Abstract
Objective
To develop and evaluate a paper-based point-of-care HPV serology test to determine if an individual has received two or more HPV immunizations.
Methods
The paper-based immunoassay was constructed using a nitrocellulose lateral flow strip with adsorbed HPV16 virus-like particles serving as the capturing moiety. Three capture zones containing virus-like particles were placed in series to allow for visual discrimination between high and low HPV16 plasma antibody concentrations. A plasma separation membrane was used to allow whole blood to be applied directly to the assay. All reagents were dried on glass fiber pads during device fabrication and were rehydrated with buffer at the time of use. A pilot study consisting of 35 subjects with a history of zero, one, two or three HPV vaccines was conducted to evaluate the immunoassay. The completed paper-based immunoassays were scanned for visual interpretation by three researchers who were blinded to the true results and separately evaluated quantitatively using MATLAB.
Results
For the 28 tests valid for analysis, fifteen subjects reported receiving two or more HPV vaccines, three reported receiving one, and ten reported having no HPV vaccinations. The paper-based immunoassays for all fifteen subjects who reported having received two or more HPV vaccines were judged positive by all researchers. Twelve of the thirteen tests from individuals reporting one or zero vaccinations were deemed negative by all observers. One test from an unvaccinated individual was judged positive by two out of three reviewers. Quantitatively, all tests were correctly separated between the two groups.
Conclusions
We successfully designed and tested a HPV serology test amenable to the point-of-care. The device showed promising results in a pilot study for discriminating between those who received two or more HPV vaccinations and those who did not. Furthermore, this device offers a platform for producing other semi-quantitative point-of-care serological tests.
Keywords: HPV, Serologic Tests, Point-of-care testing
Introduction
More than 520,000 new cases of cervical cancer and 265,000 related deaths occur annually worldwide[1]. Over 85% of cases of and deaths due to cervical cancer occur in low- and middle-income countries (LMICs), where cervical cancer is the third leading cause of cancer death among women[1]. LMICs bear a disproportional burden of cervical cancer primary due to the difficulty of implementing prevention and screening programs in these locations [2]. Infection with the human papillomavirus (HPV) is the cause of virtually all cases of cervical cancer [3]; globally, HPV16 and HPV18 types are responsible for approximately 70% of cervical cancers [4]. Vaccines to prevent HPV infection have the potential to drastically reduce the global burden of cervical cancer [5]. Three U.S. Food and Drug-Administration approved HPV vaccines, Cervarix, Gardasil, and Gardasil-9, are commercially available to protect against HPV16 and HPV18 [5–7]. Gardasil also protects against HPV6 and HPV11 which cause 90% of genital warts [8]. Randomized, prospective studies have demonstrated the efficacy of Cervarix and Gardasil to prevent HPV16 and HPV18 infections, and HPV16- or HPV18-related cervical intraepithelial neoplasia (CIN) [5,8,9]. More recently a nonavalent vaccine, Gardasil-9, was developed that provides protection against five additional oncogenic HPV types (31, 33, 45, 52 and 58) that cause approximately 15-20% of cervical cancers[10]. Thus, immunization with Gardasil-9 may potentially prevent 90% of cervical cancers [10].
Wide-scale adoption of HPV vaccines is predicted to significantly lower the incidence of cervical cancer worldwide, and reduce global disparities in cervical cancer incidence [7]. The cost of the three-dose HPV series in the United States is approximately $390 for the Cervarix Vaccine, $480 for Gardasil and $530 for Gardasil-9 [11]. However, the prices of the vaccines vary significantly by region, from as little as $5 to as much as $187 per dose [12]. Recent studies suggest that two doses of vaccine may provide protective immunity [13,14] and be more cost-effective than the three-dose series [15]. However, cost remains the biggest barrier to national implementation of the HPV vaccine in LMICs [16]. For countries that are eligible for financial assistance through the Global Alliance for Vaccines and Immunizations (GAVI), this barrier is greatly reduced. However, currently countries are only eligible for GAVI support if the three-year average of their gross national income is at or below $1580 per capita [17]. Thus, many LMICs are not eligible for GAVI support, and cost continues to be a major barrier to access. Furthermore, GAVI support is temporary and seeks to transition member countries toward government-funded vaccination programs.
Lack of comprehensive medical records in LMICs [18] presents another challenge to national HPV immunization programs. Absent medical records, providers must rely on patient self-reporting to assess whether a patient has received all recommended doses of vaccine. This can lead to re-vaccination of individuals who have previously received sufficient doses of the vaccine. In order to optimize cost-effectiveness, it is critical that vaccines are provided only to those who have not received the full series of the vaccine. The accuracy of self-reported vaccine history varies depending on the vaccine [19]. A study evaluating the accuracy of self-reported HPV vaccination history among adolescents in urban US cities revealed major inaccuracies [20]. Only 54% (36/66) of those who had received at least one dose of the vaccine correctly reported having had the vaccine, and only 35% (17/48) of those who had received all three vaccines correctly self-reported having all three doses [20]. Due to the under-reporting of HPV vaccine status and the lack of reliable medical records in many developing countries, there is a significant possibility of unnecessary re-vaccination, which wastes critical resources in both GAVI-eligible and GAVI-ineligible countries.
HPV immunization status can be determined by measuring the serum concentration of HPV antibodies. Currently, serum HPV antibody concentration can only be measured using a virus like particle (VLP) enzyme-linked immunosorbent assay (ELISA) or a neutralization assay [21,22]. However, both of these assays traditionally require sophisticated laboratory equipment and highly trained personnel. Low-cost, point-of-care alternatives to these tests are needed to help assess whether patients have previously received two or more doses of HPV vaccine in order to facilitate efficient vaccination programs. Recently, Fu et al. reported instrument-free two-dimensional paper networks (2DPNs) to perform multistep immunoassays at the point-of-care [23]. In this paper, we build on this approach to develop an equipment-free rapid paper immunoassay to detect antibodies to HPV16 from a finger prick sample of capillary blood to determine HPV immunization status at the point-of-care. We report results from a pilot study of 28 subjects to evaluate the whether an individual has received two or more doses of the Gardasil or Cervarix vaccines.
Methods
Figure 1A shows a photograph of the two-dimensional lateral flow assay to detect HPV antibodies from a drop of capillary blood. An important design consideration is to discriminate between vaccinated individuals and individuals with a history of HPV infection. Some individuals with a history of HPV infection develop detectable levels of antibodies to HPV; however, these levels are lower than levels seen in vaccinated individuals. The assay is designed to detect HPV16 antibodies. We chose HPV16 because a study using a competitive Luminex immunoassay (cLIA) demonstrated significantly higher HPV16 antibody geometric mean titers in vaccinated individuals relative to those with a history of natural HPV16 infection [24]. Using the cLIA method, Villa et al. demonstrated that this difference is much greater for HPV16 than for HPV18 [24] resulting in a better assay signal-to-noise ratio. Because all approved HPV vaccines contain both HPV16 and HPV18 VLPs, an individual vaccinated against HPV18 would also be vaccinated against HPV16, and vice versa. Measuring HPV16 serostatus infers protection against HPV18. The assay consists of a lateral flow strip with three capture zones to capture HPV16 antibodies from the plasma sample and a positive control zone. HPV16 L1 virus like particles (VLPs) are immobilized at the three capture zones and human immunoglobin (IgG) is immobilized at the positive control zone. Three test zones were included to aid in discrimination between vaccinated individuals and those with a history of natural HPV infection. This provides serial dilution on the test itself, allowing for discrimination between high and low levels of anti-HPV antibodies. Preliminary tests were performed with pooled serum from individuals with a history of HPV16 infection (provided by the National Institute of Biological Safety and Controls, Hertfordshire, England) and plasma from an individual documented to have received three doses of the Gardasil vaccine. These tests revealed that a single test zone did not allow for visual discrimination of results when the test was performed using serum from an individual with a natural HPV16 infection and one who had received three doses of the HPV vaccine. However, by using three test zones test results for these two samples were visually apparent (data not shown).
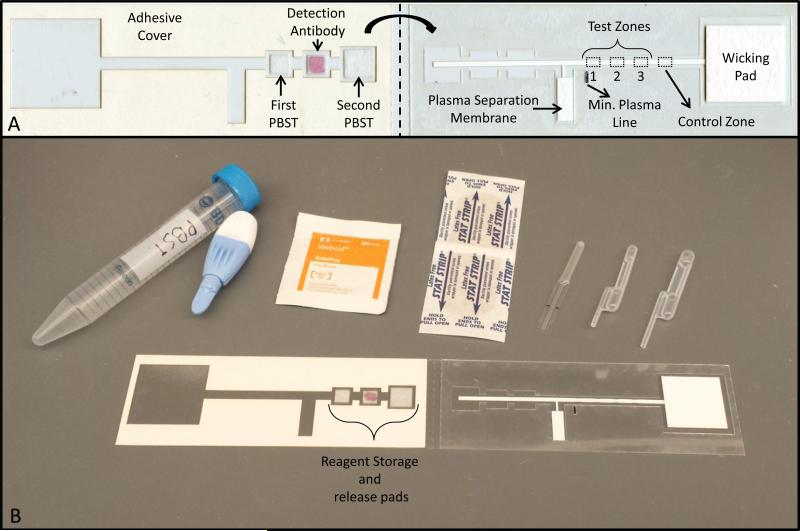
Figure 1. Two-dimensional paper network to detect human antibodies against HPV 16.
(A) Device overview. The device consists of a nitrocellulose membrane with HPV16 virus-like particles immobilized at various concentrations at three test zones (1, 2, and 3), a cellulose wicking pad on the right and a plasma separation membrane, and three glass fiber pads, one of which contains dried detection antibody, on the left. All are adhered to a thin acetate sheet. The dotted line indicates where the device is folded to start the flow of reagents through capillary action (B) All supplies needed to perform the assay at the point-of care. The supplies consist of the two-dimensional paper network device, 15 mL of phosphate-buffered saline with 0.05% Tween-20, an alcohol prep pad, a Band-Aid, a high-flow lancet, a 20 μL microsafe capillary tube and a 20μL and 40 μL exact volume transfer pipette
As shown in Figure 1A, the device consists of a nitrocellulose membrane with HPV16 VLPs immobilized at three test zones and human IgG immobilized at the positive control zone, a cellulose wicking pad, a plasma separation membrane (right side) and three glass fiber pads, one of which contains dried detection antibody (left side), all adhered to a thin acetate sheet. On the right side of the device, the plasma separation membrane is connected via a nitrocellulose leg to the main lateral flow strip. On the left side of the device, three glass fiber pads are placed on exposed adhesive. The middle pad contains dried anti-human IgG conjugated to colloidal gold. The device is operated by placing a blood sample on the plasma separation membrane and rehydrating the three glass fiber pads with phosphate buffered saline with .05% Tween 20(PBST). The plasma separation membrane allows the plasma to pass on to the nitrocellulose strip but retains red blood cells. The adhesive cover is removed from the left side of the device. Once the plasma reaches the plasma separation line, shown in Figure 1A, the device is folded in half along the midline. This places the glass fiber pads in direct contact with the main lateral flow strip and initiates sequential flow of the first wash buffer, labeled detection antibody, and final wash buffer.
Production of HPV16 L1 virus-like particles
HPV16 L1 virus-like-particles were produced by transfecting 293TT cells with a plasmid expressing a codon-modified HPV16L1 (p16L1h) gene[25]. The plasmid was supplied from Dr. Susanna Pang from the National Cancer Institute Laboratory of Cellular Oncology. The methods to produce human papillomavirus pseudoviruses have been published in detail previously [26–29].
Twenty-four hours prior to transfection, 2 × 107 293TT cells (National Cancer Institute Laboratory of Cellular Oncology) were plated on a 225cm2 flask in 50 mL of Dulbecco's Modified Eagle Medium (DMEM) with 10% fetal bovine serum, 1% non-essential amino acids and 1% Glutamax-I (Thermo Fisher Scientific). Immediately prior to transfection, 112.5 μg p16l1h DNA was added to 5.6 mL Opti-MEM (Thermo Fisher Scientific) and in a separate tube 247.5 μL of Lipofectamine 2000 (Thermo Fisher Scientific) was added to 5.6 mL of Opti-MEM. The solutions were incubated separately for 10 minutes at room temperature and then combined. After gentle mixing the combined solution was incubated for 20 minutes and then added directly to the cultured cells. The cells were transfected for 48 hours at 37°C before harvesting.
Cells were collected, centrifuged and placed in a siliconized 1.5 mL tube. Cells were lysed by resuspension in Dulbecco's phosphate buffered saline with calcium and magnesium (DPBS, Thermo Fisher Scientific) with 2.5% of 1 M ammonium sulfate, 0.5% Triton X-100 (Thermo Fisher Scientific), 0.1% Benzonase (Sigma) and 0.1% Plasmid safe (Epicentre). The lysis was then incubated overnight at 37°C to allow for capsid maturation. The following day, the solution was adjusted to 0.8 M NaCl and incubated on ice for 10 minutes. The salt lysate was double-clarified by centrifugation at 5,000 × g for five minutes. Capsids were purified by ultracentrifugation with an Optiprep gradient[26]. SDS-PAGE gels were run on each gradient fraction to determine if the fraction contained enough L1 to be visually evident. Fractions containing L1 were pooled for use in the HPV antibody immunoassay.
Fabrication of paper-based HPV antibody immunoassay
All materials for the paper-based HPV antibody immunoassay were cut using a CO2 laser cutter (Universal Laser Systems). Devices were constructed from 10 mil Dura-lar (Blick Art Supplies, Galesburg, IL) and 5 mil adhesive-backed Dura-lar (Blick Art Supplies). The lateral flow channel was cut from 2 mil backed high-flow nitrocellulose (HF090, Millipore). The reagent storage and release pads were cut glass fiber pads (Grade 8951, Alhstrom, Helsinki, Finland). The wicking pad was cut from cellulose (C083, Millipore). The plasma separation membrane was cut from a commercially available glass fiber filter (LF1, GE Healthcare Life Sciences).
The paper-based HPV antibody immunoassays were assembled as shown in Figure 1. The nitrocellulose strip has three consecutive test zones to capture anti-HPV 16 antibodies and one positive control capture location. The test zones were created by twice pipetting 0.4 μL of the HPV16 L1 VLP suspension on each test location, with drying of the devices at 37°C for ten minutes between pipettings. The first capture location was spotted with HPV16 L1 VLPs diluted one to four (25 μg/mL for a total of 20 ng VLP) in phosphate buffered saline (PBS) and the second and third locations were spotted with HPV16 L1 VLPs diluted one to two in PBS (50 μg/mL; 40 ng). The positive control location was spotted once with 0.4 μL of 44 μg/mL human IgG (Thermo Fisher Scientific, Waltham, MA) diluted in PBS.
The nitrocellulose strips were dried for one hour at 37°C. The nitrocellulose strips were then blocked by completely submerging in PBS with .05% v/v Tween 20 (Biolegend, San Diego, CA), 5% w/v sucrose (Sigma-Aldrich, St. Louis, MO) and 0.25% w/v Polyvinylpyrrolidone (40 kD, Sigma-Aldrich, St. Louis, Missouri). After blocking for 30 minutes, the strips were dried for 30 minutes at 37°C. The devices were then completely assembled by placing the glass fiber pads, nitrocellulose strips, plasma separation membrane and wicking pad as depicted in Figure 1A. To create stable detection antibody, 40 nm diameter gold conjugated goat anti-Human IgG (50 OD, BioAssay Works, Ijamsville, MD) was diluted one-to-five in PBS with 1% w/v bovine serum albumin (BSA), 5% sucrose (Sigma-Aldrich, St. Louis, Missouri) and 5% trehalose (Sigma-Aldrich, St. Louis, Missouri). Five microliters of the diluted antibody gold conjugate was spotted on the detection antibody glass fiber pad. The entire device was then dried overnight at room temperature. All devices were fabricated one day prior to clinical testing and stored at room temperature until use.
Clinical Testing
The pilot study was performed at the Baylor College of Medicine with the approval of the Institutional Review Board of Baylor College of Medicine. Informed consent was obtained from each subject. Subjects were students and staff at the Baylor College of Medicine or Rice University. Subjects were eligible for inclusion if they were 18 or older and had a history of being sexually active. Subjects were ineligible if they had a history of diagnosis of any immunodeficiency disorder, diagnosis of HIV, Hepatitis B or Hepatitis C or if they were currently using steroids or other immunosuppressive medications. After consent, subjects were asked a brief series of questions including the number of HPV vaccine dose he/she had received, the type of HPV vaccine and the number of sexual partners he/she had in the last six months.
The complete supplies required for each test are shown in Figure 1B. All testing was performed by a nurse and trained graduate research assistant. A finger prick was performed using a high-flow microtainer lancet (BD Diagnostics, Franklin Lakes, NK). Blood was collected using a 20 μL microsafe capillary tube (Safe-Tec, Ivyland, PA) and immediately dispensed onto the plasma separation membrane. While the plasma separated, PBST was dispensed onto the glass fiber reagent pads. A 20 μL exact volume transfer pipette was used to place 20 μL of PBST on both the first wash glass fiber pad and the detection antibody pad. A 40 μL exact volume transfer pipette was used to dispense PBST on the second wash pad. Next, the adhesive was exposed by peeling back the paper covering. Once the plasma reached the minimum plasma line, shown in Figure 1A, the device was folded in half to initiate the test by placing the glass fiber pads in direct contact with the main lateral flow strip. If the plasma did not reach the minimum plasma line, the test was considered invalid. Plasma reached the plasma line after one to two minutes. Completed tests were scanned at 800 dots per inch (DPI) using a flatbed color scanner, 35 minutes after folding the device.
Images were analyzed both subjectively and objectively to determine if the test result was positive or negative. The images were randomized and given to three independent reviewers blinded to the HPV vaccine status of the tested individual. The reviewers were told to determine whether each test was positive based on the presence of signal at two or more test zones. Images were also analyzed quantitatively using a custom MATLAB script; analysis was performed only on the green channel, where contrast is greatest for the gold detection system. After selecting the green channel, the image was inverted so that higher pixel intensities corresponded to higher signal. A fixed-size region of interest (ROI) was manually placed at each test zone and at the positive control site. Three background ROIs, equal in size to the test zone ROIs, were automatically placed halfway between successive capture locations. In each row of the ROI, the pixel value corresponding to the 95th percentile was calculated. The signal in each ROI was defined as the average of the 95th percentile value calculated for each row. The 95th percentile was chosen instead of the maximum value to mitigate the effect of debris in the test ROI. Signal from the three background ROIs were averaged together to define the background signal for each device. The signal-to-background ratio (SBR) was defined for each test zone location and positive control location by dividing the signal at each corresponding ROI by the mean background signal. We plotted the mean SBR for each capture zone location and compared results for unvaccinated subjects and those who reported receiving one, two or three doses of the HPV vaccine. Differences in mean signal-to-background ratios were evaluated using an unpaired two-tailed Student's t-test; p-values of less than 0.05 were considered statistically significant.
Dried Reagent Storage
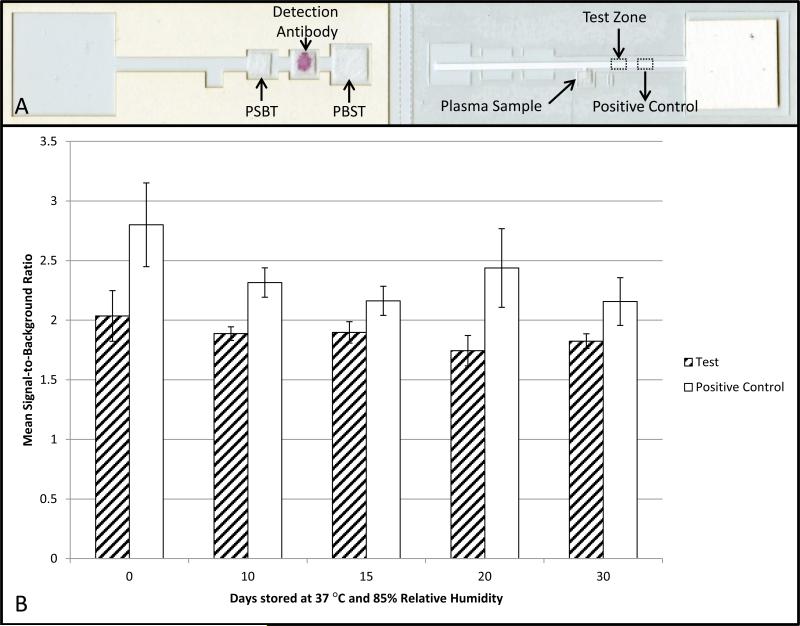
To determine the stability of the dried reagents, a simplified device with only a single test zone location, shown in Figure 3A, was fabricated. The test zone was created by pipetting 0.4 μL of 50 μg/mL HPV16 L1 VLPs in PBS on the nitrocellulose test location. The positive control was created by spotting 0.4 μL of 44 μg/mL anti-human IgG at the control location. The nitrocellulose was then dried for 10 minutes at 37°C. Another 0.4 μL of 50 μg/mL HPV16 L1 VLPs was pipetted onto the test zone and the nitrocellulose was dried for an additional 60 minutes at 37°C. The nitrocellulose was blocked and dried reagent pads were prepared identically to the paper-based HPV antibody immunoassay used for clinical testing. After the devices dried overnight, they were placed in a 4.5 mil thick Mylar foil pouch (Impak, Los Angeles) with two grams of molecular sieve (Impak, Los Angeles). Three assembled stability testing devices were placed in each bag. The foil pouches were sealed using a constant heat bag sealer. Three of the devices were tested immediately and the remaining devices were then placed in a chamber at 37°C with 85% relative humidity. The remaining devices were tested at 10, 15, 20 and 30 days after being exposed to high heat and humidity.
Figure 3. Stability testing of paper-based HPV VLP immunoassays.
Devices were stored at 37°C and 85% relative humidity for 30 days. Each device contained a test zone and positive control zone. The same sample, plasma from an individual who had received 3 HPV vaccines, was used to evaluate the performance of the devices at all time points. The mean signal-to-background ratio decreased 10% over the course of 30 days at the test zone and 23% at the positive control zone.
Plasma from an individual who had received three Gardasil HPV vaccine doses was used as the sample for all devices. Collection of blood was approved by the Rice University Institutional Review Board. Ten mL of blood was collected into a citrate dextrose solution A tube from a venous draw by a certified phlebotomist. The blood was then centrifuged at 1,000 × g for 10 minutes at 4°C. The supernatant plasma was collected and centrifuged at 10,000 × g for 10 minutes at 4°C. The plasma was then aliquoted and stored at −20°C until needed for testing.
To test the devices at each time point, the glass fiber pads were rehydrated using the exact volume transfer pipettes as described above. Then, five μL of the collected plasma was pipetted onto the sample glass fiber pad. Once the plasma reached the minimum plasma line, the sample glass fiber pad was removed, and the device was folded to initiate flow. This is to prevent the presence of the glass fiber pad from affecting the flow profile of the stability testing lateral flow strip. This step is not needed in the paper-based HPV antibody clinical test because flow from the plasma separation membrane ceases after red blood cells reach the nitrocellulose. After 35 minutes, images were obtained at 800 DPI using a flatbed scanner. Image analysis was performed similarly to the procedure used for the standard device. Fixed-size ROIs were manually placed at the test capture location and positive control location. Background ROIs of the same size as the test ROIs were automatically selected midway between the test zone and positive control location and midway between the positive control location and wicking pad. Analysis was then performed identically to the standard device.
Results and Discussion
Thirty-five subjects were enrolled in the study to evaluate the ability of the paper-based HPV antibody immunoassay to determine HPV vaccination status. After the first five subjects, the device blocking method was modified to improve device stability at 37°C. The original blocking solution included 2% BSA that degraded in the presence of high heat. The blocking procedure was modified as described in the methods. Results from the first five subjects are thus not included in the analysis. For the remaining 30 tests, the plasma failed to reach the minimum plasma line for two subjects. A summary of the data from the remaining 28 patients with valid results is provided in Table 1. After 35 minutes, the positive control signal was visible in all 28 devices, indicating completion of the assay.
Table 1.
Summary of subjects in pilot study.
| No HPV Vaccines | One Dose HPV Vaccine | Two Dose HPV Vaccines | Three Dose HPV Vaccines | |
|---|---|---|---|---|
| Number of Volunteers | 10 | 3 | 5 | 10 |
| Median age (years) (range) | 28 (23-43) | 24 (23-25) | 28.5 (22 – 34) | 25 (23 – 35) |
| Average time since last vaccine (years) (range) | - | 3.5 (0.5-8) | 7.9 (0.75-16) | 4.8 (0 – 12) |
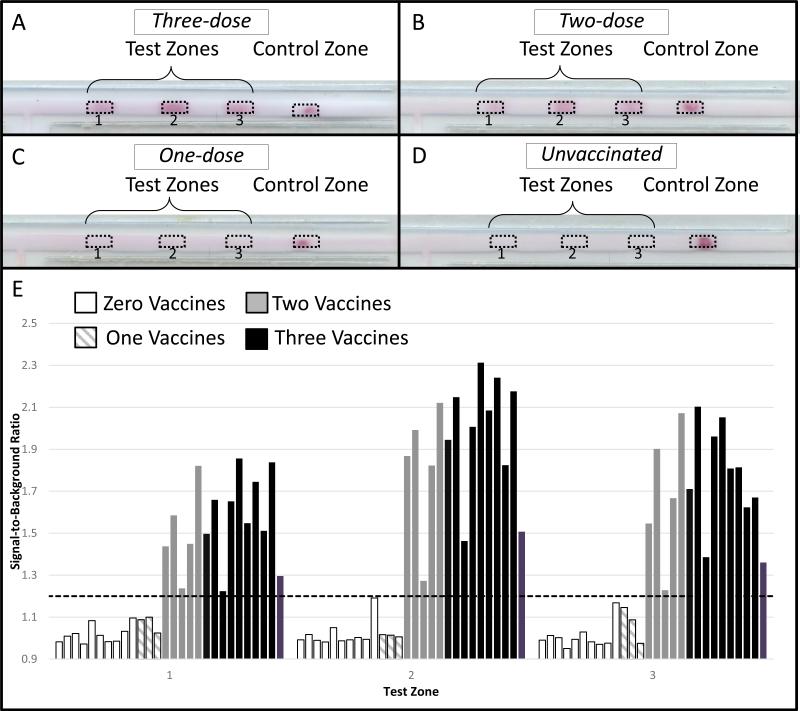
Example images of completed paper-based HPV antibody immunoassays for individuals who reported receiving zero, one, two and three HPV vaccines are provided in Figure 2(A-D). All 15 tests from subjects who reported receiving two or three doses of the HPV vaccine, were judged positive by all three observers based on the presence of signal at least two test zones (sensitivity = 100%, 95% CI = 78-100%). Twelve of the thirteen tests from subjects who reported receiving one or zero doses of the HPV vaccine were judged negative by all three observers (specificity = 92%, 95% CI = 64-100%). One test from an unvaccinated subject was judged negative by one reviewer and positive by two reviewers.
Figure 2. Results of the pilot study.
Representative images of the paper-based HPV VLP immunoassay after 35 minutes for individuals who received (A) Three doses of HPV Vaccine, (B) Two doses of HPV vaccines, (C) One dose of HPV Vaccine and (D) No HPV vaccine. (E) Average signal-to-background ratio at each test zone stratified by number of HPV vaccines received.
Figure 2E shows a quantitative comparison of the signal-to-background at each capture location for subjects stratified by the number of HPV vaccines they reported receiving. The mean SBR is significantly higher for individuals who reported receiving three or two vaccine doses than for unvaccinated individuals and individuals who reported receiving one vaccine (p<0.01 for all comparisons). There were no statistically significant differences in the mean SBR at any test zone between subjects who received two or three HPV vaccines (p = 0.53, 0.40, 0.71 at the first, second and third test zone, respectively). Similarly, there were no significant differences between the mean SBR of tests from unvaccinated subjects in comparison to subjects who had received a single HPV vaccine dose (p = 0.22, p=0.22, p= 0.82, at test zones one, two and three, respectively). By defining a positive test as any test having a signal-to-background ratio of at least 1.2 at two or more test zones, all tests from individuals with two more HPV vaccines were correctly categorized as positive and all other tests as negative. This cut-off value was chosen retrospectively to effectively separate the two groups.
The quality of the L1VLPs and appropriateness of this cut-off value were evaluated by performing the assay with a mouse monoclonal HPV16 L1 antibody (Abcam ab69, Cambridge, UK) spiked into plasma from an unvaccinated individual. To detect this mouse antibody, 40 nm diameter gold conjugated goat anti-mouse IgG (50 OD, BioAssay Works, Ijamsville, MD) was used. Two-fold serial dilutions from 32 μg/mL to 0.5 μg/mL were tested in triplicate. The results of this testing revealed that 2 μg/mL is the lowest concentration that would classify as positive based on a threshold of a signal-to-background ratio of at least 1.2 at two or more test zone locations. Results of the assay show signal-to-background ratios that are antibody dose dependent, suggesting the presence of necessary conformational epitopes on the VLPs. Using the cLIA, Villa et al[24] showed that those with natural infection had anti-HPV16 levels of 50 to 100 milli-Merck Units per milliliter (mMU/mL), and those with a history of HPV vaccination showed levels of greater than 800 mMU/mL. According to Opalka et al., 50 ng/mL is approximately equal to 4.6 mMU/mL[30]. Using this conversion, individuals with a previous HPV infection have antibody levels between 500 and 1000 ng/mL while those with full vaccination history have levels above 8 μg/mL. Therefore, the test's cutoff of 2 μg/mL is appropriate to separate these two groups. This spiking experiment was performed using a mouse antibody and some differences may exist with human antibodies, which is why the clinical pilot study was necessary to establish an appropriate SBR cut-off.
The results of stability testing are shown in Figure 3B. After 10 days of storage, the mean SBR decreased by 7% at the test zone and by 17% at the positive control zone. From day 10 to day 30, signal decreased by an additional 3.3% at the test zone and an additional 6.8% at the control zone. Because SBR both increased and decreased at measurements taken between these two time points, up-and-down fluctuations in the SBR between these two time points are likely due in part to device-to-device variability. The Eppendorf Research Plus pipette used for spotting the capture antibodies on the nitrocellulose strips has a random error of ± 6% at the volume being dispensed. Automated liquid dispensers would reduce the variability (noise) between devices. However, despite this variably the test could still discriminate unvaccinated individuals and individuals who reported having a single vaccine from those who reported receiving two or more HPV vaccine doses. The decrease in SBR over time is likely due to reduction in activity of the VLPs and gold conjugates as a result of degradation in high heat conditions. By artificially reducing the SBR determined for all test zone locations for the pilot study devices, we can examine the effect this decreased SBR would have on quantitative assay performance. Using the same 1.2 cutoff point and reducing all test zone SBRs by 10.3%, we would correctly identify all of those who reported receiving one or fewer vaccines. It would correctly identify 14 of 15 of those who reported receiving two or more HPV vaccines.
The initial results of this test are promising; however, a large-scale study is necessary to understand the repeatability and robustness of the paper-based HPV VLP immunoassay. There were only three individuals who reported receiving one HPV vaccine and five who reported receiving two HPV vaccines. Therefore, a larger study is necessary to quantify the accuracy of this test. A false-positive was recorded by two reviewers. It is possible the faint signal seen in this device was due to a previous natural HPV16 infection. We chose to include only subjects with a history of sexual activity to increase the likelihood of participants having a history of natural HPV infection; however, we do not know the natural history of infection for any participants. To reduce the likelihood of false positives, future iterations of the immunoassay could use HPV16 L1 VLPs at one test zone and HPV18 L1 VLPs at a second test zone. We hypothesize this will increase specificity because all vaccinated individuals will have antibodies to both HPV16 and HPV18; however, very few individuals will have antibodies to both types as a result of natural infection.
In future testing, device imaging or visual interpretation should occur as soon as the positive control signal is visible instead of a fixed duration of 35 minutes. This will allow for faster time to results in most cases. Additionally, when plasma does not reach the minimum plasma separation line, this indicates an insufficient volume of blood and the test should be repeated with a new device and a new finger prick. The appropriateness of this assay design also depends on ongoing research evaluating long-term efficacy one, two or three dose HPV vaccination regimens. The World Health Organization (WHO) recommends two doses spaced at least 6 months apart for those who receive their first vaccine before the age of 15[31]. While guidelines may continue to change, varying VLP capture concentrations provides the ability to tune the assay appropriately. Based on the current WHO recommendations, if the paper based immunoassay returns a negative result, the patient should receive one HPV vaccine. A follow-up appointment should be scheduled for six months later, where the immunoassay should be repeated to see if the patient has now received a total of two HPV vaccines. If the test is negative again, the patient should receive an additional vaccine.
A primary limitation of this study was reliance of self-reporting of HPV vaccine status. There are several factors which lead us to believe that the accuracy of this self-reporting is higher than that in the study reported by Stupiansky et al. [20]. The subjects in the Stupiansky et al. study were ages 14 to 17. In this study, all participants were pursuing or had completed post-graduate education in biomedical sciences and actively volunteered to participate in an HPV-vaccine related study. Two subjects reported they were unsure if they had received two or three HPV vaccines. They called their primary care physician and verified the number of HPV vaccines they had received before participating. All other subjects reported being confident in their HPV vaccination history. In future validation studies, medical records should be obtained to ensure accuracy of the participants HPV vaccination history.
The utility of this device extends beyond individual HPV vaccination status screening. With small modifications, the device could be utilized for other serological assays by substituting the appropriate antigen for the HPV VLPs. Additionally, instead of being used for individual screening, it could be used in population surveillance to estimate vaccination rates in a given region. The cost-of-goods for small-scale production of the current device prototype is $1.38, including the lancet and exact volume transfer pipettes. In order to make the device more amenable to mass manufacturing, the device should be housed in a more traditional lateral flow assay injection-molded cassette. Future improvements could include volume-metering components in the cassette, eliminating the need for exact volume pipettes. Finally, adding an HPV18 capture location could improve the specificity of the test.
Highlights.
A paper-based immunoassay for HPV serological testing is described
A pilot study of 35 patients was conducted to evaluate the device
Device platform can be used for future point-of-care serological tests
Acknowledgements
We wish to thank Dr. Cynthia Thompson of the National Cancer Institute for providing critical technical assistance in the production of HPV16 pseudoviruses.
Research reported in this publication was supported by the National Cancer Institute of the National Institutes of Health under Award Number R01CA186132. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Research reported in this publication was supported by the National Institute Of Biomedical Imaging And Bioengineering of the National Institutes of Health under Award Number U54EB015403.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
This material is based upon work supported by the National Science Foundation Graduate Research Fellowship under Grant No. 1450681. Any opinion, findings, and conclusions or recommendations expressed in this material are those of the authors(s) and do not necessarily reflect the views of the National Science Foundation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflicts of Interest: none
References
- 1.Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global Cancer Statistics, 2012. CA Cancer J Clin. 2015;65:87–108. doi: 10.3322/caac.21262. doi:10.3322/caac.21262. [DOI] [PubMed] [Google Scholar]
- 2.Denny L, Quinn M, Sankaranarayanan R. Screening for cervical cancer in developing countries. Vaccine. 2006;24:71–7. doi: 10.1016/j.vaccine.2006.05.121. Chapter 8. doi:10.1016/j.vaccine.2006.05.121. [DOI] [PubMed] [Google Scholar]
- 3.Walboomers JM, Jacobs M V, Manos MM, Bosch FX, Kummer JA, Shah KV, et al. Human Papillomavirus is a necessary cause of invasive cervical cancer worldwide. J Pathol. 1999;189:12–9. doi: 10.1002/(SICI)1096-9896(199909)189:1<12::AID-PATH431>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 4.Clifford GM, Smith JS, Plummer M, Muñoz N, Franceschi S. Human papillomavirus types in invasive cervical cancer worldwide: a meta-analysis. Br J Cancer. 2003;88:63–73. doi: 10.1038/sj.bjc.6600688. doi:10.1038/sj.bjc.6600688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pasquié J, Ph D, Scavée C, Bordachar P, Clémenty J, Haïssaguerre M. Quadrivalent Vaccine against Human Papillomavirus to Prevent High-Grade Cervical Lesions. N Engl J Med. 2007;356:1915–27. doi: 10.1056/NEJMoa061741. doi:10.1056/NEJMoa061741. [DOI] [PubMed] [Google Scholar]
- 6.Harper DM, Franco EL, Wheeler C, Ferris DG, Jenkins D, Schuind A, et al. Efficacy of a bivalent L1 virus-like particle vaccine in prevention of infection with human papillomavirus types 16 and 18 in young women: a randomised controlled trial. Lancet. 2004;364:1757–65. doi: 10.1016/S0140-6736(04)17398-4. doi:10.1016/S0140-6736(04)17398-4. [DOI] [PubMed] [Google Scholar]
- 7.Saslow D, Castle PE, Cox JT, Davey DD, Einstein MH, Ferris DG, et al. American Cancer Society Guideline for human papillomavirus (HPV) vaccine use to prevent cervical cancer and its precursors. CA Cancer J Clin. 2007;57:7–28. doi: 10.3322/canjclin.57.1.7. doi:10.1016/S0093-3619(08)70787-1. [DOI] [PubMed] [Google Scholar]
- 8.Villa LL, Costa RLR, Petta C a., Andrade RP, Ault K a., Giuliano AR, et al. Prophylactic quadrivalent human papillomavirus (types 6, 11, 16, and 18) L1 virus-like particle vaccine in young women: A randomised double-blind placebo-controlled multicentre phase II efficacy trial. Lancet Oncol. 2005;6:271–8. doi: 10.1016/S1470-2045(05)70101-7. doi:10.1016/S1470-2045(05)70101-7. [DOI] [PubMed] [Google Scholar]
- 9.Paavonen J, Naud P, Salmerón J, Wheeler CM, Chow S-N, Apter D, et al. Efficacy of human papillomavirus (HPV)-16/18 AS04-adjuvanted vaccine against cervical infection and precancer caused by oncogenic HPV types (PATRICIA): final analysis of a double-blind, randomised study in young women. Lancet. 2009;374:301–14. doi: 10.1016/S0140-6736(09)61248-4. doi:10.1016/S0140-6736(09)61248-4. [DOI] [PubMed] [Google Scholar]
- 10.Joura EA, Giuliano AR, Iversen O-E, Bouchard C, Mao C, Mehlsen J, et al. A 9-Valent HPV Vaccine against Infection and Intraepithelial Neoplasia in Women. N Engl J Med. 2015;372:711–23. doi: 10.1056/NEJMoa1405044. doi:10.1056/NEJMoa1405044. [DOI] [PubMed] [Google Scholar]
- 11. [January 1, 2016];Centers for Disease Control Vaccine Price List as of February 1, 2016. 2016 http://www.cdc.gov/vaccines/programs/vfc/awardees/vaccine-management/price-list/
- 12. [August 26, 2016];The right shot: bringing down barriers to affordable and adapted vaccines. 2015 http://www.msfaccess.org/content/right-shot-bringing-down-barriers-affordable-and-adapted-vaccines.
- 13.Kreimer AR, Rodriguez AC, Hildesheim A, Herrero R, Porras C, Schiffman M, et al. Proof-of- principle evaluation of the efficacy of fewer than three doses of a bivalent HPV16/18 vaccine. J Natl Cancer Inst. 2011;103:1444–51. doi: 10.1093/jnci/djr319. doi:10.1093/jnci/djr319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lazcano-Ponce E, Stanley M, Muñoz N, Torres L, Cruz-Valdez A, Salmerón J, et al. Overcoming barriers to HPV vaccination: Non-inferiority of antibody response to human papillomavirus 16/18 vaccine in adolescents vaccinated with a two-dose vs. a three-dose schedule at 21 months. Vaccine. 2014;32:725–32. doi: 10.1016/j.vaccine.2013.11.059. doi:10.1016/j.vaccine.2013.11.059. [DOI] [PubMed] [Google Scholar]
- 15.Laprise JF, Drolet M, Boily MC, Jit M, Sauvageau C, Franco EL, et al. Comparing the cost- effectiveness of two- and three-dose schedules of human papillomavirus vaccination: A transmission-dynamic modelling study. Vaccine. 2014;32:5845–53. doi: 10.1016/j.vaccine.2014.07.099. doi:10.1016/j.vaccine.2014.07.099. [DOI] [PubMed] [Google Scholar]
- 16.Agosti JM, Goldie SJ. Introducing HPV Vaccine in Developing Countries — Key Challenges and Issues. N Engl J Med. 2007;356:1908–10. doi: 10.1056/NEJMp078053. doi:10.1056/NEJMp078053. [DOI] [PubMed] [Google Scholar]
- 17. [January 1, 2016];Gavi Countries Elligible for Support. 2016 http://www.gavi.org/support/apply/countries-eligible- for-support/
- 18.Rotich JK, Hannan TJ, Smith FE, Bii J, Odero WW, Vu N, et al. Installing and Implementing a Computer-based Patient Record System in Sub-Saharan Africa: The Mosoriot Medical Record System. J Am Med Inform Assoc. 2003;10:295–303. doi: 10.1197/jamia.M1301. doi:10.1197/jamia.M1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rolnick SJ, Parker ED, Nordin JD, Hedblom BD, Wei F, Kerby T, et al. Self-report compared to electronic medical record across eight adult vaccines : Do results vary by demographic factors ? Vaccine. 2013;31:3928–35. doi: 10.1016/j.vaccine.2013.06.041. doi:10.1016/j.vaccine.2013.06.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stupiansky N, Zimet G, Cummings T, Fortenberry J, Shew M. Accuracy of self-reported HPV Vaccine Receipt Among Adolescent Girls and Their Mothers. J Adolesc Heal. 2012;50:103–5. doi: 10.1016/j.jadohealth.2011.04.010. doi:10.1016/j.jadohealth.2011.04.010.ACCURACY. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pastrana DV, Buck CB, Pang YYS, Thompson CD, Castle PE, FitzGerald PC, et al. Reactivity of human sera in a sensitive, high-throughput pseudovirus-based papillomavirus neutralization assay for HPV16 and HPV18. Virology. 2004;321:205–16. doi: 10.1016/j.virol.2003.12.027. doi:10.1016/j.virol.2003.12.027. [DOI] [PubMed] [Google Scholar]
- 22.Robbins HA, Kemp TJ, Porras C, Rodriguez AC, Schiffman M, Wacholder S, et al. Comparison of antibody responses to human papillomavirus vaccination as measured by three assays. Front Oncol. 2014;3:328. doi: 10.3389/fonc.2013.00328. doi:10.3389/fonc.2013.00328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Fu E, Liang T, Spicar-Mihalic P, Houghtaling J, Ramachandran S, Yager P. Two-dimensional paper network format that enables simple multistep assays for use in low-resource settings in the context of malaria antigen detection. Anal Chem. 2012;84:4574–9. doi: 10.1021/ac300689s. doi:10.1021/ac300689s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Villa LL, Ault K a., Giuliano AR, Costa RLR, Petta C a., Andrade RP, et al. Immunologic responses following administration of a vaccine targeting human papillomavirus Types 6, 11, 16, and 18. Vaccine. 2006;24:5571–83. doi: 10.1016/j.vaccine.2006.04.068. doi:10.1016/j.vaccine.2006.04.068. [DOI] [PubMed] [Google Scholar]
- 25.Leder C, Kleinschmidt JA, Wiethe C, Müller M. Enhancement of Capsid Gene Expression : Preparing the Human Papillomavirus Type 16 Major Structural Gene L1 for DNA Vaccination Purposes Enhancement of Capsid Gene Expression : Preparing the Human Papillomavirus Type 16 Major Structural Gene L1 for DNA Va. J Virol. 2001;75:9201–9. doi: 10.1128/JVI.75.19.9201-9209.2001. doi:10.1128/JVI.75.19.9201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buck CB, Pastrana D V, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. J Virol. 2004;78:751–7. doi: 10.1128/JVI.78.2.751-757.2004. doi:10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buck CB, Thompson CD, Pang Y-YS, Lowy DR, Schiller JT. Maturation of papillomavirus capsids. J Virol. 2005;79:2839–46. doi: 10.1128/JVI.79.5.2839-2846.2005. doi:10.1128/JVI.79.5.2839-2846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Buck CB, Thompson CD. Curr. Protoc. Cell Biol. John Wiley & Sons, Inc.; 2007. Production of Papillomavirus-Based Gene Transfer Vectors. doi:10.1002/0471143030.cb2601s37. [DOI] [PubMed] [Google Scholar]
- 29. [May 13, 2015];Production of Papillomaviral Vectors (Pseudoviruses) 2015 http://home.ccr.cancer.gov/LCO/pseudovirusproduction.htm.
- 30.Opalka D, Matys K, Bojczuk P, Green T, Gesser R, Saah A, et al. Multiplexed serologic assay for nine anogenital human papillomavirus types. Clin Vaccine Immunol. 2010;17:818–27. doi: 10.1128/CVI.00348-09. doi:10.1128/CVI.00348-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.WHO. Human papillomavirus vaccines: WHO position paper. 2014 Oct;89 2014. doi:10.1186/1750-9378-2-15.Voir. [Google Scholar]