Abstract
High-dose melphalan 200 mg/m2 (MEL 200) is the standard of care as a conditioning regimen for autologous hematopoietic stem cell transplantation (AHSCT) for multiple myeloma (MM). We compared a novel conditioning combination incorporating busulfan, melphalan, and bortezomib (BUMELVEL) versus standard MEL 200 in newly diagnosed patients undergoing AHSCT for MM. Between July 2009 and May 2012, 43 eligible patients received BUMELVEL conditioning followed by AHSCT. BU was administered i.v. daily for 4 days to achieve a target area under the concentration-time curve total of 20,000 mM·min based on pharmacokinetic analysis after the first dose. MEL 140 mg/m2 (MEL 140) and VEL 1.6 mg/m2 were administered i.v. on days −2 and −1, respectively. Outcomes were compared with a contemporaneous North American cohort (n = 162) receiving MEL 200 matched for age, sex, performance status, stage, interval from diagnosis to AHSCT, and disease status before AHSCT. Multivariate analysis of relapse, progression-free survival (PFS), and overall survival (OS) was performed. The median follow-up was 25 months. No transplant-related mortality was observed in the study cohort at 1 year. PFS at 1 year was superior in the BUMELVEL cohort (90%) in comparison with 77% in MEL 200 historical control subjects (P = .02). Cumulative incidence of relapse was lower in the BUMELVEL group versus the MEL 200 group (10% at 1 year versus 21%; P = .047). OS at 1 year was similar between cohorts (93% versus 93%; P =.89). BU can be safely combined with MEL 140 and VEL without an increase in toxicities or transplant-related mortality. We observed a superior PFS in the BUMELVEL cohort without maintenance therapy, warranting further trials.
Keywords: Stem cell transplantation, Multiple myeloma, Conditioning regimen, Busulfan, Melphalan, Bortezomib
INTRODUCTION
High-dose chemotherapy followed by autologous hematopoietic stem cell transplantation (AHSCT) is an effective therapy for transplant-eligible patients as consolidation after induction therapy in newly diagnosed multiple myeloma (MM). The benefit of AHSCT also extends to patients with relapsed disease who remain transplant eligible. The effectiveness of AHSCT for patients with MM remains relevant despite significant therapeutic advances achieved with the introduction of novel agents such as proteasome inhibitors and immunomodulatory agents. MM remains the most common indication for AHSCT in North America and Europe [1]. Single-agent melphalan, at a dose of 200 mg/m2 (MEL 200), is the international standard for conditioning before AHSCT for MM [2]. Other chemotherapy and chemoradiotherapy regimens have been used in preparation for AHSCT but with no clear superiority over MEL 200 [3]. These other combination regimens are generally associated with increased hematologic and nonhematologic toxicities without improvement in efficacy.
High-dose busulfan (BU) and melphalan (MEL) are myeloablative chemotherapeutic agents. Both are effective and well-tolerated agents that have been used for over 20 years in MM and other malignancies as conditioning regimens for AHSCT. The combination of BU and MEL was associated with superior progression-free survival (PFS) compared with MEL 200 in patients who had not achieved European Group for Blood and Marrow Transplantation criteria (CR) before AHSCT [4,5]. Additionally, the combination of bortezomib (VEL) and MEL appears to be synergistic, especially when VEL is administered after MEL 200 [6].
We prospectively evaluated a conditioning regimen consisting of high-dose i.v. BU and MEL followed by VEL (BUMELVEL) in an open-label, phase I/II fashion aimed at improving PFS after AHSCT for MM patients. A predefined maximum tolerated dose was used in this trial and consisted of BU 130 mg/m2 daily for 4 days and adjusted to achieve a target area under the concentration-time curve (AUC) total of 20,000 µM·min, MEL 140 mg/m2, and VEL 1.6 mg/m2. We then compared the results of patients who received the predefined maximum tolerated dose against a contemporaneous matched cohort of patients with similar characteristics who received single-agent MEL 200.
METHODS
Between July 2009 and May 2012, 43 patients received BUMELVEL conditioning followed by AHSCT in a single-center, open-label phase I/II protocol. Inclusion criteria included adults with MM who had a creatinine of less than 2.5 mg/dL, without active infections or severe obstructive and/or restrictive pulmonary disease determined by pulmonary function testing (ie, DLCO < 50% and/or FEV1 < 50% and/or FVC < 50%) and cardiac ejection fraction greater than 40%. Response criteria were assessed according to the International Myeloma Working Group Uniform Response Criteria [7].
Neutrophil and platelet engraftment were defined as the first of 3 days with a neutrophil count > .5 × 109/L and first date of 3 consecutive laboratory values with an untransfused platelet count ≥ 20 × 109/L. Because BU has been associated with the risk of sinusoidal obstructive syndrome (SOS), we monitored for SOS using the Baltimore diagnostic criteria [8]. It is known that SOS risk is higher when the total BU AUC exceeds 24,000 µM·min [9]. Therefore, BU was administered i.v. daily for a total of 4 days with the first 2 days (days −6 and −5) at fixed dose of 130 mg/m2 over 3 hours and the subsequent 2 doses (days −4 and −3) adjusted to achieve a target AUC total of 20,000 µM·min determined by pharmacokinetic analysis after the first dose of i.v. BU. MEL 140 mg/m2 and VEL 1.6 mg/m2 were administered i.v. on days −2 and −1, respectively.
Patients received prophylaxis for oral mucositis with palifermin: 2 doses of 6.25 mg were administered by i.v. bolus injection for 2 consecutive days before the first BU dose (days −8 and −7), and a third dose of 6.25 mg was administered on day 0 after stem cell infusion. This study was approved by the Loyola University Chicago Stritch School of Medicine Institutional Review Board, and all patients voluntarily signed informed consent.
STUDY DESIGN
Data from this phase I/II clinical trial in MM patients transplanted at Loyola University Chicago Medical Center using the BUMELVEL conditioning regimen were compared against a matched control cohort of contemporaneous North American MM patients (n = 162) receiving single-agent MEL 200 conditioning. Only patients who received the predefined maximum tolerated dose were included in the comparison analysis. The control subjects were identified from the Center for International Blood and Marrow Transplant Research (CIBMTR) database. The comparison was done on a 1:3 match (Loyola-to-CIBMTR). Control subjects were randomly selected and matched by age, sex, Karnofsky performance status (KPS), disease stage, interval from diagnosis to AHSCT, and disease status before AHSCT. Fifty-four centers, not including the study center, contributed with patients for the control group. Multivariate analysis of relapse, PFS, and overall survival (OS) was performed. Maintenance therapy was not administered to patients or control subjects.
Control Cohort Database
The CIBMTR is a research affiliation of the International Bone Marrow Transplant Registry and the National Marrow Donor Program (NMDP) and receives data from over 500 transplantation centers worldwide on allogeneic and autologous hematopoietic stem cell transplantation. Data are submitted to the Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis, where computerized checks for discrepancies, physicians’ reviews of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed with approval of the institutional review boards of the NMDP and the Medical College of Wisconsin.
Statistical Analysis
The primary endpoint of the study was the 1-year PFS after a myeloablative preparative regimen consisting of i.v. BUMELVEL versus MEL 200. Using a 1:3 match comparison, the study included 43 patients on the BUMELVEL regimen and 162 patients from the CIBMTR database. Descriptive statistics were used to report results including demographics, disease-related factors, transplant-related factors, incidence and severity of mucositis, incidence and severity of SOS, remission rates, and relapse rates. Survival analysis was done using a Cox proportional hazards regression to adjust for differences between the groups. P values were always 2-tailed and considered significant when <.05.
Medians and ranges are listed for continuous variables. The total number of patients and the percentage of each subgroup were calculated for categorical variables. Characteristics of patients in the 2 study cohorts were compared using the Mann-Whitney-Wilcoxon test for continuous variables and chi-square test for discrete variables. For discrete variables with small group size, the Fisher’s exact test was used for comparison. Probability of PFS and OS was calculated using the Kaplan-Meier estimator, with the variance estimated by the Greenwood’s formula. Probabilities of treatment-related mortality (TRM) and relapse were generated using cumulative incidence estimates to accommodate the competing risk event. The point-wise comparison was used to analyze outcomes of 2 study cohorts. All tests were 2-sided with a significant level of .05.
Multivariate analysis of TRM, relapse, PFS, and OS were performed using Cox proportional hazards regression models. The variables considered in the multivariable were preparative regimen, age, gender, KPS, isotype, international stage for MM, Mayo risk stratification at diagnosis, number of prior chemotherapy regimens before transplantation, chemotherapy regimens before transplantation, disease status before transplantation, time from diagnosis to transplantation, and year of transplantation. The assumption of proportional hazards for each factor in the Cox model was tested using time-dependent covariates. A backward stepwise model selection approach was used to identify all significant risk factors. Each step of model building contained the main effect for 2 different regimens. Factors significant at a 5% level were kept in the final model. The potential interactions between main effects and all significant risk factors were tested.
RESULTS
Patient Characteristics
Both cohorts were balanced for age, gender, KPS, MM isotypes, time from diagnosis to transplantation, disease stage, and disease status before transplantation (Table 1). Patient demographics in the BUMELVEL and MEL 200 groups included the following: median age 62 years and 61 years, respectively; KPS ≥ 90% in 74% and 75%, respectively; and chemotherapy-sensitive disease before transplantation in 95% and 91%, respectively. All patients underwent AHSCT within 12 months from diagnosis.
Table 1.
Characteristics of Patients Who Underwent Single Autologous Transplant with i.v. BU, MEL Followed by VEL and High-Dose MEL between 2009 and 2012
| Characteristics | BUMELVEL | MEL 200 | P |
|---|---|---|---|
| Number of patients | 43 | 162 | |
| Number of centers | 1 | 54 | |
| Age at transplant, median (range), yr |
62 (46–69) | 61 (41–69) | |
| 18–59 | 9 (21) | 56 (35) | .17 |
| 60–64 | 17 (40) | 61 (38) | |
| 65–70 | 17 (40) | 45 (28) | |
| Gender | |||
| Male | 24 (56) | 91 (56) | .97 |
| Female | 19 (44) | 71 (44) | |
| KPS at transplant | |||
| ≤80 | 11 (26) | 40 (25) | 1 |
| 90–100 | 32 (74) | 122 (75) | |
| Isotype | |||
| IgG | 26 (60) | 100 (62) | .86 |
| IgA | 8 (19) | 32 (20) | |
| Light chain | 7 (16) | 27 (17) | |
| IgD | 1 (2) | 1 (<1) | |
| Nonsecretory | 1 (2) | 2 (1) | |
| International stage at transplant | |||
| Stage I | 16 (37) | 51 (31) | .74 |
| Stage II/III | 20 (47) | 79 (53) | |
| Unknown | 7 (16) | 25 (15) | |
| Mayo risk stratification at diagnosis (mSMART) |
|||
| Standard risk | 17 (40) | 127 (78) | <.0001 |
| High risk | 4 (9) | 18 (11) | |
| Unknown | 22 (51) | 17 (10) | |
| Number of chemotherapy sessions | |||
| 1 | 20 (47) | 108 (67) | .02 |
| >1 | 23 (53) | 54 (33) | |
| Disease status before AHSCT | |||
| CR | 3 (7) | 33 (20) | .19 |
| VGPR | 15 (35) | 43 (27) | |
| PR | 23 (53) | 72 (44) | |
| Stable disease | 1 (2) | 11 (7) | |
| Relapse/progression | 1 (2) | 3 (2) | |
| Median follow-up of survivors (range), mo |
25 (2–50) | 35 (3–50) |
Values are number of cases with percents in parentheses, unless otherwise noted.
Of note, the MEL 200 control cohort had more standard-risk patients per Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) [10] (78% versus 40% in BUMELVEL, P < .0001) and more patients with only 1 prior line of therapy pre-AHSCT (67% versus 47%, P =.02). Patients in the BUMELVEL group had received induction combination regimens involving VEL, lenalidomide, and dexamethasone (51%); VEL and dexamethasone (35%); or VEL, thalidomide, and dexamethasone (14%) before AHSCT. At the time of transplantation, 3 (7%) and 15 (35%) patients were in CR and very good partial remission (VGPR), respectively. Median follow-up for the BUMELVEL and MEL 200 cohorts were 25 months and 35 months, respectively. Sixty-two percent of the control group received VEL either as a doublet or in combination with thalidomide or lenalidomide. Thirty-six percent received induction therapy with other novel agents consisting of doublets with thalidomide or lenalidomide.
Outcomes
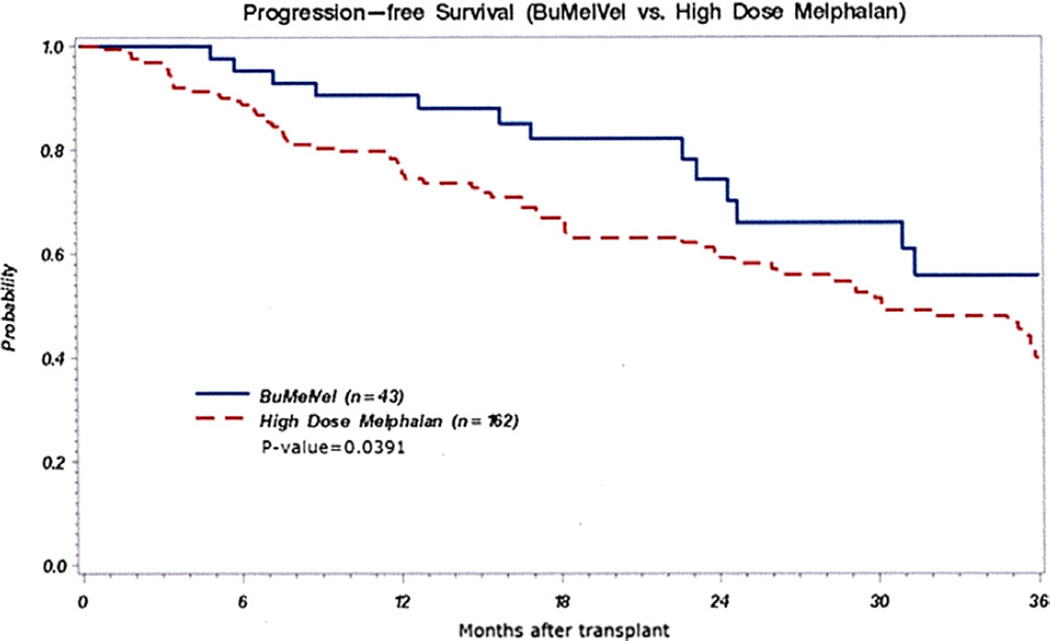
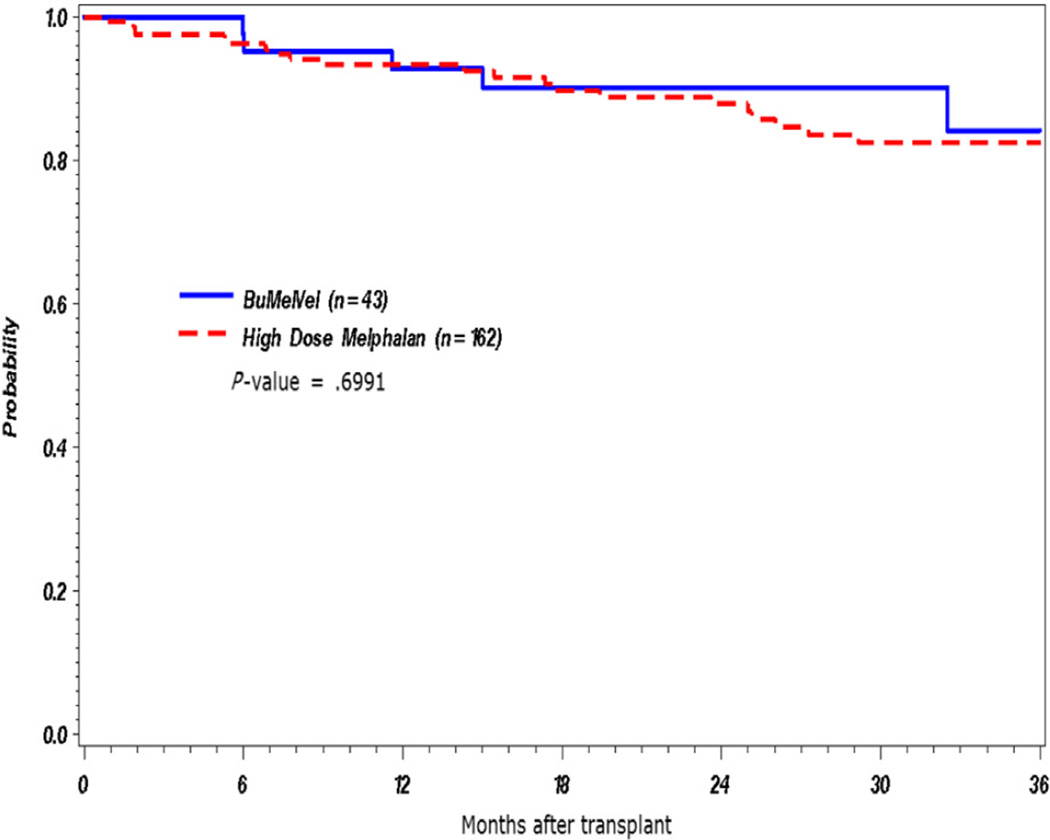
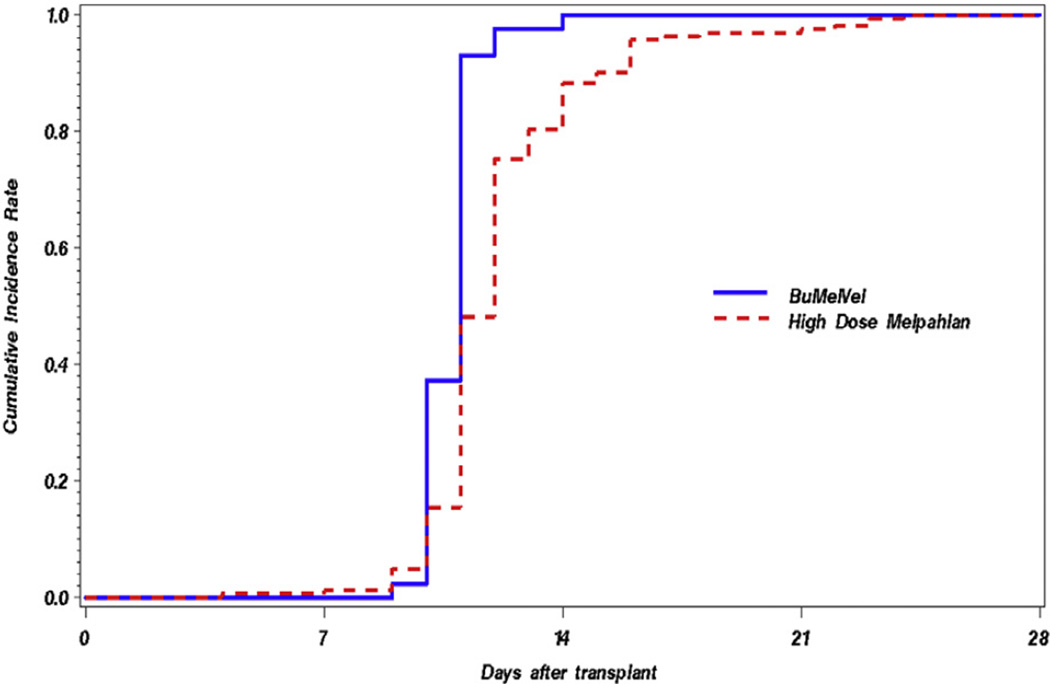
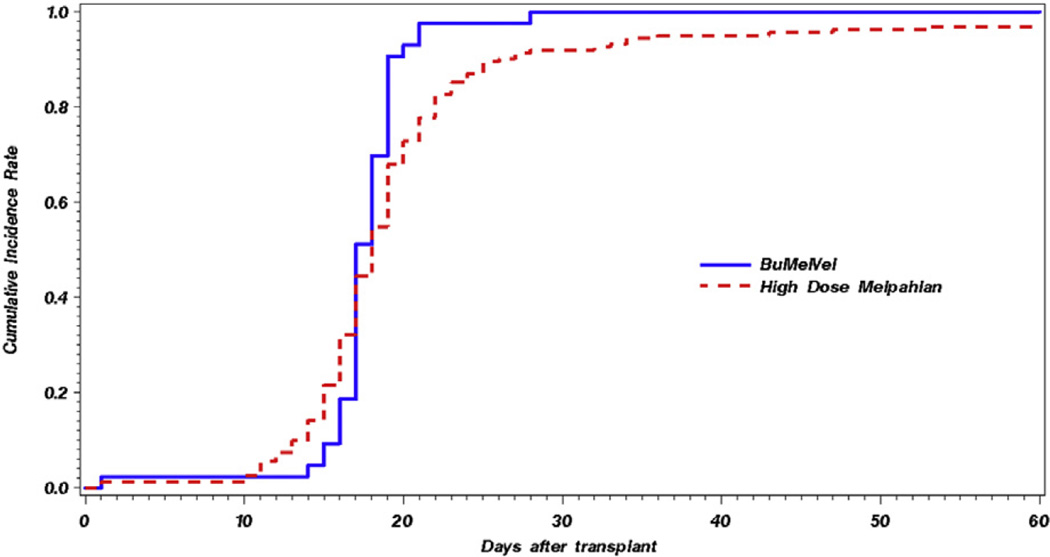
The BUMELVEL regimen resulted in an overall response rate of 98%, including at least VGPR in 70% and CR in 42% (Table 2). At 1 year post-AHSCT, 90% of patients on the BUMELVEL cohort remained progression free in comparison with 77% of MEL 200 recipients (P = .02) (Figure 1). OS was similar between both cohorts (93% versus 93% at 1 year; P =.89) (Figure 2). Cumulative incidence of relapse at 1 year was lower in the BUMELVEL group versus MEL 200 (10% versus 21%; P = .047). Neutrophil and platelet engraftment kinetics were similar between both groups (Figures 3 and 4).
Table 2.
Response Status before and after AHSCT using BUMELVEL Regimen
| Response Status | Patients before AHSCT | Patients after AHSCT |
|---|---|---|
| CR | 3 (7%) | 18 (42%) |
| VGPR | 15 (35%) | 12 (28%) |
| PR | 23 (53%) | 12 (28%) |
| Less than PR | 2 (4%) | 1 (2%) |
Figure 1.
PFS in BUMELVEL versus high-dose MEL.
Figure 2.
OS in BUMELVEL versus high-dose MEL.
Figure 3.
Cumulative incidences of neutrophil engraftment in BUMELVEL versus high-dose MEL.
Figure 4.
Cumulative incidences of platelet engraftment in BUMELVEL versus high-dose MEL.
In multivariate analysis, PFS was superior in the BUMELVEL cohort (hazard ratio for relapse/death, 1.87 for MEL 200 cohort; P =.04). BUMELVEL therapy was not associated with any difference in OS or relapse risk at the time of the analysis. Patients who achieved at least a VGPR before AHSCT had a superior PFS post-AHSCT (CR, 1.000 [95% confidence interval {CI}, 1.000 to 1.000]; VGPR, 1.983 [95% CI, .876 to 4.489]; PR, 2.668 [95% CI, 1.260 to 5.652]; and stable disease, 3.468 [95% CI, 1.337 to 8.996]), whereas lower KPS (≤80) and higher international stage were associated with inferior OS (relative risk for OS, 2.283 [95% CI,1.093 to 4.769] for KPS ≤ 80, P =.02; hazard ratio for OS, 3.568 [95% CI, 1.326 to 9.598] for stages II to III, P = .0086).
Regimen-Related Toxicity
There was no TRM in the BUMELVEL group and no episodes of SOS disease. There was a small but statistically significant TRM in the MEL cohort (relative risk for TRM, .03 [95% CI, .01 to .06]). The most common grade 3 adverse events (Table 3) included the expected febrile neutropenia, mucositis, and hypophosphatemia. Other adverse events presenting in less than 10% of the patients on the BUMELVEL group were diarrhea, nausea, hypocalcemia, transaminitis, and hyperglycemia. The median hospital stay for the BUMELVEL group was 19 days.
Table 3.
BUMELVEL Toxicities per National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03
| Toxicities | No. of Cases |
|---|---|
| Grade 3 | |
| Febrile neutropenia | 33 (77%) |
| Mucositis | 16 (37%) |
| Hypophosphatemia | 8 (19%) |
| Diarrhea | 4 (9%) |
| Nausea | 4 (9%) |
| Hypocalcemia | 3 (7%) |
| Transaminitis | 3 (7%) |
| Hyperglycemia | 2 (5%) |
| Grade 4 | |
| Hypocalcemia | 2 (5%) |
| Mucositis | 2 (5%) |
| Transaminitis | 1 (2%) |
Dose Targeting of BU
The first 2 daily infusions of BU on the BUMELVEL regimen were given at a fixed dose of 130 mg/m2 over 3 hours from days −6 to −5. This dose has been found to be safe and equivalent to the standard daily dose of 3.2 mg/kg [11]. The third and fourth daily doses of i.v. BU were adjusted to yield a systemic plasma drug exposure, represented by a targeted AUC of 5000 µM·min per dose for a total of 20,000 µM·min. Only 23% of patients had an AUC outside an acceptable range of 5000 µM·min ± 20% (<4000 or >6000 µM·min). Doses were adjusted on days −4 and −3 to achieve the total desired AUC of 20,000 µM·min.
DISCUSSION
High-dose chemotherapy followed by AHSCT is considered a standard approach by the International Myeloma Working Group for transplant-eligible MM patients. The addition of novel agents, like the immunomodulatory drugs thalidomide, lenalidomide, and pomalidomide and the proteasome inhibitors VEL and carfilzomib, in treatment paradigms has led to unprecedented survival improvement in patients with MM [12–14].
In the context of stem cell transplantation for MM, there is a relationship between the achievement of CR or VGPR and PFS or OS [15]. VEL-based induction regimens result in significant improvements in response, PFS, and OS compared with non-VEL-based induction regimens [16]. In our study 100% of patients in the BUMELVEL cohort received induction with VEL combination regimens before AHSCT versus 62% of patients in the control group. Because control subjects were matched with patients for disease status before transplantation, we believe the lack of VEL in the induction therapy is not impacting the outcomes observed. More patients in the control group were in CR prior to transplantation compared with the BUMELVEL group (20% versus 7%, respectively). The higher CR rate in the control group might be related to a higher representation of standard-risk patients per mSMART and more patients with only 1 prior line of therapy pre-AHSCT in this group.
The study group was treated before the availability of other proteasome inhibitors. Carfilzomib, which was approved by the US Food and Drug Administration in 2012 for the treatment of patients with MM who have received at least 2 prior therapies, including VEL and an immunomodulatory agent, has shown activity in patients with newly diagnosed as well as relapsed or refractory MM [17]. In a randomized, phase III, open-label, multicenter study for patients with relapsed or refractory MM, carfilzomib with dexamethasone was found to deliver better response and PFS rates when compared with VEL with dexamethasone [18]. These observations suggest that carfilzomib-based regimens could deliver better responses before AHSCT in comparison with VEL-based regimens. Obviously, this will need to be validated in prospective clinical trials while paying special attention to therapeutic index.
Despite achievements of impressive response rates after inductions with novel therapy regimes, AHSCT continues to deliver improvement in PFS and OS as consolidation of these responses [19]. A phase II study of extended treatment with carfilzomib, lenalidomide, and dexamethasone (KRd) plus AHSCT in newly diagnosed MM patients showed that this regimen resulted in higher stringent CR rates than KRd without AHSCT. There was also a higher rate of minimal residual disease negativity in the transplantation group [20]. The improvement in response rate after induction was observed in our analysis where the CR plus VGPR after BUMELVEL followed by AHSCT increased from 42% pre-transplantation to 70% post-AHSCT. Our analysis suggests that the novel preparative regimen BUMELVEL followed by AHSCT is a complementary, nonredundant therapy that can be effectively included in the management of MM supporting the trends in utilization and outcomes of autologous transplantation as a therapy for MM [2].
In an older study reported by Mansi et al. [21], a 46% response rate was observed after high-dose single-agent oral BU (16 mg/kg) followed by AHSCT in heavily pretreated patients with MM. The absorption of oral BU is unpredictable and may lead to unacceptable nonhematologic toxicity. We used an i.v. BU formulation for our regimen that has been found to deliver effective myeloablation with less nonhematologic toxicity and higher 100-day survival compared with oral BU [22]. Single-agent high-dose MEL 200 has been used almost exclusively as the preferred preparative regimen for MM since a randomized study established the superiority of this regimen over MEL 140 mg/m2 with total body radiation [23].
There is now evidence of clonal heterogeneity and clonal evolution throughout the natural history of MM [24]. Based on these observations, a response to therapy might represent the suppression of a sensitive clone, whereas resistant clones remain unperturbed and become proportionally more dominant over time, leading to inevitable relapse. This rationale supports the development of preparative regimens combining synergistic agents to achieve deeper responses to circumvent the possibility of heterogeneous resistant clones leading to relapse while maintaining an acceptable therapeutic index. The combination of BU with either MEL or cyclophosphamide has been used for over 20 years as an alternative preparative regimen in MM before AHSCT [25–28].
Proteasome inhibitors such as VEL have a consistent anti-tumor activity against chemoresistant and chemosensitive myeloma cells. The sensitivity of chemoresistant myeloma cells to this chemotherapeutic agent is markedly increased (100,000- to 1,000,000-fold) without affecting normal hematopoietic cells [29]. This observation allowed us to deliver this drug 24 hours before the stem cell infusion without potentially affecting engraftment. We did not observe graft failure or delayed engraftment in the BUMELVEL cohort.
It has been suggested that VEL up-regulates the anti-apoptotic protein MCL-1, and the sequence of administration may be critical to the combination of VEL and MEL 200 [30]. Doses of VEL were escalated from 1.0 mg/m2 up to 1.6 mg/m2. The increase in apoptosis on samples obtained from patients who were treated with MEL followed by VEL was superior to the apoptosis observed with VEL preceding MEL [31]. The combination of MEL and VEL has been found to be effective in the relapse setting as well [32,33].
Nishihori et al. [34] completed a phase I/II study of VEL in combination with MEL followed by tandem autologous transplants in primary refractory MM patients. However, with the availability of new potent novel agents, the role of tandem transplantation in patients with MM is in question.
Our novel combination of BUMELVEL delivered an impressive overall response rate of 98%, including at least a VGPR of 70% and a CR rate of 42%. These responses compare favorably with reported responses using single-agent MEL 200 (20% to 40% CR and 40% to 55% CR/VGPR) [15]. The primary endpoint of the study, 1-year PFS, was significantly improved in the BUMELVEL cohort in comparison with single-agent MEL 200 (90% versus 77%, respectively; P =.02). The improvement in PFS was achieved despite a higher proportion of standard-risk patients in the control group in comparison with the BUMELVEL cohort by mSMART criteria and more patients with >1 prior line of therapy pre-AHSCT in the BUMELVEL group. OS was similar between the 2 groups, probably due to the relatively short median duration of follow-up and the multitude of treatment options available in relapsed MM. The main adverse events were manageable and included neutropenic fever, mucositis, and hypophosphatemia. Adverse events did not translate into transplant-related mortality. The incidence of febrile neutropenia (77%) is similar to that reported by Lahuerta using BU and MEL [21]. Among recipients of high-dose chemotherapy in high-risk protocols (eg, BU, cyclophosphamide, etoposide), severe mucositis is reported in excess of 60% to 90% [35]. In our study only 37% and 2% of patients developed grade 3 and grade 4 mucositis, respectively. Thus, collectively, the addition of VEL to BUMEL does not appear to increase adverse events. The lower incidence of adverse events may be due to the use of a targeted dose of BU and the incorporation of palifermin as a mucoprotectant.
Engraftment was prompt and predictable and was not different from historical control subjects with single-agent MEL 200. Moreover, the once-daily dosing of BU allowed us to perform outpatient transplantation using the BUMELVEL regimen.
Our analysis has the limitations of being a case-control retrospective comparison with a registry population. This type of analysis could potentially introduce a selection bias through center effects. However, no center effect has ever been identified in autologous transplant studies for MM in the CIBMTR. The improvement in PFS observed in the BUMELVEL cohort could be related to the targeted BU therapy used in this regimen, the synergism observed in prior studies between MEL and VEL, or both. Randomized prospective clinical trials would probably help in answering these questions.
In conclusion, pharmacokinetic-directed dosing of BU can be safely combined with MEL 140 mg/m2 and VEL 1.6 mg/m2 (BUMELVEL) without adding nonhematologic toxicity or transplant-related mortality. This novel regimen delivered high response rates and a better PFS compared with MEL 200 and warrants further study in a prospective randomized clinical trial.
Acknowledgments
Financial disclosure: This research was supported by Otsuka Pharmaceutical Co, Ltd.
T.E.R. received research funding, consultancy, and honoraria from Otsuka and Takeda and honoraria and consultancy from Celgene and Onyx. P.H. received consultancy, honoraria, and research funding from BMS, Celgene, Onyx, and Takeda; honoraria from Janssen; and honoraria and research funding from Sanofi. P.J.S. received research funding from Amgen, Eisai, Gilead, Plasmacyclics, Incyte, Seattle Genetics, and Fate Therapeutics; consultancy and honoraria from Gilead, Plasmacyclics, Incyte, and Seattle Genetics. S.E.S. received research funding from Seattle Genetics and consultancy and honoraria from Celgene. D.H.V. received honoraria from Millennium, Takeda, and Celgene and research funding and honoraria from Onyx/Amgen.
Footnotes
Conflict of interest statement: D.S. reports no conflicts of interest.
REFERENCES
- 1.Qazilbash MH, Giralt SA. Hematopoietic cell transplantation for multiple myeloma. In: Appelbaum FR, Forman SJ, Negrin RS, Blume KG, editors. Thomas’ hematopoietic cell transplantation. 4th. West Sussex, UK: Blackwell; 2004. pp. 2295–2307. [Google Scholar]
- 2.Costa LJ, Zhang MJ, Zhong X, et al. Trends in utilization and outcomes of autologous transplantation as early therapy for multiple myeloma. Biol Blood Marrow Transplant. 2013;19:1615–1624. doi: 10.1016/j.bbmt.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schallier D, Impens N, Warson D, et al. Additive pulmonary toxicity with melphalan and busulfan therapy. Chest. 1983;84:492–493. doi: 10.1378/chest.84.4.492. [DOI] [PubMed] [Google Scholar]
- 4.Lahuerta JJ, Grande C, Blade J. Myeloablative treatments for multiple myeloma: update of a comparative study of different regimens used in patients from the Spanish registry for transplantation in multiple myeloma. Leuk Lymph. 2002;43:67–74. doi: 10.1080/10428190210194. [DOI] [PubMed] [Google Scholar]
- 5.Lahuerta JJ, Mateos MV, Martínez-Lópezet J, et al. Busulfan 12 mg/kg plus melphalan 140 mg/m2 versus melphalan 200 mg/m2 as conditioning regimens for autologous transplantation in newly diagnosed multiple myeloma patients included in the PETHEMA/GEM2000 study. Haematologica. 2010;95:1913–1920. doi: 10.3324/haematol.2010.028027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lonial S, Kaufman K, Tighiouart M, et al. A phase I/II trial combining high dose melphalan and autologous transplant with bortezomib for multiple myeloma: dose and schedule finding study. Clin Cancer Res. 2010;16:5079–5086. doi: 10.1158/1078-0432.CCR-10-1662. [DOI] [PubMed] [Google Scholar]
- 7.Durie BGM, Harousseau J-L, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–1473. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 8.Jones RJ, Lee KS, Beschorner WE, et al. Venoocclusive disease of the liver following bone marrow transplantation. Transplantation. 1987;44:778–783. doi: 10.1097/00007890-198712000-00011. [DOI] [PubMed] [Google Scholar]
- 9.Dix SP, Wingard JR, Mullins RE, et al. Association of busulfan area under the curve with veno-occlusive disease following BMT. Bone Marrow Transplant. 1996;17:225–230. [PubMed] [Google Scholar]
- 10.Kumar SK, Mikhael JR, Buadi FK, et al. Management of newly diagnosed symptomatic multiple myeloma: updated Mayo Stratification of Myeloma and Risk-Adapted Therapy (mSMART) consensus guidelines. Mayo Clin Proc. 2009;84:1095–1110. doi: 10.4065/mcp.2009.0603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Lima M, Couriel D, Shahjahan M. IV busulfan (Bu) with fludarabine (Flu) or cyclophosphamide (Cy)—comparing ablative preparative regimens for allogeneic transplantation in AML/MDS. Blood. 2004;104:11. [Google Scholar]
- 12.Kumar SK, Rajkumar SV, Dispenzieri A, et al. Improved survival in multiple myeloma and the impact of novel therapies. Blood. 2008;111:2516–2520. doi: 10.1182/blood-2007-10-116129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kumar SK, Dispenzieri A, Lacy MQ, et al. Continued improvement in survival in multiple myeloma: changes in early mortality and outcomes in older patients. Leukemia. 2014;28:1122–1128. doi: 10.1038/leu.2013.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Usmani SZ, Waheed S, Van Rhee F, et al. Total therapy 5 (TT5) for newly diagnosed high-risk multiple myeloma (HRMM): comparison with predecessor trials total therapy 3a and 3b (TT3 a/b) J Clin Oncol. 2013;31(Suppl):8539. [Google Scholar]
- 15.Harousseau JL. Induction therapy in multiple myeloma. Hematol Am Soc Hematol Educ Progr. 2008;2008:306–312. doi: 10.1182/asheducation-2008.1.306. [DOI] [PubMed] [Google Scholar]
- 16.Sonneveld P, Goldschmidt H, Rosiñol L, et al. Bortezomib-based versus nonbortezomib-based induction treatment before autologous stem-cell transplantation in patients with previously untreated multiple myeloma: a meta-analysis of phase III randomized, controlled trials. J Clin Oncol. 2013;31:3279–3287. doi: 10.1200/JCO.2012.48.4626. [DOI] [PubMed] [Google Scholar]
- 17.Moreau P, Kolb B, Attal M, et al. Phase 1/2 study of carfilzomib plus melphalan and prednisone in patients aged over 65 years with newly diagnosed multiple myeloma. Blood. 2015;125:3100–3104. doi: 10.1182/blood-2015-02-626168. [DOI] [PubMed] [Google Scholar]
- 18.Dimopoulos MA, Moreau P, Palumbo A, et al. Carfilzomib and dexamethasone versus bortezomib and dexamethasone for patients with relapsed or refractory multiple myeloma (ENDEAVOR): a randomised, phase 3, open-label, multicentre study. Lancet Oncol. 2016;17:27–38. doi: 10.1016/S1470-2045(15)00464-7. [DOI] [PubMed] [Google Scholar]
- 19.Palumbo A, Cavallo F, Gay F, et al. Autologous transplantation and maintenance therapy in multiple myeloma. N Engl J Med. 2014;371:895–905. doi: 10.1056/NEJMoa1402888. [DOI] [PubMed] [Google Scholar]
- 20.Zimmerman TM, Griffith KA, Jasielec J, et al. Phase II MMRC trial of extended treatment with carfilzomib (CFZ), lenalidomide (LEN), and dexamethasone (DEX) plus autologous stem cell transplantation (ASCT) in newly diagnosed multiple myeloma (NDMM) J Clin Oncol. 2015;33(Suppl):8510. [Google Scholar]
- 21.Mansi J, da Costa F, Viner C, et al. High-dose busulfan in patients with myeloma. J Clin Oncol. 1992;10:1569–1573. doi: 10.1200/JCO.1992.10.10.1569. [DOI] [PubMed] [Google Scholar]
- 22.Kashyap A, Wingard J, Cagnoni P, et al. Intravenous versus oral busulfan as part of a busulfan/cyclophosphamide preparative regimen for allogeneic hematopoietic stem cell transplantation: decreased incidence of hepatic venoocclusive disease (HVOD), HVOD-related mortality, and overall 100-day mortality. Biol Blood Marrow Transplant. 2002;8:493–500. doi: 10.1053/bbmt.2002.v8.pm12374454. [DOI] [PubMed] [Google Scholar]
- 23.Moreau P, Facon T, Attal M, et al. Comparison of 200 mg/m(2) melphalan and 8 Gy total body irradiation plus 140 mg/m(2) melphalan as conditioning regimens for peripheral blood stem cell transplantation in patients with newly diagnosed multiple myeloma: final analysis of the Intergroupe Francophone du Myélome 9502 randomized trial. Blood. 2002;99:731–735. doi: 10.1182/blood.v99.3.731. [DOI] [PubMed] [Google Scholar]
- 24.Keats JJ, Chesi M, Egan JB, et al. Clonal competition with alternating dominance in multiple myeloma. Blood. 2012;120:1067–1076. doi: 10.1182/blood-2012-01-405985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Toor AA, Ayers J, Strupeck J, et al. Favourable results with a single autologous stem cell transplant following conditioning with busulphan and cyclophosphamide in patients with multiple myeloma. Br J Haematol. 2004;124:769–776. doi: 10.1111/j.1365-2141.2004.04837.x. [DOI] [PubMed] [Google Scholar]
- 26.Cavo M, Bandini G, Benni M, et al. High-dose busulfan and cyclophosphamide are an effective conditioning regimen for allogeneic bone marrow transplantation in chemosensitive multiple myeloma. Bone Marrow Transplant. 1998;22:27–32. doi: 10.1038/sj.bmt.1701280. [DOI] [PubMed] [Google Scholar]
- 27.Hamon MD, Donohue SM, Franklin IM. Therapeutic progress—review XXVII. High dose chemotherapy in haematological malignancy. J Clin Pharm Ther. 1987;12:203–211. doi: 10.1111/j.1365-2710.1987.tb00529.x. [DOI] [PubMed] [Google Scholar]
- 28.Phillips GL, Shepherd JD, Barnett MJ, et al. Busulfan, cyclophosphamide, and melphalan conditioning for autologous bone marrow transplantation in hematologic malignancy. J Clin Oncol. 1991;9:1880–1888. doi: 10.1200/JCO.1991.9.10.1880. [DOI] [PubMed] [Google Scholar]
- 29.Ma MH, Yang HH, Parker K. The proteasome inhibitor PS-341 markedly enhances sensitivity of multiple myeloma tumor cells to chemotherapeutic agents. Clin Cancer Res. 2003;9:1136–1144. [PubMed] [Google Scholar]
- 30.Podar K, Gouill SL, Zhang J, et al. A pivotal role for Mcl-1 in bortezomib-induced apoptosis. Oncogene. 2008;27:721–731. doi: 10.1038/sj.onc.1210679. [DOI] [PubMed] [Google Scholar]
- 31.Lonial S, Kaufman J, Tighiouart M, et al. A phase I/II trial combining high-dose melphalan and autologous transplant with bortezomib for multiple myeloma: a dose- and schedule-finding study. Clin Cancer Res. 2010;16:5079–5086. doi: 10.1158/1078-0432.CCR-10-1662. [DOI] [PubMed] [Google Scholar]
- 32.Roussel M, Moreau P, Huynh A, et al. Bortezomib and high-dose melphalan as conditioning regimen before autologous stem cell transplantation in patients with de novo multiple myeloma: a phase 2 study of the Intergroupe Francophone du Myelome (IFM) Blood. 2010;115:32–37. doi: 10.1182/blood-2009-06-229658. [DOI] [PubMed] [Google Scholar]
- 33.Doo NW, Thompson PA, Price HM. Bortezomib with high dose melphalan conditioning for autologous transplant is safe and effective in patients with heavily pretreated and high risk multiple myeloma. Leuk Lymphoma. 2013;54:1465–1472. doi: 10.3109/10428194.2012.746682. [DOI] [PubMed] [Google Scholar]
- 34.Nishihori T, Alekshun TJ, Shain K, et al. Bortezomib salvage followed by a Phase I/II study of bortezomib plus high-dose melphalan and tandem autologous transplantation for patients with primary resistant myeloma. Br J Haematol. 2012;157:553–563. doi: 10.1111/j.1365-2141.2012.09099.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pico JL, Avila-Garavito A, Naccache P, et al. Mucositis: its occurrence, consequences, and treatment in the oncology setting. Oncologist. 1998;3:446–451. [PubMed] [Google Scholar]