Abstract
Recent structural information on ligand-gated glutamate receptors and newly-discovered clinical uses for NMDA receptor antagonists has renewed interest in understanding the mechanisms of drug action at these receptors. Although the voltage-dependence and calcium permeability of NMDA receptors are well-studied, the mechanisms affecting the time course of synaptic NMDA receptor activation may be of more therapeutic value by serving as a rheostat for the total synaptic response. The NMDA receptor-mediated EPSC time course has been thought of as a fixed parameter based simply on receptor subunit composition as variably constrained by anatomical and developmental expression patterns, albeit subject to modification by kinetic behaviors such as modal gating. However, the EPSC time course also can be manipulated by endogenous and exogenous ligands. In this commentary we discuss insights into the in situ composition and kinetic behavior of synaptic NMDA receptors and propose new opportunities to target modulatory sites on NMDA receptors and to develop useful therapeutics. The emerging data on the atomic structure of NMDA receptors and knowledge of the kinetics of native receptors in neurons provide a roadmap in this regard.
The evidence for modulation of NMDA receptors
Drugs that target ligand- or voltage-gated ion channels of excitable cells have long been used to understand how channels work. Our understanding of neuronal excitability and synaptic transmission has relied on drugs that targeted voltage-gated ion channels such as tetrodotoxin and tetramethylammonium. Likewise, drugs such as barbiturates and benzodiazepines acting on GABAA receptors prolong inhibitory postsynaptic currents (IPSCs), resulting in their efficacy as anti-convulsants and anxiolytics. As the primary mediators of excitatory synaptic transmission, ionotropic glutamate receptors in the central nervous system are the targets for a long list of agonists and antagonists. This work began with the studies of Jeff Watkins and colleagues in the early 1980s (Evans et al., 1982) and has continued in the years since the cloning of glutamate receptor subunits in the early 1990s (reviewed in Traynelis et al., 2010). NMDA receptors were considered an especially promising therapeutic target because of their unique role in synaptic transmission and plasticity, and as mediators of the after-effects of hyperexcitability in neurological diseases (Lau and Tymianski, 2010; Zhou and Sheng, 2013). As a result, considerable drug discovery efforts resulted in many potent NMDA receptor antagonists. However, the use of these competitive antagonists and channel blockers has been thwarted by undesirable side effects or therapeutic ineffectiveness. Perhaps surprisingly, relatively low efficacy NMDA receptor antagonists have found use for symptomatic treatment of Alzheimer’s disease (memantine), and the dissociative anesthetic ketamine is coming into use as an acute-acting antidepressant (reviewed in Johnson et al., 2015; Lodge and Mercier, 2015; Monteggia and Zarate, 2015). Interestingly, recent results show that the time course of the NMDA receptor activation can be manipulated by endogenous and exogenous ligands. Taking advantage of this opportunity for modulation of NMDA receptor function requires consideration of subunit composition as well as the kinetic (i.e. non-equilbrium) behavior of native receptors - the topics we highlight in this commentary.
NMDA receptors are non-selective cation channels that flux calcium and are blocked by extracellular magnesium, resulting in extrinsic voltage sensitivity. A less well-appreciated aspect of NMDA receptor function is the long-lasting duration of the synaptic response that can vary by 2-3 orders of magnitude following neurotransmitter binding. The duration has important implications for signaling imposed by these kinetic parameters. Functional NMDA receptors are multimeric proteins and their constituent subunits confer unique pharmacological, kinetic and gating properties (Moriyoshi et al., 1991; Kutsuwada et al., 1992; Meguro et al., 1992; Monyer et al., 1992; Monyer et al., 1994; Vicini et al., 1998). Cloning of NMDA receptor subunits also revealed that their expression patterns were regulated anatomically and developmentally, hinting that multiple NMDA receptor subtypes exist in vivo. Subsequent work confirmed the existence of multiple synaptic NMDA receptor subtypes with distinct functional properties and anatomical distribution (Cull-Candy and Leszkiewicz, 2004). These discoveries led to the idea that the molecular heterogeneity of NMDA receptors could be used to develop modulatory ligands targeting specific combinations of subunits or in specific neuronal populations. This attractive idea is yet to be fully realized, in part because of a lack of knowledge of the native NMDA receptor composition.
What are the native receptors?
Understanding the patterns of expression and their impact on NMDA receptor function is complex. The seven NMDA receptor subunits are grouped into three families; GluN1, GluN2 (A-D) and GluN3 (A, B). The rules of association of NMDA receptor subunits were worked out in heterologous expression systems. From these studies we know that functional receptors are composed of GluN1 and one or more of the GluN2 subunits. Receptors are tetramers (Clements and Westbrook, 1991; Laube et al., 1998; Furukawa et al., 2005) with two GluN1 subunits per receptor (Behe et al., 1995). NMDA receptors are unique among ligand-gated channels in requiring two different agonists, glutamate and glycine (or serine), for activation (Johnson and Ascher, 1987; Forsythe et al., 1988). The GluN1 subunit binds glycine (Kuryatov et al., 1994) and the GluN2 subunits bind glutamate. Each unique GluN1-GluN2 pair introduced into heterologous expression systems produces receptors with distinctive pharmacological and kinetic properties (Kutsuwada et al., 1992; Vicini et al., 1998). Expressing GluN1 and either of the GluN3 subunits produces glycine-gated channels with strikingly different properties than neuronal NMDA receptors, including a lack of sensitivity to many common NMDA receptor ligands (Chatterton et al., 2002).
GluN1 is expressed throughout the brain, in almost all neurons and in some glia. GluN2A and GluN2B have overlapping expression in the cortex and hippocampus, with expression primarily, though not exclusively, in principal neurons (Monyer et al., 1994). GluN2C is expressed in much of the midbrain and cerebellum (Takahashi et al., 1996) and GluN2D is expressed embryonically, throughout brainstem and midbrain structures (Monyer et al., 1994) with expression into the adult in some cell types (Suárez et al., 2010). The GluN2C and GluN2D subunits have been the targets for development of drugs acting as allosteric modulators, but we will base our discussion here around what is known for GluN2A and GluN2B, which are expressed at high levels and with a broad distribution throughout the cortex and hippocampus. GluN2A is barely detectable embryonically whereas GluN2B expression is high and decreases slightly during the first few postnatal weeks as GluN2A expression increases, but many neurons co-express both subunits into adulthood (Monyer et al., 1994). Expression of more than one GluN2 subunit type in single neurons is likely the rule rather than the exception, complicating the characterization of native receptors. Indeed, not long after NMDA receptor subunits were cloned, receptor complexes containing two different GluN2 subunits were biochemically detected in cortical neurons (Sheng et al., 1994). In retrospect, this suggested that the properties of in vivo NMDA receptors are unlikely to be fully explained by the properties of heterologously expressed diheteromeric receptors.
Nonetheless, many fundamental insights into the basic properties of NMDA receptors have resulted from studies of recombinant receptors, of known subunit composition, expressed in heterologous systems. This strategy was also used to find and characterize receptor/ligand interactions. Because NMDA receptors are obligate heteromers, introduction of pairs of subunit types into heterologous expression systems produces a single diheteromeric receptor type whereas introduction of three subunit types could result in three receptor types (two di- and one triheteromeric). Results from the latter type of experiment are difficult to interpret because of the inability to control the receptors produced, a limitation that only recently has been overcome (Hansen et al., 2014; Stroebel et al., 2014). In neurons, NMDA receptor subunit expression is controlled anatomically and developmentally and functional proteins are assembled and processed to synaptic sites in a non-random manner (Kumar and Huegenard, 2003; Tovar et al., 2013). Importantly, recent experiments on native receptors has revealed that triheteromeric NMDA receptors are the major contributor to excitatory postsynaptic currents (EPSCs) in hippocampal neurons (Rauner and Köhr, 2011; Gray et al., 2011; Tovar et al., 2013). Moreover, isolated triheteromeric synaptic NMDA receptors, which until then were not experimentally accessible, have novel kinetic and pharmacological properties (Tovar et al., 2013).
Modifying the NMDA receptor synaptic time course
The trigger of course for NMDA receptor activation at synapses is the time course of glutamate in the synaptic cleft. However, the glutamate transient is quite brief, rising to a peak of around 1 mM and decaying with a predominant time constant of a millisecond (Clements, 1996). Although the response associated with synaptic activation of colocalized AMPA receptors is also brief (Hestrin, 1993; Jonas and Spruston, 1994), the time course of synaptic NMDA receptor activation is orders of magnitude slower with rise times ranging from 5 to 15 milliseconds and deactivation time constants that can be hundreds of milliseconds or longer (Lester et al., 1990; Hestrin et al., 1990; Gray et al., 2011). Pulsatile neurotransmitter release emphasizes the importance of the non-equilibrium behavior of NMDA receptor activation. Because subunit composition controls this kinetic behavior, subunit composition also governs the time course of the NMDA receptor EPSC. This characteristic is more than a biophysical curiosity as the long time course is critical to synaptic function by acting as a memory device that associates a fast presynaptic signal with a much longer postsynaptic signal. This long duration can govern signaling events ranging from computation at single synapses in the olfactory bulb (Schoppa and Westbrook, 1999) to network dynamics underlying working memory in prefrontal cortex (Wang, 2001). Natural stimulus patterns from cells that are presynaptic to hippocampal pyramidal neurons (Dobrunz and Stevens, 1999) often occur at frequencies that produce overlap of synaptic NMDA receptor responses, creating an ongoing NMDA receptor ‘tone’ that given their voltage-dependence can serve as a booster device.
The time course of the NMDA response for diheteromeric receptors has been thoroughly examined (Vicini et al., 1998). These experiments revealed that the deactivation in response to brief agonist applications is highly characteristic of the diherteromeric receptor type. The primary time constant (τD) of recombinant diheteromeric receptors varied from ~20 milliseconds for A-type (GluN1/GluN2A) receptors to more than a second for D-type (GluN1/GluN2D) receptors (Vicini et al., 1998). These approaches have also been used to explore the microscopic kinetics of channel gating including modal behavior, which results from differences in transitions between fully occupied closed states (Popescu et al., 2004; Zhang et al., 2008). These approaches in heterologous expression systems have been invaluable in understanding the gating of different NMDA subunit combinations. However, because of the inability to know the amounts of each receptor type present when multiple GluN2 subunit types are heterologously expressed, these experiments do not consider the properties of triheteromeric receptors. Cultured hippocampal principal neurons from mouse lines in which GluN2A or GluN2B are genetically deleted (Sakimura et al., 1995; Kutsuwada et al., 1996) provide a way of examining EPSCs that result from pure dihetromeric NMDA receptors in neurons (Tovar et al., 2013). In those cases, EPSCs from GluN2A-lacking neurons have properties expected from B-type receptors and EPSCs from GluN2B-lacking neurons have properties expected from A-type receptors. Likewise, hippocampal neurons from mice crossed to lack both GluN2 subunits have no NMDA receptor-mediated EPSCs. However, the kinetic properties of EPSCs from wild-type neurons cannot be reconciled with combinations of A- and B-type EPSCs (Tovar et al., 2013). The same conclusions come from an analogous approach, NMDA receptor-mediated EPSCs in CA1 neurons in acute slices from mice engineered to have a conditional knockout of GluN2A or GluN2B (Gray et al., 2011).
Certain antagonists that act at the amino-terminal domain of NMDA receptors show a degree of receptor subtype specificity (Figure 1). These include ifenprodil (and other phenylethanolamine derivatives) and zinc. Ifenprodil is at least a hundred-fold more specific for B-type NMDA receptors than for other diheteromeric NMDA receptor types (Perin-Dureau et al., 2002). Similarly, A-type receptors are more than 100-fold more sensitive to zinc than to other diheteromeric receptor subtypes (Paoletti et al., 1997). Both produce a reduction in the current in ways that are independent of competitive antagonism or channel block and neither completely eliminated currents from recombinant receptors (Paoletti and Neyton, 2007). The pharmacological properties of these drugs, however, have been studied using long duration agonist applications, making it difficult to appreciate the kinetic manifestations of these allosteric modulators under the non-equilibrium conditions that exist at synapses. Under these conditions, the kinetic behavior of ligand-bound receptors is predominant. Thus drugs that are allosteric modulators would be expected to alter channel gating and thus kinetic behaviors such as deactivation.
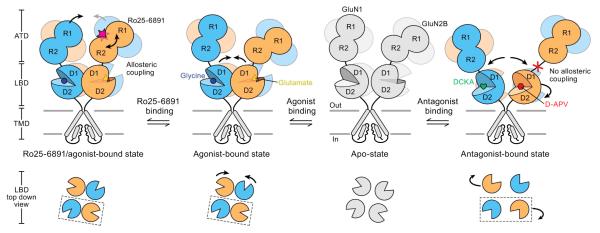
Figure 1. A model of functional interactions between ligand binding domains and amino-terminal domains.
This schematic, based on atomic structures of Xenopus GluN1-GluN2B receptors from Gouaux and colleagues, shows one pair of N1 and N2B subunits of the holoprotein; N1 is in blue and N2B is in orange. Glutamate and glycine binding stabilize the ligand binding domains (LBDs), leading to channel opening. Binding of the ifenprodil derivative Ro25-6891 to the N2 amino-terminal domain (ATD) induces clamshell closure and further affects interactions between the N1 amino-terminal domains. Occupancy and clamshell closure of the amino-terminal domains may increase the ligand binding domain interactions and coupling between the LBD and ATD, consistent with increased receptor activation time course. Competitive antagonist binding (D-APV or DCKA) prevents these allosteric coupling rearrangements. Adapted from Zhu et al., 2016 with permission.
In studying whether we could use the high subtype specificity of zinc and ifenprodil in determining the contribution of A- and B-type receptors to wild-type EPSCs, we found that in addition to reducing the peak of the response, zinc or ifenprodil prolonged A-type or B-type EPSCs, respectively (Tovar and Westbrook, 2012). Prolongation was accompanied by an increase in the latency to first opening following neurotransmitter binding and a decrease in the conditional probability of channels having opened by the EPSC peak (Po*). This result implies that prior to opening after neurotransmitter binding, these amino-terminal ligands (ATLs) increase the probability that NMDA receptor channels spend more time in an agonist-bound but non-conducting (i.e. desensitized) state. This situation is consistent with EPSC prolongation because desensitized states can lead to prolonged synaptic responses (e.g. Jones and Westbrook, 1995). EPSC prolongation by ATLs is consistent with slower neurotransmitter unbinding in which ifenprodil and zinc effectively increase the affinity of glutamate for NMDA receptors. Thus glutamate is trapped on receptors that are either open or desensitized.
NMDA receptor ligands: Drugs as rheostats for receptor function
Prolongation of NMDA receptor-mediated EPSCs by ATLs is a malleable strategy for receptor modulation. For example, in wild-type NMDA receptor-mediated EPSCs, the reduction in charge from zinc-induced decrease in EPSC peak is offset by the prolongation in the EPSC time-course, leading to an almost complete conservation of charge at some zinc concentrations (Tovar and Westbrook, 2012). Prolongation of the synaptic time course is a proven therapeutic manipulation as seen from the prolongation of synaptically-activated GABAA receptor channels by barbiturates and benzodiazepines. For AB-type receptors, the sensitivity to this form of modulation by known ATLs depends mostly on the N2A subunit (Tovar et al., 2013). Following pharmacological isolation of AB-type receptors on native receptors in hippocampal synapses, zinc decreased the EPSC peak and prolonged the deactivation, similar to its effect on pure A-type receptors (Figure 2). Ifenprodil, in contrast, decreased the peak but did not prolong the deactivation. This lack of an effect on EPSCs could result from an ifenprodil-induced decrease in neurotransmitter release probability (Delaney et al., 2012) and a small direct effect on AB-type receptors (Hatton and Paoletti, 2005). Experiments with other phenylethanolamine derivatives of ifenprodil produced similar results (Tovar, unpublished).
Figure 2. Prolongation of synaptic NMDA receptor signaling by amino-terminal ligands.
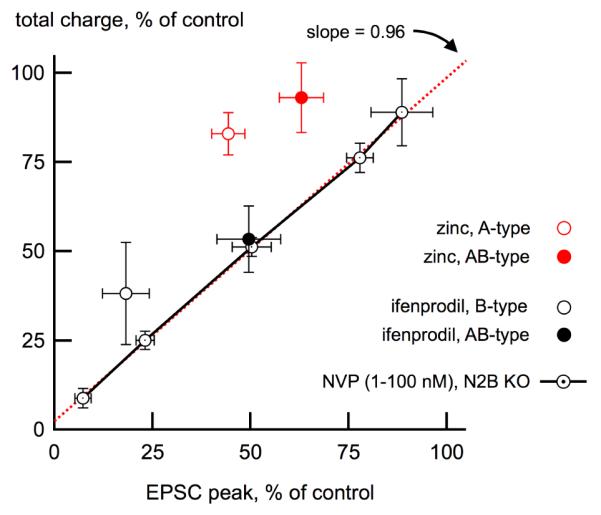
This figure illustrates the effect of NMDA receptor ligands on total synaptic charge (pA*ms) as a function of the reduction of the peak synaptic current. Competitive antagonists like NVP-AAM077 (circles and solid lines) do not affect channel kinetics because they work by preventing agonist binding as shown using a range of NVP-AAM077 concentrations in cultured hippocampal neurons expressing a homogenous NMDA receptor population (i.e. A-type receptors in neurons from GluN2B knockout mice). The points were well fitted with a straight line with a slope near unity, as expected. In contrast, because the amino-terminal ligand zinc prolongs deactivation of A- and AB-type receptors, the decrease of the EPSC peak by zinc is offset by the longer deactivation time course, resulting in only small reductions in total charge (red circles). Ifenprodil binding similarly produced a relative conservation of charge in EPSCs from B-type receptors (black open circle). However, this behavior was not seen in EPSCs from AB-type receptors, indicating that in triheteromeric receptors, occupancy of the N2B amino-terminal domain by ifenprodil results in no effective modulation of the synaptic response. Points above the unity line indicate a relative increase in charge. Adapted from Tovar et al., 2013 with permission.
Understanding the details of how ATLs produce prolongation of non-stationary macroscopic currents can be useful for finding ways of manipulating glutamate receptors clinically. Open channel blockers can be used to reveal details of channel gating mechanisms (Jahr, 1992; Rosenmund et al., 1993; Benveniste and Mayer, 1995; Tovar et al., 2013). For example, the high-affinity channel blocker MK-801, can be used to measure the latency of the NMDA receptor channel to opening for the first time after binding glutamate (Jahr, 1992; Tovar et al., 2013). A comparison of the ratio of charge from the NMDA receptor-mediated EPSC in the presence of MK-801 (25 μM) to the control EPSC, demonstrates that most of the charge resulting from synaptic activation of receptors results from reopening of liganded receptors, in some cases hundreds of milliseconds prior to sampling (Tovar et al., 2013). This technique revealed that zinc or ifenprodil on A-type or B-type receptors, respectively, increase the probability of receptors being in a ligand-bound, non-conducting state. Zinc and ifenprodil bind with high affinity to the amino-terminal domains of GluN2A and GluN2B, respectively (Zhu and Paoletti, 2015). However, the details of the interaction at the atomic level appear to be distinct (Karakas et al., 2009; Karakas et al., 2011). NMDA receptor ATDs are important for desensitization (Krupp et al., 1998; Zhu et al., 2013). Thus one inference is that pharmacologically interfering with ATD activity during gating alters the probability of neurotransmitter-bound channels residing in desensitized states.
The minor reduction by ifenprodil on pharmacologically isolated AB-type NMDA receptor EPSCs additionally demonstrates the limitations of this drug for distinguishing between NMDA receptor subtypes (Neyton and Paoletti, 2006). Ifenprodil has been used extensively to study the roles of NMDA receptor subunits in a number of neurobiological processes based on its inhibition of EPSCs (Barria and Malinow, 1992; Liu et al., 2004; Kim et al., 2005). The modest effects on AB-type receptors (Tovar et al., 2013; Hansen et al., 2014) are in keeping with reports that the efficacy of ifenprodil on triheteromeric receptors is reduced while the affinity is unchanged (Neyton and Paoletti, 2006).
In summary, at a functional level desensitized receptors are still in an agonist bound state. Thus these data highlight that glutamate unbinding from NMDA receptors can be altered by ligands that bind the ATL. The slow unbinding of glutamate from NMDA receptors results in a signal lasting many hundreds of milliseconds and thus the average rate at which glutamate unbinds controls the duration of that signal. Because the duration of NMDA receptor activation is much longer than glutamate transient, the time course of NMDA receptor activation acts as a memory device that is voltage-sensitive. Thus manipulating the glutamate unbinding rate (Lester and Jahr, 1992) provides a means to titrate the duration of this memory device in a more sensitive manner than competitive antagonists or channel blockers. An atomic structure of triheteromeric NMDA receptors, when available to add to other recent structural data (Tajima et al., 2016; Zhu et al., 2016), should provide more clues as to how to refine the existing list of NMDA receptor modulators.
Acknowledgements
Some of the work discussed in this commentary was supported by NIH grants NS 26494 and MH 46613 to GLW.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Barria A, Malinow R. Subunit-specific NMDA receptor trafficking to synapses. Neuron. 2002;35:345–353. doi: 10.1016/s0896-6273(02)00776-6. [DOI] [PubMed] [Google Scholar]
- Behe P, Stern P, Wyllie DJA, Nassar M, Schoepfer R, Colquhoun D. Determination of NMDA NR1 subunit copy number in recombinant NMDA receptors. Proc R Soc Lond B. 1995;262:205–213. doi: 10.1098/rspb.1995.0197. [DOI] [PubMed] [Google Scholar]
- Benveniste M, Meyer ML. Trapping of glutamate and glycine during open channel block of rat hippocampal neuron NMDA receptors by 9-aminoacridine. J Physiol. 1995;483:367–384. doi: 10.1113/jphysiol.1995.sp020591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterton JE, Awobuluyi M, Premkumar LS, Takahashi H, Talantova M, Shin Y, Cui J, Tu S, Sevarino KA, Nakanishi N, Tong G, Lipton SA, Zhang D. Excitatory glycine receptors containing the NR3 family of NMDA receptor subunits. Nature. 2002;415:793–798. doi: 10.1038/nature715. [DOI] [PubMed] [Google Scholar]
- Clements JD, Westbrook GL. Activation kinetics reveal the number of glutamate and glycine binding sites on the N-methyl-D-aspartate receptor. Neuron. 1991;7:605–613. doi: 10.1016/0896-6273(91)90373-8. [DOI] [PubMed] [Google Scholar]
- Clements JD. Transmitter timecourse in the synaptic cleft: its role in central synaptic function. Trends Neurosci. 1996;19:163–171. doi: 10.1016/s0166-2236(96)10024-2. [DOI] [PubMed] [Google Scholar]
- Cull-Candy SG, Leszkiewicz DN. Role of distinct NMDA receptor subtypes at central synapses. Sci STKE. 2004;2004(255):re16. doi: 10.1126/stke.2552004re16. [DOI] [PubMed] [Google Scholar]
- Delaney AJ, Power JM, Sah P. Ifenprodil reduces excitatory synaptic transmission by blocking presynaptic P/Q type calcium channels. J Neurophysiol. 2012;107:1571–1575. doi: 10.1152/jn.01066.2011. [DOI] [PubMed] [Google Scholar]
- Dobrunz LE1, Stevens CF. Response of hippocampal synapses to natural stimulation patterns. Neuron. 1999;22:157–166. doi: 10.1016/s0896-6273(00)80687-x. [DOI] [PubMed] [Google Scholar]
- Evans RH, Francis AA, Jones AW, Smith DA, Watkins JC. The effects of a series of omega-phosphonic alpha-carboxylic amino acids on electrically evoked and excitant amino acid-induced responses in isolated spinal cord preparations. Br J Pharmacol. 1982;75:65–75. doi: 10.1111/j.1476-5381.1982.tb08758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forsythe ID, Westbrook GL, Mayer ML. Modulation of excitatory synaptic transmission by glycine and zinc in cultures of mouse hippocampal neurons. J Neurosci. 1988;10:3733–3741. doi: 10.1523/JNEUROSCI.08-10-03733.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furukawa H, Singh SK, Mancusso R, Gouaux E. Subunit arrangements and function in NMDA receptors. Nature. 2005;438:185–192. doi: 10.1038/nature04089. [DOI] [PubMed] [Google Scholar]
- Gray JA, Shi Y, Usui H, During MJ, Sakimura K, Nicoll R. Distinct modes of AMPA receptor suppression at developing synapses by GluN2A and GluN2B: single-cell NMDA receptor subunit deletion in vivo. Neuron. 2011;71:1085–1101. doi: 10.1016/j.neuron.2011.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen KB, Ogden KK, Yuan H, Traynelis SF. Distinct functional and pharmacological properties of triheteromeric GluN1/GluN2A/GluN2B NMDA receptors. Neuron. 2014;81:1084–1096. doi: 10.1016/j.neuron.2014.01.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hatton CJ1, Paoletti P. Modulation of triheteromeric NMDA receptors by N-terminal domain ligands. Neuron. 2005;46:261–274. doi: 10.1016/j.neuron.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Hestrin S, Sah P, Nicoll RA. Mechanisms generating the time course of dual component excitatory synaptic currents recorded in hippocampal slices. Neuron. 1990;5:247–253. doi: 10.1016/0896-6273(90)90162-9. [DOI] [PubMed] [Google Scholar]
- Hestrin S. Different glutamate receptor channels mediate fast excitatory synaptic currents in inhibitory and excitatory cortical neurons. Neuron. 1993;11:1083–1091. doi: 10.1016/0896-6273(93)90221-c. [DOI] [PubMed] [Google Scholar]
- Jahr CE. High probability opening of NMDA receptor channels by L-glutamate. Science. 1992;255:470–472. doi: 10.1126/science.1346477. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Ascher P. Glycine potentiates the NMDA response in cultured mouse brain neurons. Nature. 1987;325(6104):529–31. doi: 10.1038/325529a0. [DOI] [PubMed] [Google Scholar]
- Johnson JW, Glasgow NG, Povysheva NV. Recent insights into the mode of action of memantine and ketamine. Curr Opin Pharmacol. 2015;20:54–63. doi: 10.1016/j.coph.2014.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jonas P, Spruston N. Mechanisms shaping glutamate-mediated excitatory synaptic currents in the CNS. Curr Opin Neurobiol. 1994;4:366–372. doi: 10.1016/0959-4388(94)90098-1. [DOI] [PubMed] [Google Scholar]
- Jones MV, Westbrook GL. Desensitized states prolong GABAA channel responses to brief agonist pulses. Neuron. 1995;15:181–91. doi: 10.1016/0896-6273(95)90075-6. [DOI] [PubMed] [Google Scholar]
- Karakas E, Simorowski N, Furukawa H. Structure of the zinc bound amino-terminal domain of the NMDA receptor NR2B subunit. EMBO J. 2009;28:3910–3920. doi: 10.1038/emboj.2009.338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karakas E, Simorowski N, Furukawa H. Subunit arrangement and phenylethanolamine binding in GluN1/GluN2B NMDA receptors. Nature. 2011;475:249–253. doi: 10.1038/nature10180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Dunah AW, Wang YT, Sheng M. Different roles of NR2A- and NR2B-containing NMDA receptors in ras-ERK signaling and AMPA receptor trafficking. Neuron. 2005;46:745–760. doi: 10.1016/j.neuron.2005.04.031. [DOI] [PubMed] [Google Scholar]
- Krupp JJ, Vissel B, Heinemann SJ, Westbrook GL. N-terminal domains in the NR2 subunit control desensitization of NMDA receptors. Neuron. 1998;20:317–327. doi: 10.1016/s0896-6273(00)80459-6. [DOI] [PubMed] [Google Scholar]
- Kumar SS, Huguenard JR. Pathway-specific differences in subunit composition of synaptic NMDA receptors on pyramidal neurons in neocortex. J Neurosci. 2003;31:10074–10083. doi: 10.1523/JNEUROSCI.23-31-10074.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuryatov A, Laube B, Betz H, Kuhse J. Mutational analysis of the glycine-binding site of the NMDA receptor: structural similarity with bacterial amino acid-binding proteins. Neuron. 1994;12:1291–1300. doi: 10.1016/0896-6273(94)90445-6. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Kashiwabuchi N, Mori H, Sakimura K, Kushiya E, Araki K, Meguro H, Masaki H, Kumanishi T, Arakawa M, Mishina M. Molecular diversity of the NMDA receptor channel. Nature. 1992;358:36–41. doi: 10.1038/358036a0. [DOI] [PubMed] [Google Scholar]
- Kutsuwada T, Sakimura K, Manabe T, Takayama C, Katakura N, Kushiya E, Natsume R, Watanabe M, Inoue Y, Yagi T, Aizawa S, Arakawa M, Takahashi T, Nakamura Y, Mori H, Mishina M. Impairment of suckling response, trigeminal neuronal pattern formation, and hippocampal LTD in NMDA receptor ε2 subunit mutant mice. Neuron. 1996;16:333–344. doi: 10.1016/s0896-6273(00)80051-3. [DOI] [PubMed] [Google Scholar]
- Lau A, Tymianski M. Glutamate receptors, neurotoxicity and neurodegeneration. Pflugers Arch. 2010;460:525–542. doi: 10.1007/s00424-010-0809-1. [DOI] [PubMed] [Google Scholar]
- Laube B, Kuhse J, Betz H. Evidence for a tetrameric structure of recombinant NMDA receptors. J Neurosci. 1998;18:2954–2961. doi: 10.1523/JNEUROSCI.18-08-02954.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lester RAJ, Clements JD, Westbrook GL, Jahr CE. Channel kinetics determine the time course of NMDA receptor-mediated synaptic currents. Nature. 1990;346:565–567. doi: 10.1038/346565a0. [DOI] [PubMed] [Google Scholar]
- Lester RAJ, Jahr CE. NMDA channel behavior depends on agonist affinity. J Neurosci. 1992;12:635–643. doi: 10.1523/JNEUROSCI.12-02-00635.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L, Wong TP, Pozza MF, Lingenhoehl K, Wang Y, Sheng M, Auberson YP, Wang YT. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- Lodge D, Mercier MS. Ketamine and phencyclidine: the good, the bad and the unexpected. Br J Pharmacol. 2015;172(17):4254–76. doi: 10.1111/bph.13222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meguro H, Mori H, Araki K, Kushiya E, Kutsuwada T, Yamazaki M, Kumanishi T, Arakawa M, Sakimura K, Mishina M. Functional characterization of a heteromeric NMDA receptor channel expressed from cloned cDNAs. Nature. 1992;357:70–74. doi: 10.1038/357070a0. [DOI] [PubMed] [Google Scholar]
- Monteggia LM, Zarate C., Jr Antidepressant actions of ketamine: from molecular mechanisms to clinical practice. Curr Opin Neurobiol. 2015;30:139–143. doi: 10.1016/j.conb.2014.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monyer H, Sprengel R, Schoepfer R, Herb A, Hiquichi Lomeli H, Burnashev N, Sakmann B, Seeburg PH. Heteromeric NMDA receptors: molecular and functional distinction of subtypes. Science. 1992;256:1217–1221. doi: 10.1126/science.256.5060.1217. [DOI] [PubMed] [Google Scholar]
- Monyer H, Burnashev N, Laurie DJ, Sakmann B, Seeburg PH. Developmental and regional expression in the rat brain and functional properties of four NMDA receptors. Neuron. 1994;12:529–40. doi: 10.1016/0896-6273(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Moriyoshi K, Masu M, Ishii T, Shigemoto R, Mizuno N, Nakanishi S. Molecular cloning and characterization of the rat NMDA receptor. Nature. 1991;354:31–37. doi: 10.1038/354031a0. [DOI] [PubMed] [Google Scholar]
- Neyton J, Paoletti P. Relating NMDA receptor function to receptor subunit composition: limitations of the pharmacological approach. J Neurosci. 2006;26:1331–1333. doi: 10.1523/JNEUROSCI.5242-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Ascher P, Neyton J. High-affinity zinc inhibition of NMDA NR1-NR2A receptors. J Neurosci. 1997;17:5711–5725. doi: 10.1523/JNEUROSCI.17-15-05711.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Neyton J. NMDA receptor subunits: function and pharmacology. Curr Opin Pharmacol. 2007;7:39–47. doi: 10.1016/j.coph.2006.08.011. [DOI] [PubMed] [Google Scholar]
- Perin-Dureau F, Rachline J, Neyton J, Paoletti P. Mapping the binding site of the neuroprotectant ifenprodil on NMDA receptors. J Neurosci. 2002;22:5955–5965. doi: 10.1523/JNEUROSCI.22-14-05955.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popescu G, Robert A, Howe JR, Auerbach A. Reaction mechanism determines NMDA receptor response to repetitive stimulation. Nature. 2004;430:790–793. doi: 10.1038/nature02775. [DOI] [PubMed] [Google Scholar]
- Rauner C, Köhr G. Triheteromeric NR1/NR2A/NR2B receptors constitute the major N-methyl-D-aspartate receptor population in adult hippocampal synapses. J Biol Chem. 2011;286:7558–7566. doi: 10.1074/jbc.M110.182600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenmund C, Clements JD, Westbrook GL. Nonuniform probability of glutamate release at a hippocampal synapse. Science. 1993;262:754–757. doi: 10.1126/science.7901909. [DOI] [PubMed] [Google Scholar]
- Sakimura K, Kutsuwada T, Ito I, Manabe T, Takayama C, Kushiya E, Yagi T, Aizawa S, Inoue Y, Sugiyama H, et al. Reduced hippocampal LTP and spatial learning in mice lacking NMDA receptor epsilon 1 subunit. Nature. 1995;373:151–155. doi: 10.1038/373151a0. [DOI] [PubMed] [Google Scholar]
- Schoppa NE, Westbrook GL. Regulation of synaptic timing in the olfactory bulb by an A-type potassium current. Nat Neurosci. 1999;12:1106–1113. doi: 10.1038/16033. [DOI] [PubMed] [Google Scholar]
- Sheng M, Cummings J, Roldan LA, Jan YN, Jan LY. Changing subunit composition of heteromeric NMDA receptors during development in rat cortex. Nature. 1994;368:144–147. doi: 10.1038/368144a0. [DOI] [PubMed] [Google Scholar]
- Stroebel D, Carvalho S, Grand T, Zhu S, Paoletti P. Controlling NMDA receptor subunit composition using ectopic retention signals. J Neurosci. 2014;34:16630–16636. doi: 10.1523/JNEUROSCI.2736-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suárez F, Zhao Q, Monaghan DT, Jane DE, Jones S, Gibb AJ. Functional heterogeneity of NMDA receptors in rat substantia nigra pars compacta and reticulata neurones. Eur J Neurosci. 2010;32:359–67. doi: 10.1111/j.1460-9568.2010.07298.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajima N, Karakas E, Grant T, Simorowski N, Diaz-Avalos R, Grigorieff N, Furukawa H. Activation of NMDA receptors and the mechanism of inhibition by ifenprodil. Nature. 2016;534:63–68. doi: 10.1038/nature17679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi T, Feldmeyer D, Suzuki N, Onodera K, Cull-Candy SG, Sakimura K, Mishina M. Functional correlation of NMDA receptor epsilon subunits expression with the properties of single-channel and synaptic currents in the developing cerebellum. J Neurosci. 1996;16:4376–82. doi: 10.1523/JNEUROSCI.16-14-04376.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, Westbrook GL. Amino-terminal ligands prolong NMDA receptor-mediated EPSCs. J Neurosci. 2012;32:8065–8073. doi: 10.1523/JNEUROSCI.0538-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tovar KR, McGinley MJ, Westbrook GL. Triheteromeric NMDA receptors at hippocampal synapses. J Neurosci. 2013;33:9150–9160. doi: 10.1523/JNEUROSCI.0829-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Traynelis SF, Wollmuth LP, McBain CJ, Menniti FS, Vance KM, Ogden KK, Hansen KB, Yuan H, Myers SJ, Dingledine R. Glutamate receptor ion channels: structure, regulation, and function. Pharmacol Rev. 2010;62(3):405–96. doi: 10.1124/pr.109.002451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicini S, Wang JF, Li JH, Zhu WJ, Wang YH, Luo JH, Wolfe BB, Grayson DR. Functional and pharmacological differences between recombinant N-methyl-D-aspartate receptors. J Neurophysiol. 1998;79:555–566. doi: 10.1152/jn.1998.79.2.555. [DOI] [PubMed] [Google Scholar]
- Wang XJ. Synaptic reverberation underlying mnemomic persistent activity. Trends Neurosci. 2001;24:455–463. doi: 10.1016/s0166-2236(00)01868-3. [DOI] [PubMed] [Google Scholar]
- Zhang W, Howe JR, Popescu GK. Distinct gating modes determine the biphasic relaxation of NMDA receptor currents. Nat Neurosci. 2008;11:1373–1375. doi: 10.1038/nn.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Q, Sheng M. NMDA receptors in nervous system diseases. Neuropharmacology. 2013;74:69–75. doi: 10.1016/j.neuropharm.2013.03.030. [DOI] [PubMed] [Google Scholar]
- Zhu S, Stroebel D, Yao CA, Taly A, Paoletti P. Allosteric signaling and dynamics of the clamshell-like NMDA receptor GluN1 N-terminal domain. Nat Struct Mol Biol. 2013;20:477–485. doi: 10.1038/nsmb.2522. [DOI] [PubMed] [Google Scholar]
- Zhu S, Paoletti P. Allosteric modulators of NMDA receptors: multiple sites and mechanisms. Curr Opin Pharmacol. 2015;20:14–23. doi: 10.1016/j.coph.2014.10.009. [DOI] [PubMed] [Google Scholar]
- Zhu S, Stein RA, Yoshioka C, Lee C-H, Goehring A, Mchaourab HS, Gouaux E. Mechanism of NMDA receptor inhibition and activation. Cell. 2016;165:704–714. doi: 10.1016/j.cell.2016.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]