Abstract
Introduction
Epidermal Growth Factor Receptor (EGFR) is a therapeutic target in Non-Small Cell Lung Cancer (NSCLC) for EGFR mutant patients. Proximity ligation assay (PLA) is a method to detect functional signaling associated protein complexes. GRB2 is an adaptor protein which binds to the phosphorylated residues of active EGFR. EGFR and GRB2 interaction, correlates with active EGFR signaling and leads to activation of MAPK/ERK pathway.
Methods
A PLA developed to detect EGFR:GRB2 interaction was measured by quantitative immunofluorescence (QIF) using AQUA® technology. EGFR pathway activation was assessed in NSCLC patients with different mutation status along with overall EGFR expression. Additionally, PLA EGFR:GRB2 was evaluated as a prognostic marker in two lung adenocarcinoma patient cohorts.
Results
PLA EGFR:GRB2 was unrelated to overall EGFR expression or mutation in a series of NSCLC patients with known mutation status. EGFR mutant (p=0.04) and EGFR/KRAS wild type tumors (p=0.0049) had significantly higher EGFR pathway activation compared to KRAS mutant cases, with no significant difference shown between mutation sites. In two series of lung adenocarcinoma patients, PLA EGFR:GRB2 was independently associated with longer survival (HR=0.46, CI 95% 0.2–0.78, p=0.0085 and HR=0.48, CI 95% 0.2–0.85, p=0.017). Total EGFR protein expression alone was not correlated with outcome.
Conclusions
EGFR co-localization with GRB2 as assessed by PLA is not correlated to EGFR expression levels or mutation status, defining a patient group that may show EGFR pathway activation, as illustrated by its prognostic value. Future studies may determine if this group is more likely to respond to EGFR targeted therapies.
Keywords: egfr, grb2, proximity ligation assay
INTRODUCTION
Lung cancer is the second most frequently diagnosed malignancy. It is estimated that over 200,000 cases will be diagnosed in 2016 and over 100,000 patients will die, making it the most common cause of cancer-related death in the United States 1. Non-Small Cell Lung Cancer (NSCLC) accounts for approximately 85% of lung cancer. While novel agents that target specific signaling molecules of cell pathways have proved effective in treating NSCLC, only 15.9% of newly diagnosed patients will still be alive after 5 years or more 2.
The epidermal growth factor receptor (EGFR) is a 170 kDa transmembrane protein, member of the ErbB family, that is expressed in different levels in 50% to 90% of NSCLC tumors 3 depending on the method of assessment. Its activation leads to downstream pathway activation and plays an important role in the regulation of cell proliferation and survival 4. Growth factor receptor bound protein 2 (GRB2) is an adaptor protein that forms stable complexes -mediated via SH2 domain- with tyrosine phosphorylated EGFR 5, leading to the activation of RAS and its downstream kinases, ERK1/2. EGFR tyrosine kinase inhibitors (TKIs), erlotinib and gefitinib, are small molecules that bind the ATP-binding pocket of the receptor to prevent ligand-induced phosphorylation and downstream signaling. Many clinic-pathological factors have been assessed for prediction of response to EGFR targeted therapies. The most compelling is the presence of EGFR activating mutations 6 that are found in about 10% of patients with NSCLC in Western countries and almost 50% of Asian populations7. EGFR TKIs are the first line treatment option of choice in advanced NSCLC patients harboring EGFR-activating mutations in exons 18–21, and are also approved for locally advanced or metastatic NSCLC after the failure of at least one prior chemotherapy regimen, regardless of EGFR status 8.
The role of EGFR expression level in NSCLC as a predictive or prognostic biomarker is controversial. There are conflicting data about the prognostic value of EGFR measured by immunohistochemisty (IHC) with most of the studies showing no association between EGFR abundance and prognosis 9, 10. EGFR gene copy number assessment by fluorescent in situ hybridization (FISH), although more easily standardized than IHC, has also failed to show a consistent association between increased EGFR gene copy number and survival 11. The lack of consensus regarding the prognostic value of EGFR protein expression or EGFR copy number may reflect the inability to measure the underlying biological processes, like the functional and signaling activity of the receptor.
The Duolink® Proximity Ligation Assay, PLA, uses antibodies conjugated to oligonucleotides that can only hybridize when the conjugated antibody targets are within 40nm of each other. This enables the detection of molecular binding events in situ 12. This method has been used in previous studies to detect signaling associated protein complexes, receptor dimerization and phosphorylation 13–16. Unlike antibodies targeting the phosphorylated form of a protein, which are often not reliable due to pre-analytic effects 17 and difficult to use in formalin fixed paraffin embedded tissue (FFPE), PLA detects the signaling activation by directly targeting the protein complexes. In a previous study, it was shown that PLA targeting EGFR and its adaptor protein GRB2 correlated with active EGFR signaling 16. We developed a PLA to detect EGFR and GRB2 complexes that could be quantified using the AQUA® method of automated quantitative immunofluorescence (QIF) 18, eliminating any subjective interpretation of the results and allowing the objective and reproducible measurement of the obtained signal. EGFR:GRB2 PLA was compared to total EGFR expression levels and mutational status in a series of NSCLC patient tumors, and its role as a prognostic marker was assessed in two NSCLC adenocarcinoma cohorts.
MATERIALS AND METHODS
Cell lines and Western Blots
The cell lines MCF7, A431, H1975, H1650, HCC193, HT29, SW480, H2126 and H2882 were purchased from the American Type Culture Collection (Manassas, VA) or donated by other labs. Culture conditions were as described previously 19. Cell lines selected to represent a range of EGFR expression and different mutation status were MCF7, A431, H1975, H1650, HCC193 and H2882. Whole-cell lysates were prepared, and total protein concentration was measured using the Pierce BCA Protein Assay (Thermo Fischer Scientific, Waltham, MA). Fifteen micrograms total protein for each lysate was resolved using SDS-PAGE on an 8% Bis-Tris gel (NuPAGE Invitrogen Corp., Carlsbad, CA) using NuPAGE MOPS [3-(N-morpholino) propane sulfonic acid] SDS running buffer at 45 mA. Resolved protein was transferred using NuPAGE transfer buffer at 50V for 2 hours. Immunoblots were probed with EGFR (D38B1) rabbit monoclonal antibody diluted 1:1000 (Cell Signaling Technology, Inc.), anti-GRB2 (clone 81) mouse monoclonal antibody 1:1000 (BD Biosciences), phospho-EGFR (Tyr1068) D7A5 rabbit monoclonal antibody 1:1000 (Cell Signaling Technology, Inc.) and β-Tubulin (Cell Signaling Technology, Inc.) diluted 1:1000 as a loading control. Western Blots have been performed twice as independent experiments. Bands were quantified using ImageJ software (National Institutes of Health, Washington, DC), and were normalized to β-tubulin.
Proximity Ligation Assay
Fresh 5μm tissue microarray (TMA) sections were deparaffinized at 60°C for 30 minutes, incubated in xylene (soaking twice for 20 minutes) and rehydrated with ethanol (twice in 100% ethanol for 1 minute, and then in 70% ethanol for 1 minute). Antigen retrieval was performed in a PT module (LabVision, Fremont, CA) with EDTA buffer (Sigma-Aldrich, St Louis, MO) pH 8.0 for 20 minutes at 97°C. After blocking of endogenous peroxidase with 30% hydrogen peroxide in methanol, slides were incubated with a blocking solution containing 0.3% bovine serum albumin in Tris-buffered saline solution and 0.05% Tween solution for 30 minutes at room temperature. Slides were then incubated overnight with a cocktail of EGFR (D38B1) rabbit monoclonal antibody (Cell Signaling Technology, Inc.) and anti-GRB2 (clone 81) mouse monoclonal antibody (BD Biosciences). Anti-rabbit MINUS and anti-mouse PLUS PLA probes (1:5 dilution, Duolink, Sigma-Aldrich) were used to incubate the slides for 60 minutes at 37°C. Following a 30 minute incubation with 5X Ligation Stock (1:5 dilution, Duolink, Sigma-Aldrich) and Ligase (1:40 dilution, Duolink, Sigma-Aldrich) at 37°C, Amplification Stock 5X (1:5 dilution, Duolink, Sigma-Aldrich) and Polymerase (1:80 dilution, Duolink, Sigma-Aldrich) were added for 120 minutes at 37°C. To generate an amplified signal, TMA sections were incubated for 60 minutes at room temperature with 5X Detection Stock (1:5 dilution, Duolink, Sigma-Aldrich), which contains oligonucleotides complementary to the Rolling Circle Amplification product conjugated with Horseradish Peroxidase. Cyanine 5 (Cy5) directly conjugated to tyramide (FP1117; Perkin-Elmer) at a 1:50 dilution for 10 minutes was used for signal generation. Conjugated anti-pan cytokeratin eFluor 570 (clone AE1/AE3, eBioscience) was used to demarcate the epithelial compartment. Finally, ProLong mounting medium (ProLong Gold; Molecular Probes) containing 4,6-diamidino-2-phenyl-indole (DAPI) was used to detect tissue nuclei. All primary antibodies and the PLA kit are commercially available. To address reproducibility, slides were stained and analyzed on different days using the same protocol and sections from controls slides were run alongside each slide staining.
Quantitative Immunofluorescence (QIF)
Tissue sections were subjected to the same deparaffinization, antigen retrieval, and blocking protocol mentioned above and incubated overnight with a cocktail of EGFR (D38B1, Cell Signaling Technology) rabbit monoclonal antibody and mouse monoclonal cytokeratin antibody clone AE1/AE3 (M3515, Dako). Next, a mixture of Alexa 546 conjugated goat anti-mouse secondary antibody (Molecular Probes, Eugene, OR, USA) diluted 1:100 in rabbit EnVision reagent (K4003, Dako) was applied to the slides for 60 minutes at room temperature. Cyanine 5 (Cy5) directly conjugated to tyramide (FP1117; Perkin-Elmer) at a 1:50 dilution for 10 minutes was used for target detection and ProLong mounting medium (ProLong Gold; Molecular Probes) containing DAPI was used to stain nuclei. Control slides were run for reproducibility alongside each experimental slide-staining run.
Quantitative measurements of EGFR:GRB2 PLA and EGFR immunofluorescence (IF) were performed using AQUA method (Automated Quantitative Analysis) (Genoptix Medical Laboratory), quantifying fluorescent signal within subcellular compartments, as described previously 18. A tumor mask was created by binarizing the cytokeratin signal and creating an epithelial compartment. Quantitative immunofluorescence (QIF) score was calculated by dividing the target pixel intensity by the area of cytokeratin compartment. QIF scores were normalized to the exposure time and bit depth at which the images were captured, allowing scores collected at different exposure times to be comparable. All patient tumor spots were visually evaluated and cases with staining artifacts or presence of less than 2% tumor area were systematically excluded.
Tissue Microarray and Patient Cohorts
Tissue specimens were prepared in a tissue microarray format as previously described 20. Representative tumor areas were obtained from formalin-fixed, paraffin-embedded specimens of the primary tumor, and 0.6mm cores from each tumor block were arrayed in a recipient block. Formalin-fixed, paraffin-embedded cell line pellets were used as controls.
A NSCLC array, termed YTMA 310, that consists of 139 tumor cores from patients with different mutation status was constructed after pathology reports from 2011–2013 were retrieved. NSCLC patient cases that had complete surgical resection of primary tumor and subsequently molecular testing for common NSCLC mutations were selected for further review. This cohort was explicitly designed and used here to assess the relationship between biomarker expression and mutation status. It is not a serial collection, and thus outcome information is not obtained. The cases that had adequate residual tumor from primary site were selected and cored. YTMA 310 includes 30 EGFR mutant, 43 KRAS mutant, 66 EGFR and KRAS wild type patient cases as well as 6 cell line cores (HCC193, A431, HT29, SW480, MCF7, H2126). YTMA 310 and an additional custom index TMA containing FFPE cell lines, including A431, MCF7, H1975, H1650, HCC193, H2882, were used for assay validation and reproducibility assessment.
A serial collected cohort of patient tissues obtained in the Yale Surgical Pathology suite, called YTMA 250 (Cohort A), comprises a sample collection from 314 NSCLC patients that had surgical resection of their primary tumor between 2004 and 2011. An older previously described Yale cohort 21, YTMA 79 (Cohort B), consists of 202 formalin-fixed, paraffin-embedded, primary NSCLC tumors from patients seen at Pathology Department of Yale University between 1988 and 2003. These cohort predate routine mutation testing, but are valuable for assessment of outcome. From those two cohorts, only the adenocarcinoma patients with follow up, n=125 and n=98 respectively, were assessed for EGFR:GRB2 PLA and EGFR expression. Clinicopathological information from patients in both cohorts was collected from clinical records and pathology reports. Detailed characteristics of the cohorts’ adenocarcinoma cases in each cohort are presented in Table 1. All tissue was used after approval from the Yale Human Investigation Committee protocol #9505008219, which approved the patient consent forms or in some cases waiver of consent.
Table 1.
Clinico-pathological characteristics of the adenocarcinoma patients in Yale cohorts A and B.
| Yale Cohort A | Yale Cohort B | |||
|---|---|---|---|---|
| n=125 | n=98 | |||
|
| ||||
| Age | (44–91 years) | (42–84 years) | ||
|
| ||||
| <70 years | 73 | 58.4% | 59 | 60.2% |
| ≥70 years | 52 | 41.6% | 39 | 39.8% |
|
| ||||
| Sex | ||||
|
| ||||
| Male | 42 | 33.6% | 42 | 42.9% |
| Female | 83 | 66.4% | 56 | 57.1% |
|
| ||||
| Stage at Diagnosis | ||||
|
| ||||
| 0/I | 80 | 64.0% | 54 | 55.1% |
| II | 26 | 20.8% | 10 | 10.2% |
| III | 14 | 11.2% | 26 | 26.5% |
| IV | 5 | 4.0% | 8 | 8.2% |
|
| ||||
| Smoking History | ||||
|
| ||||
| Non-Smoker | 24 | 19.2% | 12 | 12.2% |
| Former/Current Smoker | 95 | 70.0% | 83 | 84.7% |
| Unknown | 6 | 4.8% | 3 | 3.1% |
Statistical Analysis
Pearson’s correlation coefficient (R) was used to assess the correlation between QIF scores from redundant tumor cores as well as from serial cuts of the same cores of the selected series NSCLC array (YTMA-310). To test for QIF score differences between mutation status and site, p-values were calculated by Mann-Whitney analysis. For the statistical analysis, the maximal marker score obtained from two available cores of each case was used. Stratification of the cohort patients according to the marker levels for survival analysis was performed by obtaining EGFR:GRB2 PLA QIF scores of the patient cohorts and splitting them by quartiles. The higher segments were shown to collapse defining two distinct groups, one including the lowest quartile and another comprising the higher three segments (Suppl. Fig. 2A).
Patient characteristics were compared using chi-square test as categorical variables. Overall-survival curves were constructed using the Kaplan-Meier analysis with a follow up of 60 months and statistical significance was determined using the log-rank test. Univariate and multivariable Cox proportional hazards models including age, gender, smoking status, and clinical stage as covariables were performed to determine which factors had a significant impact on overall survival and assess the independent prognostic significance of EGFR:GRB2 PLA. All p-values were based on two-sided tests and all p-values <0.05 were considered statistically significant. Statistical analyses were performed using IBM SPSS Version 20 (IBM Corp., Armonk, NY) and GraphPad Prism v6.0 for Windows (GraphPad Software, Inc, San Diego, CA).
RESULTS
Assay reproducibility and assessment of EGFR:GRB2 PLA in FFPE cell lines
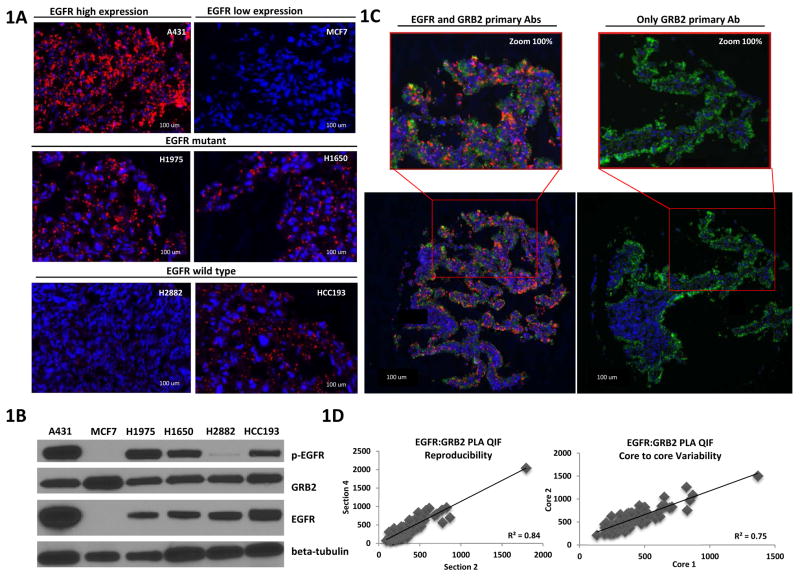
Initially, EGFR:GRB2 PLA was performed in FFPE cell lines with different EGFR expression levels and mutation status (Fig. 1A). A431 and MCF7 were used, as high and low EGFR expression cell line controls. As expected, no PLA signal was detected in MCF7 cells that have very low levels of EGFR. In two EGFR mutant cell lines, H1975 and H1650, that harbor the EGFR tyrosine kinase domain mutations L858R and DeltaE746–A750 respectively, robust EGFR:GRB2 PLA signal was detected, indicative of numerous EGFR:GRB2 complexes. The results are consistent with the known ligand-independent EGFR activity in those cell lines 22. To evaluate if EGFR:GRB2 PLA correlates with the presence of EGFR mutation or EGFR expression, two NSCLC EGFR wild-type cell lines H2882 and HCC193 were assessed. H2882 cells showed low EGFR:GRB2 PLA signal while increased levels were found in the HCC193 cell line, indicating that EGFR:GRB2 signaling complexes abundance is not the same between different wild-type cell lines and is not merely reflected by EGFR mutation status. All NSCLC cell lines expressed both EGFR and GRB2 proteins (Fig. 1B) which indicates that low level of EGFR:GRB2 PLA signal is not due to absence of either protein. In addition, EGFR:GRB2 PLA signal intensity as measured by quantitative fluorescence is correlated with the abundance of Tyr1068 p-EGFR (Suppl. Fig 1A) showing a concordance between the presence of EGFR activation signaling complexes and EGFR phosphorylation.
Figure 1.
Validation of EGFR:GRB2 PLA in NSCLC cell lines. (A) AQUA® images of FFPE cell lines with different EGFR expression levels and NSCLC FFPE cell lines with different EGFR mutation status. EGFR:GRB2 PLA (red), cytokeratin 42, DAPI (4′,6-diamidino-2-phenylindole) (blue). (B) Western blots of phosphorylated Tyr1068 EGFR, total EGFR, and GRB2. β-tubulin was used as a loading control. Blots are representative of two independent experiments. (C) Technical control of EGFR:GRB2 PLA. AQUA® images of EGFR mutant patient case with complete PLA reaction and without EGFR primary antibody. EGFR:GRB2 PLA (red), cytokeratin 42, DAPI (4′,6-diamidino-2-phenylindole) (blue). Images in (A) and (C) are representative of two independent experiments (D) Reproducibility and core-to-core variability of EGFR:GRB2 quantified by AQUA®.
To further validate the EGFR:GRB2 PLA reaction, we compared a complete PLA reaction with both EGFR and GRB2 primary antibodies and an incomplete PLA reaction in the absence of EGFR antibody. There was no PLA signal detected in the absence of one primary antibody as representatively shown in an example of an EGFR mutant patient case (Fig. 1C). Moreover, EGFR:GRB2 PLA measured by QIF is a reproducible method when performed on serial cuts of the same tumor core but also EGFR:GRB2 PLA has low variability between duplicate cores (Fig. 1D).
The relationship between EGFR:GRB2 PLA, EGFR expression and mutation status in NSCLC patients
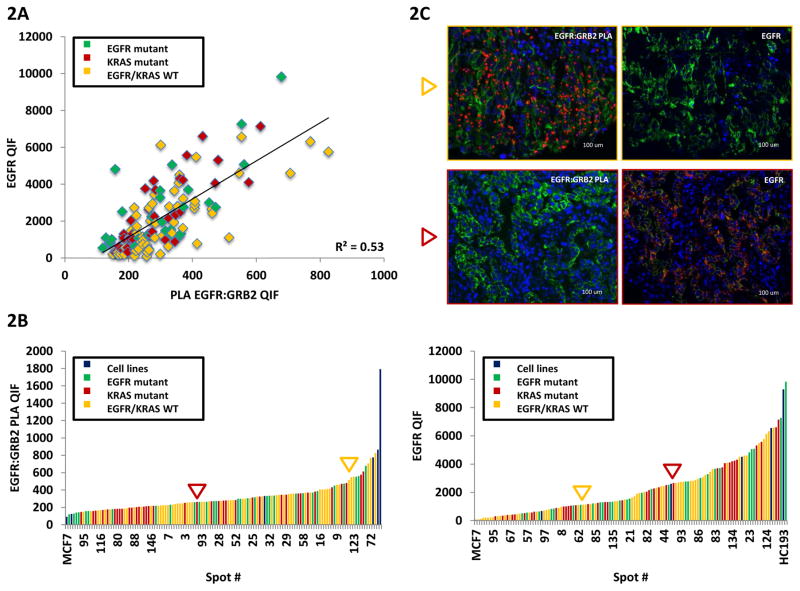
EGFR:GRB2 PLA and total EGFR expression relationship was evaluated in serial cuts of the YTMA 310 array containing tumor cores from NSCLC patients with different mutational status. EGFR:GRB2 PLA was found not to be correlated with total EGFR expression indicating that EGFR:GRB2 PLA was not solely driven by the abundance of EGFR (Fig. 2A). Similarly, mutation status does not correlate with activation as assessed by EGFR:GRB2 PLA in that both mutated and wild type EGFR show both high and low levels of activation. Figure 2 B and C illustrate that both EGFR:GRB2 PLA and EGFR QIF scores were distributed independently of the mutation status (Fig. 2B). The discordance between EGFR:GRB2 PLA and EGFR expression is shown in two representative cases (Fig. 2C). In the first case (yellow arrow) of an EGFR wild type tumor, EGFR:GRB2 signaling complexes are abundant, while expression of the receptor is low. In contrast, EGFR high expression was not reflected into strong EGFR:GRB2 PLA signal in a KRAS mutant patient case (red arrow). Core-to-core variability was low for EGFR:GRB2 PLA in both EGFR and KRAS mutant cases (Suppl. Fig 1B).
Figure 2.
EGFR:GRB2 PLA and total EGFR correlation in an array with different mutational status NSCLC patients. (A) QIF score regression chart of EGFR:GRB2 PLA and EGFR in NSCLC patients with known mutation status. (B) EGFR:GRB2 PLA and EGFR QIF score distribution. Cell lines (blue), EGFR mutant cases 42, KRAS mutant cases (red), EGFR/KRAS wild type cases (yellow). (C) Images of FFPE tumor sections of two patient cases with EGFR:GRB2 PLA and EGFR expression discordance. Yellow arrow points at an EGFR wild type tumor with high EGFR:GRB2 PLA QIF scores but low EGFR expression. Red arrow points at a KRAS mutant case with high EGFR expression, but low EGFR:GRB2 PLA signal. EGFR:GRB2 PLA (red), cytokeratin 42, DAPI (4′,6-diamidino-2-phenylindole) (blue).
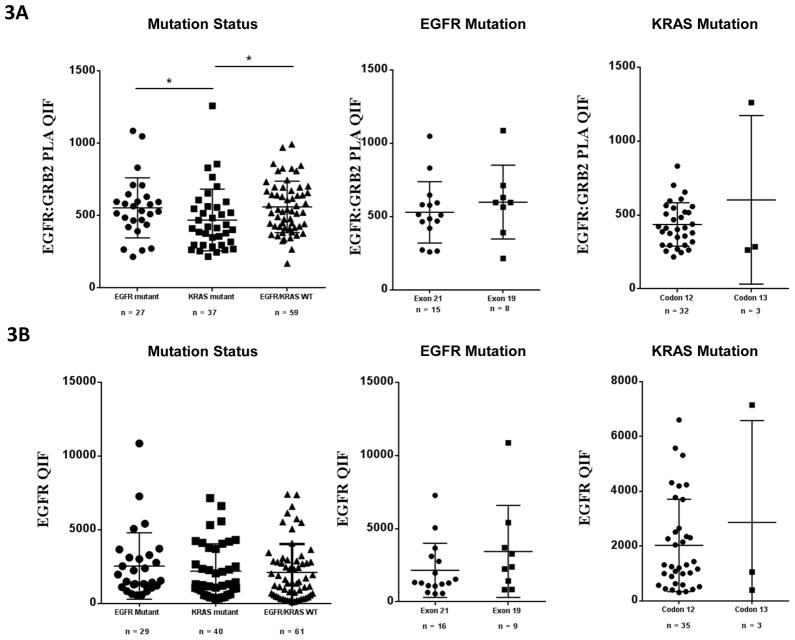
To determine if specific site mutations were associated with activation, we assessed EGFR:GRB2 signaling complexes split by mutation site and gene (Fig. 3). EGFR:GRB2 PLA scores were significantly higher in EGFR mutant and EGFR/KRAS wild type patients compared to KRAS mutant patients (p=0.04 and p=0.0049 respectively), indicating a stronger EGFR activation in those groups compared to KRAS mutant patients. No difference was observed across mutation hotspots. Similarly, there was no difference in EGFR expression levels when split by mutation in any gene or site.
Figure 3.
EGFR:GRB2 PLA and EGFR QIF scores across mutations and mutation sites in NSCLC tumors. (A) EGFR:GRB2 PLA QIF scores in NSCLC tumors with different mutation status. EGFR mutant (p=0.04) and EGFR/KRAS wild type tumors (p=0.0049) have significantly higher EGFR:GRB2 PLA QIF scores compared to KRAS mutant tumors. (B) Total EGFR QIF scores in NSCLC tumors with different mutation status. No statistically significant difference across different mutations or mutation sites. Two-tailed Mann-Whitney U test, bars represent means with standard deviation. * Denotes statistical significance p<0.05.
EGFR:GRB2 PLA QIF as a prognostic marker in NSCLC adenocarcinoma patients
The potential correlation between EGFR:GRB2 PLA and patient characteristics was assessed for adenocarcinoma patients of the Yale cohorts A and B in parallel. The clinic-pathologic parameters analyzed included age, gender, stage, and smoking status. Both cohorts are from a time period where testing for mutations was not the standard of care, so the correlation with mutation status is not possible (and was shown previously with YTMA-310). No consistent association between EGFR:GRB2 PLA level and major clinico-pathological variables was found (Suppl. Fig 2C) in either cohort.
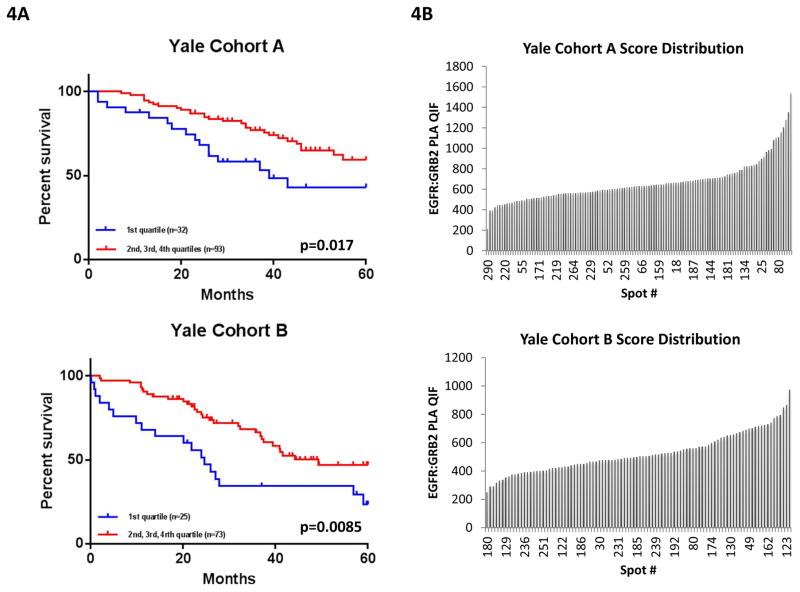
Since the EGFR:GRB2 score is a continuous value and essentially all cases are greater than the MCF7 negative control, we divided the cohort into quartiles for outcome analysis. Examination of the level the EGFR:GRB2 PLA scores split by quartiles showed a clear clustering of the top 3 quartiles and separation of the 4th quartile (see Methods and Suppl. Fig 2A). So for statistical analysis, populations were split in two groups where the low group is the first quartile and the high group is the top 3 quartiles. Validation of the collapsed quartile cut-point was achieved since the defined division of Yale cohort A was applied to Yale cohort B as an independent validation set. As shown in Figure 4, high (2nd, 3rd and 4th high quartiles) EGFR:GRB2 PLA was statistically significantly associated with longer 5-year survival in both Yale cohorts A and B compared to patients classified as low EGFR:GRB2 PLA (1st low quartile) (log-rank p=0.017 for Yale cohort A and p= 0.0085 for Yale cohort B).
Figure 4.
EGFR:GRB2 PLA as a prognostic marker. (A)Kaplan-Meier 5-year survival curves of adenocarcinoma patients in Yale cohort A and B, stratified according to EGFR:GRB2 PLA QIF scores. P=0.017 (cohort A) and p=0.0085 (cohort B), log-rank(Mantel-Cox) test. (B) Score distribution of EGFR:GRB2 QIF scores of adenocarcinoma patients in Yale cohorts A and B.
Multivariate Cox proportional hazards regression analysis was performed to derive risk estimates related to survival for all clinic-pathologic characteristics and EGFR:GRB2 complexes (Table 2); cases with missing values were excluded from analysis. Low EGFR:GRB2 PLA group (1st quartile) was independently associated with worse survival in both Yale cohorts A and B. Gender and stage were shown to be independent prognostic markers only in Yale cohort A.
Table 2.
Univariate and multivariate analysis by Cox proportional hazards model in adenocarcinoma patients of Yale cohorts A and B. EGFR:GRB2 PLA is an independent prognostic marker of survival. Gender and stage were independent prognostic markers only in Yale cohort A.
| Yale Cohort A
| |||||||
|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | ||||||
| Variables | Hazard Ratio 95% CI | P value | Hazard Ratio 95% CI | P value | |||
| Age | <70 yr vs ≥70yr | 1.178 | 0.652 – 2.131 | 0.587 | 1.01 | 0.516 – 1.974 | 0.978 |
| Gender | Female vs Male | 2.336 | 1.297 – 4.205 | *0.005 | 2.826 | 1.524 – 5.242 | *0.001 |
| Stage | I–IIIA vs IIIB–IV | 4.689 | 1.836 – 11.976 | *0.001 | 9.599 | 2.94 – 31.342 | *<0.000 |
| Smoking Status | Never Smoker vs Former/Current Smoker | 1.22 | 0.581 – 2.564 | 0.599 | 1.127 | 0.536 – 2.372 | 0.752 |
| EGFR:GRB2 PLA | 1st quartile vs 2nd, 3rd, 4th quartile | 0.486 | 0.263 – 0.896 | *0.021 | 0.439 | 0.229 – 0.841 | *0.013 |
| Yale Cohort B
| |||||||
|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariate Analysis | ||||||
| Variables | Hazard Ratio 95% CI | P value | Hazard Ratio 95% CI | P value | |||
| Age | <70 yr vs ≥70yr | 0.76 | 0.418 – 1.383 | 0.369 | 0.732 | 0.387 – 1.386 | 0.338 |
| Gender | Female vs Male | 1.631 | 0.93 – 2.859 | 0.088 | 1.321 | 0.709 – 2.461 | 0.381 |
| Stage | I–IIIA vs IIIB–IV | 1.746 | 0.84 – 3.630 | 0.136 | 1.577 | 0.716 – 3.471 | 0.258 |
| Smoking Status | Never Smoker vs Former/Current Smoker | 1.483 | 0.531 – 4.137 | 0.452 | 1.109 | 0.383 – 3.208 | 0.849 |
| EGFR:GRB2 PLA | 1st quartile vs 2nd, 3rd, 4th quartile | 0.466 | 0.26 – 0.834 | *0.01 | 0.44 | 0.234 – 0.829 | *0.011 |
DISCUSSION
The use of the PLA assay to detect EGFR binding to GRB2 as a surrogate for activation has been previously described [17] but never quantitatively measured. We found that the addition of a tyramide signal amplification step 23 resulted in a sufficiently large amplification of signal that could be objectively, linearly and reproducibly measured by the AQUA method of QIF. Using this method, the assessment of EGFR signaling activity in FFPE cell lines with different mutation status revealed that the abundance of EGFR:GRB2 PLA signal was not driven by EGFR expression level nor by the presence of EGFR activating mutations. This was further confirmed in NSCLC tumor cores from patients with different mutational status of the receptor. The level of PLA signal and EGFR expression in serial sections were only weakly correlated, further indicating a patient group that shows EGFR pathway dependence unrelated to overall EGFR expression. The difference in the QIF score range of EGFR:GRB2 PLA and overall EGFR expression is attributed to the different levels of signal amplification of the QIF and PLA protocols. In the PLA QIF protocol oligonucleotide probes carrying an HRP molecule hybridize to the Rolling Circle Amplification product. In the protein QIF protocol, the use of secondary antibodies carrying a dextran backbone, to which multiple enzyme molecules are attached, results to the generation of a stronger signal.
Although recent meta-analyses investigating the role of EGFR TKIs in wild type patients, confirmed the superiority of chemotherapy over EGFR TKIs in terms of PFS 24, 25, they also revealed the presence of a subgroup of patients not carrying EGFR activating mutations that derived clinical benefit from EGFR targeted treatment. A recent collective study 26 reported that EGFR wild-type patients receiving EGFR-TKIs achieved a response rate of 11% in all-stage NSCLC. This suggests that there are factors other than EGFR mutation that lead to TKI sensitivity in a small number of unselected patients. No obvious relationship between EGFR protein expression assessed by IHC and tumor sensitivity to TKIs has been found in multiple retrospective analyses 27–31. Similarly, prospective studies have not validated EGFR FISH as a useful biomarker. EGFR gene amplification as assessed by FISH was unrelated to outcome with cetuximab, a chimeric monoclonal antibody against EGFR, in FLEX and BMS-099 trials 32, 33.
The observations in the literature, combined with the findings in this study suggest that EGFR:GRB2 PLA could select tumors with increased EGFR signaling in the absence of activating mutations, and thus, perhaps identify patients that would benefit from an EGFR targeted therapy. However, in a previous study by Smith et al 16, EGFR:GRB2 PLA was not found to be predictive of response to cetuximab in targeted analysis of NSCLC patient derived xenograft (PDX) tumors. This could be attributed to the lack of a quantitative method of assessment or simply the absence of an association. However, the MCC3 cohort assessed by Smith and colleagues shows better survival in patients classified as EGFR:GRB2 PLA positive by their method. Furthermore, they found that 30% of the cases with high EGFR:GRB2 PLA were also wild type EGFR. These findings, combined with our observations, further support the hypothesis that EGFR activation-based classification of NSCLC may have clinical value.
Both the work of Smith et al and our work show that not all EGFR mutant tumors had strongly active EGFR. While not yet proven, this observation could be associated with the reported 75% response rate of EGFR mutant patients to EGFR TKIs 34. Additionally, EGFR:GRB2 PLA signal was higher in EGFR mutant and EGFR wild type patients compared to KRAS mutant patients, although there were some KRAS mutant tumors with robust EGFR:GRB2 PLA signal, indicative of numerous EGFR:GRB2 complexes. Two meta-analyses 35, 36 have reported that KRAS mutant compared to wild-type tumors (including both EGFR mutant and EGFR wild type) were significantly associated with lack of response to EGFR TKIs, although the objective response rate of KRAS mutant patients was found to be 3% 35.
EGFR:GRB2 PLA was found to be an independent prognostic marker of survival in adenocarcinoma patients of two NSCLC patient cohorts. Consistent with the existing literature 9, 10, EGFR protein expression level was not found to be associated with survival in those patients (data not shown). Additionally, while there was a trend towards higher EGFR:GRB2 PLA scores in squamous cell carcinoma tumors, EGFR expression only was found to be statistically significantly higher only in squamous cell carcinoma compared to adenocarcinoma patients (p<0.0001) in the two NSCLC cohorts (Suppl Fig S2B). Patients harboring EGFR activating mutations have been reported to exhibit improved response rate and prolonged survival compared to those without mutations, even when they received chemotherapy alone (without TKIs) 37, 38 or placebo 39, 40. Similarly, in our study, patients with high EGFR:GRB2 complexes abundance, indicative of EGFR activation, had superior outcome compared to the EGFR:GRB2 low PLA group. However, no molecular testing results were available in the adenocarcinoma patients included, making a further stratification based on underlying mutational status impossible.
There are a number of limitations to this study. Perhaps the most significant is that the cohorts tested were not treated with EGFR inhibitors. While this would clearly be optimal, access to the valuable clinical trial often requires proof of concept, as shown in this work, prior to release of the tissue. A second limitation of this work is the fact that the assays were done entirely on tissue microarrays. While tissue microarrays are often used for large cohort studies, and considered representative, they are never used in a true clinical setting. Furthermore, in other studies 41, we have shown that whole slides can be easily measured using quantitative fluorescence in the same manner used here for tissue microarrays. A third limitation of this work is that it uses a technique (QIF) that is not commonly used in the CLIA lab setting. Another interpretation of this limitation is that it is an advantage, since more information is obtained than can be derived from conventional IHC. While it is not commonly performed in clinical labs, the same could have been said of mutation detection 10 years ago. We believe that if this test were shown to be a sensitive and specific method for prediction of response to EGFR inhibitors, labs would learn to adopt this technology.
In conclusion, PLA is a powerful method to detect signaling-associated protein complexes in situ. After further validation on clinical trial tissue, this tool could provide a method to classify tumors with EGFR signaling activity and guide therapeutic decisions in different patient subgroups (i.e. EGFR/KRAS wild type patients).
Supplementary Material
Acknowledgments
Support sources: This work was supported by grants from the NIH including the Yale SPORE in Lung Cancer, P50-CA196530, the Yale Cancer Center Support Grant, P30-CA016359, the OKIBEE support grant (KS) and support from Gilead Sciences Inc.
This work was supported by grants from the NIH including the Yale SPORE in Lung Cancer, P50-CA196530, the Yale Cancer Center Support Grant, P30-CA016359 and support from Gilead Sciences Inc. The authors also acknowledge the expert assistance of Lori Charette and her staff in the Yale Tissue Microarray Facility division of Yale Pathology Tissue Services for construction of the tissue microarrays used in the study.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERRENCES
- 1.Howlader N, Noone A, Krapcho M, et al. SEER Cancer Statistics Review, 1975–2012. National Cancer Institute; Bethesda, MD: 2015. based on November 2014 SEER data submission, posted to the SEER web site, April 2015. [Google Scholar]
- 2.Ettinger DS, Akerley W, Borghaei H, et al. Non-small cell lung cancer, version 2. 2013. Journal of the National Comprehensive Cancer Network: JNCCN. 2013;11:645–653. doi: 10.6004/jnccn.2013.0084. quiz 653. [DOI] [PubMed] [Google Scholar]
- 3.Giaccone G. HER1/EGFR-targeted agents: predicting the future for patients with unpredictable outcomes to therapy. Annals of oncology: official journal of the European Society for Medical Oncology/ESMO. 2005;16:538–548. doi: 10.1093/annonc/mdi129. [DOI] [PubMed] [Google Scholar]
- 4.Roberts PJ, Der CJ. Targeting the Raf-MEK-ERK mitogen-activated protein kinase cascade for the treatment of cancer. Oncogene. 2007;26:3291–3310. doi: 10.1038/sj.onc.1210422. [DOI] [PubMed] [Google Scholar]
- 5.Lowenstein EJ, Daly RJ, Batzer AG, et al. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992;70:431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- 6.Lynch TJ, Bell DW, Sordella R, et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. The New England journal of medicine. 2004;350:2129–2139. doi: 10.1056/NEJMoa040938. [DOI] [PubMed] [Google Scholar]
- 7.Hirsch FR, Bunn PA., Jr EGFR testing in lung cancer is ready for prime time. The Lancet Oncology. 2009;10:432–433. doi: 10.1016/S1470-2045(09)70110-X. [DOI] [PubMed] [Google Scholar]
- 8.Zornosa C, Vandergrift JL, Kalemkerian GP, et al. First-line systemic therapy practice patterns and concordance with NCCN guidelines for patients diagnosed with metastatic NSCLC treated at NCCN institutions. Journal of the National Comprehensive Cancer Network: JNCCN. 2012;10:847–856. doi: 10.6004/jnccn.2012.0088. [DOI] [PubMed] [Google Scholar]
- 9.Berghmans T, Meert AP, Martin B, et al. Prognostic role of epidermal growth factor receptor in stage III nonsmall cell lung cancer. Eur Respir J. 2005;25:329–335. doi: 10.1183/09031936.05.00060804. [DOI] [PubMed] [Google Scholar]
- 10.Meert AP, Martin B, Delmotte P, et al. The role of EGF-R expression on patient survival in lung cancer: a systematic review with meta-analysis. Eur Respir J. 2002;20:975–981. doi: 10.1183/09031936.02.00296502. [DOI] [PubMed] [Google Scholar]
- 11.Peled N, Yoshida K, Wynes MW, et al. Predictive and prognostic markers for epidermal growth factor receptor inhibitor therapy in non-small cell lung cancer. Therapeutic advances in medical oncology. 2009;1:137–144. doi: 10.1177/1758834009347923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Soderberg O, Gullberg M, Jarvius M, et al. Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nature methods. 2006;3:995–1000. doi: 10.1038/nmeth947. [DOI] [PubMed] [Google Scholar]
- 13.Gajadhar AS, Bogdanovic E, Munoz DM, et al. In situ analysis of mutant EGFRs prevalent in glioblastoma multiforme reveals aberrant dimerization, activation, and differential response to anti-EGFR targeted therapy. Molecular cancer research: MCR. 2012;10:428–440. doi: 10.1158/1541-7786.MCR-11-0531. [DOI] [PubMed] [Google Scholar]
- 14.Halle C, Lando M, Sundfor K, et al. Phosphorylation of EGFR measured with in situ proximity ligation assay: relationship to EGFR protein level and gene dosage in cervical cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2011;101:152–157. doi: 10.1016/j.radonc.2011.05.052. [DOI] [PubMed] [Google Scholar]
- 15.Chen TC, Liu YW, Huang YH, et al. Protein phosphorylation profiling using an in situ proximity ligation assay: phosphorylation of AURKA-elicited EGFR-Thr654 and EGFR-Ser1046 in lung cancer cells. PloS one. 2013;8:e55657. doi: 10.1371/journal.pone.0055657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Smith MA, Hall R, Fisher K, et al. Annotation of human cancers with EGFR signaling-associated protein complexes using proximity ligation assays. Science signaling. 2015;8:ra4. doi: 10.1126/scisignal.2005906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bai YL, Tolles J, Cheng H, et al. Quantitative assessment shows loss of antigenic epitopes as a function of pre-analytic variables. Laboratory Investigation. 2011;91:1253–1261. doi: 10.1038/labinvest.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Camp RL, Chung GG, Rimm DL. Automated subcellular localization and quantification of protein expression in tissue microarrays. Nature medicine. 2002;8:1323–1327. doi: 10.1038/nm791. [DOI] [PubMed] [Google Scholar]
- 19.Dimou A, Neumeister V, Agarwal S, et al. Measurement of aldehyde dehydrogenase 1 expression defines a group with better prognosis in patients with non-small cell lung cancer. The American journal of pathology. 2012;181:1436–1442. doi: 10.1016/j.ajpath.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 20.McCabe A, Dolled-Filhart M, Camp RL, et al. Automated quantitative analysis (AQUA) of in situ protein expression, antibody concentration, and prognosis. Journal of the National Cancer Institute. 2005;97:1808–1815. doi: 10.1093/jnci/dji427. [DOI] [PubMed] [Google Scholar]
- 21.Anagnostou VK, Syrigos KN, Bepler G, et al. Thyroid transcription factor 1 is an independent prognostic factor for patients with stage I lung adenocarcinoma. J Clin Oncol. 2009;27:271–278. doi: 10.1200/JCO.2008.17.0043. [DOI] [PubMed] [Google Scholar]
- 22.Sharma SV, Bell DW, Settleman J, et al. Epidermal growth factor receptor mutations in lung cancer. Nature reviews Cancer. 2007;7:169–181. doi: 10.1038/nrc2088. [DOI] [PubMed] [Google Scholar]
- 23.Bobrow MN, Shaughnessy KJ, Litt GJ. Catalyzed reporter deposition, a novel method of signal amplification. II. Application to membrane immunoassays. Journal of immunological methods. 1991;137:103–112. doi: 10.1016/0022-1759(91)90399-z. [DOI] [PubMed] [Google Scholar]
- 24.Lee JK, Hahn S, Kim DW, et al. Epidermal growth factor receptor tyrosine kinase inhibitors vs conventional chemotherapy in non-small cell lung cancer harboring wild-type epidermal growth factor receptor: a meta-analysis. Jama. 2014;311:1430–1437. doi: 10.1001/jama.2014.3314. [DOI] [PubMed] [Google Scholar]
- 25.Zhao N, Zhang XC, Yan HH, et al. Efficacy of epidermal growth factor receptor inhibitors versus chemotherapy as second-line treatment in advanced non-small-cell lung cancer with wild-type EGFR: a meta-analysis of randomized controlled clinical trials. Lung cancer. 2014;85:66–73. doi: 10.1016/j.lungcan.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 26.Lindeman NI, Cagle PT, Beasley MB, et al. Molecular testing guideline for selection of lung cancer patients for EGFR and ALK tyrosine kinase inhibitors: guideline from the College of American Pathologists, International Association for the Study of Lung Cancer, and Association for Molecular Pathology. Journal of thoracic oncology: official publication of the International Association for the Study of Lung Cancer. 2013;8:823–859. doi: 10.1097/JTO.0b013e318290868f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bailey LR, Janas M, Schmidt K, et al. Evaluation of epidermal growth factor receptor (EGFR) as a predictive marker in patients with non-small-cell lung cancer (NSCLC) receiving first-line gefitinib combined with platinum-based chemotherapy. Journal of Clinical Oncology. 2004;22:620s–620s. [Google Scholar]
- 28.Miller VA, Zakowski M, Riely GJ, et al. EGFR mutation and copy number, EGFR protein expression and KRAS mutation as predictors of outcome with erlotinib in bronchioloalveolar cell carcinoma (BAC): Results of a prospective phase II trial. Journal of Clinical Oncology. 2006;24:364s–364s. [Google Scholar]
- 29.Parra HS, Cavina R, Latteri F, et al. Analysis of epidermal growth factor receptor expression as a predictive factor for response to gefitinib (‘Iressa’, ZD1839) in non-small-cell lung cancer. British journal of cancer. 2004;91:208–212. doi: 10.1038/sj.bjc.6601923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Perez-Soler R, Chachoua A, Hammond LA, et al. Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. Journal of Clinical Oncology. 2004;22:3238–3247. doi: 10.1200/JCO.2004.11.057. [DOI] [PubMed] [Google Scholar]
- 31.Tsao MS, Sakurada A, Cutz JC, et al. Erlotinib in lung cancer - molecular and clinical predictors of outcome. The New England journal of medicine. 2005;353:133–144. doi: 10.1056/NEJMoa050736. [DOI] [PubMed] [Google Scholar]
- 32.O’Byrne KJ, Gatzemeier U, Bondarenko I, et al. Molecular biomarkers in non-small-cell lung cancer: a retrospective analysis of data from the phase 3 FLEX study. The Lancet Oncology. 2011;12:795–805. doi: 10.1016/S1470-2045(11)70189-9. [DOI] [PubMed] [Google Scholar]
- 33.Khambata-Ford S, Harbison CT, Hart LL, et al. Analysis of potential predictive markers of cetuximab benefit in BMS099, a phase III study of cetuximab and first-line taxane/carboplatin in advanced non-small-cell lung cancer. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2010;28:918–927. doi: 10.1200/JCO.2009.25.2890. [DOI] [PubMed] [Google Scholar]
- 34.Pao W, Chmielecki J. Rational, biologically based treatment of EGFR-mutant non-small-cell lung cancer. Nature reviews Cancer. 2010;10:760–774. doi: 10.1038/nrc2947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mao C, Qiu LX, Liao RY, et al. KRAS mutations and resistance to EGFR-TKIs treatment in patients with non-small cell lung cancer: a meta-analysis of 22 studies. Lung cancer. 2010;69:272–278. doi: 10.1016/j.lungcan.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 36.Linardou H, Dahabreh IJ, Kanaloupiti D, et al. Assessment of somatic k-RAS mutations as a mechanism associated with resistance to EGFR-targeted agents: a systematic review and meta-analysis of studies in advanced non-small-cell lung cancer and metastatic colorectal cancer. The Lancet Oncology. 2008;9:962–972. doi: 10.1016/S1470-2045(08)70206-7. [DOI] [PubMed] [Google Scholar]
- 37.Bell DW, Lynch TJ, Haserlat SM, et al. Epidermal growth factor receptor mutations and gene amplification in non-small-cell lung cancer: molecular analysis of the IDEAL/INTACT gefitinib trials. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23:8081–8092. doi: 10.1200/JCO.2005.02.7078. [DOI] [PubMed] [Google Scholar]
- 38.Eberhard DA, Johnson BE, Amler LC, et al. Mutations in the epidermal growth factor receptor and in KRAS are predictive and prognostic indicators in patients with non-small-cell lung cancer treated with chemotherapy alone and in combination with erlotinib. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2005;23:5900–5909. doi: 10.1200/JCO.2005.02.857. [DOI] [PubMed] [Google Scholar]
- 39.Shepherd FA, Tsao MS. Unraveling the mystery of prognostic and predictive factors in epidermal growth factor receptor therapy. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2006;24:1219–1220. doi: 10.1200/JCO.2005.04.4420. author reply 1220–1211. [DOI] [PubMed] [Google Scholar]
- 40.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. Erlotinib in previously treated non-small- cell lung cancer. The New England journal of medicine. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 41.McLaughlin J, Han G, Schalper KA, et al. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA oncology. 2015:1–9. doi: 10.1001/jamaoncol.2015.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.von Seidlein L, Walraven G, Milligan PJ, et al. The effect of mass administration of sulfadoxine-pyrimethamine combined with artesunate on malaria incidence: a double-blind, community-randomized, placebo-controlled trial in The Gambia. Transactions of the Royal Society of Tropical Medicine and Hygiene. 2003;97:217–225. doi: 10.1016/s0035-9203(03)90125-8. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.