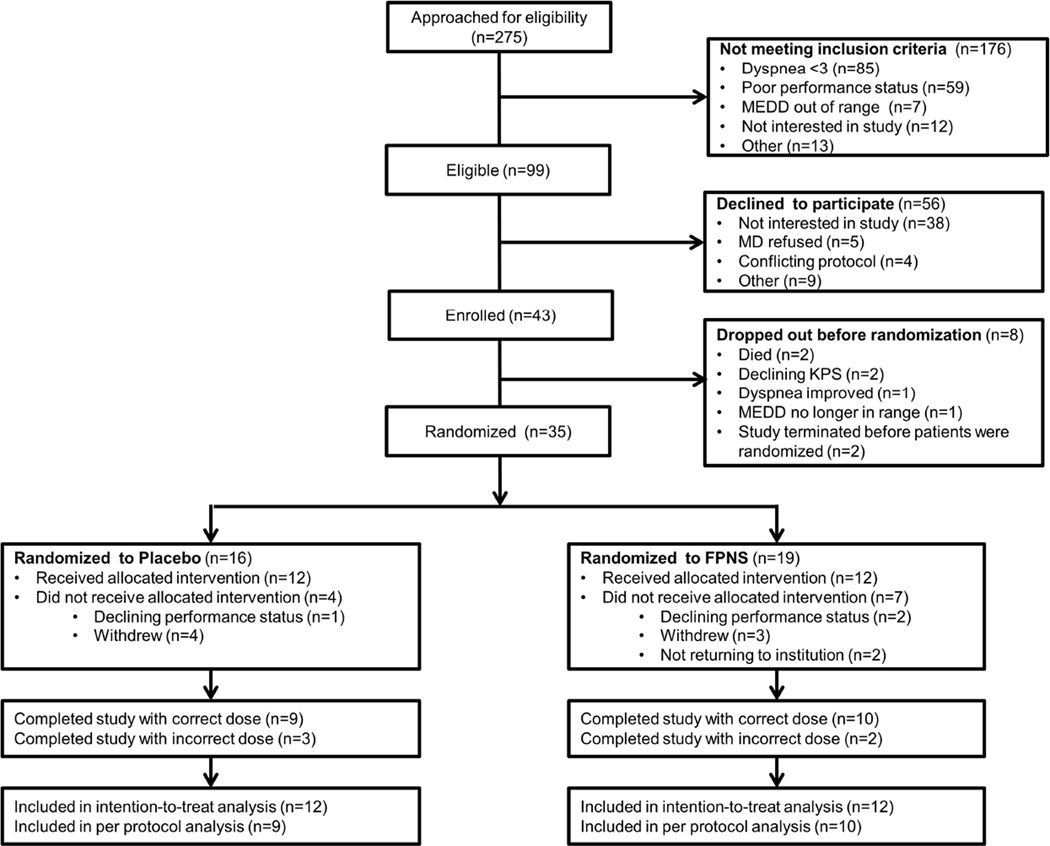
Recruitment occurred between 7/16/2013 and 3/23/2015. We identified 99 eligible patients, 43 (43%) were willing to participate, although 19 (19%) never started mostly because of declining health status or interest (Fig. 1). Among the 24 enrolled patients who initiated study procedures, 23 (96%) completed all 3 walk tests, and 1 (4%) completed only 2 6MWTs. The mean baseline Cancer Dyspnea Scale was 12.8 (SD 7.1) in the FPNS arm and 9.3 (SD 5.8) in the placebo arm. Table 1 shows the baseline characteristics of study participants.
Fig. 1.
CONSORT Diagram
Table 1.
Baseline patient characteristics
| FPNS N=12 (%)a |
Placebo N=12 (%)a |
All patients N=24 (%)a |
|
|---|---|---|---|
| Average age (range) | 51.5 (44.7, 58.3) | 53.3 (45.0, 61.6) | 52.4 (47.5, 57.4) |
| Female sex | 9 (75.0) | 4 (33.3) | 13 (54.2) |
| Race | |||
| Caucasian | 8 (66.7) | 8 (66.7) | 16 (66.7) |
| Black | 3 (25) | 2 (16.7) | 5 (20.8) |
| Hispanic | 1 (8.3) | 2 (16.7) | 3 (12.5) |
| Education | |||
| High school or less | 4 (33.3) | 2 (16.7) | 6 (25.0) |
| College | 8 (66.7) | 7 (58.3) | 15 (62.5) |
| Advanced degree | 0 | 3 (25.0) | 3 (12.5) |
| Cancer type | |||
| Breast | 3 (25.0) | 2 (16.7) | 5 (20.8) |
| Gastrointestinal | 2 (16.7) | 4 (33.3) | 6 (25.0) |
| Genitourinary | 2 (16.7) | 0 | 2 (8.3) |
| Gynecologic | 2 (16.7) | 0 | 2 (8.3) |
| Lung | 1 (8.3) | 2 (16.7) | 3 (12.5) |
| Hematologic | 0 | 2 (16.7) | 2 (8.3) |
| Others | 2 (16.7) | 2 (16.7) | 4 (16.7) |
| Cancer stage | |||
| Metastatic/refractory | 8 (66.7) | 10 (83.4) | 18 (75.0) |
| Localized/Locally advanced | 4 (33.3) | 2 (16.6) | 6 (25.0) |
| *Cancer Dyspnea Scale, mean (SD) | |||
| Effort | 6.3 (3.7) | 4.6 (2.7) | 5.5 (3.3) |
| Anxiety | 3.6 (3.0) | 2.3 (3.7) | 3.0 (3.4) |
| Discomfort | 2.8 (1.5) | 2.4 (1.4) | 2.6 (1.4) |
| Total | 12.8 (7.1) | 9.3 (5.8) | 11.1 (6.6) |
| Average dyspnea NRS during breakthrough episodes over the last week, mean (SD) |
4.4 (1.4) | 4 (1.0) | 4.2 (1.2) |
| Co-morbidities | |||
| COPD | 2 (16.7) | 2 (16.7) | 4 (16.7) |
| Heart failure | 1 (8.3) | 0 | 1 (4.2) |
| Asthma | 5 (41.7) | 0 | 5 (20.8) |
| Bronchiectasis | 0 | 0 | 0 |
| Concurrent therapies | |||
| Opioids | 12 (100.0) | 12 (100.0) | 24 (100.0) |
| Bronchodilators | 6 (50.0) | 5 (41.7) | 11 (45.8) |
| Steroids | 0 | 0 | 0 |
| Supplemental oxygen | 1 (8.3) | 1 (8.3) | 2 (8.3) |
| Morphine equivalent daily doses, median (interquartile range) in mg |
137.5 (125, 200) | 175 (135, 325) | 158 (133, 235) |
| Karnofsky performance status, mean (SD) |
75.8 (9.0) | 75.8 (10.8) | 75.8 (9.7) |
Abbreviations: COPD, chronic obstructive pulmonary disease; NRS, numeric rating scale; SD, standard deviation
unless otherwise specified
Dyspnea and Walk Distance
Compared to the first walk, within-arm analyses show that FPNS was associated with a significant mean reduction in dyspnea NRS at the end of 6MWT (second 6MWT −2.0; third 6MWT −2.3) and dyspnea NRS at rest (i.e. 0 minutes) immediately before the 6MWTs (second 6MWT −0.9; third 6MWT −1.3), with an increase in 6 minute walk distance (second 6MWT +23.8 m) (Table 2). However, the difference in dyspnea NRS between 0 and 6 minutes did not reach statistical significance (second 6MWT −1.1, 95% confidence interval [CI] −2.4 to 0.2; third 6MWT −1.1, 95% CI −2.7 to 0.5).
Table 2.
Change in Dyspnea, Walk Distance and Fatigue Between with Fentanyl Pectin Nasal Spray and Placeboa
| Baseline walk test |
Second walk test |
Difference between first walk test and second walk test |
Third walk test |
Difference between first walk test and third walk test |
|
|---|---|---|---|---|---|
| Variable | Mean (SD) | Mean (SD) | Mean change (95% CI)a |
Mean (SD) |
Mean change (95% CI)a |
|
Dyspnea numeric rating scale |
|||||
| Placebo: at time of study drug admin |
- | 2.0 (1.2) | - | 1.6 (1.4) | - |
| FPNS: at time of study drug admin |
- | 3.3 (1.4) | - | 2.0 (1.9) | - |
| Placebo: 0 minutes | 1.7 (1.2) | 1.2 (1.4) | −0.5 (−1.3, 0.3) |
1.1 (1.3) | −0.5 (−1.4, 0.3) |
| FPNS: 0 minutesb | 2.4 (1.7) | 1.5 (1.8) | −0.9 (−1.7,− 0.1) |
1.2 (1.7) | −1.3 (−2.0, −0.5) |
| Placebo: 6 minutes | 5.4 (2.0) | 3.8 (2.7) | −1.7 (−3.3,− 0.1) |
2.8 (2.5) | −2.5 (−4.2, −0.9) |
| FPNS: 6 minutes | 6.2 (1.9) | 4.1 (2.6) | −2.0 (−3.5,− 0.6) |
3.8 (2.6) | −2.3 (−4.0, −0.7) |
| Walk distance at 6 minutes | |||||
| Placebo: 6 minutes | 371.2 (114.2) |
387.5 (111.3) | 16.3 (−8.6, 41.3) |
391.3 (112.1) |
14.6 (−11.0, 40.3) |
| FPNS: 6 minutes | 321.8 (80.6) |
345.5 (79.7) |
23.8 (1.3, 46.2) |
345.0 (80.5) |
23.3 (−1.7, 48.2) |
| Dyspnea Borg Scale | |||||
| Placebo: 0 minutes | 1.3 (1.0) | 0.8 (1.2) | −0.4 (− 1.0,0.1) |
1.0 (1.3) | −0.2 (−0.9,0.5) |
| FPNS: 0 minutesb | 2.0 (1.3) | 1.2 (1.5) | −0.8 (−1.6,− 0.1) |
1.0 (1.6) | −1.0 (−1.9,−0.2) |
| Placebo: 6 minutes | 4.4 (1.8) | 2.7 (2.5) | −1.7 (−3.3,− 0.1) |
2.3 (2.2) | −2.4 (−4.2,−0.6) |
| FPNS: 6 minutes | 5.0 (2.0) | 3.3 (2.3) | −1.8 (−3.1,− 0.4) |
3.1 (2.9) | −1.7 (−3.5,0) |
| Fatigue Borg Scale | |||||
| Placebo: 0 minutes | 1.5 (1.1) | 1.5 (1.4) | 0 (−0.8, 0.8) | 1.4 (1.8) | −0.1 (−1.3, 1.1) |
| FPNS: 0 minutesb | 3.0 (1.0) | 2.0 (1.8) | −1.0 (−1.9,− 0.1) |
2.6 (1.9) | −0.5 (−1.6, 0.7) |
| Placebo: 6 minutes | 3.4 (2.9) | 2.9 (2.7) | −0.5 (−1.6, 0.7) |
2.3 (2.1) | −0.9 (−2.3, 0.5) |
| FPNS: 6 minutes | 3.7 (2.5) | 3.2 (2.4) | −0.5 (−1.7, 0.8) |
2.8 (2.4) | −0.7 (−2.1, 0.6) |
Abbreviations: CI, confidence interval; FPNS, fentanyl pectin nasal spray; SD, standard deviation
Statistically significant values are highlighted in bold
The moment just prior to the 6 minute walk test, 15 minutes after fentanyl/placebo administration (i.e. dyspnea at rest)
Placebo was also observed to have a significant within-arm reduction in dyspnea NRS at the end of 6MWT (mean reduction: second 6MWT −1.7; third 6MWT −2.5) but not dyspnea NRS at rest (i.e. 0 minutes) immediately before the 6MWTs or walk distance (Table 2). We also found a significant reduction in dyspnea NRS between 0 and 6 minutes between the first and third 6MWT (mean −2.0, 95% CI −3.6 to −0.4) but not between the first and second 6MWT (mean −1.2 95% CI −2.8 to 0.4).
Dyspnea Borg scale showed similar findings in within-arm analysis (Table 2). Per protocol analysis also yielded the similar conclusions. This study was not powered for between-arm comparison, and we detected no significant differences in dyspnea or walk distance between FPNS and placebo in linear mixed model analysis.
Physiologic Variables, Neurocognitive Function
As shown in Table 3, some changes in heart rate, respiratory rate, systolic and diastolic blood pressure and oxygen saturation in both FPNS and placebo arms were statistically significant but the magnitude was not clinically significant. We also found no significant within arm differences in neurocognitive tests before and after drug administration.
Table 3.
Change in Physiologic Variables and Neurocognitive Testing Between with Fentanyl Pectin Nasal Spray and Placeboa
| Mean change (95% CI) between first walk test and second walk test |
Mean change (95% CI) between first walk test and third walk test |
|||
|---|---|---|---|---|
| Variable | FPNS | Placebo | FPNS | Placebo |
| Respiratory rate | ||||
| 0 minuteb | 0.9 (−0.2, 2.0) | −0.2 (−1.5, 1.2) | 1.2 (0.1, 2.3) | −0.5 (−1.7, 0.6) |
| 6 minute | 0.3 (−1.3, 1.8) | −1.3 (−2.4, −0.2) | 0.3 (−1.1, 1.6) | −1.6 (−2.6, −0.6) |
| Oxygen saturation | ||||
| 0 minuteb | −0.5 (−1.6, 0.6) | 0.1 (−1.0, 1.1) | 0.2 (−0.8, 1.2) | 1.0 (−0.4, 2.4) |
| 6 minute | 0.4 (−0.7, 1.5) | −0.2 (−1.3, 1.0) | −0.2 (−1.2, 0.8) | −1.1 (−2.3, 0.1) |
| Heart rate | ||||
| 0 minuteb | 0.8 (−3.3, 4.9) | −0.3 (−6.3, 5.6) | −0.7 (−6.4, 4.9) | 0.8 (−6.3, 8.0) |
| 6 minute | 1.3 (−2.1, 4.6) | 1.3 (−3.7, 6.2) | 0.8 (−4.2, 5.7) | −1.4 (−6.4, 3.7) |
| Systolic Blood Pressure | ||||
| 0 minuteb | 1.5 (−11.7, 14.8) | 3.1 (−2.0, 8.2) | 4.6 (−6.3, 15.5) | −0.7 (−7.8, 6.3) |
| 6 minute | 10.4 (3.0, 17.9) | −1.9 (−9.2, 5.3) | 2.3 (−3.6, 8.1) | −3.1 (−10.8, 4.6) |
| Diastolic Blood Pressure | ||||
| 0 minuteb | −4.4 (−8.1, −0.7) | 1.3 (−0.6, 3.2) | −2.2 (−8.9, 4.5) | 1.2 (−1.9, 4.2) |
| 6 minute | 4.7 (−0.4, 9.7) | 0.3 (−3.0, 3.5) | 1.6 (−1.9, 5.0) | 0.2 (−3.7, 4.0) |
| Neurocognitive testing | ||||
| Tapping | 3.3 (−1.5, 8.2) | 1.8 (−0.6, 4.1) | 2.9 (−0.6, 6.3) | 2.3 (−2.3, 6.9) |
| Arithmetic | 9.9 (−17.6, 37.5) | 7.0 (−21.5, 35.4) | 11.2 (−29, 51.4) | −6.7 (−28.1, 14.7) |
| Reverse digits | −0.2 (−1.2, 0.9) | −0.1 (−1.5, 1.3) | −0.3 (−1.1, 0.4) | −0.2 (−1.3, 1.0) |
| Visual memory | −0.2 (−0.8, 0.5) | 0.5 (−0.7, 1.7) | −0.2 (−1.0, 0.7) | 0.4 (−1.2, 1.9) |
Abbreviations: CI, confidence interval; FPNS, fentanyl pectin nasal spray; SD, standard deviation
Statistically significant values are in bold
The moment just prior to the 6 minute walk test, 15 minutes after fentanyl/placebo administration
Adverse Effects
Table 4 shows that few patients reported adverse effects. Between the first and third 6MWT, 5 patients reported more drowsiness in the placebo arm compared to 1 patient in the FPNS arm (P=0.04); furthermore 5 patients reported more dizziness in FPNS and 1 with placebo (P=0.07).
Table 4.
Adverse Effects in FPNS and Placebo Armsa
| Second walk test | Third walk test | |||
|---|---|---|---|---|
| Variable | FPNS N (%) |
Placebo N (%) |
FPNS N (%) |
Placebo N (%) |
| Dizziness | 2 (18.2) | 2 (18.2) | 5 (45.5) | 1 (10) |
| Drowsiness | 1 (8.3) | 2 (16.7) | 1 (8.3) | 5 (45.5) |
| Nausea | 0 | 0 | 2 (16.7) | 0 |
| Stuffy nose | 0 | 2 (16.7) | 0 | 0 |
| Runny nose | 0 | 0 | 0 | 0 |
| Itchy nose | 0 | 0 | 0 | 0 |
| Nose Dryness | 0 | 2 (16.7) | 0 | 2 (18.2) |
| Cough | 1 (8.3) | 0 | 0 | 1 (9.1) |
| Sore throat | 0 | 1 (8.3) | 0 | 0 |
| Taste change | 0 | 0 | 0 | 0 |
Abbreviations: FPNS, fentanyl pectin nasal spray
Each adverse effect was measured using an 11-point numeric rating scale (0=none, 10=worst) immediate before drug administration and immediately after the walk test (approximately 30 minutes later). The number of patients who reported worsening of adverse effects after drug administration by at least 1 point are shown.
Discussion
This is the first randomized trial to examine FPNS’s effect on dyspnea. Our primary objective was to examine the within arm effects of prophylactic FPNS—we found it to be well tolerated, and was associated with a reduction in dyspnea and greater walk distance. We also observed a large placebo effect. The results of this pilot study can inform the design of the next generation of trials to further investigate FPNS’s effect on dyspnea and function.
We observed a significant reduction of dyspnea 20 minutes after administration of FPNS and immediately prior to initiating the 6MWT but not in the placebo arm, suggesting that FPNS may provide rapid relief of dyspnea at rest. Interestingly, the same effect was observed in our previous study using subcutaneous fentanyl.(13) This is consistent with the literature suggesting that opioids may be considered for dyspnea relief on an as needed basis.(38, 39)
Although FPNS was associated with a significant within-arm reduction in dyspnea after 6 minutes of exertion, this was no longer statistically significant after adjusting for the dyspnea level at the beginning of the walk. This is likely because dyspnea at the beginning of walk was already lowered in the FPNS arm, and thus there was little room for further improvement. Encouragingly, FPNS was associated with a statistically significant increase in 6MWT distance but not placebo. The within-arm improvement of 23–24 m is considered clinically significant,(40) although further studies are needed to confirm this finding.
This study is not powered to directly compare between FPNS and placebo; however, the magnitude of effect between FPNS and placebo was comparable, raising doubts about the efficacy of FPNS. One potential explanation is that FPNS may be more effective at managing dyspnea at rest than preventing exertional dyspnea induced by the 6MWT. Another possibility is that the proportional dosing strategy with FPNS, despite the repeated dosing, was suboptimal because of the significant variability in bioavailability among individuals with rapid onset opioids.(41, 42) Future studies may consider adopting a titration approach similar to management of breakthrough pain.(43) The inclusion of the placebo arm highlights the fact that many factors could contribute to an observed reduction, such as nasal mucosal cooling effect, training effect, obsequiousness bias, regression to the mean, and response shift,(44) which justifies the importance of placebo-controlled Phase II studies instead of single arm trials when the study outcome is subjective in nature.(45) We observed that a few individuals had an unusually large reduction in dyspnea in the placebo arm, which could skew the findings. Indeed, the large placebo effect precluded us from estimating the appropriate sample size based on dyspnea. Before Phase III studies can be conducted, our study urges for further pilot studies to distinguish the opioid effect from placebo effect. Specifically, randomized trials with a placebo run-in phase in which patients with large placebo response or training effect are excluded prior to randomization may be helpful while maintaining the internal validity of the study.(46–48) Alternatively, a pilot study with a titration phase to identify the optimal dose among responders to FPNS prior to randomization similar to pain trials may be considered.(43)
Another interesting issue is regarding the study outcomes for exertional dyspnea trials. Because patients were asked to maximize their walking distance during the 6MWTs, FPNS’s main benefit on exertional dyspnea may be enabling patients to walk further rather than to achieve a lower level of dyspnea, as observed in this study. Thus, future pilot studies on exertional dyspnea should consider walk distance as the primary outcome.
Reassuringly, FPNS was well tolerated at the doses used in this study. We observed no major changes in vital signs, neurocognitive tests or adverse effects despite 2 doses separated approximately 30 minutes apart. Some clinicians are concerned about the effect of respiratory depression with opioids, which was not observed in our study. This is the first randomized controlled trial to formally assess the short term effects of rapid onset opioids on cognitive function. In fact, we observed nocebo effect with some more drowsiness in the placebo group, which is observed in other clinical trials.(49) Our study suggests that higher doses of FPNS should be tested to identify its therapeutic window and toxicity ceiling.
This study has several limitations. First, the sample size was too small for us to conduct any between arm comparisons, and precluded us from conducting subgroup analysis to identify patients who may derive a greater benefit. Second, we conducted multiple statistical tests for secondary outcomes as part of the pre-planned exploratory analysis. This could increase the chance of false positives. Third, we only enrolled cancer patients who were opioid-tolerant and had a good performance status. The study findings may not be generalizable to other populations. Fourth, 5 patients received the incorrect dose resulting in protocol violation. We were reassured that per protocol analysis was similar to intention to treatment analysis. Finally, there was an imbalance of co-morbidities between arms, which may potentially affect dyspnea response to FPNS. Future studies may consider stratification by co-morbidities.
Our study provided preliminary evidence to support the role of FPNS for both dyspnea at rest, dyspnea with exertion and walk distance with no significant toxicities despite repeated dosing. A large placebo effect was seen with exertional dyspnea. Future study designs may consider incorporation of a placebo run-in period and/or titration strategy to better delineate the treatment effect from the placebo effect.
Supplementary Material
Acknowledgments
This research is supported by an investigator-initiated study grant from Depomed to Dr. Hui. Dr. Hui is supported in part by an American Cancer Society Mentored Research Scholar Grant in Applied and Clinical Research (MRSG-14-1418-01-CCE) and a National Institutes of Health grant (R21CA186000-01A1). Dr. Bruera is supported in part by National Institutes of Health grants R01NR010162-01A1, R01CA122292-01, and R01CA124481-01. The funding sources were not involved in the conduct of the study or development of the submission.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures
The authors have no conflicts of interest.
References
- 1.Simon ST, Weingartner V, Higginson IJ, Voltz R, Bausewein C. Definition, categorization, and terminology of episodic breathlessness: consensus by an international Delphi survey. J Pain Symptom Manage. 2014;47:828–838. doi: 10.1016/j.jpainsymman.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 2.Reddy SK, Parsons HA, Elsayem A, Palmer JL, Bruera E. Characteristics and correlates of dyspnea in patients with advanced cancer. J Palliat Med. 2009;12:29–36. doi: 10.1089/jpm.2008.0158. [DOI] [PubMed] [Google Scholar]
- 3.Simon ST, Bausewein C, Schildmann E, et al. Episodic breathlessness in patients with advanced disease: a systematic review. J Pain Symptom Manage. 2013;45:561–578. doi: 10.1016/j.jpainsymman.2012.02.022. [DOI] [PubMed] [Google Scholar]
- 4.Mercadante S, Aielli F, Adile C, et al. Epidemiology and characteristics of episodic breathlessness in advanced cancer patients: an observational study. J Pain Symptom Manage. 2016;51:17–24. doi: 10.1016/j.jpainsymman.2015.07.020. [DOI] [PubMed] [Google Scholar]
- 5.Weingartner V, Bausewein C, Higginson IJ, et al. Characterizing episodic breathlessness in patients with advanced disease. J Palliat Med. 2013;16:1275–1279. doi: 10.1089/jpm.2013.0087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Weingartner V, Scheve C, Gerdes V, et al. Characteristics of episodic breathlessness as reported by patients with advanced chronic obstructive pulmonary disease and lung cancer: results of a descriptive cohort study. Palliat Med. 2015;29:420–428. doi: 10.1177/0269216314563428. [DOI] [PubMed] [Google Scholar]
- 7.Charles MA, Reymond L, Israel F. Relief of incident dyspnea in palliative cancer patients: a pilot, randomized, controlled trial comparing nebulized hydromorphone, systemic hydromorphone, and nebulized saline. J Pain Symptom Manage. 2008;36:29–38. doi: 10.1016/j.jpainsymman.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 8.Johnson MJ, Hui D, Currow DC. Opioids, exertion, and dyspnea: a review of the evidence. Am J Hosp Palliat Care. 2016;33:194–200. doi: 10.1177/1049909114552692. [DOI] [PubMed] [Google Scholar]
- 9.Benitez-Rosario MA. Fentanyl for episodic dyspnoea in cancer patients. Ann Palliat Med. 2014;3:4–6. doi: 10.3978/j.issn.2224-5820.2014.01.02. [DOI] [PubMed] [Google Scholar]
- 10.Benitez-Rosario MA, Martin AS, Feria M. Oral transmucosal fentanyl citrate in the management of dyspnea crises in cancer patients. J Pain Symptom Manage. 2005;30:395–397. doi: 10.1016/j.jpainsymman.2005.10.002. [DOI] [PubMed] [Google Scholar]
- 11.Sitte T, Bausewein C. Intranasal fentanyl for episodic breathlessness. J Pain Symptom Manage. 2008;36:e3–e6. doi: 10.1016/j.jpainsymman.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 12.Gauna AA, Kang SK, Triano ML, Swatko ER, Vanston VJ. Oral transmucosal fentanyl citrate for dyspnea in terminally ill patients: an observational case series. J Palliat Med. 2008;11:643–648. doi: 10.1089/jpm.2007.0161. [DOI] [PubMed] [Google Scholar]
- 13.Hui D, Xu A, Frisbee-Hume S, et al. Effects of prophylactic subcutaneous fentanyl on exercise-induced breakthrough dyspnea in cancer patients: a preliminary double-blind, randomized, controlled trial. J Pain Symptom Manage. 2014;47:209–217. doi: 10.1016/j.jpainsymman.2013.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jensen D, Alsuhail A, Viola R, et al. Inhaled fentanyl citrate improves exercise endurance during high-intensity constant work rate cycle exercise in chronic obstructive pulmonary disease. J Pain Symptom Manage. 2012;43:706–719. doi: 10.1016/j.jpainsymman.2011.05.007. [DOI] [PubMed] [Google Scholar]
- 15.Kotrach HG, Bourbeau J, Jensen D. Does nebulized fentanyl relieve dyspnea during exercise in healthy man? J Appl Physiol. 2015;118:1406–1414. doi: 10.1152/japplphysiol.01091.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Simon ST, Koskeroglu P, Gaertner J, Voltz R. Fentanyl for the relief of refractory breathlessness: a systematic review. J Pain Symptom Manage. 2013;46:874–886. doi: 10.1016/j.jpainsymman.2013.02.019. [DOI] [PubMed] [Google Scholar]
- 17.Fisher A, Watling M, Smith A, Knight A. Pharmacokinetics and relative bioavailability of fentanyl pectin nasal spray 100 – 800 microg in healthy volunteers. Int J Clin Pharmacol Ther. 2010;48:860–867. doi: 10.5414/cpp48860. [DOI] [PubMed] [Google Scholar]
- 18.Fisher A, Watling M, Smith A, Knight A. Pharmacokinetic comparisons of three nasal fentanyl formulations; pectin, chitosan and chitosan-poloxamer 188. Int J Clin Pharmacol Ther. 2010;48:138–145. doi: 10.5414/cpp48138. [DOI] [PubMed] [Google Scholar]
- 19.Portenoy RK, Burton AW, Gabrail N, Taylor D. A multicenter, placebo-controlled, double-blind, multiple-crossover study of Fentanyl Pectin Nasal Spray (FPNS) in the treatment of breakthrough cancer pain. Pain. 2010;151:617–624. doi: 10.1016/j.pain.2010.07.028. [DOI] [PubMed] [Google Scholar]
- 20.Kress HG, Oronska A, Kaczmarek Z, et al. Efficacy and tolerability of intranasal fentanyl spray 50 to 200 microg for breakthrough pain in patients with cancer: a phase III, multinational, randomized, double-blind, placebo-controlled, crossover trial with a 10-month, open-label extension treatment period. Clin Ther. 2009;31:1177–1191. doi: 10.1016/j.clinthera.2009.05.022. [DOI] [PubMed] [Google Scholar]
- 21.Fallon M, Reale C, Davies A, et al. Efficacy and safety of fentanyl pectin nasal spray compared with immediate-release morphine sulfate tablets in the treatment of breakthrough cancer pain: a multicenter, randomized, controlled, double-blind, double-dummy multiple-crossover study. J Support Oncol. 2011;9:224–231. doi: 10.1016/j.suponc.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 22.Davies A, Sitte T, Elsner F, et al. Consistency of efficacy, patient acceptability, and nasal tolerability of fentanyl pectin nasal spray compared with immediate-release morphine sulfate in breakthrough cancer pain. J Pain Symptom Manage. 2011;41:358–366. doi: 10.1016/j.jpainsymman.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 23.Portenoy RK, Raffaeli W, Torres LM, et al. Long-term safety, tolerability, and consistency of effect of fentanyl pectin nasal spray for breakthrough cancer pain in opioid-tolerant patients. J Opioid Manag. 2010;6:319–328. doi: 10.5055/jom.2010.0029. [DOI] [PubMed] [Google Scholar]
- 24.Chen C, Bujanover S, Gupta A. Effect of dosing interval on pharmacokinetics of fentanyl pectin nasal spray from a crossover study. J Opioid Manag. 2015;11:139–146. doi: 10.5055/jom.2015.0263. [DOI] [PubMed] [Google Scholar]
- 25.Hui D, Park M, Shamieh O, et al. Personalized symptom goals and response in patients with advanced cancer. Cancer. 2016;122:1774–1781. doi: 10.1002/cncr.29970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mercadante S, Villari P, Ferrera P, et al. Transmucosal fentanyl vs intravenous morphine in doses proportional to basal opioid regimen for episodic-breakthrough pain. Br J Cancer. 2007;96:1828–1833. doi: 10.1038/sj.bjc.6603811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mercadante S, Gatti A, Porzio G, et al. Dosing fentanyl buccal tablet for breakthrough cancer pain: dose titration versus proportional doses. Curr Med Res Opin. 2012;28:963–968. doi: 10.1185/03007995.2012.683112. [DOI] [PubMed] [Google Scholar]
- 28.Dorman S, Byrne A, Edwards A. Which measurement scales should we use to measure breathlessness in palliative care? A systematic review. Palliat Med. 2007;21:177–191. doi: 10.1177/0269216307076398. [DOI] [PubMed] [Google Scholar]
- 29.Tanaka K, Akechi T, Okuyama T, Nishiwaki Y, Uchitomi Y. Development and validation of the Cancer Dyspnoea Scale: a multidimensional, brief, self-rating scale. Br J Cancer. 2000;82:800–805. doi: 10.1054/bjoc.1999.1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gift AG, Narsavage G. Validity of the numeric rating scale as a measure of dyspnea. Am J Crit Care. 1998;7:200–204. [PubMed] [Google Scholar]
- 31.Hui D, Morgado M, Vidal M, et al. Dyspnea in Hospitalized Advanced Cancer Patients: Subjective and Physiologic Correlates. J Palliat Med. 2013;16:274–280. doi: 10.1089/jpm.2012.0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Laboratories ATSCoPSfCPF. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 33.Hui D, Shamieh O, Paiva C, et al. Minimal clinically important differences in the Edmonton Symptom Assessment Scale in cancer patients: a prospective study. Cancer. 2015;121:3027–3035. doi: 10.1002/cncr.29437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ries AL. Minimally clinically important difference for the UCSD Shortness of Breath Questionnaire, Borg Scale, and Visual Analog Scale. COPD. 2005;2:105–110. doi: 10.1081/copd-200050655. [DOI] [PubMed] [Google Scholar]
- 35.Bruera E, Miller MJ, Macmillan K, Kuehn N. Neuropsychological effects of methylphenidate in patients receiving a continuous infusion of narcotics for cancer pain. Pain. 1992;48:163–166. doi: 10.1016/0304-3959(92)90053-E. [DOI] [PubMed] [Google Scholar]
- 36.Redelmeier DA, Guyatt GH, Goldstein RS. Assessing the minimal important difference in symptoms: a comparison of two techniques. J Clin Epidemiol. 1996;49:1215–1219. doi: 10.1016/s0895-4356(96)00206-5. [DOI] [PubMed] [Google Scholar]
- 37.Guyatt GH, Feeny DH, Patrick DL. Measuring health-related quality of life. Ann Intern Med. 1993;118:622–629. doi: 10.7326/0003-4819-118-8-199304150-00009. [DOI] [PubMed] [Google Scholar]
- 38.Bruera E, MacEachern T, Ripamonti C, Hanson J. Subcutaneous morphine for dyspnea in cancer patients. Ann Intern Med. 1993;119:906–907. doi: 10.7326/0003-4819-119-9-199311010-00007. [DOI] [PubMed] [Google Scholar]
- 39.Jennings AL, Davies AN, Higgins JP, Gibbs JS, Broadley KE. A systematic review of the use of opioids in the management of dyspnoea. Thorax. 2002;57:939–944. doi: 10.1136/thorax.57.11.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Granger CL, Holland AE, Gordon IR, Denehy L. Minimal important difference of the 6-minute walk distance in lung cancer. Chron Respir Dis. 2015;12:146–154. doi: 10.1177/1479972315575715. [DOI] [PubMed] [Google Scholar]
- 41.Coluzzi PH, Schwartzberg L, Conroy JD, et al. Breakthrough cancer pain: a randomized trial comparing oral transmucosal fentanyl citrate (OTFC) and morphine sulfate immediate release (MSIR) Pain. 2001;91:123–130. doi: 10.1016/s0304-3959(00)00427-9. [DOI] [PubMed] [Google Scholar]
- 42.Parikh N, Goskonda V, Chavan A, Dillaha L. Pharmacokinetics and dose proportionality of fentanyl sublingual spray: a single-dose 5-way crossover study. Clin Drug Investig. 2013;33:391–400. doi: 10.1007/s40261-013-0079-8. [DOI] [PubMed] [Google Scholar]
- 43.Hagen NA, Fisher K, Victorino C, Farrar JT. A titration strategy is needed to manage breakthrough cancer pain effectively: observations from data pooled from three clinical trials. J Palliat Med. 2007;10:47–55. doi: 10.1089/jpm.2006.0151. [DOI] [PubMed] [Google Scholar]
- 44.Cook C. Mode of administration bias. Journal of Manual and Manipulative Therapy. 2010;18:61–63. doi: 10.1179/106698110X12640740712617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hui D, Bruera E. The essential role of feasibility studies in supportive care: reply to Sanz Rubiales and del Valle. J Pain Symptom Manage. 2014;48:e5–e6. doi: 10.1016/j.jpainsymman.2014.05.001. [DOI] [PubMed] [Google Scholar]
- 46.Pablos-Mendez A, Barr RG, Shea S. Run-in periods in randomized trials: implications for the application of results in clinical practice. JAMA. 1998;279:222–225. doi: 10.1001/jama.279.3.222. [DOI] [PubMed] [Google Scholar]
- 47.Berger VW, Rezvani A, Makarewicz VA. Direct effect on validity of response run-in selection in clinical trials. Control Clin Trials. 2003;24:156–166. doi: 10.1016/s0197-2456(02)00316-1. [DOI] [PubMed] [Google Scholar]
- 48.Davis CE, Applegate WB, Gordon DJ, Curtis RC, McCormick M. An empirical evaluation of the placebo run-in. Control Clin Trials. 1995;16:41–50. doi: 10.1016/0197-2456(94)00027-z. [DOI] [PubMed] [Google Scholar]
- 49.de la Cruz M, Hui D, Parsons HA, Bruera E. Placebo and nocebo effects in randomized double-blind clinical trials of agents for the therapy for fatigue in patients with advanced cancer. Cancer. 2010;116:766–774. doi: 10.1002/cncr.24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.