Abstract
INTRODUCTION
The functional aspects of programmed death 1 (PD-1) and PD ligand 1 (PD-L1) immune checkpoints in malignant mesothelioma have not been studied.
METHODS
Tumor samples from 65 patients with mesothelioma were evaluated for PD-L1 expression by immunohistochemistry and its prognostic significance. Malignant effusions from patients with pleural and peritoneal mesothelioma were evaluated for PD-1+ and PD-L1+ infiltrating lymphocytes and their role in inducing tumor cell PD-L1 expression. Antibody dependent cellular cytotoxicity (ADCC) of avelumab, a fully humanized IgG1 anti PD-L1 antibody towards primary mesothelioma cell lines was evaluated in presence of autologous and allogeneic NK cells.
RESULTS
Of 65 pleural and peritoneal mesothelioma tumors examined, 41 (63%) were PD-L1 positive, which was associated with slightly inferior overall survival compared to patients with PD-L1 negative tumors (median 23.0 vs. 33.3 months; p=0.35). The frequency of PD-L1 expression was similar in pleural and peritoneal mesothelioma patients with 62% and 64% of samples positive, respectively. Of nine mesothelioma effusion samples evaluated, the fraction of cells expressing PD-L1 ranged from 12 to 83%. Of 7 patients with paired malignant effusion and peripheral blood mononuclear cells (PBMC) samples, PD-L1 expression was significantly higher on CD3+ T cells present in malignant effusions as compared with PBMC (p=0.016). In addition, CD14+PD-1+ cells were elevated in malignant effusions compared with PBMC (p=0.031). The lymphocytes present in malignant effusions recognized autologous tumor cells and induced IFN-γ-mediated PD-L1 expression on the tumor cell surface. Of the three primary mesothelioma cell lines tested, two were susceptible to avelumab mediated ADCC in presence of autologous NK cells.
CONCLUSION
The majority of pleural as well as peritoneal mesothelioma express PD-L1. Malignant effusions in this disease are characterized by presence of tumor cells and CD3+ T cells that highly express PD-L1. In addition, mesothelioma tumor cells are susceptible to ADCC by anti-PD-L1 antibody avelumab.
Keywords: PD-1-PD-L1, mesothelioma, avelumab, ADCC
INTRODUCTION
Mesothelioma is an aggressive cancer of serosal surfaces such as pleura and peritoneum associated with a poor prognosis.1 Pleural mesothelioma often invades lungs and adjacent thoracic structures and presents with pleural effusions in a majority of patients2, whereas peritoneal mesothelioma often presents with ascites. For patients with unresectable pleural mesothelioma, chemotherapy using the regimen of cisplatin plus pemetrexed is the standard of care with a median overall survival of 1 year.3 Clearly, there is a need for newer therapeutic approaches for patients with mesothelioma.
Although generally considered a non-immunogenic tumor, several lines of evidence suggest that mesothelioma is subject to immune-surveillance in humans.4 First, tumor antigen-specific humoral and cellular immune responses have been observed in patients with mesothelioma.5, 6 Second, significant tumoral lymphoid infiltration has been reported in mesothelioma and has been associated with a better prognosis.7–9 However, despite evidence for human immune reactivity, outside of rare instances10, immune responses do not lead to spontaneous regressions, suggesting that these immune responses are ineffectual. The possibilities, which could explain the failure of the immune system to clear the tumor, include the locally immunosuppressive effects of the tumor itself. The PD-1 and PD-L1 pathway is an immune checkpoint required for protecting the normal tissues from immune attack by curbing the effector T-cell responses.11–13 In peripheral tissues, the binding of PD-1 on T cells with PD-L1 on antigen-presenting cells (APCs) prevents the immune damage to self-normal and healthy tissues. However, tumor cells also take advantage of this checkpoint to down-regulate the T cell effector function by expressing PD-L1, which interacts with PD-1 on T cells and blocks its cytolytic activity by inhibition of its proliferation and cytokine release. The induction of PD-L1 on tumor cells can also be mediated by IFN-γ released by PD-1 expressing T cells by a mechanism known as adaptive immune resistance.14 Given the encouraging clinical activity observed in various tumors of blocking the interaction of PD-1 and PD-L115–17 and the context dependency of these interactions, which vary greatly depending on the tissue of origin and the underlying genetic landscape, further studies are needed to better understand this pathway in mesothelioma and explore strategies to target this pathway using the patient’s own immune system.
Avelumab is a fully humanized IgG1 anti-PD-L1 antibody that is currently in clinical trials for treating solid tumors.18 By binding to PD-L1 on tumor cells avelumab blocks PD-L1 interaction with PD-1 on T cells, which activates these cytotoxic T cells against the tumor. In addition, avelumab could also mediate direct anti-tumor effect by antibody dependent cellular cytotoxicity (ADCC) due to natural killer (NK) cells binding to the Fc region of the antibody via their FcϒRIII receptor (CD16) and kill tumor cells19 by the release of granzymes and perforins. In this study, we show that PD-1 and PD-L1 expressing T cells characterize malignant mesothelioma effusions and these lymphocytes can induce tumor cell PD-L1 expression, which could play an important role in preventing anti-tumor immune responses. Also, we show that NK cells cultured with autologous or allogeneic tumor cells from mesothelioma patients mediate ADCC in the presence of avelumab.
MATERIAL AND METHODS
Patients
Patients with malignant pleural and peritoneal mesothelioma who provided malignant effusions and peripheral blood were enrolled in the National Cancer Institute (NCI) study of Tissue Procurement and Natural History Study of Patients With Malignant Mesothelioma (Clinicaltrials.gov. NCT01950572). Patients with malignant pleural and peritoneal mesothelioma who were evaluated at the NCI between 1993 and 2012 with available tumor tissue or unstained slides were included in the immunohistochemistry (IHC) analysis for PD-L1 expression. These patients were enrolled in various Institutional Review Board approved protocols, which allowed the use of clinical information and biological specimens.
PD-L1 expression in tumors
Sections of formalin-fixed, paraffin-embedded (FFPE) mesothelioma tumors were tested for PD-L1 antigen expression using the Ventana BenchMark XT automated staining platform with the ultraView Universal DAB Detection Kit (Roche Diagnostics GmbH 760-500) according to the manufacturer’s instructions. Two pathologists independently evaluated the mesothelioma samples in a blinded, randomized way. For each slide, three to five random fields were evaluated; for each field, the percentage of membrane DAB-positive tumor cells was calculated as: [(number of DAB-positive tumor cells/total number of tumor cells) × 100], and the relative staining intensity was scored as weak (+) for pale brown intensity, moderate (++) for intermediate brown intensity, and strong (+++) for intense, dark brown immune-precipitate. Staining was recorded as negative if <5% of the tumor stained positive for membrane PD-L1 expression.
Peripheral blood, peritoneal, and pleural effusion collection
Blood samples were collected in BD Vacutainer CPT™ tubes (BD Biosciences 362761). Malignant effusion samples (ascites and pleural fluid) were obtained from patients who underwent drainage for diagnostic purposes or for symptomatic relief. Samples were collected in Interventional Radiology at the National Institutes of Health (NIH) Clinical Center in an evacuated 1 L container with a drainage needle inserted to it.
Isolation of mononuclear cells from peripheral blood, peritoneal, and pleural effusions
For PBMC isolation, whole blood in a BD Vacutainer CPT™ tube was centrifuged at 1800 g for 30 minutes with brakes off. The cells were washed thrice with PBS and stored in liquid nitrogen in PBMC freezing medium. The ascites and pleural effusion sample was centrifuged at 330 g for 10 minutes and washed thrice with PBS. If required, the red blood cells (RBCs) were lysed using 1× BD Pharm Lyse Buffer (BD Biosciences 555899). The cells were suspended in freezing medium containing 70% RPMI, 20% FBS and 10% DMSO and stored in liquid nitrogen.
PD-L1 expression on cells in malignant effusions
The basal PD-L1 expression on nine different ascites and pleural effusion samples was determined by staining the cells with APC anti-PD-L1/CD274 (Clone-29E.2A3, Biolegend 329708) antibody. APC mouse IgG2b antibody (Biolegend 400322) was used as an isotype control. All effusion samples tested, except for NCI-Meso37, were frozen. The cells were acquired on an LSR-II (BD Biosciences) and the data was analyzed on FlowJo (Treestar). The cells were gated based on their scatter properties and cells with very low SSC vs. FSC were gated out followed by gating on singlets. The percentage of PD-L1+ cells was expressed as percentage of those singlets.
PD-L1 expression on T cells in PBMC and malignant effusions
Paired PBMC and effusion samples from the patient were tested for PD-L1 expression on T cells. The samples were stained with the following antibodies: FITC anti CD3 (Clone-HIT3a, BD Biosciences 555339) and APC anti–PD-L1 (Clone-29E.2A3, Biolegend 329708). The flow cytometry data was acquired on LSR-II.
PD-1 expression on immune cells in PBMC and in malignant effusions
Paired PBMC and malignant effusions from the same patient were tested for PD-1 expression on CD4+ and CD8+ T cells and CD14+ monocytes. The cells were stained with the following antibodies: FITC anti CD14 (Clone-M5E2, Biolegend 301804), V450 anti CD4 (Clone-RPA-T4, BD Biosciences 560345), APC/Cy7 anti CD8a (Clone-RPA-T8, Biolegend 301016), PE anti PD-1 (Clone-MIH4, BD Biosciences 557946). Far red live/dead fixable (Life Technologies L34960) was used as a live/dead marker to gate out the dead cells. The flow cytometry data was acquired on LSR-II.
Co-culture of autologous lymphocytes and tumor cells present in ascites
Before setting up the co-cultures, the presence of tumor cells in NCI-Meso29 ascites was ascertained by the cell surface expression of the tumor differentiation antigen mesothelin that is highly expressed in mesothelioma.20 For this, the cells from the ascites of mesothelioma patient NCI-Meso29 were stained with mouse anti-mesothelin primary antibody (Rockland Immunochemicals Inc. 200-301-A88) followed by goat anti-mouse IgG-PE secondary antibody (Life Technologies P852). The stained cells were acquired on BD FACSCalibur (BD Biosciences). For co-culture studies, ascites from a mesothelioma patient (NCI-Meso29) was used as a source of both lymphocytes and tumor cells. After lysis of erythrocytes, cells were pelleted by centrifugation and seeded in three different 24 multi-well plates, at 2 × 107 cells per well in complete RPMI supplemented with 20% FBS. Two plates were supplemented with 106 IU/mL recombinant human IL-2 (Peprotech AF-200-02) to foster the growth of lymphoid populations, and another plate was cultured in the absence of IL-2. After 2 weeks in culture, all plates contained a monolayer of epithelioid cells growing in adherence, but only the plates supplemented with IL-2 also contained lymphoid cells growing in suspension. At this point, the cells growing in suspension were removed from one of the IL-2 treated plates and adherent cells were cultured for another week in the absence of IL-2. The other two plates were cultured for 1 additional week in the same conditions as in the previous 2 weeks. Upon completion of the 3-week period, tumor cells were tested for surface PD-L1 expression by flow cytometry using APC anti–PD-L1 (described earlier). Lymphoid cells growing in suspension were tested for recognition of autologous tumor cells by IFN-γ release upon overnight co-culture. 105 NCI-Meso29 tumor cells (1×105) were plated alone or in combination with 1×105 lymphocytes per well in a 96-multiwell plate. The concentration of IFN-γ in the supernatants was measured 16 h later by human IFN-γ assay kit (Thermo Scientific EHIFNG).
Antibody dependent cellular cytotoxicity experiments
For ADCC experiments three primary mesothelioma cell lines NCI-Meso21, NCIMeso29 and NCI-Meso49 were used. H441 lung adenocarcinoma cell line was used as a positive control. The primary mesothelioma cell lines were derived either from ascites or pleural effusions of mesothelioma patients. In allogeneic setting the NK cells were obtained from peripheral blood of healthy donors and in autologous setting from the ascites or pleural effusions of same patients whose tumor cells were being tested in ADCC experiments. The NK cells were isolated from blood or from malignant effusions using NK cell isolation kit from Miltenyi Biotech (130-092-657). Isolated NK cells were allowed to rest overnight in RPMI supplemented with 10% human serum (Valley Biomedicals HP1022). Primary tumor cells (NCI-Meso29 and NCI-Meso49) were treated with 10 ng/mL recombinant human IFN-γ (Gibco PHC4031) for induction of surface PDL1 expression for 24 hours before setting up the experiment. NCI-Meso21 cell lines expressing basal levels of PD-L1 were used for the experiments. This was to ascertain that high PD-L1 expression on tumor cells is a pre-requisite for efficient ADCC. After this, NK cells were stained with CFSE (Life Technologies C34554) to distinguish between effector and target cells and cultured in RPMI supplemented with 10% FBS at different ratios (3.125:1, 6.25:1, 12.5:1, 25:1) with autologous or allogeneic tumor cells in the presence of anti PD-L1 antibody avelumab (EMD Serono MSB0010718C) for 4 hours in a 24-well plate. We optimized the amount of antibody to be added to the ADCC experiments by testing three different concentrations, 10, 20 and 40ng/mL, on the NCIMeso21 cell line. NCI-Meso21 tumor cells with NK cells from healthy donors without the addition of avelumab were used as controls. NK cells could not effectively mediate ADCC in the absence of anti-PD-L1 antibody avelumab at all three E: T ratios studied (data not shown). The percent specific lysis obtained with different concentrations of avelumab was very similar (data not shown) and thus we decided to use 20 ng/mL antibody in all our experiments. After 4 hours, the supernatants were collected for determining the concentration of granzyme B and IFN-γ released by NK cells and the cells were stained with 7-AAD (BD Biosciences 559925) for 20 minutes to identify the apoptotic tumor cells21. Tumor cells cultured alone without drug were used as a negative control. The samples were acquired immediately on LSR Fortessa (Becton Dickinson). granzyme B and IFN-γ levels in the supernatants were determined by using human granzyme B platinum ELISA kit (ebioscience BMS2027) and human IFN-γ ELISA Ready-SET Go kit (ebioscience 88-7316-22) according to the manufacturer’s protocol. 237 The following formula was used for calculating percentage specific lysis of tumor cells 238 by NK cells:
where basal lysis is tumor cell lysis in the absence of NK cells and sample lysis is the 240 tumor cell lysis in the presence of NK cells at different E: T ratio.
Statistical analysis
The significance of comparisons between dichotomous categorical parameters and positive or negative PD-L1 was determined by Fisher’s exact test. An exact form of the Wilcoxon rank sum test was used to determine the significance of comparisons between two groups with respect to a continuous parameter. Survival was estimated using the Kaplan-Meier method, with the significance of the difference in curves determined by the log-rank test. All p-values were two-tailed and presented without any adjustments for the number of tests presented. The significance of the difference in the paired effusion and PBMC samples for PD-1 and PD-L1 expression was determined by a Wilcoxon signed rank test. P values of <0.05 were considered as significant. The analyses were performed using SAS version 9.3 (SAS Institute) and Graph Pad Prism 6 software.
RESULTS
Tumor PD-L1 expression and correlation with clinical characteristics
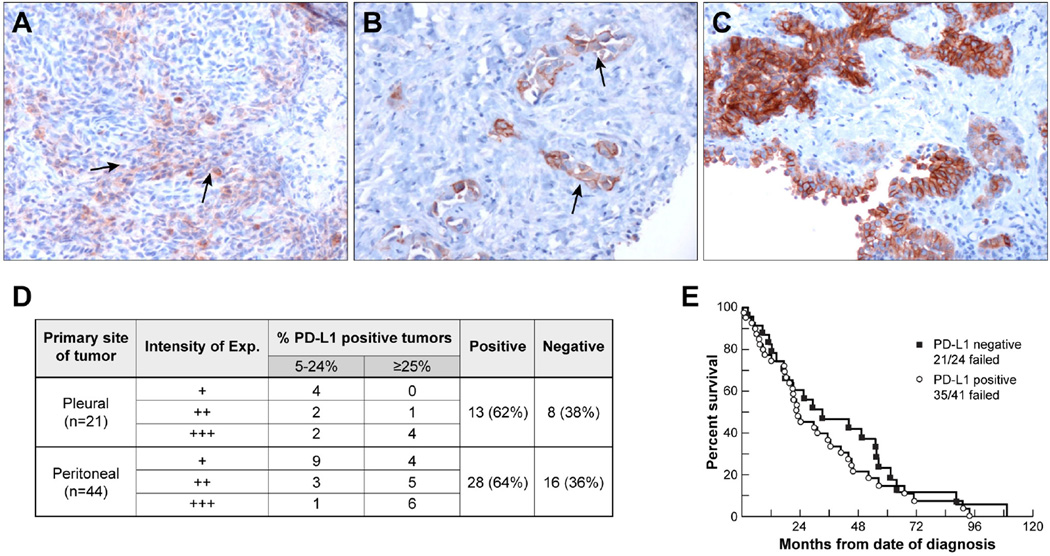
The expression of PD-L1 was analyzed by immunohistochemistry with a rabbit anti-PD-L1 monoclonal recombinant antibody in 65 cases of mesothelioma. The population was predominantly male (63%), and consisted mostly of patients who were younger than 60 (63%) years of age and those with epithelioid histology (85%). The primary site of the tumor was peritoneal in 44 (68%) cases. Forty-one of 65 (63%) mesothelioma samples were positive (≥5%) for PD-L1 expression. Representative cases are shown in Fig. 1(A–C). The expression of PD-L1 was observed predominantly on the membrane of mesothelioma cells. The levels of membrane PD-L1 expression in positive mesothelioma tissues ranged from 5% to 80% of the tumor cells, with intensities that varied from weak (+) to strong (+++). Focal staining of varying intensity was detected in 10 (15%) cases. Twenty-four (37%) cases were negative for PD-L1 expression (Fig. 1D). A higher proportion of males had tumoral PD-L1 expression than females (73% vs. 46%; p=0.04). There was no association between PD-L1 expression and primary site, age, race, histology and distant metastasis. Patients with PD-L1 positive tumors had a trend towards inferior overall survival compared to patients with PD-L1 negative tumors (median, 23.0 months for PD-L1 positive tumors vs. 33.3 months for PD-L1-negative tumors) [hazard ratio (HR) =1.30; 95% confidence interval (CI), 0.75–2.26]. However, this difference was not statistically significant as the curves were not dissimilar overall (p=0.35) (Fig. 1E).
Figure 1. PD-L1 expression on tumor samples from patients with pleural and peritoneal mesothelioma.
Immunohistochemical analysis showing different degrees of PD-L1 expression in mesothelioma. (A) Sarcomatoid mesothelioma with 10% cell membrane positivity and weak (+) intensity (arrow). (B) Epithelioid mesothelioma with 10% cell membrane positivity and strong (+++) intensity (arrow). (C) Epithelioid mesothelioma with 60% cell membrane positivity and strong (+++) intensity. All images were acquired at a magnification of 200×. (D) Table summarizing tumor PD-L1 expression by immunohistochemistry. (E) Kaplan-Meier plot showing overall survival of patients with PD-L1-expressing and PD-L1 negative tumors. Patients with PD-L1 positive tumors had a slightly inferior overall survival compared to that of patients with PD-L1 negative tumors (median survival 23.0 months for PD-L1 positive tumors vs. 33.3 months for PD-L1 negative tumors; p=0.35).
Malignant effusions of mesothelioma patients are characterized by high percentage of PD-L1 positive cells
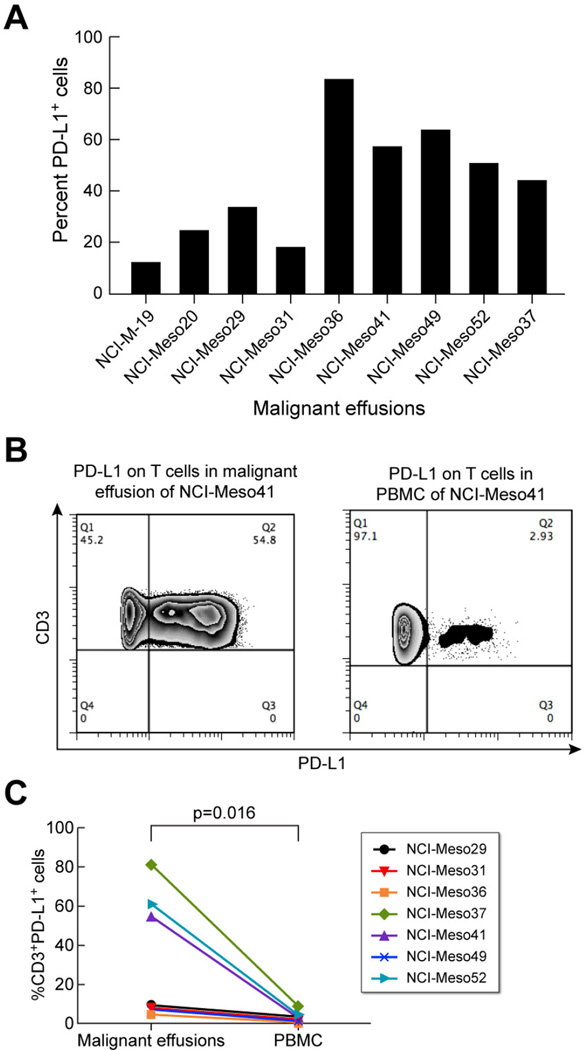
Ascites from six patients with peritoneal mesothelioma and pleural fluid from three patients with pleural mesothelioma were evaluated for basal PD-L1 expression. Out of the nine samples tested, eight were frozen effusion samples and one was fresh. All samples had basal PD-L1 expression with mean fluorescence intensity (MFI) ranging from ~ 220–1870 (data not shown). Fig. 2A shows the percentage of cells expressing PDL1 in malignant effusions, which varied from ~ 12–83%. NCI-Meso36 ascites cells had the highest basal PD-L1 expression. Of the nine malignant effusions and six primary cell cultures studied, we had paired malignant effusions and primary cell lines for three patients: NCI-Meso19, NCI-Meso29 and NCI-Meso31. The % PD-L1+ cells in malignant effusions from NCI-Meso19, NCI-Meso29 and NCI-Meso31 were 11.7, 32.9 and 17.5, respectively. The % PD-L1+ cells (basal level) in primary cell lines derived from the same patients i.e., NCI-Meso19, NCI-Meso29 and NCI-Meso31, were 4.7, 5.4 and 9.2, respectively (data not shown). Thus, the cells in malignant effusions have higher basal PD-L1 expression than the same patient-derived primary mesothelioma cell line.
Figure 2. PD-L1 expression on cells present in malignant mesothelioma effusions.
(A)Percentage of basal PD-L1 expressing cells present in malignant effusions from nine different patients. (B) Representative example of PD-L1 expression on CD3+ T cells in malignant effusion and PBMC of patient NCI-Meso41. (C) Percentage of CD3+PD-L1+ cells in the malignant effusions and PBMCs of seven patients with paired effusion and PBMC samples.
PD-L1 expression is higher on T cells in malignant effusions of mesothelioma patients compared to peripheral blood
Since immune cells such as T cells, monocytes and macrophages are known to express PD-L1, we evaluated paired malignant effusion and PBMC samples from seven patients for the presence of PD-L1 positive CD3+ T cells. Fig. 2B shows a representative example for PD-L1 expressing T cells in malignant effusion and PBMC of patient NCIMeso41. In this case the effusion samples contained 54.8% CD3+PD-L1+ T cells whereas only 2.9% CD3+PD-L1+ T cells were present in PBMC. Fig. 2C shows the percentage of CD3+PD-L1+ cells in malignant effusions and PBMCs of the seven patients with paired effusion and PBMC samples. The percentage of CD3+ T cells expressing PD-L1 in malignant effusions varied between 7–82%. The frequencies of CD3+ T cells in peripheral blood expressing PD-L1 were very low ranging from 0.5–9%. These values are significantly lower than those found on T cells in malignant effusions from the same patients (p=0.016; Fig. 2C).
Immune cells with high PD-1 expression are present in malignant effusion of mesothelioma patients
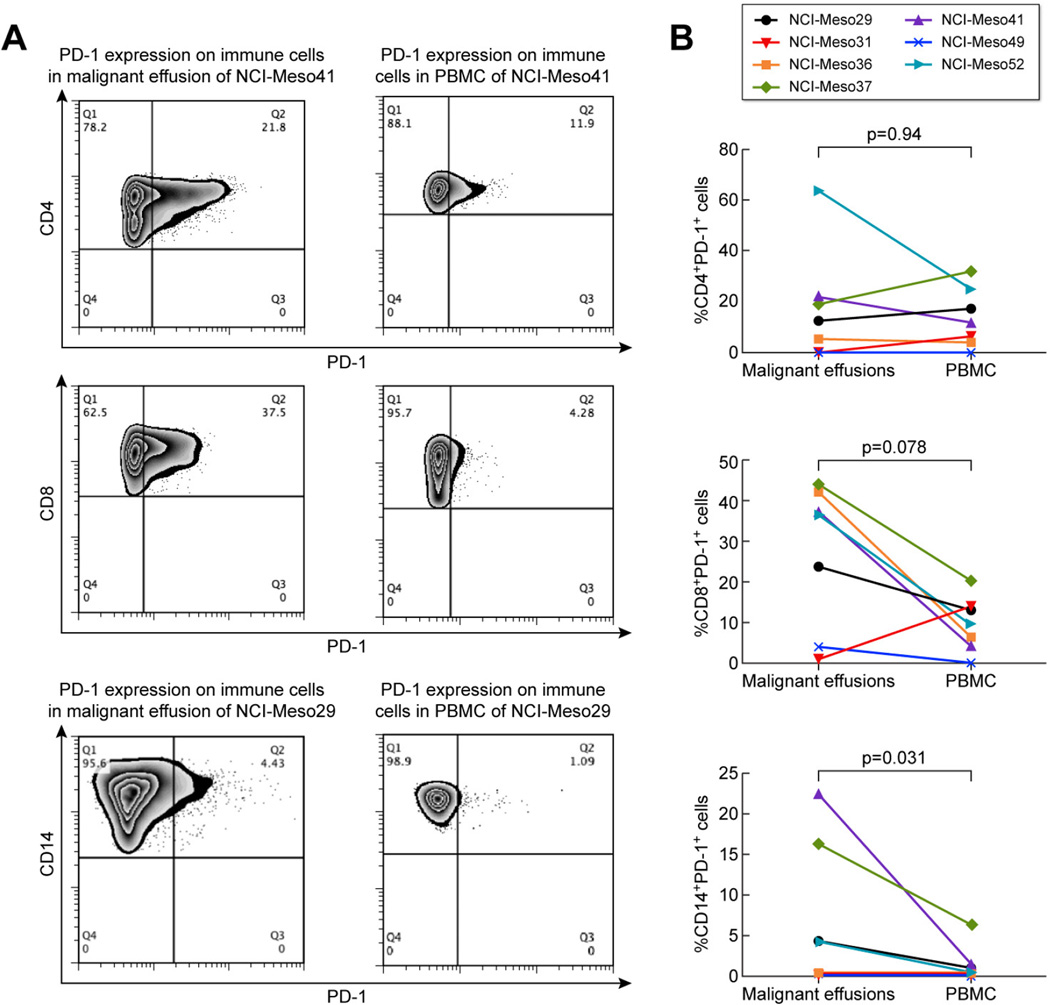
In addition to determining the presence of PD-L1+ cells in malignant effusions of mesothelioma patients we evaluated these effusions for the presence of PD-1 expression on immune cells. PD-1 expression on CD4+ T cells, CD8+ T cells and CD14+ monocytes was tested in effusion and PBMC samples of the seven patients with paired samples. Fig. 3A depicts the difference in expression of PD-1 on these immune cells in malignant effusions and PBMC of patient NCI-Meso41 (for CD4+ and CD8+ T cells) and NCIMeso29 (for CD14+cells) as representative examples. In this case, the effusion samples contained 21.8, 37.5 and 4.4% CD4+PD-1+, CD8+PD-1+ and CD14+PD-1+ cells, respectively. The paired PBMC sample on the other hand had 11.9, 4.23, 1.1% CD4+PD1+, CD8+PD-1+ and CD14+PD-1+ cells, respectively. Fig. 3B shows the percentage of CD4+PD-1+, CD8+PD-1+ and CD14+PD-1+ cells in malignant effusion and PBMCs of the seven patients with paired samples. The difference in PD-1 expression on CD4+ T cells in effusion and PBMC was not statistically significant (p=0.94). Although there was higher PD-1 expression on CD8+ T cells in malignant effusions than in PBMC for six of the seven patients this difference was also not statistically significant (p=0.078). The percentage of PD-1+CD14+ cells were 0.2–22% vs. 0–6% in malignant effusions and PBMC, respectively. These PD-1 expressing CD14+ monocytes were significantly elevated in malignant effusions as compared to PBMC from the same patients (p=0.031).
Figure 3. PD-1 expression on immune cells in malignant effusions and PBMC of mesothelioma patients.
(A) Representative example of PD-1 expression on CD4+ T cells and CD8+ T cells in malignant effusions and PBMC for patient NCI-Meso41 and on CD14+ monocytes for patient NCI-Meso29. (B) Percentage of CD4+PD-1+ cells, CD8+PD-1+ and CD14+PD-1+ cells in malignant effusions and PBMCs of seven patients with paired effusion and PBMC samples.
Autologous lymphocytes in malignant effusion induce tumor cell PD-L1 expression
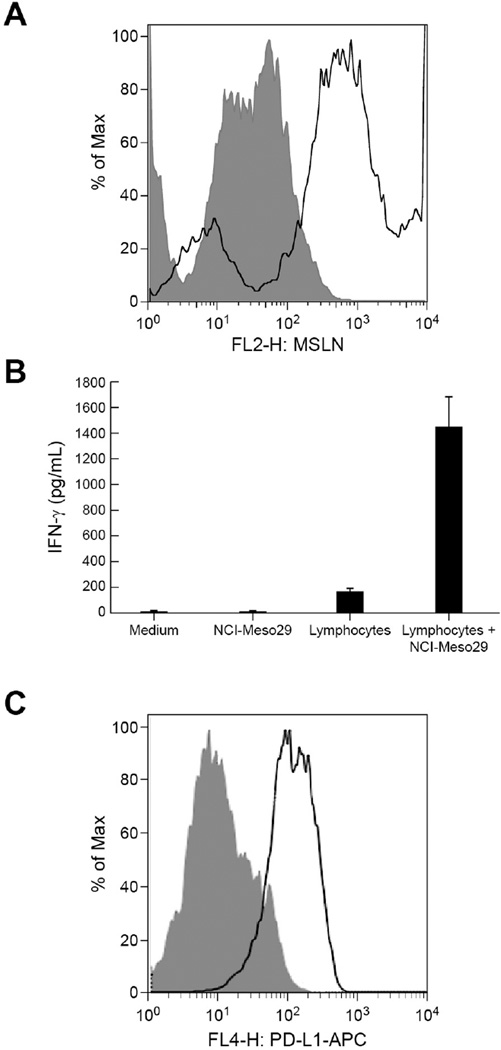
To study the role of lymphocytes in malignant effusions on inducing the PD-L1 expression on tumor cells, we used ascites from mesothelioma patient NCI-Meso29. The presence of tumor cells in ascites was ascertained by their expression of the tumor antigen mesothelin, before setting up in-vitro cultures of tumor cells with lymphocytes. Flow cytometry analysis revealed that 22.5% of the total cells in ascites expressed mesothelin (Fig. 4A). Fig. 4B compares the IFN-γ released by NCI-Meso29 tumor cells alone, autologous lymphocytes alone and NCI-Meso29 tumor cell-lymphocyte co-cultures and depicts a clear induction of IFN-γ production by lymphocytes in the presence of autologous tumor cells, with a mean value of 1448 pg/mL. This finding indicates that in-vitro cultured lymphocytes contained tumor reactive cells. Tumor cells grown in the presence of IL-2 and lymphocytes for 3 weeks presented a marked overexpression of PD-L1 compared with tumor cells grown in the absence of IL-2 and lymphocytes (Fig. 4C). Interestingly, tumor cells lost expression of PD-L1 when IL-2 and lymphocytes were removed after the second week in culture (data not shown).
Figure 4. Induction of tumor PD-L1 expression by co-culture of autologous lymphocytes and tumor cells present in ascites of mesothelioma patient NCI-Meso29.
(A) Tumor cells present in ascites were evaluated by their positivity for the tumor differentiation antigen mesothelin by flow cytometry using mouse anti-mesothelin primary antibody followed by goat anti-mouse IgG PE labeled secondary antibody. Shaded histogram represents the binding of isotype control antibody and solid histograms represent the binding of anti-mesothelin antibody. (B) Reactivity of lymphocytes with autologous tumor cells was evaluated by measuring IFN-γ released in the supernatants of co-cultures of lymphocytes and tumor cells. (C) Flow cytometry analysis of PD-L1 expression on autologous tumor cells obtained from ascites of patient NCI-Meso29 grown in the presence (solid) or absence (shaded) of autologous lymphocytes cultured from ascites and 106 IU/mL IL-2.
NK cells mediate ADCC of both autologous and allogeneic mesothelioma tumor cells in the presence of the anti PD-L1 antibody avelumab
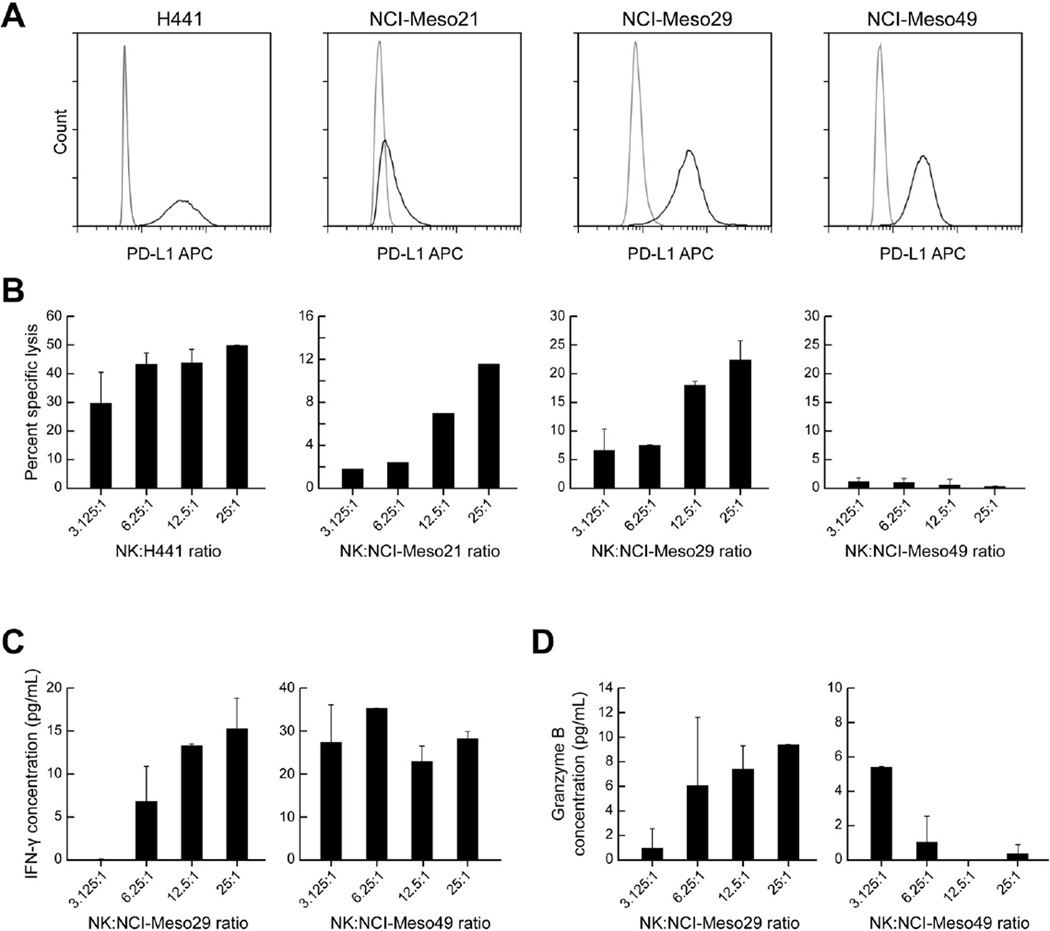
Since avelumab is an IgG1 anti-PD-L1 antibody, we evaluated its ability to mediate ADCC against primary mesothelioma cell lines NCI-Meso21, NCI-Meso29 and NCI-Meso49. As a control for these experiments we used the PD-L1 expressing lung adenocarcinoma cell line H441 (Fig. 5A). Fig. 5B depicts the % specific lysis of H441 cells by allogeneic NK cells from healthy donors in the presence of 20 ng/mL of avelumab. These cells have high basal PD-L1 expression and no external IFN-γ was added to the cells for inducing PD-L1 expression (Fig. 5A). The number of H441 cells killed by NK cells was proportional to the effector to target (E: T) ratio (Fig. 5B). The highest % specific killing detected was 49.7 ±0.2% at the highest E: T ratio of 25:1. In case of NCI-Meso21 cells, which weakly express PD-L1, the maximum % specific killing in the presence of avelumab was 13% at the highest E: T ratio. In NCI-Meso29 and NCI-Meso49 ADCC experiments, we used autologous NK cells of the patients and also tumor cells cultured in the presence of IFN-γ to induce PD-L1 expression. NCIMeso29 cells were sensitive to ADCC in presence of avelumab with % specific lysis of 23% at a 25:1 E: T ratio. However, avelumab did not mediate ADCC of NCI-Meso49 cells even though they have PD-L1 expression similar to that of NCI-Meso29 cells. In order to understand the reason behind this observation, we evaluated IFN-γ and granzyme B levels in co-culture supernatants at the end of co-culture experiments by ELISA. We found that the percent specific lysis of NCI-Meso29 at different E: T ratios correlated with an increased release of both IFN-γ and granzyme B in the supernatant, which indicates that NK cells could recognize autologous tumor cells and mediate ADCC in the presence of avelumab by secreting granzyme B (Fig. 5C and 5D). However, in case of autologous NCI-Meso49 tumor and NK cells, there was increase in IFN-γ levels in the culture supernatants with an increasing E: T ratio indicating that the autologous NK cells were activated but very low levels of granzyme B were released in the supernatant, which could explain their inability to kill the PD-L1+ NCI-Meso49 cells (Fig. 5C and 5D).
Figure 5. Antibody dependent cellular cytotoxicity of avelumab against primary mesothelioma cell lines.
(A) Strong basal PD-L1 expression on the lung adenocarcinoma cell line H441 and weak PD-L1 expression on the NCI-Meso21 cell line. Strong expression of PD-L1 on NCI-Meso29 and NCI-Meso49 cell lines after treatment with IFN-γ for 24 hours. (B) Percent specific lysis of H441 and NCI-Meso21 cells by allogeneic NK cells and for NCI-Meso29 and NCI-Meso49 cells by autologous NK cells at different E:T ratios in the presence of 20 µg/mL avelumab. (C) IFN-γ released in the supernatants after 4 h co-cultures of autologous NCI-Meso29 tumor and NK cells, and autologous NCI-Meso49 tumor cells and NK cells. (D) Granzyme B levels in supernatants of autologous co-culture of tumor cells and NK cells for NCI-Meso29 and NCI-Meso49.
DISCUSSION
In this study we evaluated the relevance of the PD-1 and PD-L1 immune checkpoints in patients with mesothelioma using archival tumor samples, malignant ascites and pleural effusions and PBMCs. Our results show that two-thirds of pleural and peritoneal mesotheliomas express PD-L1 and PD-L1 expression is associated with a trend towards decreased overall survival. In addition, malignant effusions of patients with mesothelioma are characterized by PD-L1 positive tumor cells and infiltration with both PD-1 and PD-L1 positive immune cells. The lymphocytes present in these effusions induce PD-L1 expression on tumor cells, which in turn could be targeted using the patient’s own NK cells in the presence of anti-PD-L1 antibody avelumab.
Two prior studies, one by Mansfield et al. and the other by Cedres et al., have previously looked at PD-L1 expression in malignant mesothelioma by immunohistochemistry.22, 23 However, we would like to point out that our study is the first study to report PD-L1 expression in peritoneal mesothelioma, a type of mesothelioma with different prognosis and treatments than pleural mesothelioma. In contrast to our findings, the above mentioned studies reported PD-L1 being associated with decreased survival. It is possible that the difference in survival between our study and the two earlier studies could be related to underlying patient characteristics, since our study had patients with both pleural and peritoneal mesothelioma, or due to the differences in reagents and assays used to determine PD-L1 expression.
A major hurdle to evaluating the tumor immune microenvironment is the limited availability of tissue samples collected by biopsies, which hampers concurrent phenotyping, quantification, and functional studies of distinct immune cells within tumor tissue. However, malignant pleural effusions and ascites, a consequence of tumor invasion of pleura and peritoneum, respectively, allow phenotypic and functional evaluation of the interactions between immune cells and cancer cells in the tumor microenvironment. Our results show that mesothelioma effusions are characterized by the presence of not only PD-L1 positive tumor cells but also PD-L1+ and PD-1+ T cells. On testing paired malignant effusion samples and PBMC samples from seven patients, we found that PD-L1 was more highly expressed on T cells present in effusion samples than in PBMC. Our results are similar to the results by Herbst et al., who studied 732 different tumor tissues for the presence of PD-L1+ tumor cells and PD-L1+ tumor infiltrating immune cells and observed PD-L1 positivity on both tumor cells and immune cells.17 The high expression of PD-L1 on T cells is probably a mechanism by which the immune system tries to counteract the immunosuppressive tumor microenvironment24 and is probably a marker for recovery of the exhausted PD-1 expressing T cells. However, we would like to point out that the PD-L1 positive CD3+ T cells in effusions and peripheral blood could also include immunosuppressive regulatory T cells (Treg).
We observed a higher percentage of CD8+ T cells expressing PD-1 in six of the seven malignant effusions samples than in PBMC. This indicates that the CD8+ T cells in effusions are more exhausted than the CD8+ T cells in peripheral blood due to high PD-1+ expression. A similar trend was observed in earlier studies of non-small cell lung cancer, renal cell carcinoma and melanoma25, 26, wherein a higher frequency of PD-1+ CD8+ T cells was found in tumor than in PBMC. These results suggest that the PD-1-PD-L1 pathway may have greater effect on CD8+ T cells in tumor microenvironment than in peripheral blood.
To better characterize the involvement of lymphocytes on the induction of PD-L1 by tumor cells, we co-cultured autologous lymphocytes and tumor cells present in ascites of a mesothelioma patient and showed an IFN-γ-mediated induction of PD-L1 expression on tumor cells. These findings suggest that lymphoid populations present in the ascites of mesothelioma patients have the ability to recognize tumor cells present in the ascites, and induce PD-L1 expression on tumor cells upon IFN-γ release. A prior study noted an increase in PD-L1 positivity of mouse ovarian cancer cells co-cultured with activated lymphocytes.27 However, to our knowledge this is the first study looking at interactions between PD-1 and PD-L1 in malignant effusions in patients, and this is clinically relevant since mesothelioma patients often present with pleural effusions and ascites.
The expression of PD-L1 in mesothelioma tumors suggests that therapies targeting either PD-1 or PD-L1 could potentially have clinical benefit. Preliminary results of small trials have shown evidence of anti-tumor activity in some patients.28–30 The main mechanism of activity of immune checkpoint inhibitors is blocking the interaction between PD-1 and PD-L1 leading to T cell mediated killing. Avelumab, an IgG1 antiPD-L1 antibody, currently in clinical trials to treat many different cancers can potentially also kill tumor cells by NK cell mediated ADCC. The clinical efficacy of avelumab was recently assessed in phase Ib clinical trial in which patients with unresectable pleural or peritoneal mesothelioma received avelumab (10 mg/ kg) every 2 weeks intravenously.29 The patients who had progressed after a platinum-pemetrexed-containing regimen were not selected for tumor PD-L1 expression. Unconfirmed responses were observed in 9.4% (5 of 53) of patients and median progression free survival was 17.1 weeks. Responses were seen both in patients with PD-L1 positive and PD-L1 negative tumors.
Using either autologous NK cells (obtained from patient effusions) or allogeneic NK cells (from healthy donors), we demonstrate that avelumab induced specific killing in two of the three PD-L1 expressing primary mesothelioma cell lines, which was mediated by granzyme release. This result is in agreement with an earlier study, which showed that PD-L1 expression is an indicator of susceptibility of tumor cells to ADCC by avelumab.31 Indeed, recent studies32 have also shown that ipilimumab can engage FcγRIIIA (CD16)-expressing, non-classical monocytes resulting in ADCC-mediated lysis of regulatory T cells (Tregs). In contrast, classical CD14++ CD16− monocytes are unable to do so. Moreover, patients responding to ipilimumab display significantly higher baseline peripheral frequencies of non-classical monocytes compared with non-responder patients. In the tumor microenvironment, responders have higher CD68+/CD163+ macrophage ratios at baseline and show decreased Treg infiltration after treatment.
Our study also has some limitations. Firstly, although malignant effusions could be considered a surrogate for tumor, it is not known whether they fully reflect the biology of the tumor microenvironment. The interaction of PD-1 and PD-L1 on immune cells and tumor cells in malignant effusions may not be reflective of their interactions within the tumor. Additional studies are needed to fully explore how the phenotypic and functional assessments of tumor and immune cells in malignant effusions correlate with that of the cells in the tumor microenvironment. Secondly, the prognostic and predictive role of PD-L1 expression remains an important clinical question across different tumors. Whereas initial studies indicated that expression of PD-L1 on primary tumors or on tumor infiltrating lymphocytes is a predictor for worse prognosis,33 subsequent studies have yielded conflicting results.34–37 Additionally, the use of PD-L1 immunohistochemistry as a predictive biomarker has been confounded by multiple unresolved issues including variability in antibody characteristics, tissue processing and immunohistochemistry cutoffs in positivity, as well as those that relate to expression on tumor cells vs. immune cells.38 Finally, these results should be interpreted with the caveat that PD-L1 expression is variable over time and is regulated by a number of factors including cytokines, exogenous stimuli delivered by pathogen associated molecular patterns and receptor-mediated signaling molecules among others, rendering the assessments of its expression a snapshot in time.39
CONCLUSIONS
In summary, our results show that malignant mesothelioma is characterized by the high expression of PD-L1 and malignant effusions of these patients have high expression of PD-L1 on tumor cells and infiltration with PD-L1 and PD-1 positive immune cells. Our results also show that lymphocytes within malignant effusions could dampen the immune response to the tumors by inducing PD-L1 expression. At the same time, however, the presence of these immune checkpoints could make mesothelioma tumors susceptible to treatment with agents targeting PD-L1 or PD-1.
Supplementary Material
Acknowledgments
This work was supported by Intramural Research Program of the Center for Cancer Research, National Cancer Institute, National Institutes of Health, as well as a Cooperative Research and Development Agreement (CRADA) between EMD Serono and the National Cancer Institute. Partially supported by the European Social Fund, Italian Ministry of Education, University and Research (MIUR) PON03PE_00146_1/10 BIBIOFAR (CUP B88F12000730005) to F.G.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
The authors disclose no potential conflicts of interest
REFERENCES
- 1.Robinson BW, Lake RA. Advances in malignant mesothelioma. N Engl J Med. 2005;353:1591–1603. doi: 10.1056/NEJMra050152. [DOI] [PubMed] [Google Scholar]
- 2.Boutin C, Schlesser M, Frenay C, et al. Malignant pleural mesothelioma. Eur Respir J. 1998;12:972–981. doi: 10.1183/09031936.98.12040972. [DOI] [PubMed] [Google Scholar]
- 3.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–2644. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 4.Thomas A, Hassan R. Immunotherapies for non-small-cell lung cancer and mesothelioma. Lancet Oncol. 2012;13:e301–e310. doi: 10.1016/S1470-2045(12)70126-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho M, Hassan R, Zhang J, et al. Humoral immune response to mesothelin in mesothelioma and ovarian cancer patients. Clin Cancer Res. 2005;11:3814–3820. doi: 10.1158/1078-0432.CCR-04-2304. [DOI] [PubMed] [Google Scholar]
- 6.Robinson C, Robinson BW, Lake RA. Sera from patients with malignant mesothelioma can contain autoantibodies. Lung Cancer. 1998;20:175–184. doi: 10.1016/s0169-5002(98)00014-2. [DOI] [PubMed] [Google Scholar]
- 7.Leigh RA, Webster I. Lymphocytic infiltration of pleural mesothelioma and its significance for survival. S Afr Med J. 1982;61:1007–1009. [PubMed] [Google Scholar]
- 8.Ujiie H, Kadota K, Nitadori JI, et al. The tumoral and stromal immune microenvironment in malignant pleural mesothelioma: A comprehensive analysis reveals prognostic immune markers. Oncoimmunology. 2015;4:e1009285. doi: 10.1080/2162402X.2015.1009285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suzuki K, Kadota K, Sima CS, et al. Chronic inflammation in tumor stroma is an independent predictor of prolonged survival in epithelioid malignant pleural mesothelioma patients. Cancer Immunol Immunother. 2011;60:1721–1728. doi: 10.1007/s00262-011-1073-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Robinson BW, Robinson C, Lake RA. Localised spontaneous regression in mesothelioma -- possible immunological mechanism. Lung Cancer. 2001;32:197–201. doi: 10.1016/s0169-5002(00)00217-8. [DOI] [PubMed] [Google Scholar]
- 11.Dong H, Zhu G, Tamada K, et al. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5:1365–1369. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 12.Freeman GJ, Long AJ, Iwai Y, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192:1027–1034. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keir ME, Liang SC, Guleria I, et al. Tissue expression of PD-L1 mediates peripheral T cell tolerance. J Exp Med. 2006;203:883–895. doi: 10.1084/jem.20051776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Taube JM, Anders RA, Young GD, et al. Colocalization of inflammatory response with B7-h1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci Tansl Med. 2012;4:127ra137. doi: 10.1126/scitranslmed.3003689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med. 2012;366:2443–2454. doi: 10.1056/NEJMoa1200690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med. 2012;366:2455–2465. doi: 10.1056/NEJMoa1200694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Herbst RS, Soria JC, Kowanetz M, et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature. 2014;515:563–567. doi: 10.1038/nature14011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Heery CR, O'Sullivan Coyne HG, Madan RA, et al. Phase I open-label, multiple ascending dose trial of MSB0010718C, an anti-PD-L1 monoclonal antibody, in adavnced solid malignancies. ASCO Meeting Abstract. 2014:3064. [Google Scholar]
- 19.Nimmerjahn F, Ravetch JV. Fc gamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 20.Ordonez NG. Value of mesothelin immunostaining in the diagnosis of mesothelioma. Mod Pathol. 2003;16:192–197. doi: 10.1097/01.MP.0000056981.16578.C3. [DOI] [PubMed] [Google Scholar]
- 21.Lecoeur H, Fevrier M, Garcia S, et al. A novel flow cytometric assay for quantitation and multiparametric characterization of cell-mediated cytotoxicity. J Immunol Methods. 2001;253:177–187. doi: 10.1016/s0022-1759(01)00359-3. [DOI] [PubMed] [Google Scholar]
- 22.Mansfield AS, Roden AC, Peikert T, et al. B7-H1 expression in malignant pleural mesothelioma is associated with sarcomatoid histology and poor prognosis. J Thorac Oncol. 2014;9:1036–1040. doi: 10.1097/JTO.0000000000000177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cedres S, Ponce-Aix S, Zugazagoitia J, et al. Analysis of expression of programmed cell death 1 ligand 1 (PD-L1) in malignant pleural mesothelioma (MPM) PLoS One. 2015;10:e0121071. doi: 10.1371/journal.pone.0121071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ahmad SM, Larsen SK, Svane IM, et al. Harnessing PD-L1-specific cytotoxic T cells for anti-leukemia immunotherapy to defeat mechanisms of immune escape mediated by the PD-1 pathway. Leukemia. 2014;28:236–238. doi: 10.1038/leu.2013.261. [DOI] [PubMed] [Google Scholar]
- 25.Zhang Y, Huang S, Gong D, et al. Programmed death-1 upregulation is correlated with dysfunction of tumor-infiltrating CD8+ T lymphocytes in human non-small cell lung cancer. Cell Mol Immunol. 2010;7:389–395. doi: 10.1038/cmi.2010.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Blank C, Kuball J, Voelkl S, et al. Blockade of PD-L1 (B7-H1) augments human tumor-specific T cell responses in vitro. Int J Cancer. 2006;119:317–327. doi: 10.1002/ijc.21775. [DOI] [PubMed] [Google Scholar]
- 27.Abiko K, Mandai M, Hamanishi J, et al. PD-L1 on tumor cells is induced in ascites and promotes peritoneal dissemination of ovarian cancer through CTL dysfunction. Clin Cancer Res. 2013;19:1363–1374. doi: 10.1158/1078-0432.CCR-12-2199. [DOI] [PubMed] [Google Scholar]
- 28.Alley EW, Molife LR, Santoro A, et al. Clinical safety and efficacy of pembrolizumab (MK-3475) in patients with malignant pleural mesothelioma: Preliminary results from KEYNOTE-028. Proceedings of the 106th Annual Meeting of the American Association for Cancer Research. 2015 [Google Scholar]
- 29.Hassan R, Thomas A, Patel MR, et al. Avelumab (MSB0010718C-anti-PD-L1) in patients with advanced unresectable mesothelioma from the JAVELIN solid tumor phase Ib trial: Safety, clinical activity, and PD-L1 expression. ASCO 2016. J Clin Oncol. 2016;34 [Google Scholar]
- 30.Hassan R, Thomas A, Patel M, et al. Safety and clinical activity of avelumab (MSB0010718C), an anti-PD-L1 antibody, in patients with advanced, unresectable mesothelioma: A phase 1B trial. European Cancer Congress 2015. 2015 [Google Scholar]
- 31.Boyerinas B, Jochems C, Fantini M, et al. Antibody-Dependent Cellular Cytotoxicity Activity of a Novel Anti-PD-L1 Antibody Avelumab (MSB0010718C) on Human Tumor Cells. Cancer Immunol Res. 2015;3:1148–1157. doi: 10.1158/2326-6066.CIR-15-0059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Romano E, Kusio-Kobialka M, Foukas PG, et al. Ipilimumab-dependent cell-mediated cytotoxicity of regulatory T cells ex vivo by nonclassical monocytes in melanoma patients. Proc Natl Acad Sci U S A. 2015;112:6140–6145. doi: 10.1073/pnas.1417320112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Thompson RH, Gillett MD, Cheville JC, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101:17174–17179. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hamanishi J, Mandai M, Iwasaki M, et al. Programmed cell death 1 ligand 1 and tumor-infiltrating CD8+ T lymphocytes are prognostic factors of human ovarian cancer. Proc Natl Acad Sci U S A. 2007;104:3360–3365. doi: 10.1073/pnas.0611533104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ghebeh H, Mohammed S, Al-Omair A, et al. The B7-H1 (PD-L1) T lymphocyte-inhibitory molecule is expressed in breast cancer patients with infiltrating ductal carcinoma: correlation with important high-risk prognostic factors. Neoplasia. 2006;8:190–198. doi: 10.1593/neo.05733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hino R, Kabashima K, Kato Y, et al. Tumor cell expression of programmed cell death-1 ligand 1 is a prognostic factor for malignant melanoma. Cancer. 2010;116:1757–1766. doi: 10.1002/cncr.24899. [DOI] [PubMed] [Google Scholar]
- 37.Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med. 2015;373:123–135. doi: 10.1056/NEJMoa1504627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McLaughlin J, Han G, Schalper KA, et al. Quantitative Assessment of the Heterogeneity of PD-L1 Expression in Non-Small-Cell Lung Cancer. JAMA Oncol. 2016;2:46–54. doi: 10.1001/jamaoncol.2015.3638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sheng J, Fang W, Yu J, et al. Expression of programmed death ligand-1 on tumor cells varies pre and post chemotherapy in non-small cell lung cancer. Sci Rep. 2016;6:20090. doi: 10.1038/srep20090. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.