Abstract
Aims
To examine the incremental usefulness of adding alanine aminotransferase to established risk factors for predicting future diabetes.
Methods
The study population of the Insulin Resistance Atherosclerosis Study included 724 people aged 40–69 years. We excluded people who had excessive alcohol intake or were treated with lipid-lowering agents. Incident diabetes was assessed after a mean follow-up period of 5.2 years.
Results
Alanine aminotransferase had a non-linear relationship with incident diabetes (Wald chi-squared test, P <0.001; P for linearity = 0.005) independent of demographic variables, family history of diabetes, BMI and fasting glucose; therefore, we used Youden’s J statistic to dichotomize alanine aminotransferase [threshold ≥0.43 μkat/L (≥26 IU/l)]. Dichotomized alanine aminotransferase increased the area under the receiver-operating characteristic curve (0.805 vs. 0.823; P = 0.007) of a model that included demographic variables, family history of diabetes, BMI and fasting glucose as independent variables. The net reclassification improvement was 9.6% (95% CI 1.8–17.4; P = 0.016), and the integrated discrimination improvement was 0.031 (95% CI 0.011–0.050; P = 0.002). Dichotomized alanine aminotransferase reclassified a net of 9.6% of individuals more appropriately.
Conclusions
Alanine aminotransferase may be useful for classifying individuals who are at risk of future diabetes after accounting for the effect of other risk factors, including family history, adiposity and plasma glucose.
Introduction
Non-alcoholic fatty liver disease, the hepatic manifestation of insulin resistance syndrome, may account for 75% of cases of chronic liver disease [1]. People with non-alcoholic fatty liver disease tend to have hepatic insulin resistance, which is characterized by the inability to suppress hepatic glucose production with increased insulin concentration [2]. Markers of liver injury including aspartate aminotransferase (AST), alanine aminotransferase (ALT) and γ-glutamyltransferase (GGT) are often elevated in people with non-alcoholic fatty liver disease [3] and insulin resistance [4]. These serum markers have been shown to predict future development of Type 2 diabetes [5,6]; however, few studies have examined whether markers of liver injury contribute substantially to the identification of people who are at risk of developing diabetes [7–11].
Risk prediction has been one of the leading topics in the recent cardiovascular literature, but the effort to identify novel markers of cardiovascular risk (e.g. C-reactive protein) has often given equivocal results [12,13]. These studies have used the area under the receiver-operating characteristic curve (ROC) or the related C-statistic as the test of discrimination [14]; however, the area under the ROC is insensitive to model improvement [14] and is uninformative in terms of both absolute predicted risk (which is of primary clinical interest) and cross-tabulation of risk-strata [15]. To overcome these limitations, new statistical methods based on risk reclassification have been developed, such as the net reclassification improvement (NRI) and the integrated discrimination improvement (IDI) tests [16]. These tests have been shown to be useful for analysing the discriminatory value of novel markers of cardiovascular risk (e.g. C-reactive protein) [17]. Several studies have also used these tests to assess the clinical utility of novel risk markers for predicting diabetes [8,9,18,19].
It has been shown that ALT, which is often measured in clinical practice, has a strong correlation with directly measured liver fat using magnetic resonance spectroscopy (r = 0.46 in women and 0.62 in men) [20]. Its ability to predict future development of diabetes has been tested in the context of readily available clinical information [8,18]; however, it is not known whether ALT adds substantial prognostic information. The aim of the present study, the Insulin Resistance Atherosclerosis Study (IRAS), was to investigate the incremental value of ALT to a prediction model of established risk factors for Type 2 diabetes. This large epidemiological study was designed to examine the relationships between insulin sensitivity and cardiovascular disease risk factors in different ethnic groups and varying states of glucose tolerance [21].
Subjects and methods
Study population
The design and methods of the IRAS have been described in detail [21]. Briefly, the study was conducted at four clinical centres. At the centres in Oakland and Los Angeles (CA, USA), non-Hispanic white and African-American people were recruited from Kaiser Permanente, a nonprofit health maintenance organization. Centres in San Antonio (TX, USA) and San Luis Valley (CO, USA) recruited non-Hispanic white and Hispanic people from two ongoing population-based studies (the San Antonio Heart Study and the San Luis Valley Diabetes Study). A total of 1625 people aged 40–69 years were enrolled in the IRAS (56% women), which was carried out between October 1992 and April 1994. After a mean (range) of 5.2 (4.6–6.6) years, diabetes status was ascertained in 85.3% of participants without diabetes at the baseline visit. The IRAS protocol complied with the ethical guidelines of the 1975 Declaration of Helsinki and was approved by local institutional review committees. All participants provided written informed consent.
Among the 1043 people without diabetes at baseline, 169 were excluded because of excessive alcohol intake (≥28 and ≥14 g/day in men and women, respectively), treatment with lipid-lowering agents, or ALT ≥3 times the normal limit. We also excluded 150 individuals because of lack of data on ALT at baseline or diabetes status at follow-up; therefore, the present report analysed data on 724 people (270 non-Hispanic white, 185 African-American and 269 Hispanic people). These participants did not differ from those with missing information on incident diabetes in terms of age, sex, BMI or ALT, or fasting and 2-h glucose concentrations (P >0.38 for all variables).
Acquisition of data and definition of variables and outcome
Age, sex, race/ethnicity, family history of diabetes and treatment with glucose- and lipid-lowering medications were obtained from self-report. Height, weight and waist circumference were measured using standardized protocols. A 75-g oral glucose tolerance test was administered both at the baseline and the follow-up visits to assess glucose tolerance status. Participants were asked before each visit to fast for 12 h, to abstain from heavy exercise and alcohol for 24 h and to refrain from smoking on the morning of the examination. Laboratory analyses of plasma glucose took place at the University of Southern California (Los Angeles, CA, USA). Serum ALT was measured at the central IRAS laboratory using a Paramax PLA instrument (Baxter, Deerfield, IL, USA). The interassay coefficient of variation was <7%.
Diabetes, the outcome of interest, was defined as fasting plasma glucose ≥7.0 mmol/l and/or 2-h plasma glucose ≥11.1 mmol/l, impaired glucose tolerance as 2-h glucose ≥7.8 and <11.1 mmol/l, and impaired fasting glucose as fasting glucose ≥5.6 and <7.0 mmol/l. People who were taking glucose-lowering medications were considered to have diabetes. BMI was used to define normal weight (<25 kg/m2), overweight (25–29.9 kg/m2) and obesity (≥30 kg/m2).
Statistical analyses
Statistical analyses were performed using the SAS statistical software (version 9.2, SAS Institute Inc. Cary, NC, USA) and R project statistical software (version 2.9.2, The R Foundation for Statistical Computing, Vienna, Austria). Baseline characteristics by diabetes status at follow-up were investigated by one-way ANCOVA for continuous variables or logistic regression for dichotomous variables. We modelled incident diabetes with a restricted cubic polynomial spline for ALT to estimate the varying effects of ALT over its full range. The linearity assumption was also tested by restricted cubic splines. As the relationship between ALT and incident diabetes appeared to be non-linear and with a point of inflection, we used Youden’s J statistic to dichotomize ALT in order to identify people who were at higher risk of diabetes. In separate logistic regression models, appropriate interaction terms were introduced to examine the effect of age, sex, race/ethnicity, family history of diabetes, BMI and fasting glucose on the relationship between dichotomized ALT and incident diabetes.
We used the area under the ROC to determine the predictive discrimination of ALT [14]. Areas under the ROC were compared by bootstrap sampling. To determine the discriminative value of ALT [16,22], risk reclassification (NRI, category-free NRI and IDI) was evaluated using the %add_predictive macro (http://analytics.ncsu.edu/sesug/2010/SDA07.Kennedy.pdf). Category-free NRI and IDI are independent of risk categories. The IDI test assesses the change in the estimated prediction probabilities for all possible thresholds. Category-free NRI measures only the direction of change in people with events and in those without events [22]. The NRI examines the change in the estimated prediction probabilities that involve a change from one ‘a priori’ meaningful category to another [16]. We have previously reported meaningful categories as follows: low (<1%), intermediate (1–5.9%) and high (≥6%) yearly risks of developing diabetes [19]. The low and high yearly risks of future diabetes matched the risk in people with normal fasting and 2-h plasma glucose concentrations and in those with impaired glucose tolerance, respectively. To assess the calibration of these models, we used the Hosmer–Lemeshow goodness-of-fit test. To develop a parsimonious and accurate prediction model, we used a forward selection procedure (level of significance to entry and stay <0.25 and <0.05, respectively). As independent variables, we chose categorized measures (age, sex, race/ethnicity, BMI, fasting glucose and ALT) that may be readily available in the clinical setting. The natural log transformation of ALT was used to improve discrimination and calibration of the models. A two-sided P value <0.05 was taken to indicate statistical significance.
Results
After a mean follow-up of 5.2 years, 115 of the 724 (15.9%) study participants developed incident diabetes. Family history of diabetes, older age and elevations in BMI and fasting plasma glucose and ALT levels were associated with an increased incidence of diabetes (Table 1).
Table 1.
Baseline characteristics by diabetes status at the follow-up visit
| People who did not develop diabetes | People who developed diabetes | P | |
|---|---|---|---|
| Number of people | 609 | 115 | - |
| Mean ± SD age*, years | 53.8 ± 0.3 | 56.3 ± 0.8 | 0.004 |
| Female*, % | 56.7 (52.9, 60.4) | 63.4 (54.6, 71.4) | 0.166 |
| Race/ethnicity*, % | 0.940 | ||
| African-American | 25.8 (22.5, 29.4) | 24.3 (17.4, 33.0) | 0.747 |
| Hispanic | 36.9 (33.2, 40.9) | 38.3 (29.9, 47.4) | 0.788 |
| Non-Hispanic white | 37.3 (33.5, 41.2) | 37.8 (33.5, 41.2) | 0.981 |
| Family history of diabetes*, % | 39.5 (35.6–43.4) | 56.2 (47.0–65.1) | <0.001 |
| Mean ± SD BMI, kg/m2 | 28.0 ± 0.2 | 31.5 ± 0.5 | <0.001 |
| Mean ± SD waist circumference, cm | 89.0 ± 0.5 | 96.2 ± 1.1 | <0.001 |
| Mean ± SD fasting glucose, mmol/l | 5.33 ± 0.02 | 5.84 ± 0.05 | <0.001 |
| Impaired fasting glucose, % | 31.4 (27.6–35.4) | 69.7 (60.2–77.7) | <0.001 |
| Mean ± SD 2-h glucose, mmol/l | 6.53 ± 0.07 | 8.46 ± 0.16 | <0.001 |
| Impaired glucose tolerance, % | 29.6 (21.8–38.9) | 76.7 (73.1–80.0) | <0.001 |
| Mean ± SD ALT†, μkat/l | 0.27 ± 0.01 | 0.33 ± 0.02 | <0.001 |
ALT, alanine aminotransferase.
Results adjusted for age, sex, race/ethnicity, and clinic
Non-adjusted results.
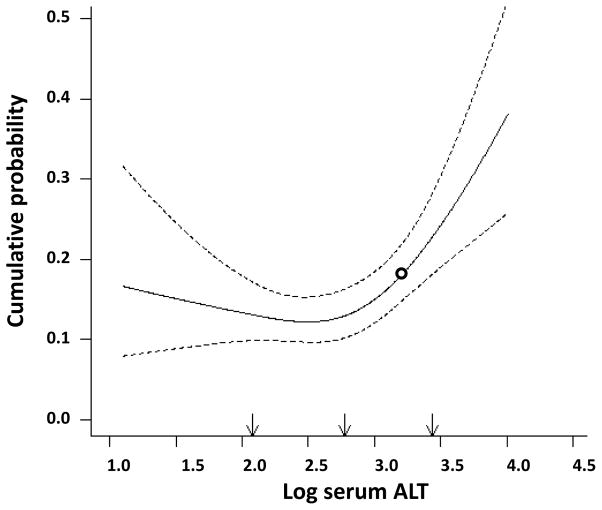
Figure 1 shows a logistic regression fitted to data to model incident diabetes, with a restricted cubic polynomial spline for log ALT to estimate the varying effects of log ALT over its full range. The relationship between log ALT and incident diabetes was significant (Wald chi-squared test, P <0.001) and not linear (Wald for linearity, P = 0.005). The relationship remained significant and not linear even after the adjustment for age, sex, race/ethnicity, clinic, family history of diabetes, BMI and fasting glucose (Wald chi-squared test, P = 0.005; Wald for linearity, P = 0.011). Adding 2-h glucose to the model did not change the relationship between log ALT and incident diabetes (Wald chi-squared test, P = 0.003; Wald for linearity, P = 0.005).
Figure 1. Relationship between ALT and incident diabetes.
Relationship between the 5-year risk of Type 2 diabetes and log alanine aminotransferase (ALT) modelled by a smooth function. White circle: log ALT threshold (≥ −0.84), equivalent to ALT [≥0.43 μkat/l (≥26 IU/l)] based on Youden’s J statistic (sensitivity of 32.2% and specificity of 85.7%).
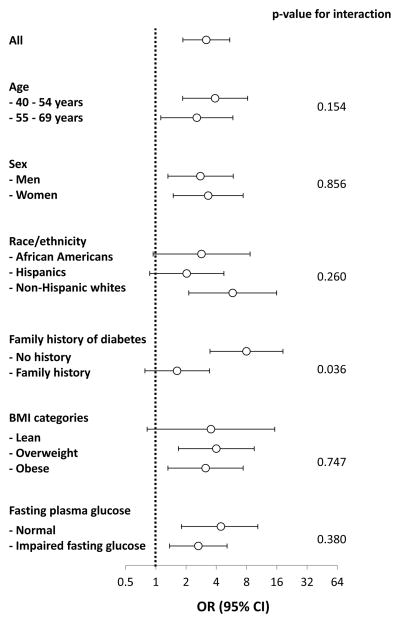
Because of the existence of a point of inflection in the relationship between ALT and incident diabetes, we used Youden’s J statistic to dichotomize ALT (log ALT ≥ −0.84 vs. < −0.84, which was equivalent to ALT ≥0.43 vs. <0.43 μkat/l [≥26 vs. <26 IU/l]). A total of 124 people (17.1%) had an ALT level of ≥0.43 μkat/l. ALT ≥0.43 μkat/l was associated with a threefold greater risk of developing diabetes [odds ratio 2.87 (95% CI 1.83–4.52)]. Analyses in men and women are shown in Table S1. This highly significant relationship remained unchanged [odds ratio 3.03 (95% CI 1.71–5.36)], even after accounting for the effect of age, sex, race/ethnicity, clinic, family history of diabetes, BMI, fasting glucose and 2-h glucose. The relationship between dichotomized ALT and incident diabetes was quite similar in all categories of age, sex, race/ethnicity, BMI and fasting glucose (Fig. 2). None of these variables had a significant effect on the relationship between dichotomized ALT and incident diabetes (P for interaction >0.15 for all); however, the interaction term family history of diabetes × dichotomized ALT was statistically significant (P for interaction = 0.036), because dichotomized ALT was not significantly related to incident diabetes in people with a family history of diabetes [odds ratio 1.63 (95% CI 0.78–3.42)]. An ALT level of ≥0.43 μkat/l was associated with a higher risk of future diabetes, regardless of glucose tolerance status [odds ratio 2.46 (95% CI 1.47–4.11); Fig. S1].
Figure 2. Relationship between ALT (≥0.43 vs. <0.43 μkat/L [≥26 vs. <26 IU/L]) and incident diabetes.
Relationship between alanine aminotransferase [ALT; ≥0.43 vs. <0.43 μkat/l (≥26 vs. <26 IU/l)] and incident diabetes across categories of age, sex, race/ethnicity, family history of diabetes, BMI and fasting plasma glucose. Categories of age, sex, race/ethnicity, clinic, family history of diabetes, BMI, and fasting glucose were included in all models except for the measure of interest. OR, odds ratio.
We also tested the predictive discrimination and risk reclassification properties of dichotomized ALT (≥0.43 vs. <0.43 μkat/l) in a model that included age, sex, race/ethnicity, clinic, family history of diabetes, BMI and fasting glucose as covariates (Table 2). Adding dichotomized ALT and interaction term family history of diabetes × dichotomized ALT increased the area under the ROC of the model (0.795 vs. 0.812; P = 0.136). The added predictive value was relatively small for people at low risk of developing diabetes (reclassification of 10.5% of people), but more substantial for those at intermediate and high risks (reclassification of 21.3 and 20.0% of people, respectively). The NRI was 9.6% (95% CI 1.8–27.7; P = 0.016), and category-free NRI was 45.4% [95% CI 25.8– 64.9; P <0.001 (Table 3)]. The integrated sensitivity curve (for the overall performance among people who developed diabetes) was moved to the right (area change 0.026; P = 0.010), and the integrated 1-specificity curve (for the overall performance among people who remained without diabetes) to the left (area change - 0.005; P = 0.027). The IDI (the sum of the areas between the integrated sensitivity curves and the integrated 1-specificity curves) was 0.031 (95% CI 0.011–0.050; P = 0.002). The prediction model was well calibrated before (Hosmer–Lemeshow test: chi-squared 15.6; P = 0.049) and after the inclusion of dichotomized ALT (chi-squared 4.5; P = 0.805).
Table 2.
Observed and predicted risk of 5-year incidence of diabetes
| Prediction model plus dichotomized ALT and interaction terms family history of diabetes × dichotomized ALT § | ||||
|---|---|---|---|---|
| Prediction model* | <1% yearly risk | 1 – 5.9% yearly risk | ≥6% yearly risk | % Reclassified |
| <1% yearly risk | ||||
| People who developed diabetes, n | 2 | 3 | 0 | - |
| People who did not develop diabetes, n | 152 | 15 | 0 | - |
| Total, n | 154 | 18 | 0 | - |
| Percentage† | 89.5 | 10.5 | - | 10.5 |
| Predicted yearly risk with prediction model, % | 0.6 | 0.8 | - | - |
| Predicted yearly risk by adding ALT, % | 0.4 | 1.9 | - | - |
| Observed yearly risk‡, % | 0.3 | 3.3 | - | - |
| 1–5.9% yearly risk | ||||
| People who developed diabetes, n | 1 | 51 | 6 | - |
| People who did not develop diabetes, n | 66 | 289 | 19 | - |
| Total, n | 67 | 340 | 25 | - |
| % | 15.5 | 78.7 | 5.8 | 21.3 |
| Predicted yearly risk with prediction model, % | 1.2 | 2.8 | 4.1 | - |
| Predicted yearly risk by adding ALT, % | 0.8 | 2.7 | 7.4 | - |
| Observed yearly risk, % | 0.3 | 3.0 | 4.8 | - |
| ≥6% yearly risk | ||||
| People who developed diabetes, n | 0 | 6 | 43 | - |
| People who did not develop diabetes, n | 0 | 16 | 45 | - |
| Total, n | 0 | 22 | 88 | - |
| % | - | 20.0 | 80.0 | 20.0 |
| Predicted yearly risk with prediction model, % | - | 6.7 | 9.5 | - |
| Predicted yearly risk by adding ALT, % | - | 5.4 | 9.9 | - |
| Observed yearly risk, % | - | 5.5 | 9.8 | - |
ALT, alanine aminotransferase.
The prediction model included age, sex, race/ethnicity, clinic, family history of diabetes, BMI and fasting glucose as independent variables.
Percent classified in each risk stratum by the prediction model with dichotomized ALT and interaction terms family history of diabetes × dichotomized ALT.
Observed proportion of people developing diabetes in each category.
ALT categorized as a dichotomous variable (≥0.43 vs. <0.43 μkat/l).
Table 3.
Added predictive value of dichotomized alanine aminotransferase (≥0.43 vs. <0.43 μkat/l) to a prediction model
| Prediction model | P | Prediction model + dichotomized ALT and interaction terms family history of diabetes × dichotomized ALT | P | |
|---|---|---|---|---|
| Area under the ROC* (95% CI) | 0.805 (0.731–0.870) | 0.823 (0.753–0.889) | 0.007 | |
| Calibration†, chi-squared | 15.6 | 0.049 | 4.5 | 0.805 |
| NRI‡, % | ||||
| Overall | Referent | 9.6 (1.8–17.4) | 0.016 | |
| For persons who developed diabetes, n | Referent | 2 | 0.617 | |
| For persons who did not develop diabetes, n | Referent | 8 | <0.001 | |
| Category-free NRI§ | ||||
| Overall, % (range) | Referent | 45.4 (25.8–64.9) | <0.001 | |
| For people who developed diabetes, n | Referent | 11 | 0.257 | |
| For people who did not develop diabetes, n | Referent | 56 | <0.001 | |
| IDI | ||||
| Overall, % (range) | Referent | 0.031 (0.011–0.050) | 0.002 | |
| For persons who developed diabetes, n | Referent | 0.026 | ||
| For persons who did not develop diabetes, n | Referent | 0.005 | ||
| Integrated sensitivity | 0.295 | 0.321 | 0.010 | |
| Integrated 1-specificity | 0.131 | 0.127 | 0.027 |
ALT, alanine aminotransferase; ROC, receiver-operating curve; NRI, net reclassification improvement; IDI, integrated discrimination improvement.
The prediction model included age, sex, race/ethnicity, clinic, family history of diabetes, BMI and fasting glucose as independent variables.
NRI for persons with events + NRI for persons without events.
Category-free NRI for persons with events + category-free NRI for persons without events.
The 95% CIs are shown in parentheses.
Areas under the ROC were compared by bootstrap sampling.
Hosmer–Lemeshow goodness of fit.
A simple score sheet was developed to predict the 5-year incidence of diabetes from the β coefficients of a multiple logistic regression model (Table 4). Because of the interaction between family history of diabetes and dichotomized ALT, family history added little to our model; therefore, we excluded family history from the prediction model. For example, a 50-year-old obese man with normal fasting glucose and ALT ≥0.43 μkat/l has a score of 2.5 points. Dividing this persons’ yearly risk by the yearly risk of a man with no risk factors (score of 0 points) provides an estimate of relative risk (3%/0.5% = 6).
Table 4.
Diabetes score sheet developed from participants in the Insulin Resistance Atherosclerosis Study
| A. β coefficients and score points | ||
|---|---|---|
| β coefficient | Score sheet | |
| Intercept | − 3.36 | - |
| Age ≥60 years | 0.61 | 1 |
| Female gender | 0.55 | 1 |
| Impaired fasting glucose | 1.26 | 1.5 |
| Obesity (BMI ≥30 kg/m2) | 0.75 | 1 |
| ALT ≥0.43 μkat/l | 1.05 | 1.5 |
| B. Predictive discrimination | ||
|---|---|---|
| Prediction model | Total score | |
| Area under the ROC | 0.761 | 0.756 |
| C. Diabetes Prediction Sheet | ||
|---|---|---|
| Total score | Yearly risk of diabetes (%) | Relative risk |
| 0–1 | 0.5 | Reference |
| 1.5–2.5 | 3 | 6 |
| 3–4 | 6 | 9 |
| ≥ 4.5 | 9 | 18 |
ALT, alanine aminotransferase; ROC, receiver-operating curve.
We used a forward selection procedure to develop a parsimonious and accurate prediction model. Level of significance to entry and stay were <0.25 and <0.05, respectively. The prediction model included the following variables: age categories (40–49, 50–59, 60–69 years), sex, ethnicity (Hispanic, African-American and non-Hispanic white), IFG (yes vs. no), BMI categories (<25, 25–29.9, ≥30 kg/m2), and ALT [≥0.43 vs. <0.43 μkat/l (≥26 vs. 26 IU/l)].
Discussion
The present study has several novel findings. ALT has a non-linear relationship with incident diabetes. This may allow the dichotomization of ALT [≥0.43 vs. <0.43 μkat/l (≥26 vs. <26 IU/l)] for risk prediction. A total of 17.1% of the IRAS participants without diabetes had an ALT concentration ≥0.43 μkat/l. This threshold adds substantial prognostic value to a prediction model of established risk factors (including family history of diabetes and measures of adiposity and glucose tolerance).
It has been shown that ALT can predict future diabetes independent of adiposity and direct measures of insulin sensitivity and secretion [6]. In a recent study from the Netherlands, liver function tests, including ALT, AST, GGT and albumin, modestly improved the prediction of incident diabetes in models with fasting glucose and other readily available clinical variables [8]. In that report, liver function tests had little incremental predictive value after adding HbA1c to the model [8]. In the present study, ALT improved discrimination after considering glucose tolerance. Among men participants in the Epidemiological Study on the Insulin Resistance Syndrome, GGT and fasting glucose improved the predictive discrimination of a model that included smoking, waist circumference and blood pressure [7]. In the European Prospective Investigation into Cancer and Nutrition-Potsdam study, HDL cholesterol, triglycerides, ALT and GGT enhanced the predictive discrimination of a model that included lifestyle characteristics and fasting glucose [9]. In the British Regional Heart Study and the British Women’s Heart and Health Study, HbA1c and GGT (which can be measured in non-fasting samples) were as accurate a tool as triglycerides and fasting blood glucose in identifying those at increased risk [10]. In the Atherosclerosis Risk in Communities Study, a large panel of novel risk factors for Type 2 diabetes that included ALT and GGT was associated with small improvements in risk prediction beyond that provided by standard risk factors [18]. Disparities between studies may be in part related to differences in terms of participant characteristics, availability of markers of risk, ascertainment of outcome (criteria for diagnosis of diabetes) and statistical methods.
To the list of reasons for disparities, we may add the non-linear relationship between ALT and incident diabetes. In the IRAS, the existence of a point of inflection in the relationship between ALT and incident diabetes has been of help to dichotomize ALT to improve risk stratification. In the present data, an ALT threshold of 0.43 μkat/l was useful for detecting people at risk of future diabetes. An ALT concentration ≥0.43 μkat/l was present in 17.1% of the people who did not have diabetes and is associated with a threefold increase in diabetes risk independent of family history of diabetes, BMI and IFG. In addition, dichotomized ALT increases the predictive discrimination of a clinical risk prediction model that includes BMI and fasting glucose, and helps to reclassify a significant proportion of people at intermediate or high risk of developing diabetes (17.6 and 28.9%, respectively). ALT is associated with peripheral and hepatic insulin resistance, increased insulin secretion, decreased hepatic insulin clearance, atherogenic dyslipidaemia and elevated C-reactive protein levels [6,23]. Whether one or more of these associations accounts for the relationship between ALT and progression to Type 2 diabetes needs to be tested in future studies.
There are several indices that have been developed to identify individuals with non-alcoholic fatty liver disease, such as the Fatty Liver Index [24] and the non-alcoholic fatty liver disease liver fat score [25]. These indices have been shown to predict incident diabetes [7]. This is not surprising (these indices are computed using measures of adiposity and/or insulin resistance), but it was beyond the scope of the present study to determine their predictive discriminatory value. Non-alcoholic fatty liver disease liver fat score is more strongly related to insulin resistance, adiposity and dyslipidaemia than is ALT [26]; however, relatively unaffected by adiposity and plasma glucose, ALT can be used in the design of a simple diabetes risk score that has an acceptable predictive discrimination (area under the ROC 0.756).
Dichotomized ALT adds discriminatory value to a prediction model of readily available risk factors. The requirements for model improvement are met [16]: the marker is related to the outcome; the marker increases the discrimination as measured by area under the ROC; the model is well calibrated, suggesting that the marker can be applied to an external population; and risk prediction is improved within clinically relevant categories (as measured by NRI and IDI tests). Conceivably, people who may benefit most are those with moderate and high pretest probabilities. As ALT is commonly available in many clinical settings, future studies need to confirm these results and test other measures of liver fat.
The present study has several strengths. ALT is associated with incident diabetes with no significant interaction effects of age, sex, race/ethnicity, BMI and plasma glucose levels. In addition, the IRAS included a well characterized sample population with the aim of studying risk factors for diabetes. The study also has some limitations, however, including the fact that there was no information on GGT and hepatitis B and C serologies. Our ALT threshold may not be applicable in other studies because of differences in either participant characteristics or analytical procedures used by the laboratories [27]. Nevertheless, its clinical application may be important. Serum ALT levels (and other liver enzymes) can change with pharmacological and lifestyle interventions [28,29]. In the National Health and Nutrition Examination Survey, 1999–2002, the prevalence of elevated ALT activity [>0.72 μkat/l (>43 IU/l)] was 8.9% overall (a twofold increase from previously available estimates) and 7.3% among people without excessive alcohol intake or viral hepatitis [30]. Finally, risk stratification was carried out on the basis of a single measurement of ALT. We would therefore point out the need to replicate the results in other studies. Nevertheless, the present data suggest that a single measurement of ALT is useful for classifying people at risk of future diabetes.
In summary, ALT is a useful marker for detecting people at risk of future diabetes independent of family history of diabetes, adiposity and fasting glucose. A non-linear relationship between ALT and incident diabetes may partially explain previous conflicting information on the discriminatory value of ALT. Although most people with ALT ≥0.43 μkat/l (≥26 IU/l) have normal ALT values, this threshold adds discriminative value to a model of established risk factors, particularly among people with a moderate or high risk of diabetes.
Supplementary Material
What’s new?
We tested the added value of alanine aminotransferase for predicting diabetes in the context of readily available clinical information.
The results suggest that alanine aminotransferase may be useful for classifying individuals who are at risk of future diabetes.
Alanine aminotransferase has a non-linear relationship with incident diabetes. This may allow the dichotomization of alanine aminotransferase for risk prediction.
Acknowledgments
Funding sources
This study was supported by National Heart, Lung, and Blood Institute grants HL-47887, HL-47889, HL-47890, HL-47892 and HL-47902, and the General Clinical Research Centers Program (NCRR GCRC, M01 RR431 and M01 RR01346). The funding bodies had no involvement in study design, data collection, data analysis, manuscript preparation and/or publication decisions.
Footnotes
Competing interests
None declared.
Additional Supporting Information may be found in the online version of this article:
Figure S1. Effect of alanine aminotransferase (ALT) ≥0.43 μkat/l (≥26 IU/l) on risk of future diabetes by glucose tolerance status. Risk among individuals with ALT <0.43 μkat/l (white circles) and ALT ≥0.43 μkat/l (black circles). ALT ≥0.43 μkat/l was associated with an increased risk [odds ratio 2.46 (95% CI 1.47–4.11)] regardless of glucose tolerance status.
Table S1. Risk of diabetes associated with alanine aminotransferase (ALT) 0.43 μkat/l (26 IU/l) in men and women.
References
- 1.Younossi ZM, Stepanova M, Afendy M, Fang Y, Younossi Y, Mir H, et al. Changes in the prevalence of the most common causes of chronic liver diseases in the United States from 1988 to 2008. Clin Gastroent Hepatology. 2011;9:524–530. doi: 10.1016/j.cgh.2011.03.020. [DOI] [PubMed] [Google Scholar]
- 2.Firth RG, Bell PM, Marsh HM, Hansen I, Rizza RA. Postprandial hyperglycemia in patients with noninsulin-dependent diabetes mellitus. Role of hepatic and extrahepatic tissues. J Clin Invest. 1986;77:1525–1532. doi: 10.1172/JCI112467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kelley DE, McKolanis TM, Hegazi RA, Kuller LH, Kalhan SC. Fatty liver in type 2 diabetes mellitus: relation to regional adiposity, fatty acids, and insulin resistance. Am J Physiol Endocrinol Metab. 2003;285:E906–E916. doi: 10.1152/ajpendo.00117.2003. [DOI] [PubMed] [Google Scholar]
- 4.Hanley AJ, Wagenknecht LE, Festa A, D’Agostino RB, Jr, Haffner SM. Alanine aminotransferase and directly measured insulin sensitivity in a multiethnic cohort: the Insulin Resistance Atherosclerosis Study. Diabetes Care. 2007;30:1819–1827. doi: 10.2337/dc07-0086. [DOI] [PubMed] [Google Scholar]
- 5.Hanley AJ, Williams K, Festa A, Wagenknecht LE, D’Agostino RB, Jr, Kempf J, et al. Elevations in markers of liver injury and risk of type 2 diabetes: The Insulin Resistance Atherosclerosis Study. Diabetes. 2004;53:2623–2632. doi: 10.2337/diabetes.53.10.2623. [DOI] [PubMed] [Google Scholar]
- 6.Vozarova B, Stefan N, Lindsay RS, Saremi A, Pratley RE, Bogardus C, et al. High alanine aminotransferase is associated with decreased hepatic insulin sensitivity and predicts the development of type 2 diabetes. Diabetes. 2002;51:1889–1895. doi: 10.2337/diabetes.51.6.1889. [DOI] [PubMed] [Google Scholar]
- 7.Balkau B, Lange C, Vol S, Fumeron F, Bonnet F Group Study D.E.S.I.R. Nine-year incident diabetes is predicted by fatty liver indices: the French D.E.S.I.R. study. BMC Gastroenterol. 2010;10:56. doi: 10.1186/1471-230X-10-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Abbasi A, Bakker SJ, Corpeleijn E, van der ADL, Gansevoort RT, Gans RO, et al. Liver function tests and risk prediction of incident type 2 diabetes: evaluation in two independent cohorts. PLoS One. 2012;7:e51496. doi: 10.1371/journal.pone.0051496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schulze MB, Weikert C, Pischon T, Bergmann MM, Al-Hasani H, Schleicher E, et al. Use of multiple metabolic and genetic markers to improve the prediction of type 2 diabetes: the EPIC-Potsdam Study. Diabetes Care. 2009;32:2116–2119. doi: 10.2337/dc09-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wannamethee SG, Papacosta O, Whincup PH, Thomas MC, Carson C, Lawlor DA, et al. The potential for a two-stage diabetes risk algorithm combining non-laboratory-based scores with subsequent routine non-fasting blood tests: results from prospective studies in older men and women. Diabet Med. 2011;28:23–30. doi: 10.1111/j.1464-5491.2010.03171.x. [DOI] [PubMed] [Google Scholar]
- 11.Kashima S, Inoue K, Matsumoto M, Akimoto K. Do non-glycaemic markers add value to plasma glucose and hemoglobin a1c in predicting diabetes? Yuport Health Checkup Center Study. PLoS One. 2013;8:e66899. doi: 10.1371/journal.pone.0066899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Folsom AR, Aleksic N, Catellier D, Juneja HS, Wu KK. C-reactive protein and incident coronary heart disease in the Atherosclerosis Risk in Communities (ARIC) study. Am Heart J. 2002;144:233–238. doi: 10.1067/mhj.2002.124054. [DOI] [PubMed] [Google Scholar]
- 13.Ridker PM, Rifai N, Rose L, Buring JE, Cook NR. Comparison of C-reactive protein and low-density lipoprotein cholesterol levels in the prediction of first cardiovascular events. N Engl J Med. 2002;347:1557–1565. doi: 10.1056/NEJMoa021993. [DOI] [PubMed] [Google Scholar]
- 14.Pepe MS, Janes H, Longton G, Leisenring W, Newcomb P. Limitations of the odds ratio in gauging the performance of a diagnostic, prognostic, or screening marker. Am J Epidemiol. 2004;159:882–890. doi: 10.1093/aje/kwh101. [DOI] [PubMed] [Google Scholar]
- 15.Cook NR. Assessing the incremental role of novel and emerging risk factors. Curr Cardiovasc Risk Rep. 2010;4:112–119. doi: 10.1007/s12170-010-0084-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pencina MJ, D’Agostino RB. Overall C as a measure of discrimination in survival analysis: model specific population value and confidence interval estimation. Stat Med. 2004;23:2109–2123. doi: 10.1002/sim.1802. [DOI] [PubMed] [Google Scholar]
- 17.Ridker PM, Paynter NP, Rifai N, Gaziano JM, Cook NR. C-reactive protein and parental history improve global cardiovascular risk prediction: the Reynolds Risk Score for men. Circulation. 2008;118:2243–2251. doi: 10.1161/CIRCULATIONAHA.108.814251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Raynor LA, Pankow JS, Duncan BB, Schmidt MI, Hoogeveen RC, Pereira MA, et al. Novel risk factors and the prediction of type 2 diabetes in the Atherosclerosis Risk in Communities (ARIC) study. Diabetes Care. 2013;36:70–76. doi: 10.2337/dc12-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lorenzo C, Williams K, Haffner SM. Insulin secretion based on the late oral glucose tolerance test period and incident diabetes: the San Antonio Heart Study. Diabet Med. 2012;29:e151–e158. doi: 10.1111/j.1464-5491.2012.03660.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kotronen A, Yki-Järvinen H, Sevastianova K, Bergholm R, Hakkarainen A, Pietiläinen KH, et al. Comparison of the relative contributions of intra-abdominal and liver fat to components of the metabolic syndrome. Obesity (Silver Spring) 2011;19:23–28. doi: 10.1038/oby.2010.137. [DOI] [PubMed] [Google Scholar]
- 21.Wagenknecht LE, Mayer EJ, Rewers M, Haffner S, Selby J, Borok GM, et al. The Insulin Resistance Atherosclerosis Study: Design, Objectives and Recruitment Results. Ann Epidemiol. 1995;5:464–472. doi: 10.1016/1047-2797(95)00062-3. [DOI] [PubMed] [Google Scholar]
- 22.Maarten JG, Leening MJ, Vedder MM, Witteman JC, Pencina MJ, Steyerberg EW. Net reclassification improvement: computation, interpretation, and controversies: a literature review and clinician’s guide. Ann Intern Med. 2014;160:122–131. doi: 10.7326/M13-1522. [DOI] [PubMed] [Google Scholar]
- 23.Kerner A, Avizohar O, Sella R, Bartha P, Zinder O, Markiewicz W, et al. Association between elevated liver enzymes and C-reactive protein: possible hepatic contribution to systemic inflammation in the metabolic syndrome. Arterioscler Thromb Vasc Biol. 2005;25:193–197. doi: 10.1161/01.ATV.0000148324.63685.6a. [DOI] [PubMed] [Google Scholar]
- 24.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The Fatty Liver Index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137:865–872. doi: 10.1053/j.gastro.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 26.Lorenzo C, Hanley AJ, Rewers MJ, Haffner SM. The association of alanine aminotransferase within the normal and mildly elevated range with lipoproteins and apolipoproteins: the Insulin Resistance Atherosclerosis Study. Diabetologia. 2013;56:746–757. doi: 10.1007/s00125-012-2826-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Myara A, Guechot J, Lasnier E, Imbert-Bismut F, Voitot H, Ferard G. The use of enzyme results for liver fibrosis evaluation necessitates standardization. Hepatology. 2008;47:1100–1101. doi: 10.1002/hep.22128. [DOI] [PubMed] [Google Scholar]
- 28.Belfort R, Harrison SA, Brown K, Darland C, Finch J, Hardies J, et al. A placebo-controlled trial of pioglitazone in subjects with nonalcoholic steatohepatitis. N Engl J Med. 2006;355:2297–2307. doi: 10.1056/NEJMoa060326. [DOI] [PubMed] [Google Scholar]
- 29.St George A, Bauman A, Johnston A, Farrell G, Chey T, George J. Independent effects of physical activity in patients with nonalcoholic fatty liver disease. Hepatology. 2009;50:68–76. doi: 10.1002/hep.22940. [DOI] [PubMed] [Google Scholar]
- 30.Ioannou GN, Boyko EJ, Lee SP. The prevalence and predictors of elevated serum aminotransferase activity in the United States in 1999–2002. Am J Gastroenterol. 2006;101:76–82. doi: 10.1111/j.1572-0241.2005.00341.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.