Abstract
The diagnostic approach to a possible pancreatic mass lesion relies first upon various non-invasive imaging modalities, including computed tomography, ultrasound, and magnetic resonance imaging techniques. Once a suspect lesion has been identified, tissue acquisition for characterization of the lesion is often paramount in developing an individualized therapeutic approach. Given the high prevalence and mortality associated with pancreatic cancer, an ideal approach to diagnosing pancreatic mass lesions would be safe, highly sensitive, and reproducible across various practice settings. Tools, in addition to radiologic imaging, currently employed in the initial evaluation of a patient with a pancreatic mass lesion include serum tumor markers, endoscopic retrograde cholangiopancreatography, and endoscopic ultrasound-guided fine needle aspiration (EUS-FNA). EUS-FNA has grown to become the gold standard in tissue diagnosis of pancreatic lesions.
Keywords: Endoscopic ultrasound, Fine needle aspiration, Pancreatic cancer, Pancreatic mass, Endoscopy
Core tip: Evidence-based techniques to increase the diagnostic yield during endoscopic ultrasound-guided fine needle aspiration (FNA) of pancreatic masses include: (1) use of general anesthesia; (2) use smaller (22 or 25G) needles for transduodenal FNA; (3) use If histology is desired, use 19G or core biopsy needles; (4) use suction; (5) use the “fanning technique”; and (6) use on-site cytopathologist or perform 7 needle passes.
INTRODUCTION
Peripheral blood tumor markers, among the least invasive diagnostic tests, are not yet useful in the initial evaluation of a patient with a pancreatic mass. Cancer antigen (CA) 19-9, the leading tumor marker used to monitor pancreatic adenocarcinoma, has sensitivity and specificity as low as 70% and 68% respectively in diagnosing pancreatic adenocarcinoma, which has led to recommendations that it not be used routinely for diagnosis of this condition[1,2]. The CA 19-9 marker is, however, useful for post-surgical cancer surveillance[3]. Because of this, a CA 19-9 level may be checked prior to any surgical intervention with curative intent and serum concentrations of the marker followed thereafter to detect disease recurrence.
Prior to the introduction of the EUS-FNA technique in the early 1990’s, pancreatic masses were diagnosed using ERCP and percutaneous biopsy techniques (Figure 1). ERCP is limited by a sensitivity of 49%-66% with pancreatic duct brushing, and a reported complication rate of pancreatitis up to 6%[4,5]. Use of CT or ultrasound guided biopsy carries a sensitivity of 62%-90% and specificity up to 100% with a randomized study demonstrating higher sensitivity (84%) for EUS-FNA compared to CT or ultrasound-guided biopsy (62%)[6-10]. In addition, the risk of tumor seeding into the peritoneum or along the percutaneous needle tract has led to avoidance of the percutaneous approach to tissue diagnosis, and studies have suggested a significantly lower risk of peritoneal carcinomatosis using EUS-FNA[11].
Figure 1.
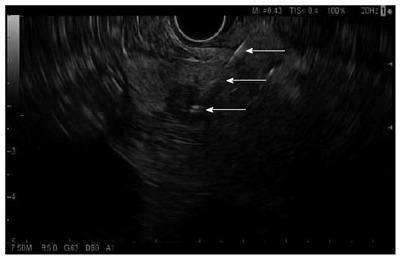
Endoscopic ultrasound-guided fine needle aspiration of a mass in the pancreatic head. Arrows show the needle course to the tip of the needle within the hypoechoic mass (bottommost arrow).
STANDARD OF CARE: EUS-FNA
EUS-FNA is a safe, effective and efficient diagnostic tool in the evaluation of pancreatic mass lesions (Figure 2). Cytopathological specimens, and more recently core biopsies, may be obtained with high sensitivity (75%-98%), specificity (71%-100%), positive predictive value (96%-100%), negative predictive value (33%-85%) and accuracy (79%-98%) in the diagnosis of pancreatic cancer as compared to other modalities (Table 1)[12-16]. The one caveat to the high diagnostic yield of EUS-FNA is in the presence of chronic pancreatitis where sensitivity decreases to 74% compared to 91% with normal surrounding pancreatic parenchyma[17]. Studies have shown that repeating EUS-FNA does improve diagnostic yield by enabling definitive diagnosis in about 63%-84% of patients[18-20]. Thus, EUS-FNA is the standard of care approach with repeat procedure recommended when the initial procedure is nondiagnostic.
Figure 2.
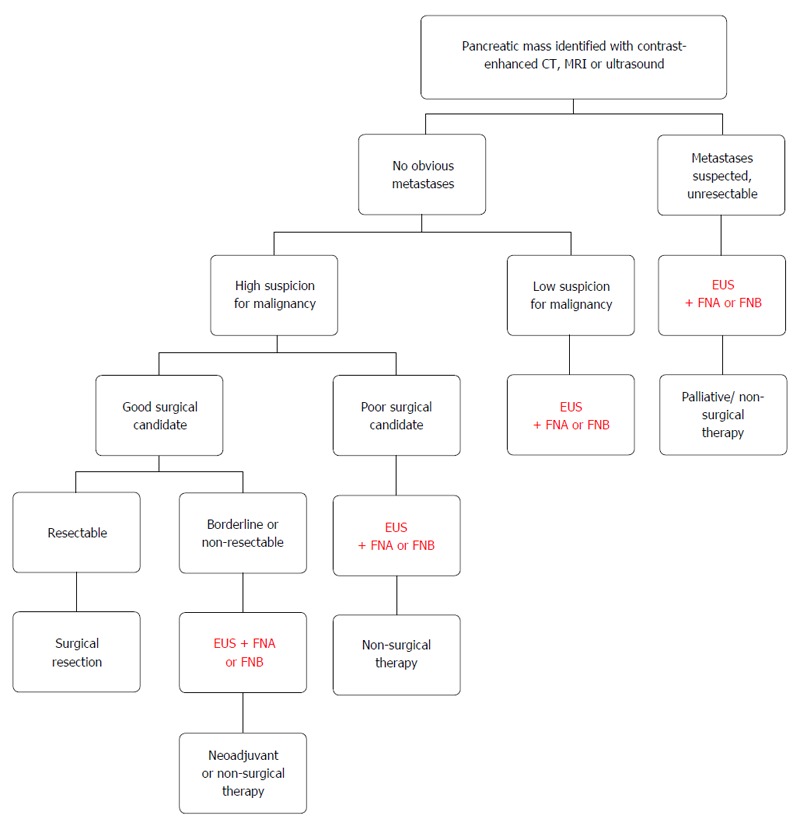
Algorithmic approach to a pancreatic mass.
Table 1.
Sensitivity and specificity of various diagnostic approaches to a pancreatic mass lesion
| Modality | Sensitivity | Specificity |
| CA 19-9 | 70%-92% | 68%-92% |
| CT | 77%-97% | 56%-89% |
| Transabdominal ultrasound | 89% | 99% |
| Percutaneous FNA | 62%-90% | 98%-100% |
| ERCP | 49%-66% | 96% |
| EUS-FNA | 75%-98% | 71%-100% |
| EUS-FNB | 85%-95% | 86%-100% |
EUS-FNA: Endoscopic ultrasound-guided fine needle aspiration; EUS-FNB: Endoscopic ultrasound-guided fine needle biopsy; ERCP: Endoscopic retrograde cholangiopancreatography.
EUS-FNA TECHNIQUE
Numerous studies have aimed at determining the ideal EUS-FNA equipment and techniques to obtain a diagnosis when evaluating a pancreatic mass. In basic principal, the target lesion is visualized by EUS, the most ideal lesion puncture approach is located, a chosen needle is advanced to puncture the lesion, the stylet is removed (if used), suction is applied (or not), the needle is advanced and withdrawn through the lesion to obtain cellular material, and finally the needle is removed and the tissue is collected for cytopathological examination.
Within this basic technique, more complex issues of scope positioning, selection of the puncture site, selection of the FNA needle, use of a stylet and suction, the technique of needle puncture, the number of needle punctures and use of an on-site cytopathologist have been studied (Table 2).
Table 2.
Techniques to increase diagnostic yield and decrease complications during endoscopic ultrasound-guided fine needle aspiration of a pancreatic mass
| Pre-procedural considerations | General anesthesia may increase yield |
| Goal platelet count greater than 50000 and INR less than 1.5 to reduce risk of bleeding | |
| Hold antiplatelet and antithrombotic agents except aspirin or NSAIDS | |
| Procedural Considerations | Take caution when duodenal diverticulum is present to reduce risk of perforation |
| Use Doppler to identify vasculature prior to needle advancement to avoid bleeding | |
| Use smaller (22 or 25) gauge needles for transduodenal FNA of the pancreatic head and uncinate | |
| If core histology samples needed, use 19G (in body or tail) or core biopsy needles | |
| Use suction | |
| Use the “fanning technique” during FNA | |
| Traverse the least amount of normal pancreatic tissue to reduce pancreatitis | |
| Specimen Processing | Use on-site cytopathology or perform 7 needle passes |
EUS-FNA: Endoscopic ultrasound-guided fine needle aspiration.
Scope positioning and puncture site
The first task in performing high quality EUS-FNA involves locating the target tissue and determining the ideal needle approach. Perhaps for this reason, use of general anesthesia has shown to be associated with increased diagnostic yield (83% with vs 73% without) during EUS-FNA of pancreatic mass lesions[21]. However this was a single center retrospective study and further study is required to confirm these results.
Limitations in approaching a pancreatic mass include difficult location, small size, necrosis and vascularity. Ideally the mass should be located in the six o’ clock position with the ultrasound transducer firmly applied to the luminal wall with suction. When possible, a transgastric approach is usually simplest as this avoids angulation of the scope permitting the needle to more easily pass through the biopsy channel. It may be difficult to advance the needle through the thicker gastric wall, which may be countered by suctioning the gastric wall, increasing the angle at which the needle passes through the gastric wall, and briskly advancing the needle. Acute angulation of the scope is often required when performing transduodenal FNA. Thus in these cases advancing the needle out of the biopsy channel may be more challenging, which makes smaller gauge needles (22 or 25 gauge) preferred with a transduodenal approach[22]. The needle should not be forced out of the echoendoscope, which may need to be withdrawn to reduce any loops in the instrument to advance the needle forward. A site with minimal intervening vasculature should be chosen through use of Doppler imaging to avoid bleeding complications, discussed later in this review.
Selection of FNA needle
There are a wide variety of EUS-FNA needles on the market, with the main differentiating factor being gauge (G) (Figure 3). These range from highly flexible and smaller 25G needles, to commonly used 22G and even larger 19G needles. Contrary to the mantra “bigger is better” studies have repeatedly shown that larger gauge needles may not provide more adequate diagnostic samples of target tissue within the pancreas[23-25]. In fact in one study, biopsy of lesions located within the pancreatic head and uncinate process showed a trend towards better diagnostic success with 25G needles over 22G needles[24]. Additionally, a meta-analysis comparing 22G and 25G needles for FNA of pancreatic masses found that sensitivity was significantly higher (93% vs 85%, P = 0.0003) with a 25G needle[25]. Numerous other studies have suggested that the diagnostic yield is not statistically different between 22G and 25G FNA needles[24,26-29]. One theme that rings true throughout the literature is that smaller gauge needles (22G and 25G) should be chosen when performing transduodenal FNA of the head and uncinate process of the pancreas given the significant bend and tension on the distal scope limiting movement of the needle. Larger needles, particularly 19G, carry higher technical failure rates in this situation, though without increase in rate of complications[23,30]. A prospective study evaluated the use of an algorithmic approach to choosing needle size in EUS-FNA, which recommended using 25G needles for transduodenal approach, 22G or 25G for transgastric approach and 19G or core needles when more tissue is required[31]. Following this algorithmic approach led to improved technical outcomes and cost savings without negatively impacting diagnostic accuracy. In general, for a transduodenal approach, a 22G or 25G FNA needle should be used while for a transgastric route, a 19G FNA needle may also be used especially if more tissue is desired. Core biopsies will be discussed further below, however, if the oncologists desire more tissue for molecular marker testing, the corresponding gauge core biopsy needle can be used.
Figure 3.
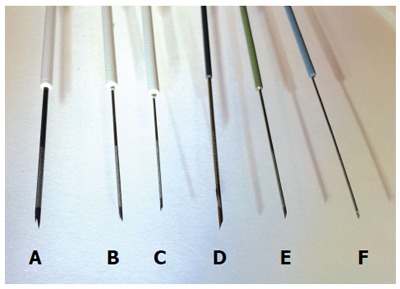
Representative endoscopic ultrasound biopsy and fine needle aspiration needles. A: 19G core biopsy needle; B: 22G core biopsy needle; C: 25G core biopsy needle; D: 19G FNA needle; E: 22G FNA needle; F: 25G FNA needle.
Different commercially available FNA needles have different echogenicity and appearance under EUS guidance. Readily visualizing the needle tip is critical to performing FNA. To improve visibility, needle tips are tailored by different techniques including laser etching, mechanical dimpling, or sandblasting. One large multicenter study involved multiple experienced endosonographers internationally who evaluated and ranked 10 different EUS needles in a bench top model based on their echogenicity and sharpness of distinction from the surrounding phantom. A prototype needle with polymeric coating had significantly higher overall ranking, which suggested that this coating to the needle tip and shaft may improve visualization[32].
Use of stylet and suction
A stylet is pre-loaded within all EUS-FNA needles with the intent of preventing sample contamination from the needle passing through other tissue prior to penetrating the target lesion. However, studies suggest that there is no difference in diagnostic success with or without use of the stylet[33-35]. Some EUS centers perform no-stylet FNA procedures, which means that the stylet is completely removed and not replaced during FNA. The stylet may be useful to the nurse assistant for advancing a tissue sample out of the needle after removal from the echoendoscope especially if air flushing fails. A randomized study found no difference in diagnostic samples or accuracy between air flushing or using the stylet to express the aspirate from the needle[36]. Other centers replace the stylet in between passes, withdraw it a little to sharpen the needle before puncture, and after entering the lesion, push the stylet in completely to expel any tissue collected at the tip of the needle.
Use of suction is also variable, though multiple studies agree that suction will increase the amount of target cellular material at the expense of a bloodier specimen[37,38]. If the cytology samples prove bloody, subsequent FNA passes should be performed with minimal or no suction. Also, the syringe vacuum suction must be turned off before withdrawing the needle from the lesion. One randomized study found that during EUS-FNA of solid lesions including pancreatic masses, sensitivity was significantly improved from 67% without suction to 86% with the use of 10 mL of suction[37]. Another prospective study of only pancreatic masses confirmed higher diagnostic samples with the use of suction during EUS-FNA[36]. Higher negative pressure suction (50 mL negative pressure) showed a trend toward increased diagnostic yield as compared to lower negative pressure (10 mL negative pressure) that did not reach significance[39]. A “wet suction” technique, where the stylet is removed, the FNA needle is preloaded with saline and then 10 mL of syringe vacuum applied during FNA has been proposed. In a prospective randomized trial of solid lesions with the majority being pancreatic masses, this wet suction technique significantly improved specimen adequacy compared with standard 10 mL syringe vacuum suction (86% vs 75%, P < 0.035)[40]. The “slow pull” technique, whereby the stylet is slowly withdrawn as the needle passes through the lesion to provide gentle capillary suction, has not proven superior when compared to standard syringe vacuum suction during FNA[41-43]. Suction does seem to improve diagnostic yield although whether different methods of suction application (simple syringe, “wet suction,” “slow pull”) makes a difference remains unclear.
Technique of needle puncture
The methods used during FNA are also hotly debated. Given that inner portions of a pancreatic tumor may be necrotic, targeting the peripheral areas of the mass may improve diagnostic yield. However, dense desmoplastic reaction at the periphery may also pose challenges to obtaining adequate tissue for diagnosis. Therefore, endosonographers advocate for the “fanning” technique, which involves adjusting the trajectory of the needle typically using the elevator and/or dials on the head of the echoendoscope (Figure 4), Thus, instead of advancing the needle back and forth through the same portion of the mass, it samples different areas. A randomized trial comparing fanning with standard tissue acquisition during EUS-FNA reported superiority of the fanning technique after a single pass (86% diagnostic yield) as compared to standard technique (58% diagnostic yield)[44]. Therefore, after puncturing the lesion, the needle should be advanced back and forth through as much of the lesion as possible about 12-15 times using the fanning technique, if possible. Other methods include the “door knocking method” where after puncturing the mass, the needle stopper is locked at a distance just short of the length from the tip of the needle to the most distal extent of the lesion, the needle quickly advanced through the mass until it hits the stopper, and slowly withdrawn to the opposite side of the mass. This sequence of rapid insertion and slow pullback is repeated until that needle pass is completed. A multicenter prospective assessment of the role of this technique in diagnostic yield found no difference in reaching a histologic diagnosis between the door knocking and conventional needle puncture methods[45].
Figure 4.
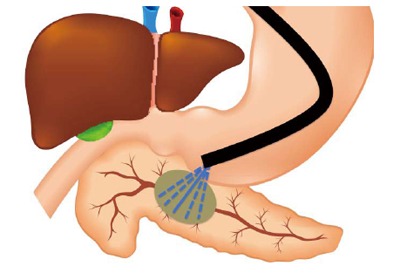
Schematization of the “fanning” technique for endoscopic ultrasound-guided fine needle aspiration. Dashed lines represent the change in course of the aspiration needle during each needle pass.
Role of on-site cytopathology
The diagnostic accuracy of EUS-FNA is reported to be over 90% in most studies when rapid on-site evaluation (ROSE) for cytopathology samples is employed[46-48]. With ROSE, the endosonographer typically makes one to two passes and then allows the pathologist to evaluate the sample smears for diagnostic yield. Further passes may be made as needed in order to achieve diagnostic success. If bloody aspirates are consistently seen by an onsite cytologist, switch to a smaller gauge needle without using suction. Older retrospective studies suggested decrease in nondiagnostic samples as well as need for repeat EUS with ROSE. Limitations of ROSE include increased cost due to cytopathologist time commitment, as well as limited access to cytopathologists and low reimbursements for ROSE[49].
In the absence of ROSE, several studies have concluded that 5-7 needle passes are ideal in order to achieve high diagnostic success[50-54]. In one large study, 5-6 passes achieved ROSE-level yields of 90%[50]. Another study using only 22G needles found that the sensitivity increased from 17% after the first pass to nearly 90% after the seventh pass, thus suggesting that 7 passes with a 22G needle may be required[51]. Yet another study using 25G needles suggested that four needle passes are sufficient[52]. Recently 2 randomized studies evaluated the diagnostic yield of performing 7 passes using a 22G or 25G FNA needle without ROSE to cytologist-guided FNA in pancreatic masses. Both studies found no significant difference in diagnostic yield. Therefore, if onsite cytology review is not available, 7 FNA passes into the pancreatic mass should be performed[53,54].
FINE NEEDLE BIOPSY
In theory a fine needle biopsy (FNB), or core needle biopsy, contains a superior tissue sample with preserved cellular architecture as compared to that from FNA. It has been hypothesized that this will yield increased diagnostic accuracy with tissue processing and testing more easily accomplished through routine histology specimen processing. Three randomized studies of 22G FNA and 22G core biopsy needle (EchoTip ProCore, Cook Medical, Bloomington, IN) produced 3 different conclusions[55-57]. One study reported comparable diagnostic yield (89%-100%), number of passes needed for diagnosis, and complications while another suggested significantly worse diagnostic capability (94% FNA vs 28% core needle) and ease of use with the core biopsy needle. The most recent study found significantly higher diagnostic yield with the core biopsy needle (90%) compared to the FNA needle (67%)[57]. A metaanalysis of ProCore compared with FNA needles found no difference in diagnostic yield although the ProCore needle obtained diagnosis with fewer passes[58]. Another prospective randomized comparison of 22G fenestrated core biopsy needle to standard 22G FNA needle in solid pancreatic lesions showed similar accuracy between the two needle types, though the fenestrated needle required, on average, one less pass (two instead of three) to achieve a diagnosis[59].
A retrospective study of a newer FNB needle (SharkCore, Medtronic, Minneapolis, MN) compared with FNA needles reported higher yield of tissue sufficient for histology with the core needle (95% SharkCore vs 59% FNA needle) with fewer median passes to achieve this (2 passes FNA vs 4 passes for SharkCore)[60]. Comparison of 2 different 19G core biopsy needles (ProCore and Quick-Core, both Cook Medical, Bloomington, IN) in a randomized study found the ProCore had significantly higher diagnostic histology (85% vs 57%)[61,62]. Another study compared 22G and 25G core biopsy needles and found no statistical difference between diagnostic accuracy of one needle size over another[63]. Given the increased use of molecular studies on tissue samples required for gene-specific oncologic therapy, obtaining histologic sized specimens, rather than cytopathology, will be of importance in the future. FNB may also play a critical role in rescue procedures when EUS-FNA is nondiagnostic. It may also change our practice if proven more efficacious than FNA needles whereby only FNB needles may be necessary without ROSE to obtain adequate specimens. Ongoing study of EUS-FNB regarding the clinical effectiveness as compared to FNA and cost analyses are required.
POTENTIAL ADVERSE EVENTS
Compared to other interventional procedures like ERCP, EUS-FNA procedures are very safe with reported overall complication rates ranging from 0.3% to 2.2%[64,65]. Smaller (≤ 20 mm) and pancreatic neuroendocrine lesions were associated with increased risk of complications including pancreatitis, abdominal pain, and bleeding in a retrospective single center study[66]. Several complications of EUS-FNA and best-practice tips to avoid them are discussed.
Pancreatitis
The most common serious complication of EUS-FNA of pancreatic mass lesions is pancreatitis, which can occur in anywhere from 0.29% to 2% of cases[66-68]. Needle gauge has no impact on the development of pancreatitis, which is thought to occur when the needle traverses normal pancreatic parenchyma and ducts to reach the target lesion. When discussing this risk with patients, it may be helpful to note that the risk of pancreatitis reported during percutaneous FNA is slightly higher at 3%[69]. To avoid pancreatitis after EUS-FNA, it is recommended to select a needle path that will traverse the least amount of normal pancreas as possible. Whether administration of rectal indomethacin in potentially higher risk EUS-FNA procedures reduces the risk of post-EUS-FNA pancreatitis requires further study[70].
Hemorrhage
Bleeding during and after EUS-FNA procedures has been reported to occur from 1.0%-4.4% of cases[62-71]. Bleeding may be intraluminal or extraluminal and in most cases is self-limited. Steps to avoid procedure-related hemorrhage include avoidance of antithrombotic medications when possible, though generally aspirin and NSAIDs may be continued. A minimum platelet count of 50000 and INR less than 1.5 is also recommended as with many endoscopic procedures[72]. Additionally, use of Doppler ultrasonography to avoid intervening bleed vessels during needle puncture is advised. If blood is seen filling the suction syringe during EUS-FNA, FNA should be stopped.
Perforation
Perforation of the esophagus is reported to occur in 0.009% to 0.15% of procedures[73,74]. This is likely related to the larger diameter (generally 12-14 mm) of the echoendoscope, oblique position of the endoscopic camera limiting visualization of esophageal intubation, and blunt tip of some echoendoscopes. Duodenal perforation is more common, occurring in 0.02% to 0.86% in different series, and is often attributed to the presence of duodenal diverticula[65,75]. Avoidance of perforation is achieved through scope lubrication, careful intubation, avoidance of undue pressure and awareness of any risky anatomic features including a diverticulum.
Infection
Infection is rare, though several studies have shown that there may be a small risk of bacteremia comparable to the risk associated with routine endoscopic procedures at about 2%[76-78]. Clinically significant infection is exceedingly rare, therefore, routine prophylactic antibiotics are not advised[79]. If the solid lesion has a significant cystic component, prophylactic antibiotics should be administered as recommended by the American Society for Gastrointestinal Endoscopy[80].
Tumor seeding
Tumor seeding is perhaps the most feared complication, however there are only limited single case reports of EUS-FNA associated tumor seeding and thus the risk is thought to be extremely low[81]. To avoid this complication, it is important to ensure the echoendoscope is as close to the suspected malignancy as possible to limit the amount of tissue traversed. It is also important to perform EUS-FNA only when the results of the procedure will impact management of the patient, and to send patients straight to exploratory or curative surgery when appropriate. A retrospective study evaluating the impact of preoperative EUS-FNA found no difference in postoperative complications and overall or recurrence-free survival between patients who had and had not undergone preoperative EUS-FNA[82].
STAGING OF PANCREATIC MASSES
Staging pancreatic cancer is of paramount importance in determining the resectability of any given cancer. Only approximately 10%-15% of patients with a pancreatic cancer will be candidates for surgical resection; therefore, an evaluation for distant metastases, vascular invasion, and lymphatic spread are considered. While pancreatic protocol CT scan of the abdomen is generally recommended as first line for this purpose, other modalities have been evaluated and play a role in staging. In addition to providing diagnostic information about the pancreatic mass, EUS is also important in detecting metastatic disease not seen on ultrasound or CT imaging. An older study found that 12% of patients with pancreatic masses had metastatic disease involving lymph nodes, liver, ascites, and the retroperitoneum identified by EUS-FNA that were not visualized by abdominal ultrasound or CT[83]. Whether this would still hold true with improved abdominal imaging technology today is unclear.
With advancements in cross-sectional imaging, CT and MRI are now comparable to EUS for T-staging with accuracy ranging from 62% to 94%[84]. A systematic review of the literature suggested that nodal staging also has similar accuracy between EUS (62%) and CT scan (63%)[85]. Presence of malignant celiac lymph nodes may preclude resection, therefore, this area should be examined carefully by EUS. EUS also seems comparable to CT scan for detecting vascular invasion. For determination of vascular invasion, sensitivity of EUS varies depending on the vessels involved. EUS is superior to CT for assessing vascular invasion of the portal vein (60%-100% sensitive) while inferior for judging involvement of the SMV, SMA, and celiac artery (17%-83% sensitive)[86-89]. There is no consensus regarding the EUS criteria used to assess vascular invasion. Complete vascular obstruction, venous collaterals and visible tumor in the vessel have the highest specificity for vascular invasion and therefore, are the best criteria to use[90]. Regarding resectability, a systematic review inclusive of 678 patients demonstrated that EUS was 63%-93% accurate in identifying surgically curable cases, which was generally similar to or better than CT scan (60%-83% sensitive)[85]. Routine cross-sectional imaging is still recommended in order to evaluate for other intraperitoneal and hepatic metastases that may not be well evaluated with EUS.
ANCILLARY EUS TECHNIQUES
In an effort to further push the diagnostic accuracy of EUS to 100%, several complementary technologies have been developed including elastography, contrast-harmonic EUS and fluorescence in situ hybridization (FISH). Elastography during EUS may be used to calculate tissue stiffness, which may be of utility given that the properties of normal pancreatic vs cancerous tissue differ. Most cancerous lesions will be “harder” showing less elasticity, while benign lesions are generally “soft.” Meta-analyses of elastography have reported sensitivity in detecting pancreatic cancer of 95%, though use of the technology for this indication is not yet mainstream and only available on certain ultrasound processors[91-93].
Contrast harmonic ultrasonography involves use of intravenous microbubble contrast to enhance visualization of the microvasculature during EUS, theoretically improving the ability to detect malignancies. Lesions may be differentiated based on their enhancement with this microbubble contrast, whereby most carcinomas show hypoenhancement and normal tissue is non-enhancing. A systematic review of 82 reports using contrast harmonic EUS for solid pancreatic lesions found that the heterogeneous hypoenhancement pattern was 89%-96% sensitive and 69%-94% specific compared to a hyperenhancing pattern in diagnosing pancreatic adenocarcinoma[94]. The accuracy of this technique was comparable to EUS-FNA, and whether the concomitant use of contrast harmonic EUS with EUS-FNA significantly improves overall diagnostic sensitivity compared to using each technique alone requires further study. In addition, interobserver agreement ranges from fair to good, which may improve with the advent of quantitative contrast harmonic EUS[94].
Several tissue-based techniques may improve diagnosis of pancreatic masses. FISH uses pre-specified fluorescently labeled DNA probes and has been shown to improve the diagnostic yield of indeterminate cytology from EUS-FNA samples of pancreatic masses, but is not readily available[95,96]. For inconclusive EUS-FNA specimens from pancreatic solid masses, a metaanalysis of 931 patients found that the addition of K-ras mutation analysis significantly increased sensitivity from 81% to 89% and reduced the false-negative rate by 56%[97]. This was associated with a concomitant reduction in specificity from 97% to 92% and an 11% increase in false-positive rate. RNA sequencing of EUS-FNA samples has also been recently reported in a proof-of-principle study with 87% sensitivity and 75% specificity in diagnosing pancreatic adenocarcinoma[98]. A 5 microRNA panel was found to augment cytologic diagnosis of pancreatic ductal adenocarcinoma from 79% to 91% and out of 39 cytologically benign, indeterminate, or nondiagnostic samples, 22 were correctly diagnosed as malignant by the microRNA classifier[99]. This requires further study and is not yet available clinically.
OTHER TOOLS ON THE HORIZON
Probe based confocal laser endomicroscopy
As probe based confocal endomicroscopy has been further miniaturized, needle confocal endomicroscopy, or nCLE (AQ-Flex 19 miniprobe, Mauna Kea, Paris, France), has become available for clinical use (Figure 5). The nCLE miniprobe has 0.85 mm diameter and may be inserted through a 19G EUS-FNA needle to provide real-time cellular level imaging. The probe can be preloaded into the FNA needle before performing EUS-FNA or loaded after the mass has been punctured with the FNA needle and stylet removed. After administering 2.5-5 mL of 10% fluorescein sodium intravenously, the probe is advanced about 3-5 mm beyond the tip of the needle to image the mass. A pilot study of nCLE for diagnosis of pancreatic mass lesions has reported findings of dark clumps measuring greater than 40 µm associated with malignancy, no complications, and good interobserver agreement amongst three endosonographers blinded to all clinincal data. However, this technology will require further evaluation to determine its place in diagnosis of solid pancreatic masses[100].
Figure 5.

Confocal laser endomicroscopy miniprobe through a 19G FNA needle. Photo provided with permissions by Mauna Kea, Paris, France.
Through-the-needle biopsy forceps
A new miniaturized 0.75 mm biopsy forceps is available that can be advanced through a 19G EUS-FNA needle to obtain histology (Figure 6). The stylet is removed from the FNA needle and the biopsy forceps preloaded into the needle with the end positioned about 2-3 mm proximal to the needle tip. After puncturing the lesion with the FNA needle, the biopsy forceps is advanced out of the needle and 2-3 bites obtained before removing it. FNA can then be performed in the usual manner. The forceps can also be advanced through the needle after puncturing the mass. If difficulty is encountered in pushing out the forceps, it should be opened and closed by the assistant while the endoscopist continues advancing it forward. Using the mini-forceps through an FNA needle has been proven feasible and safe for pancreatic tissue acquisition[101]. While only a pilot study has been completed, this initial report suggested high diagnostic sensitivity with no device failures or complications. This may offer an attractive alternative for the future.
Figure 6.
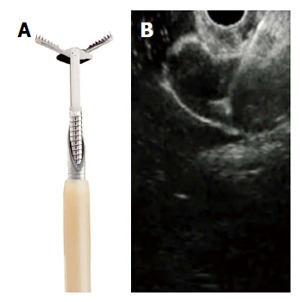
Miniature biopsy forceps. A: In open position, passing through a 19G FNA needle; B: EUS view of open biopsy forceps through the FNA needle. Photo provided with permissions by US Endoscopy, Mentor, OH.
CONCLUSION
EUS-FNA has overtaken all other technologies in the diagnosis of unknown pancreatic mass lesions. While it is clearly the single best test for elucidation of a pancreatic mass, cross-sectional imaging plays an important role in the initial evaluation and staging of pancreatic cancer. EUS-FNA is minimally invasive, safe, and highly effective in tissue acquisition. Diagnostic accuracy is enhanced with attention to the ideal technique through the choice of needle, biopsy technique and number of passes. When EUS-FNA does fail to provide a diagnosis, there are several adjunctive technologies currently under study which may assist in obtaining necessary diagnostic information including novel core biopsy needles, elastography, contrast harmonic EUS, through the needle confocal imaging probes and biopsy forceps, and tissue-based technology including FISH, DNA and RNA analysis.
Footnotes
Manuscript source: Invited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: United States
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): 0
Grade C (Good): C
Grade D (Fair): 0
Grade E (Poor): 0
Conflict-of-interest statement: Both authors have no conflicts of interest to disclose including no pharmaceutical or industry support.
Peer-review started: July 4, 2016
First decision: August 8, 2016
Article in press: September 14, 2016
P- Reviewer: Fabbri C S- Editor: Qi Y L- Editor: A E- Editor: Wang CH
References
- 1.Pleskow DK, Berger HJ, Gyves J, Allen E, McLean A, Podolsky DK. Evaluation of a serologic marker, CA19-9, in the diagnosis of pancreatic cancer. Ann Intern Med. 1989;110:704–709. doi: 10.7326/0003-4819-110-9-704. [DOI] [PubMed] [Google Scholar]
- 2.Cwik G, Wallner G, Skoczylas T, Ciechanski A, Zinkiewicz K. Cancer antigens 19-9 and 125 in the differential diagnosis of pancreatic mass lesions. Arch Surg. 2006;141:968–973; discussion 974. doi: 10.1001/archsurg.141.10.968. [DOI] [PubMed] [Google Scholar]
- 3.van den Bosch RP, van Eijck CH, Mulder PG, Jeekel J. Serum CA19-9 determination in the management of pancreatic cancer. Hepatogastroenterology. 1996;43:710–713. [PubMed] [Google Scholar]
- 4.Uchida N, Kamada H, Tsutsui K, Ono M, Aritomo Y, Masaki T, Kushida Y, Haba R, Nakatsu T, Kuriyama S. Utility of pancreatic duct brushing for diagnosis of pancreatic carcinoma. J Gastroenterol. 2007;42:657–662. doi: 10.1007/s00535-007-2071-7. [DOI] [PubMed] [Google Scholar]
- 5.Yamaguchi T, Shirai Y, Nakamura N, Sudo K, Nakamura K, Hironaka S, Hara T, Denda T. Usefulness of brush cytology combined with pancreatic juice cytology in the diagnosis of pancreatic cancer: significance of pancreatic juice cytology after brushing. Pancreas. 2012;41:1225–1229. doi: 10.1097/MPA.0b013e31825d60fc. [DOI] [PubMed] [Google Scholar]
- 6.DelMaschio A, Vanzulli A, Sironi S, Castrucci M, Mellone R, Staudacher C, Carlucci M, Zerbi A, Parolini D, Faravelli A. Pancreatic cancer versus chronic pancreatitis: diagnosis with CA 19-9 assessment, US, CT, and CT-guided fine-needle biopsy. Radiology. 1991;178:95–99. doi: 10.1148/radiology.178.1.1984331. [DOI] [PubMed] [Google Scholar]
- 7.Johnson DE, Pendurthi TK, Balshem AM, Ross E, Litwin S, Eisenberg BL, Hoffman JP. Implications of fine-needle aspiration in patients with resectable pancreatic cancer. Am Surg. 1997;63:675–679; discussion 679-680. [PubMed] [Google Scholar]
- 8.Erturk SM, Mortelé KJ, Tuncali K, Saltzman JR, Lao R, Silverman SG. Fine-needle aspiration biopsy of solid pancreatic masses: comparison of CT and endoscopic sonography guidance. AJR Am J Roentgenol. 2006;187:1531–1535. doi: 10.2214/AJR.05.1657. [DOI] [PubMed] [Google Scholar]
- 9.Horwhat JD, Paulson EK, McGrath K, Branch MS, Baillie J, Tyler D, Pappas T, Enns R, Robuck G, Stiffler H, et al. A randomized comparison of EUS-guided FNA versus CT or US-guided FNA for the evaluation of pancreatic mass lesions. Gastrointest Endosc. 2006;63:966–975. doi: 10.1016/j.gie.2005.09.028. [DOI] [PubMed] [Google Scholar]
- 10.Volmar KE, Vollmer RT, Jowell PS, Nelson RC, Xie HB. Pancreatic FNA in 1000 cases: a comparison of imaging modalities. Gastrointest Endosc. 2005;61:854–861. doi: 10.1016/s0016-5107(05)00364-0. [DOI] [PubMed] [Google Scholar]
- 11.Micames C, Jowell PS, White R, Paulson E, Nelson R, Morse M, Hurwitz H, Pappas T, Tyler D, McGrath K. Lower frequency of peritoneal carcinomatosis in patients with pancreatic cancer diagnosed by EUS-guided FNA vs. percutaneous FNA. Gastrointest Endosc. 2003;58:690–695. doi: 10.1016/s0016-5107(03)02009-1. [DOI] [PubMed] [Google Scholar]
- 12.Puli SR, Bechtold ML, Buxbaum JL, Eloubeidi MA. How good is endoscopic ultrasound-guided fine-needle aspiration in diagnosing the correct etiology for a solid pancreatic mass?: A meta-analysis and systematic review. Pancreas. 2013;42:20–26. doi: 10.1097/MPA.0b013e3182546e79. [DOI] [PubMed] [Google Scholar]
- 13.Hartwig W, Schneider L, Diener MK, Bergmann F, Büchler MW, Werner J. Preoperative tissue diagnosis for tumours of the pancreas. Br J Surg. 2009;96:5–20. doi: 10.1002/bjs.6407. [DOI] [PubMed] [Google Scholar]
- 14.Hewitt MJ, McPhail MJ, Possamai L, Dhar A, Vlavianos P, Monahan KJ. EUS-guided FNA for diagnosis of solid pancreatic neoplasms: a meta-analysis. Gastrointest Endosc. 2012;75:319–331. doi: 10.1016/j.gie.2011.08.049. [DOI] [PubMed] [Google Scholar]
- 15.Harewood GC, Wiersema MJ. Endosonography-guided fine needle aspiration biopsy in the evaluation of pancreatic masses. Am J Gastroenterol. 2002;97:1386–1391. doi: 10.1111/j.1572-0241.2002.05777.x. [DOI] [PubMed] [Google Scholar]
- 16.Ardengh JC, Lopes CV, de Lima LF, de Oliveira JR, Venco F, Santo GC, Modena JL. Diagnosis of pancreatic tumors by endoscopic ultrasound-guided fine-needle aspiration. World J Gastroenterol. 2007;13:3112–3116. doi: 10.3748/wjg.v13.i22.3112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Varadarajulu S, Tamhane A, Eloubeidi MA. Yield of EUS-guided FNA of pancreatic masses in the presence or the absence of chronic pancreatitis. Gastrointest Endosc. 2005;62:728–736; quiz 51, 53. doi: 10.1016/j.gie.2005.06.051. [DOI] [PubMed] [Google Scholar]
- 18.Eloubeidi MA, Varadarajulu S, Desai S, Wilcox CM. Value of repeat endoscopic ultrasound-guided fine needle aspiration for suspected pancreatic cancer. J Gastroenterol Hepatol. 2008;23:567–570. doi: 10.1111/j.1440-1746.2007.05119.x. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki R, Lee JH, Krishna SG, Ramireddy S, Qiao W, Weston B, Ross WA, Bhutani MS. Repeat endoscopic ultrasound-guided fine needle aspiration for solid pancreatic lesions at a tertiary referral center will alter the initial inconclusive result. J Gastrointestin Liver Dis. 2013;22:183–187. [PubMed] [Google Scholar]
- 20.DeWitt J, McGreevy K, Sherman S, LeBlanc J. Utility of a repeated EUS at a tertiary-referral center. Gastrointest Endosc. 2008;67:610–619. doi: 10.1016/j.gie.2007.09.037. [DOI] [PubMed] [Google Scholar]
- 21.Ootaki C, Stevens T, Vargo J, You J, Shiba A, Foss J, Borkowski R, Maurer W. Does general anesthesia increase the diagnostic yield of endoscopic ultrasound-guided fine needle aspiration of pancreatic masses? Anesthesiology. 2012;117:1044–1050. doi: 10.1097/ALN.0b013e31826e0590. [DOI] [PubMed] [Google Scholar]
- 22.Itoi T, Itokawa F, Kurihara T, Sofuni A, Tsuchiya T, Ishii K, Tsuji S, Ikeuchi N, Kawai T, Moriyasu F. Experimental endoscopy: objective evaluation of EUS needles. Gastrointest Endosc. 2009;69:509–516. doi: 10.1016/j.gie.2008.07.017. [DOI] [PubMed] [Google Scholar]
- 23.Song TJ, Kim JH, Lee SS, Eum JB, Moon SH, Park DY, Seo DW, Lee SK, Jang SJ, Yun SC, et al. The prospective randomized, controlled trial of endoscopic ultrasound-guided fine-needle aspiration using 22G and 19G aspiration needles for solid pancreatic or peripancreatic masses. Am J Gastroenterol. 2010;105:1739–1745. doi: 10.1038/ajg.2010.108. [DOI] [PubMed] [Google Scholar]
- 24.Siddiqui UD, Rossi F, Rosenthal LS, Padda MS, Murali-Dharan V, Aslanian HR. EUS-guided FNA of solid pancreatic masses: a prospective, randomized trial comparing 22-gauge and 25-gauge needles. Gastrointest Endosc. 2009;70:1093–1097. doi: 10.1016/j.gie.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 25.Madhoun MF, Wani SB, Rastogi A, Early D, Gaddam S, Tierney WM, Maple JT. The diagnostic accuracy of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of solid pancreatic lesions: a meta-analysis. Endoscopy. 2013;45:86–92. doi: 10.1055/s-0032-1325992. [DOI] [PubMed] [Google Scholar]
- 26.Bang JY, Varadarajulu S. Procore and flexible 19 gauge needle can replace trucut biopsy needle? Clin Endosc. 2013;46:503–505. doi: 10.5946/ce.2013.46.5.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lee JH, Stewart J, Ross WA, Anandasabapathy S, Xiao L, Staerkel G. Blinded prospective comparison of the performance of 22-gauge and 25-gauge needles in endoscopic ultrasound-guided fine needle aspiration of the pancreas and peri-pancreatic lesions. Dig Dis Sci. 2009;54:2274–2281. doi: 10.1007/s10620-009-0906-1. [DOI] [PubMed] [Google Scholar]
- 28.Affolter KE, Schmidt RL, Matynia AP, Adler DG, Factor RE. Needle size has only a limited effect on outcomes in EUS-guided fine needle aspiration: a systematic review and meta-analysis. Dig Dis Sci. 2013;58:1026–1034. doi: 10.1007/s10620-012-2439-2. [DOI] [PubMed] [Google Scholar]
- 29.Brugge WR. EUS. Gastrointest Endosc. 2013;78:414–420. doi: 10.1016/j.gie.2013.07.002. [DOI] [PubMed] [Google Scholar]
- 30.Larghi A, Verna EC, Stavropoulos SN, Rotterdam H, Lightdale CJ, Stevens PD. EUS-guided trucut needle biopsies in patients with solid pancreatic masses: a prospective study. Gastrointest Endosc. 2004;59:185–190. doi: 10.1016/s0016-5107(03)02538-0. [DOI] [PubMed] [Google Scholar]
- 31.Bang JY, Hawes RH, Varadarajulu S. Objective evaluation of a new endoscopic ultrasound processor. Dig Endosc. 2013;25:554–555. doi: 10.1111/den.12148. [DOI] [PubMed] [Google Scholar]
- 32.Tang SJ, Vilmann AS, Saftoiu A, Wang W, Streba CT, Fink PP, Griswold M, Wu R, Dietrich CF, Jenssen C, et al. EUS Needle Identification Comparison and Evaluation study (with videos) Gastrointest Endosc. 2016;84:424–433.e2. doi: 10.1016/j.gie.2016.01.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rastogi A, Wani S, Gupta N, Singh V, Gaddam S, Reddymasu S, Ulusarac O, Fan F, Romanas M, Dennis KL, et al. A prospective, single-blind, randomized, controlled trial of EUS-guided FNA with and without a stylet. Gastrointest Endosc. 2011;74:58–64. doi: 10.1016/j.gie.2011.02.015. [DOI] [PubMed] [Google Scholar]
- 34.Sahai AV, Paquin SC, Gariépy G. A prospective comparison of endoscopic ultrasound-guided fine needle aspiration results obtained in the same lesion, with and without the needle stylet. Endoscopy. 2010;42:900–903. doi: 10.1055/s-0030-1255676. [DOI] [PubMed] [Google Scholar]
- 35.Wani S, Early D, Kunkel J, Leathersich A, Hovis CE, Hollander TG, Kohlmeier C, Zelenka C, Azar R, Edmundowicz S, et al. Diagnostic yield of malignancy during EUS-guided FNA of solid lesions with and without a stylet: a prospective, single blind, randomized, controlled trial. Gastrointest Endosc. 2012;76:328–335. doi: 10.1016/j.gie.2012.03.1395. [DOI] [PubMed] [Google Scholar]
- 36.Lee JK, Choi JH, Lee KH, Kim KM, Shin JU, Lee JK, Lee KT, Jang KT. A prospective, comparative trial to optimize sampling techniques in EUS-guided FNA of solid pancreatic masses. Gastrointest Endosc. 2013;77:745–751. doi: 10.1016/j.gie.2012.12.009. [DOI] [PubMed] [Google Scholar]
- 37.Puri R, Vilmann P, Săftoiu A, Skov BG, Linnemann D, Hassan H, Garcia ES, Gorunescu F. Randomized controlled trial of endoscopic ultrasound-guided fine-needle sampling with or without suction for better cytological diagnosis. Scand J Gastroenterol. 2009;44:499–504. doi: 10.1080/00365520802647392. [DOI] [PubMed] [Google Scholar]
- 38.Bang JY, Ramesh J, Trevino J, Eloubeidi MA, Varadarajulu S. Objective assessment of an algorithmic approach to EUS-guided FNA and interventions. Gastrointest Endosc. 2013;77:739–744. doi: 10.1016/j.gie.2012.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kudo T, Kawakami H, Hayashi T, Yasuda I, Mukai T, Inoue H, Katanuma A, Kawakubo K, Ishiwatari H, Doi S, et al. High and low negative pressure suction techniques in EUS-guided fine-needle tissue acquisition by using 25-gauge needles: a multicenter, prospective, randomized, controlled trial. Gastrointest Endosc. 2014;80:1030–1037.e1. doi: 10.1016/j.gie.2014.04.012. [DOI] [PubMed] [Google Scholar]
- 40.Attam R, Arain MA, Bloechl SJ, Trikudanathan G, Munigala S, Bakman Y, Singh M, Wallace T, Henderson JB, Catalano MF, et al. “Wet suction technique (WEST)”: a novel way to enhance the quality of EUS-FNA aspirate. Results of a prospective, single-blind, randomized, controlled trial using a 22-gauge needle for EUS-FNA of solid lesions. Gastrointest Endosc. 2015;81:1401–1407. doi: 10.1016/j.gie.2014.11.023. [DOI] [PubMed] [Google Scholar]
- 41.Kin T, Katanuma A, Yane K, Takahashi K, Osanai M, Takaki R, Matsumoto K, Gon K, Matsumori T, Tomonari A, et al. Diagnostic ability of EUS-FNA for pancreatic solid lesions with conventional 22-gauge needle using the slow pull technique: a prospective study. Scand J Gastroenterol. 2015;50:900–907. doi: 10.3109/00365521.2014.983155. [DOI] [PubMed] [Google Scholar]
- 42.Nakai Y, Isayama H, Chang KJ, Yamamoto N, Hamada T, Uchino R, Mizuno S, Miyabayashi K, Yamamoto K, Kawakubo K, et al. Slow pull versus suction in endoscopic ultrasound-guided fine-needle aspiration of pancreatic solid masses. Dig Dis Sci. 2014;59:1578–1585. doi: 10.1007/s10620-013-3019-9. [DOI] [PubMed] [Google Scholar]
- 43.Tarantino I, Di Mitri R, Fabbri C, Pagano N, Barresi L, Granata A, Liotta R, Mocciaro F, Maimone A, Baccarini P, et al. Is diagnostic accuracy of fine needle aspiration on solid pancreatic lesions aspiration-related? A multicentre randomised trial. Dig Liver Dis. 2014;46:523–526. doi: 10.1016/j.dld.2014.02.023. [DOI] [PubMed] [Google Scholar]
- 44.Bang JY, Magee SH, Ramesh J, Trevino JM, Varadarajulu S. Randomized trial comparing fanning with standard technique for endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic mass lesions. Endoscopy. 2013;45:445–450. doi: 10.1055/s-0032-1326268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Mukai S, Itoi T, Ashida R, Tsuchiya T, Ikeuchi N, Kamada K, Tanaka R, Umeda J, Tonozuka R, Fukutake N, et al. Multicenter, prospective, crossover trial comparing the door-knocking method with the conventional method for EUS-FNA of solid pancreatic masses (with videos) Gastrointest Endosc. 2016;83:1210–1217. doi: 10.1016/j.gie.2015.10.025. [DOI] [PubMed] [Google Scholar]
- 46.Chen J, Yang R, Lu Y, Xia Y, Zhou H. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration for solid pancreatic lesion: a systematic review. J Cancer Res Clin Oncol. 2012;138:1433–1441. doi: 10.1007/s00432-012-1268-1. [DOI] [PubMed] [Google Scholar]
- 47.Iglesias-Garcia J, Dominguez-Munoz JE, Abdulkader I, Larino-Noia J, Eugenyeva E, Lozano-Leon A, Forteza-Vila J. Influence of on-site cytopathology evaluation on the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration (EUS-FNA) of solid pancreatic masses. Am J Gastroenterol. 2011;106:1705–1710. doi: 10.1038/ajg.2011.119. [DOI] [PubMed] [Google Scholar]
- 48.Hébert-Magee S, Bae S, Varadarajulu S, Ramesh J, Frost AR, Eloubeidi MA, Eltoum IA. The presence of a cytopathologist increases the diagnostic accuracy of endoscopic ultrasound-guided fine needle aspiration cytology for pancreatic adenocarcinoma: a meta-analysis. Cytopathology. 2013;24:159–171. doi: 10.1111/cyt.12071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Layfield LJ, Bentz JS, Gopez EV. Immediate on-site interpretation of fine-needle aspiration smears: a cost and compensation analysis. Cancer. 2001;93:319–322. doi: 10.1002/cncr.9046. [DOI] [PubMed] [Google Scholar]
- 50.Erickson RA, Sayage-Rabie L, Beissner RS. Factors predicting the number of EUS-guided fine-needle passes for diagnosis of pancreatic malignancies. Gastrointest Endosc. 2000;51:184–190. doi: 10.1016/s0016-5107(00)70416-0. [DOI] [PubMed] [Google Scholar]
- 51.LeBlanc JK, Ciaccia D, Al-Assi MT, McGrath K, Imperiale T, Tao LC, Vallery S, DeWitt J, Sherman S, Collins E. Optimal number of EUS-guided fine needle passes needed to obtain a correct diagnosis. Gastrointest Endosc. 2004;59:475–481. doi: 10.1016/s0016-5107(03)02863-3. [DOI] [PubMed] [Google Scholar]
- 52.Suzuki R, Irisawa A, Bhutani MS, Hikichi T, Takagi T, Sato A, Sato M, Ikeda T, Watanabe K, Nakamura J, et al. Prospective evaluation of the optimal number of 25-gauge needle passes for endoscopic ultrasound-guided fine-needle aspiration biopsy of solid pancreatic lesions in the absence of an onsite cytopathologist. Dig Endosc. 2012;24:452–456. doi: 10.1111/j.1443-1661.2012.01311.x. [DOI] [PubMed] [Google Scholar]
- 53.Lee LS, Nieto J, Watson RR, Hwang AL, Muthusamy VR, Walter L, Jajoo K, Ryou MK, Saltzman JR, Saunders MD, et al. Randomized Noninferiority Trial Comparing Diagnostic Yield of Cytopathologist-guided versus 7 passes for EUS-FNA of Pancreatic Masses. Dig Endosc. 2015 doi: 10.1111/den.12594. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 54.Wani S, Mullady D, Early DS, Rastogi A, Collins B, Wang JF, Marshall C, Sams SB, Yen R, Rizeq M, et al. The clinical impact of immediate on-site cytopathology evaluation during endoscopic ultrasound-guided fine needle aspiration of pancreatic masses: a prospective multicenter randomized controlled trial. Am J Gastroenterol. 2015;110:1429–1439. doi: 10.1038/ajg.2015.262. [DOI] [PubMed] [Google Scholar]
- 55.Bang JY, Hebert-Magee S, Trevino J, Ramesh J, Varadarajulu S. Randomized trial comparing the 22-gauge aspiration and 22-gauge biopsy needles for EUS-guided sampling of solid pancreatic mass lesions. Gastrointest Endosc. 2012;76:321–327. doi: 10.1016/j.gie.2012.03.1392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Strand DS, Jeffus SK, Sauer BG, Wang AY, Stelow EB, Shami VM. EUS-guided 22-gauge fine-needle aspiration versus core biopsy needle in the evaluation of solid pancreatic neoplasms. Diagn Cytopathol. 2014;42:751–758. doi: 10.1002/dc.23116. [DOI] [PubMed] [Google Scholar]
- 57.Aadam AA, Wani S, Amick A, Shah JN, Bhat YM, Hamerski CM, Klapman JB, Muthusamy VR, Watson RR, Rademaker AW, et al. A randomized controlled cross-over trial and cost analysis comparing endoscopic ultrasound fine needle aspiration and fine needle biopsy. Endosc Int Open. 2016;4:E497–E505. doi: 10.1055/s-0042-106958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bang JY, Hawes R, Varadarajulu S. A meta-analysis comparing ProCore and standard fine-needle aspiration needles for endoscopic ultrasound-guided tissue acquisition. Endoscopy. 2016;48:339–349. doi: 10.1055/s-0034-1393354. [DOI] [PubMed] [Google Scholar]
- 59.Alatawi A, Beuvon F, Grabar S, Leblanc S, Chaussade S, Terris B, Barret M, Prat F. Comparison of 22G reverse-beveled versus standard needle for endoscopic ultrasound-guided sampling of solid pancreatic lesions. United European Gastroenterol J. 2015;3:343–352. doi: 10.1177/2050640615577533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kandel P, Tranesh G, Nassar A, Bingham R, Raimondo M, Woodward TA, Gomez V, Wallace MB. EUS-guided fine needle biopsy sampling using a novel fork-tip needle: a case-control study. Gastrointest Endosc. 2016 doi: 10.1016/j.gie.2016.03.1405. Epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 61.DeWitt J, Cho CM, Lin J, Al-Haddad M, Canto MI, Salamone A, Hruban RH, Messallam AA, Khashab MA. Comparison of EUS-guided tissue acquisition using two different 19-gauge core biopsy needles: a multicenter, prospective, randomized, and blinded study. Endosc Int Open. 2015;3:E471–E478. doi: 10.1055/s-0034-1392222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Eloubeidi MA, Chen VK, Eltoum IA, Jhala D, Chhieng DC, Jhala N, Vickers SM, Wilcox CM. Endoscopic ultrasound-guided fine needle aspiration biopsy of patients with suspected pancreatic cancer: diagnostic accuracy and acute and 30-day complications. Am J Gastroenterol. 2003;98:2663–2668. doi: 10.1111/j.1572-0241.2003.08666.x. [DOI] [PubMed] [Google Scholar]
- 63.Park SW, Chung MJ, Lee SH, Lee HS, Lee HJ, Park JY, Park SW, Song SY, Kim H, Chung JB, et al. Prospective Study for Comparison of Endoscopic Ultrasound-Guided Tissue Acquisition Using 25- and 22-Gauge Core Biopsy Needles in Solid Pancreatic Masses. PLoS One. 2016;11:e0154401. doi: 10.1371/journal.pone.0154401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Jani BS, Rzouq F, Saligram S, Lim D, Rastogi A, Bonino J, Olyaee M. Endoscopic Ultrasound-Guided Fine-Needle Aspiration of Pancreatic Lesions: A Systematic Review of Technical and Procedural Variables. N Am J Med Sci. 2016;8:1–11. doi: 10.4103/1947-2714.175185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Jenssen C, Faiss S, Nürnberg D. Complications of endoscopic ultrasound and endoscopic ultrasound-guided interventions - results of a survey among German centers. Z Gastroenterol. 2008;46:1177–1184. doi: 10.1055/s-2008-1027334. [DOI] [PubMed] [Google Scholar]
- 66.Katanuma A, Maguchi H, Yane K, Hashigo S, Kin T, Kaneko M, Kato S, Kato R, Harada R, Osanai M, et al. Factors predictive of adverse events associated with endoscopic ultrasound-guided fine needle aspiration of pancreatic solid lesions. Dig Dis Sci. 2013;58:2093–2099. doi: 10.1007/s10620-013-2590-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Gress F, Michael H, Gelrud D, Patel P, Gottlieb K, Singh F, Grendell J. EUS-guided fine-needle aspiration of the pancreas: evaluation of pancreatitis as a complication. Gastrointest Endosc. 2002;56:864–867. doi: 10.1067/mge.2002.129602. [DOI] [PubMed] [Google Scholar]
- 68.Eloubeidi MA, Gress FG, Savides TJ, Wiersema MJ, Kochman ML, Ahmad NA, Ginsberg GG, Erickson RA, Dewitt J, Van Dam J, et al. Acute pancreatitis after EUS-guided FNA of solid pancreatic masses: a pooled analysis from EUS centers in the United States. Gastrointest Endosc. 2004;60:385–389. doi: 10.1016/s0016-5107(04)01714-6. [DOI] [PubMed] [Google Scholar]
- 69.Mueller PR, Miketic LM, Simeone JF, Silverman SG, Saini S, Wittenberg J, Hahn PF, Steiner E, Forman BH. Severe acute pancreatitis after percutaneous biopsy of the pancreas. AJR Am J Roentgenol. 1988;151:493–494. doi: 10.2214/ajr.151.3.493. [DOI] [PubMed] [Google Scholar]
- 70.Elmunzer BJ, Serrano J, Chak A, Edmundowicz SA, Papachristou GI, Scheiman JM, Singh VK, Varadurajulu S, Vargo JJ, Willingham FF, et al. Rectal indomethacin alone versus indomethacin and prophylactic pancreatic stent placement for preventing pancreatitis after ERCP: study protocol for a randomized controlled trial. Trials. 2016;17:120. doi: 10.1186/s13063-016-1251-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Affi A, Vazquez-Sequeiros E, Norton ID, Clain JE, Wiersema MJ. Acute extraluminal hemorrhage associated with EUS-guided fine needle aspiration: frequency and clinical significance. Gastrointest Endosc. 2001;53:221–225. doi: 10.1067/mge.2001.111391. [DOI] [PubMed] [Google Scholar]
- 72.Acosta RD, Abraham NS, Chandrasekhara V, Chathadi KV, Early DS, Eloubeidi MA, Evans JA, Faulx AL, Fisher DA, Fonkalsrud L, et al. The management of antithrombotic agents for patients undergoing GI endoscopy. Gastrointest Endosc. 2016;83:3–16. doi: 10.1016/j.gie.2015.09.035. [DOI] [PubMed] [Google Scholar]
- 73.Das A, Sivak MV, Chak A. Cervical esophageal perforation during EUS: a national survey. Gastrointest Endosc. 2001;53:599–602. doi: 10.1067/mge.2001.113385. [DOI] [PubMed] [Google Scholar]
- 74.Mortensen MB, Fristrup C, Holm FS, Pless T, Durup J, Ainsworth AP, Nielsen HO, Hovendal C. Prospective evaluation of patient tolerability, satisfaction with patient information, and complications in endoscopic ultrasonography. Endoscopy. 2005;37:146–153. doi: 10.1055/s-2005-861142. [DOI] [PubMed] [Google Scholar]
- 75.Raut CP, Grau AM, Staerkel GA, Kaw M, Tamm EP, Wolff RA, Vauthey JN, Lee JE, Pisters PW, Evans DB. Diagnostic accuracy of endoscopic ultrasound-guided fine-needle aspiration in patients with presumed pancreatic cancer. J Gastrointest Surg. 2003;7:118–126; discussion 127-128. doi: 10.1016/S1091-255X(02)00150-6. [DOI] [PubMed] [Google Scholar]
- 76.Barawi M, Gottlieb K, Cunha B, Portis M, Gress F. A prospective evaluation of the incidence of bacteremia associated with EUS-guided fine-needle aspiration. Gastrointest Endosc. 2001;53:189–192. doi: 10.1067/mge.2001.108966. [DOI] [PubMed] [Google Scholar]
- 77.Levy MJ, Norton ID, Wiersema MJ, Schwartz DA, Clain JE, Vazquez-Sequeiros E, Wilson WR, Zinsmeister AR, Jondal ML. Prospective risk assessment of bacteremia and other infectious complications in patients undergoing EUS-guided FNA. Gastrointest Endosc. 2003;57:672–678. doi: 10.1067/mge.2003.204. [DOI] [PubMed] [Google Scholar]
- 78.Janssen J, König K, Knop-Hammad V, Johanns W, Greiner L. Frequency of bacteremia after linear EUS of the upper GI tract with and without FNA. Gastrointest Endosc. 2004;59:339–344. doi: 10.1016/s0016-5107(03)02707-x. [DOI] [PubMed] [Google Scholar]
- 79.Hirota WK, Petersen K, Baron TH, Goldstein JL, Jacobson BC, Leighton JA, Mallery JS, Waring JP, Fanelli RD, Wheeler-Harbough J, et al. Guidelines for antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2003;58:475–482. doi: 10.1067/s0016-5107(03)01883-2. [DOI] [PubMed] [Google Scholar]
- 80.Khashab MA, Chithadi KV, Acosta RD, Bruining DH, Chandrasekhara V, Eloubeidi MA, Fanelli RD, Faulx AL, Fonkalsrud L, Lightdale JR, et al. Antibiotic prophylaxis for GI endoscopy. Gastrointest Endosc. 2015;81:81–89. doi: 10.1016/j.gie.2014.08.008. [DOI] [PubMed] [Google Scholar]
- 81.Fujii LL, Levy MJ. Basic techniques in endoscopic ultrasound-guided fine needle aspiration for solid lesions: Adverse events and avoiding them. Endosc Ultrasound. 2014;3:35–45. doi: 10.4103/2303-9027.123006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Beane JD, House MG, Coté GA, DeWitt JM, Al-Haddad M, LeBlanc JK, McHenry L, Sherman S, Schmidt CM, Zyromski NJ, et al. Outcomes after preoperative endoscopic ultrasonography and biopsy in patients undergoing distal pancreatectomy. Surgery. 2011;150:844–853. doi: 10.1016/j.surg.2011.07.068. [DOI] [PubMed] [Google Scholar]
- 83.Mortensen MB, Pless T, Durup J, Ainsworth AP, Plagborg GJ, Hovendal C. Clinical impact of endoscopic ultrasound-guided fine needle aspiration biopsy in patients with upper gastrointestinal tract malignancies. A prospective study. Endoscopy. 2001;33:478–483. doi: 10.1055/s-2001-14966. [DOI] [PubMed] [Google Scholar]
- 84.Varadarajulu S, Wallace MB. Applications of endoscopic ultrasonography in pancreatic cancer. Cancer Control. 2004;11:15–22. doi: 10.1177/107327480401100103. [DOI] [PubMed] [Google Scholar]
- 85.Dewitt J, Devereaux BM, Lehman GA, Sherman S, Imperiale TF. Comparison of endoscopic ultrasound and computed tomography for the preoperative evaluation of pancreatic cancer: a systematic review. Clin Gastroenterol Hepatol. 2006;4:717–725; quiz 664. doi: 10.1016/j.cgh.2006.02.020. [DOI] [PubMed] [Google Scholar]
- 86.Yasuda K, Mukai H, Nakajima M, Kawai K. Staging of pancreatic carcinoma by endoscopic ultrasonography. Endoscopy. 1993;25:151–155. doi: 10.1055/s-2007-1010274. [DOI] [PubMed] [Google Scholar]
- 87.Brugge WR. Pancreatic cancer staging. Endoscopic ultrasonography criteria for vascular invasion. Gastrointest Endosc Clin N Am. 1995;5:741–753. [PubMed] [Google Scholar]
- 88.Kala Z, Válek V, Hlavsa J, Hana K, Vánová A. The role of CT and endoscopic ultrasound in pre-operative staging of pancreatic cancer. Eur J Radiol. 2007;62:166–169. doi: 10.1016/j.ejrad.2007.01.039. [DOI] [PubMed] [Google Scholar]
- 89.Soriano A, Castells A, Ayuso C, Ayuso JR, de Caralt MT, Ginès MA, Real MI, Gilabert R, Quintó L, Trilla A, et al. Preoperative staging and tumor resectability assessment of pancreatic cancer: prospective study comparing endoscopic ultrasonography, helical computed tomography, magnetic resonance imaging, and angiography. Am J Gastroenterol. 2004;99:492–501. doi: 10.1111/j.1572-0241.2004.04087.x. [DOI] [PubMed] [Google Scholar]
- 90.Rösch T, Dittler HJ, Strobel K, Meining A, Schusdziarra V, Lorenz R, Allescher HD, Kassem AM, Gerhardt P, Siewert JR, et al. Endoscopic ultrasound criteria for vascular invasion in the staging of cancer of the head of the pancreas: a blind reevaluation of videotapes. Gastrointest Endosc. 2000;52:469–477. doi: 10.1067/mge.2000.106682. [DOI] [PubMed] [Google Scholar]
- 91.Pei Q, Zou X, Zhang X, Chen M, Guo Y, Luo H. Diagnostic value of EUS elastography in differentiation of benign and malignant solid pancreatic masses: a meta-analysis. Pancreatology. 2012;12:402–408. doi: 10.1016/j.pan.2012.07.013. [DOI] [PubMed] [Google Scholar]
- 92.Mei M, Ni J, Liu D, Jin P, Sun L. EUS elastography for diagnosis of solid pancreatic masses: a meta-analysis. Gastrointest Endosc. 2013;77:578–589. doi: 10.1016/j.gie.2012.09.035. [DOI] [PubMed] [Google Scholar]
- 93.Kongkam P, Lakananurak N, Navicharern P, Chantarojanasiri T, Aye K, Ridtitid W, Kritisin K, Angsuwatcharakon P, Aniwan S, Pittayanon R, et al. Combination of EUS-FNA and elastography (strain ratio) to exclude malignant solid pancreatic lesions: A prospective single-blinded study. J Gastroenterol Hepatol. 2015;30:1683–1689. doi: 10.1111/jgh.13067. [DOI] [PubMed] [Google Scholar]
- 94.Fusaroli P, Napoleon B, Gincul R, Lefort C, Palazzo L, Palazzo M, Kitano M, Minaga K, Caletti G, Lisotti A. The clinical impact of ultrasound contrast agents in EUS: a systematic review according to the levels of evidence. Gastrointest Endosc. 2016;84:587–596.e10. doi: 10.1016/j.gie.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 95.Reicher S, Boyar FZ, Albitar M, Sulcova V, Agersborg S, Nga V, Zhou Y, Li G, Venegas R, French SW, et al. Fluorescence in situ hybridization and K-ras analyses improve diagnostic yield of endoscopic ultrasound-guided fine-needle aspiration of solid pancreatic masses. Pancreas. 2011;40:1057–1062. doi: 10.1097/MPA.0b013e3182200201. [DOI] [PubMed] [Google Scholar]
- 96.Kubiliun N, Ribeiro A, Fan YS, Rocha-Lima CM, Sleeman D, Merchan J, Barkin J, Levi J. EUS-FNA with rescue fluorescence in situ hybridization for the diagnosis of pancreatic carcinoma in patients with inconclusive on-site cytopathology results. Gastrointest Endosc. 2011;74:541–547. doi: 10.1016/j.gie.2011.04.043. [DOI] [PubMed] [Google Scholar]
- 97.Fuccio L, Hassan C, Laterza L, Correale L, Pagano N, Bocus P, Fabbri C, Maimone A, Cennamo V, Repici A, et al. The role of K-ras gene mutation analysis in EUS-guided FNA cytology specimens for the differential diagnosis of pancreatic solid masses: a meta-analysis of prospective studies. Gastrointest Endosc. 2013;78:596–608. doi: 10.1016/j.gie.2013.04.162. [DOI] [PubMed] [Google Scholar]
- 98.Rodriguez SA, Impey SD, Pelz C, Enestvedt B, Bakis G, Owens M, Morgan TK. RNA sequencing distinguishes benign from malignant pancreatic lesions sampled by EUS-guided FNA. Gastrointest Endosc. 2016;84:252–258. doi: 10.1016/j.gie.2016.01.042. [DOI] [PubMed] [Google Scholar]
- 99.Brand RE, Adai AT, Centeno BA, Lee LS, Rateb G, Vignesh S, Menard C, Wiechowska-Kozłowska A, Bołdys H, Hartleb M, et al. A microRNA-based test improves endoscopic ultrasound-guided cytologic diagnosis of pancreatic cancer. Clin Gastroenterol Hepatol. 2014;12:1717–1723. doi: 10.1016/j.cgh.2014.02.038. [DOI] [PubMed] [Google Scholar]
- 100.Kongkam P, Pittayanon R, Sampatanukul P, Angsuwatcharakon P, Aniwan S, Prueksapanich P, Sriuranpong V, Navicharern P, Treeprasertsuk S, Kullavanijaya P, et al. Endoscopic ultrasound-guided needle-based confocal laser endomicroscopy for diagnosis of solid pancreatic lesions (ENES): a pilot study. Endosc Int Open. 2016;4:E17–E23. doi: 10.1055/s-0034-1393183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Nakai Y, Isayama H, Chang KJ, Yamamoto N, Mizuno S, Mohri D, Kogure H, Matsubara S, Tada M, Koike K. A pilot study of EUS-guided through-the-needle forceps biopsy (with video) Gastrointest Endosc. 2016;84:158–162. doi: 10.1016/j.gie.2015.12.033. [DOI] [PubMed] [Google Scholar]


