Abstract
AIM
To determine the association of p53, carcinoembryonic antigen (CEA) and CA19-9 protein expression with esophageal carcinogenesis.
METHODS
An iodine staining endoscopic screening program of esophageal lesions was carried out in the high-incidence area of Feicheng County, China. Seventy-seven patients with basal cell hyperplasia (BCH), 247 with low-grade dysplasia (LGD), 51 with high-grade dysplasia (HGD), 134 with invasive cancer, and 80 normal controls diagnosed by mucous membrane biopsy pathology were enrolled. Immunohistochemical detection of p53, CEA and CA19-9 proteins was performed. In the ROC curve analysis, the expression of a single biomarker and the expression of a combination of biomarkers were used to predict the risk of these four esophageal lesions.
RESULTS
The positive rates of p53 protein expression in invasive cancer, HGD, LGD, BCH and the normal control groups were 53.0%, 52.9%, 35.6%, 27.3% and 20.0%, respectively; the positive rates of CA19-9 protein expression were 44.0%, 33.3%, 16.5%, 9.2% and 6.2%, respectively; the positive rates of CEA protein expression were 74.6%, 60.8%, 23.3%, 23.7% and 16.2%, respectively. The positive rates of the combined expression of the three biomarkers were 84.3%, 76.5%, 47.6%, 42.9% and 27.5%, respectively. In the receiver operating characteristic curves of the combination of the three biomarkers, the specificity was 88.8% for the normal controls, and the sensitivity was 58.2% for invasive cancer, 25.5% for HGD, 11.2% for LGD, and 6.5% for BCH.
CONCLUSION
p53, CEA and CA19-9 protein expression was correlated with esophageal carcinogenesis, and testing for the combination of these biomarkers is useful for identifying high-risk patients with precancerous lesions.
Keywords: Esophageal squamous cell cancer, Esophageal squamous cell dysplasia, p53, Carcinoembryonic antigen, CA19-9, Immunohistochemistry, Prediction
Core tip: Immunohistochemical detection of p53, carcinoembryonic antigen (CEA) and CA19-9 proteins was carried out in patients with basal cell hyperplasia, low-grade dysplasia, high-grade dysplasia, invasive cancer, and normal controls from an area with a high incidence of esophageal lesions. Our data suggest that p53, CEA, and CA19-9 protein expression correlated with the stages of esophageal carcinogenesis. In an endoscopic screening program, the expression of these three biomarkers will be a useful panel for identifying high-risk patients with precancerous lesions, and the results will provide a basis for targeted prevention in a high-incidence area of esophageal carcinoma.
INTRODUCTION
Esophageal squamous cell carcinoma (ESCC) is one of the most lethal malignancies[1]. Due to the lack of effective clinical methods for early detection, most patients are at an advanced stage at the time of diagnosis[2-4]. ESCC is the fourth most common cause of cancer death in China[5,6].
The pathogenesis of ESCC involves a stepwise progression from basal cell hyperplasia (BCH) to low-grade dysplasia (LGD), high-grade dysplasia (HGD), carcinoma in situ, and finally invasive carcinoma. Esophageal dysplasia is a precancerous lesion[7-11], but the development from dysplasia to carcinoma is by no means inevitable[12]. Long-term epidemiological studies have indicated that severe-dysplasia is related to the risk of ESCC development, but LGD transforms into HGD only in a small number of patients, and can revert to normal mucosa or does not transform into malignant lesions in the majority of patients[13-16].
Endoscopic screening is effective in early esophageal cancer and to ascertain the stages of esophageal carcinogenesis[17-21]. Current management guidelines promote endoscopic screening in individuals with esophageal dysplasia, thus it is very important to identify biomarkers to screen high-risk subjects who should undergo endoscopic examination.
The accumulation of p53 protein appears in the very early stage of esophageal carcinogenesis and culminates in malignant transformation, and increased p53 expression observed on immunostaining is associated with a higher risk of histological progression[22-27]. Carcinoembryonic antigen (CEA) is normally produced during fetal development and is used as a classic tumor marker[28,29]. Carbohydrate antigen sialyl Lewis a (CA19-9) is associated with cancers of the colon, stomach, pancreas and bile duct, but it is also associated with noncancerous conditions[30-33]. As far as we know there are few studies on CEA and CA19-9 protein expression in biopsy tissue from precancerous lesions of the esophagus in a high-incidence area.
Therefore, we determined whether CEA and CA19-9 protein expression is also related to the early stage of esophageal carcinogenesis transition, whether there are associations between these three biomarkers, and whether the combined expression of these biomarkers may lead to a more thorough understanding of the evolving process of ESCC. The aim was to prove a relationship between the three biomarkers and the stages in the transition to esophageal carcinoma, and to evaluate the combination of these protein biomarkers in predicting the malignant transition of LGD and HGD in an endoscopic screening program.
MATERIALS AND METHODS
Subjects
An iodine staining endoscopic screening program of esophageal lesions in the high-incidence area of Feicheng County was carried out from January 2004 to December 2007. A questionnaire was used to interview all subjects to obtain basic information. The endoscopic screening test was performed in a small mobile car in villages. For persons with a non-staining area of the mucosa, random 4-quadrant biopsy specimens were obtained. Histopathologic diagnosis was carried out by two pathologists independently. The ethics committee of Shandong Academy of Medical Sciences approved the study protocol and all participants gave their written informed consent.
In this study, subjects with liver diseases and cardiovascular diseases were excluded. Immunohistochemical analysis was performed to determine the expression of p53, CEA and CA19-9 in histological sections of endoscopic biopsies from 603 persons who had free iodine staining regions of the esophagus. Based on pathological diagnosis of the biopsies, BCH was diagnosed in 77 persons, LGD in 247 (mild dysplasia in 167 and moderate dysplasia in 80), HGD in 51 (severe dysplasia in 35 and carcinoma in situ in 16), and early invasive carcinoma in 134. Eighty persons had no abnormal lesions and acted as normal controls. The data of 14 persons were excluded from the analysis due to failure of the immunohistochemical test (5 for p53, 4 for CEA and 5 for CA19-9).
Immunohistochemistry
The immunohistochemical detection of p53, CEA and CA19-9 proteins was performed using enzyme-linked immunosorbent assay (ELISA) kits (Zymed Laboratories, Inc., South San Francisco, CA, United States).
Reviewing and scoring of the sections
The stained sections were reviewed and scored independently by two investigators using an Olympus microscope. Sections stained for p53, CEA and CA19-9 protein expression were scored from 0 to 4: 0, negative (no staining); 1, weakly positive (positive cells were ≤ 10%); 2, positive (positive cells were > 10% but ≤ 25%); 3, strongly positive (positive cells were > 25% but ≤ 50%); and 4, very strongly positive (positive cells were > 50%). In the multinomial logistic model analysis, each biomarker was used as a dependent variable and we defined 0 as negative and 1 as positive in order to avoid a zero number in one or more groups.
Statistical analysis
The χ2 and Kruskal-Wallis H tests were used in the univariate analysis. The Spearman correlation test was performed to determine the association between the three biomarkers. Odds ratios (ORs) were calculated in the multinomial logistic model analysis. Receiver operating characteristic (ROC) curve analysis was used to discriminate the sensitivity and specificity between each lesion group and the normal control group for positive expression of the three biomarkers. All statistical analyses were performed using SPSS (version 17.0), and P < 0.05 (two-sided) was accepted as statistically significant.
RESULTS
p53, CEA and CA19-9 protein expression
The univariate analysis indicated that there were significant differences in age, school year, income per year-person, alcohol drinking, and smoking among the five groups, therefore these five variables were adjusted in the multinomial logistic analysis as potential confounding factors. Table 1 shows the characteristics of the variables in the five groups.
Table 1.
Distribution of selected variables in the esophageal basal cell hyperplasia, low-grade dysplasia, high-grade dysplasia, esophageal squamous cell cancer and control groups1 n (%)
| Variables | ESCC | HGD | LGD | BCH | Normal control |
| Gender | |||||
| Male | 89 (66.4) | 32 (62.7) | 146 (59.1) | 48 (62.3) | 40 (50.0) |
| Female | 45 (3.6) | 19 (37.3) | 101 (40.9) | 29 (37.7) | 40 (50.0) |
| Age (yr) | |||||
| 40-50 | 52 (38.8) | 16 (31.4) | 60 (24.3) | 20 (26.0) | 7 (8.8) |
| 50-60 | 60 (44.8) | 25 (49.0) | 128 (51.8) | 34 (44.2) | 29 (36.3) |
| ≥ 60 | 22 (16.4) | 10 (19.6) | 59 (23.9) | 23 (29.9) | 44 (55.0) |
| School year (yr) | |||||
| ≤ 6 | 79 (59.0) | 20 (39.3) | 105 (42.5) | 32 (41.6) | 23 (28.8) |
| 7-11 | 50 (37.3) | 22 (43.1) | 98 (39.7) | 30 (39.0) | 36 (45.0) |
| ≥ 12 | 5 (3.7) | 9 (17.6) | 44 (17.8) | 15 (19.5) | 21 (26.3) |
| Income per year-person ($) | |||||
| < 150 | 60 (44.8) | 22 (43.1) | 101 (40.9) | 26 (33.8) | 8 (10.0) |
| 150-350 | 56 (41.8) | 17 (33.3) | 92 (37.2) | 24 (31.2) | 21 (26.3) |
| ≥ 350 | 18 (13.4) | 12 (23.5) | 54 (21.9) | 27 (35.1) | 51 (63.8) |
| Family history of esophageal cancer | |||||
| Yes | 19 (14.2) | 12 (23.5) | 50 (20.2) | 16 (20.8) | 8 (10.0) |
| No | 115 (85.8) | 39 (76.5) | 197 (79.8) | 61 (79.2) | 72 (90.0) |
| Smoking index2 | |||||
| ≥ 450 | 52 (38.8) | 23 (45.1) | 69 (27.8) | 31 (40.0) | 14 (17.7) |
| < 450 | 25 (18.7) | 4 (7.8) | 40 (16.3) | 12 (16.0) | 16 (20.3) |
| None | 57 (42.5) | 24 (47.1) | 138 (55.9) | 34 (44.0) | 49 (62.0) |
| Alcohol drinking index3 | |||||
| ≥ 120 | 56 (42.0) | 16 (31.4) | 77 (31.0) | 22 (28.0) | 6 (7.5) |
| < 120 | 28 (20.6) | 11 (21.6) | 44 (18.0) | 17 (22.7) | 29 (36.3) |
| None | 50 (37.4) | 24 (47.1) | 126 (51.0) | 38 (49.3) | 45 (56.3) |
1In the five groups, χ2 test values for gender, age, school year, family history of esophageal cancer, income per year-person, smoking index, and alcohol drinking index were 6.046 (P = 0.196), 52.858 (P < 0.01), 45.436 (P < 0.01), 76.476 (P < 0.01), 7.137 (P = 0.129), 21.682 (P = 0.006), and 34.085 (P < 0.01), respectively;
Smoking index = cigarette/day × number of smoking years;
Alcohol drinking ≥ 120 g/d represents heavy drinking. ESCC: Esophageal squamous cell cancer; HGD: High-grade dysplasia; LGD: Low-grade dysplasia; BCH: Basal cell hyperplasia.
The positive rates of p53 protein expression in the ESCC, HGD, LGD, BCH and normal control groups were 53.0%, 52.9%, 35.6%, 27.3% and 20.0%, respectively; the positive rates of CA19-9 protein expression were 44.0%, 33.3%, 16.5%, 9.2% and 6.2%, respectively; the positive rates of CEA protein expression were 74.6%, 60.8%, 23.3%, 23.7% and 16.2%, respectively (Table 2). The differences in the expression of the three biomarkers in the five groups were significant, and there were also significant linear trends in the increase in positive ratios with the transformation from normal to carcinoma.
Table 2.
Distribution of p53, CA19-9 and carcinoembryonic antigen expression in the five groups1 n (%)
| Protein expression | ESCC | HGD | LGD | BCH | Normal control |
| p53 | |||||
| Negative | 63 (47.0) | 24 (47.1) | 159 (64.4) | 56 (72.7) | 64 (80.0) |
| Positive | |||||
| 1%-10% | 18 (13.4) | 17 (33.3) | 62 (25.1) | 17 (22.1) | 11 (13.8) |
| 10%-25% | 20 (14.9) | 8 (15.7) | 20 (8.1) | 3 (3.9) | 5 (6.3) |
| 25%-50% | 24 (17.9) | 2 (3.9) | 6 (2.4) | 1 (1.3) | 0 (0) |
| ≥ 50% | 9 (6.8) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| CA19-9 | |||||
| Negative | 75 (56.0) | 34 (66.7) | 206 (83.5) | 70 (90.8) | 75 (93.8) |
| Positive | |||||
| 1%-10% | 43 (32.1) | 15 (29.4) | 40 (16.1) | 6 (7.9) | 4 (5.0) |
| 10%-25% | 13 (9.7) | 1 (2.0) | 1 (0.4) | 0 (0) | 1 (1.3) |
| ≥ 25% | 3 (2.2) | 1 (2.0) | 0 (0) | 1 (1.3) | 0 (0) |
| CEA | |||||
| Negative | 34 (25.4) | 20 (39.2) | 189 (76.7) | 59 (76.3) | 67 (83.8) |
| Positive | |||||
| 1%-10% | 47 (35.1) | 27 (52.9) | 56 (22.5) | 16 (21.1) | 7 (8.8) |
| 10%-25% | 31 (23.2) | 4 (7.8) | 2 (0.8) | 1 (1.3) | 6 (7.5) |
| 25%-50% | 19 (14.2) | 0 (0) | 0 (0) | 1 (1.3) | 0 (0) |
| ≥ 50% | 3 (2.2) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
| Three biomarkers combined2 | |||||
| Negative | 21 (15.7) | 12 (23.5) | 129 (52.4) | 44 (57.1) | 58 (72.5) |
| Positive | |||||
| 1%-10% | 35 (26.1) | 26 (51.0) | 90 (36.4) | 28 (36.4) | 13 (16.3) |
| 10%-25% | 30 (22.4) | 10 (19.6) | 22 (8.8) | 4 (5.2) | 9 (11.3) |
| 25%-50% | 36 (26.8) | 3 (5.9) | 6 (2.4) | 1 (1.3) | 0 (0) |
| ≥ 50% | 12 (9.0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) |
1In the five groups, the Kruskal-Wallis H test values of the differences in positive protein expression ratios of p53, CA19-9, CEA, and the three biomarkers combined were 50.279 (P < 0.001), 68.660 (P < 0.001), 160.453 (P < 0.001), and 142.393 (P < 0.001), respectively. The values of the linear-by-linear association test were 68.737 (P < 0.001), 61.076 (P < 0.001), 128.738 (P < 0.001), and 141.591 (P < 0.001), respectively;
If the expression of any one of the three biomarkers was positive, the combined expression was counted as positive; if there was an overlap in expression, the score of the strongest expression was taken as the score of the combination. ESCC: Esophageal squamous cell cancer; HGD: High-grade dysplasia; LGD: Low-grade dysplasia; BCH: Basal cell hyperplasia.
The positive rates of the combined expression of the three biomarkers (if the expression of any one of the three biomarkers was positive, the combined expression was counted as positive;if there was an overlap in expression, the score of the strongest expression was taken as the score of the combination) were 84.3%, 76.5%, 47.6%, 42.9% and 27.5% in the five groups, respectively.
Correlations between the three biomarkers and their correlations with other factors
The correlation coefficients of p53 and CA19-9, p53 and CEA, and CA19-9 and CEA were 0.325, 0.374 and 0.503, respectively (all P < 0.01). The correlation coefficients of p53, CA19-9 and CEA with the degree of esophageal lesions were 0.287, 0.326 and 0.455, respectively (all P < 0.01). Both p53 and CEA, but not CA 19-9, had significant correlations with age (both P < 0.05). The three biomarkers had no significant correlations with school year, alcohol drinking index, smoking index and family history of esophageal cancer (Table 3).
Table 3.
The correlation coefficients for p53, CA19-9, CEA and selected variables
| Variables |
Spearman's correlation coefficient |
||
| p53 | CA19-9 | CEA | |
| Age | -0.0811 | -0.065 | -0.0931 |
| School year | 0.197 | 0.139 | 0.213 |
| Alcohol drinking index | -0.050 | -0.056 | -0.021 |
| Smoking index | -0.038 | -0.050 | -0.003 |
| Family history of esophageal cancer | 0.020 | -0.078 | -0.035 |
| Degree of esophageal lesions3 | 0.2872 | 0.3262 | 0.4552 |
| p53 | 1 | 0.3252 | 0.3742 |
| CA19-9 | 1.000 | 0.5032 | |
| CEA | 1.000 | ||
Correlation is significant at the 0.05 level (2-tailed);
Correlation is significant at the 0.01 level (2-tailed);
The variable definitions for the degree of esophageal lesions were: 1, normal control; 2, basal cell hyperplasia; 3, low-grade dysplasia; 4, high-grade dysplasia; 5, invasive squamous cell cancer.
Relationships of the three biomarkers with the stages of transformation from basal squamous cell hyperplasia to invasive carcinoma
The normal control group was regarded as the baseline (OR = 1.0), and both the ORs and 95% CIs of the three biomarkers in the other four groups were calculated using multinomial logistic models. The positive protein expression of p53, CEA and CA19-9 was significantly associated with the four esophageal lesions. As shown in Table 4, almost all ORs (95%CIs) of p53, CEA and CA19-9 increased with the stages of transition. The strongest relationship was seen in the ESCC group. BCH had no significant association with the positive protein expression of the three biomarkers. However, the combination of the three biomarkers had significant relationships with all four esophageal lesions.
Table 4.
The associations of the positive expression of p53, CA19-9 and CEA proteins with basal cell hyperplasia, low-grade dysplasia, high-grade dysplasia, and esophageal squamous cell cancer [OR (95%CI)]
| Variables | ESCC | HGD | LGD | BCH |
| p53 | ||||
| Model 11 | 4.51 (2.38-8.59) | 4.50 (2.07-9.78) | 2.21 (1.21-4.06) | 1.50 (0.71-3.15) |
| Model 22 | 4.52 (2.17-4.92) | 4.88 (2.10-11.33) | 2.31 (1.17-4.56) | 1.60 (0.72-3.57) |
| CA19-9 | ||||
| Model 11 | 15.15 (7.45-30.84) | 7.99 (3.53-18.10) | 1.57 (0.81-3.04) | 1.60 (0.72-3.54) |
| Model 22 | 23.57 (9.27-56.86) | 12.01 (4.61-31.32) | 2.05 (0.91-4.63) | 1.99 (0.81-4.93) |
| CEA | ||||
| Model 11 | 11.80 (4.49-31.05) | 7.50 (2.56-22.00) | 2.96 (1.13-7.76) | 1.52 (0.46-5.02) |
| Model 22 | 17.40 (4.76-63.59) | 11.06 (2.80-43.71) | 3.80 (1.06-13.66) | 1.81 (0.41-7.96) |
| Three biomarkers combined3 | ||||
| Model 11 | 14.19 (7.21-27.91) | 8.57 (3.80-19.30) | 2.40 (1.38-4.15) | 1.98 (1.02-3.85) |
| Model 22 | 16.64 (7.68-36.05) | 10.11 (4.20-24.32) | 2.59 (1.38-4.86) | 2.15 (1.04-4.45) |
In the regression model, ORs were calculated without adjustments for any variables;
In the regression model, ORs were calculated after adjustment for age, school year, income per year-person, smoking, alcohol drinking, and family history of esophageal cancer;
If the expression of any one of the three biomarkers was positive, the combined expression was counted as positive; if there was an overlap in expression, the score of the strongest expression was taken as the score of the combination. ESCC: Esophageal squamous cell cancer; HGD: High-grade dysplasia; LGD: Low-grade dysplasia; BCH: Basal cell hyperplasia.
ROC curves and the possibility of predicting the malignant development of the esophageal lesions based on the positive expression of the three biomarkers
In the ROC curve analysis, we used a single biomarker and the combination of the three biomarkers to predict the risk of the four esophageal lesions. As shown in Table 5, the areas of ROC curves for the combination of the three biomarkers were 0.837, 0.740, 0.590 and 0.562 in the ESCC, HGD, LGD and BCH groups, respectively. Statistical significance was found in the ESCC, HGD and LGD groups, but not in the BCH group.
Table 5.
Area of receiver operating characteristic curves for p53, CA19-9, CEA and the three biomarkers combined in the four esophageal lesions
| Protein expression | Area | SE | 95%CI | Z value | P value |
| p53 | |||||
| ESCC | 0.696 | 0.0357 | 0.629-0.756 | 5.483 | 0.001 |
| HGD | 0.669 | 0.0495 | 0.581-0.749 | 3.416 | 0.006 |
| LGD | 0.578 | 0.0356 | 0.523-0.632 | 2.197 | 0.028 |
| BCH | 0.533 | 0.0461 | 0.452-0.613 | 0.725 | 0.468 |
| CA19-9 | |||||
| ESCC | 0.650 | 0.0359 | 0.625-0.751 | 5.288 | < 0.001 |
| HGD | 0.638 | 0.0509 | 0.549-7.200 | 2.714 | 0.007 |
| LGD | 0.554 | 0.0363 | 0.498-0.609 | 1.484 | 0.138 |
| BCH | 0.515 | 0.0464 | 0.433-0.595 | 0.317 | 0.751 |
| CEA | |||||
| ESCC | 0.802 | 0.0293 | 0.742-0.853 | 10.302 | < 0.001 |
| HGD | 0.712 | 0.0479 | 0.260-0.788 | 4.423 | < 0.001 |
| LGD | 0.532 | 0.0369 | 0.476-0.558 | 0.872 | 0.383 |
| BCH | 0.531 | 0.0463 | 0.449-0.611 | 0.668 | 0.504 |
| Three biomarkers combined1 | |||||
| ESCC | 0.837 | 0.0260 | 0.780-0.884 | 212.656 | < 0.001 |
| HGD | 0.740 | 0.0460 | 0.656-0.813 | 5.220 | < 0.001 |
| LGD | 0.590 | 0.0352 | 0.535-0.644 | 2.586 | 0.010 |
| BCH | 0.562 | 0.0458 | 0.0481-0.641 | 1.360 | 0.174 |
If the expression of any one of the three biomarkers was positive, the combined expression was counted as positive; if there was an overlap in expression, the score of the strongest expression was taken as the score of the combination. ESCC: Esophageal squamous cell cancer; HGD: High-grade dysplasia; LGD: Low-grade dysplasia; BCH: Basal cell hyperplasia.
When the positive expression score of 10%-25% was taken as the cut-off value, the sensitivities and specificities of the four ROC curves were determined and are shown in Table 6 and Figure 1. In the ROC curves of the combination of the three biomarkers, the specificity was 88.8% for the normal control group, the sensitivity was 58.2% for the ESCC group, 25.5% for the HGD group, 11.2% for the LGD group, and 6.5% for the BCH group.
Table 6.
Diagnostic values of predicting the four esophageal lesions based on the positive expression of the three biomarkers combined at the cut off value of 10%-25%1 (%)
| Group | Sensitivity | 95%CI | +PV | 95%CI |
| ESCC | 58.2 | 49.4-66.7 | 89.7 | 81.3-95.1 |
| HGD | 25.5 | 14.3-39.6 | 59.1 | 36.4-79.3 |
| LGD | 11.2 | 7.6-15.8 | 75.7 | 58.8-88.2 |
| BCH | 6.5 | 2.2-14.5 | 35.7 | 12.9-64.8 |
1If the expression of any one of the three biomarkers was positive, the combined expression was counted as positive; if there was an overlap in expression, the score of the strongest expression was taken as the score of the combination. The specificity was 88.8% for the normal control group, with a 95%CI of 79.7%-94.7%. ESCC: Esophageal squamous cell cancer; HGD: High-grade dysplasia; LGD: Low-grade dysplasia; BCH: Basal cell hyperplasia.
Figure 1.
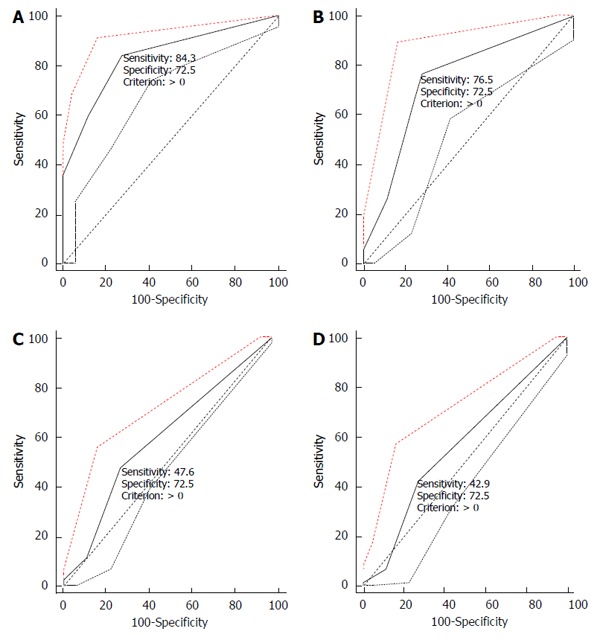
Curves for predicting esophageal lesions based on positive expression of the three biomarkers. These ROC curves are for ESCC (A), HGD (B), LGD (C), and BCH (D), respectively. Note: If the expression of any one of the three biomarkers was positive, the combined expression was counted as positive; if there was an overlap in expression, the score of the strongest expression was taken as the score of the combination. ESCC: Esophageal squamous cell cancer; HGD: High-grade dysplasia; LGD: Low-grade dysplasia; BCH: Basal cell hyperplasia; ROC: Receiver operating characteristic curve.
DISCUSSION
Wild-type p53 suppresses cell proliferation in normal tissues[34], and p53 overexpression is regarded as a potential tumor prognostic factor[35-39]. Increased p53 expression in the pathogenesis of ESCC or adenocarcinoma indicates that p53 overexpression is involved in the initial steps of esophageal carcinogenesis and contributes to the development of precancerous lesions. van Dekken et al[40] studied histologic sections of endoscopic biopsies from patients with Barrett’s esophagus, and found a significant trend for p53 protein overexpression during malignant progression. Kim et al[23] reported that positively stained p53 protein was observed in 87% of ESCC, 80% of esophageal dysplasia, and was not observed in normal mucosa. Bellini et al[26] reported that positive p53 immunohistochemistry progressively increased with pathology severity: Chagas disease (7.7%), chagasic megaesophagus (26.1%), chronic esophagitis (52.2%) and ESCC (100%). We also found that p53 expression showed a significant linear trend with the transition from normal to cancer.
An increase in CEA or CA19-9 level is associated with a more advanced tumor stage[32,41], and their combination may provide more information for diagnosis and prognosis. Bagaria et al[42] reported that with the specificity set at 100%, the sensitivity for esophageal cancer was 28%, 18% and 42% for CEA, CA19-9 and their combination, respectively. Scarpa et al[43] reported that the two biomarkers should be considered when evaluating candidates for esophagectomy.
Setoyama et al[44] found that CEA mRNA expression was positively related to tumor depth and lymph node metastasis in ESCC patients. In another study, CEA mRNA was expressed in the blood, even though CEA and CA19-9 were normal in patients with relapse[45]. From these studies, we suggest that the serum level of CEA or CA19-9 protein expression in patients with early stages of carcinoma may be too low to be detected. In contrast, CEA or CA19-9 protein expression in biopsies from patients with the initial stages of esophageal carcinogenesis can easily be detected by immunohistochemistry. In the present study, the main finding was that the positive expression of CEA and CA19-9 proteins increased with the severity of BCH, dysplasia and carcinoma of the esophagus. These results are useful in understanding the mechanism of the evolution of esophageal cancer at the molecular level of protein expression.
Positive protein expression of p53, CEA and CA19-9 was associated with esophageal carcinogenesis, and a moderate association was found between the three biomarkers in the present study. Interestingly, we discovered that when the positive expressions of the three biomarkers were combined, the specificity for diagnosis of esophageal lesions was 88.8%, which was the target level for screening high-risk individuals, and the sensitivities markedly increased with severity of the esophageal lesions.
However, the identification of patients with dysplasia who will then develop malignant lesions is very important. It is known that p53, CA19-9 and CEA proteins are secreted by malignant cells, and the simultaneous positive expression of these biomarkers in benign pathological lesions of the esophagus may possibly indicate that these lesions will become malignant. Our results indicate that an endoscopic screening program to detect these three biomarkers is beneficial to identify high-risk individuals with esophageal diseases. This information will be useful for doctors to plan the follow-up interval for endoscopic biopsy surveillance, and to decide on appropriate treatment.
It is difficult to obtain a biopsy from the free iodine staining area exactly at the site of the lesion, thus there may be some misclassification of diagnosis, and the controls may not have normal esophageal mucosa. Therefore, the expression rates of the three biomarkers were much higher in the controls. It is possible that the predictive values of the three biomarkers for the stages of ESCC may have been underestimated.
In addition, the expression of other biomarkers, such as p21[46], Ki-67, ProExC[47] and cyclin D1[48], is associated with ESCC, thus the relatively low sensitivity of the three biomarkers combined in the present study may have been influenced by other mechanisms involved in ESCC carcinogenesis.
ACKNOWLEDGMENTS
We appreciate Dr. Guo-Qing Wang and Gui-Qi Wang of the Cancer Institute and Hospital, Chinese Academy of Medical Science for guidance on the endoscopic screening of esophageal diseases in Feicheng County.
COMMENTS
Background
Esophageal squamous cell carcinoma (ESCC) is one of the most lethal malignancies, and the pathogenesis involves a stepwise progression from basal cell hyperplasia (BCH) to low-grade dysplasia (LGD), high-grade dysplasia (HGD), carcinoma in situ, and finally invasive carcinoma. It is very important to identify biomarkers to screen high-risk subjects.
Research frontiers
The accumulation of p53 protein appears in the very early stage of esophageal carcinogenesis and culminates in malignant transformation. There are few studies on carcinoembryonic antigen (CEA) and CA19-9 protein expression in biopsy tissue from precancerous lesions of the esophagus in a high-incidence area. Therefore, the authors determined whether the combined expression of these three biomarkers would lead to a more thorough understanding of the development of ESCC.
Innovations and breakthroughs
The positive protein expression of p53, CEA and CA19-9 was associated with esophageal carcinogenesis. When the positive expressions of the three biomarkers were combined, the specificity achieved the target level for screening high-risk individuals, and the sensitivities markedly increased with the severity of esophageal lesions.
Applications
The results of this study indicate that an endoscopic screening program to determine these three biomarkers is beneficial to identify high-risk individuals with esophageal diseases. Information on the expression of these three biomarkers will be useful for doctors to plan the follow-up interval for endoscopic biopsy surveillance, and to decide on appropriate treatment.
Terminology
CA19-9 is a high-molecular-mass mucin glycoprotein complex and is used as a tumor marker for cancers of the colon, stomach, pancreas and bile duct.
Peer-review
The authors revealed that the combined assessment of p53, CEA and CA19-9 could predict the malignant potential of esophageal lesions. The concept of the study is clinically relevant.
Footnotes
Manuscript source: Unsolicited manuscript
Specialty type: Gastroenterology and hepatology
Country of origin: China
Peer-review report classification
Grade A (Excellent): 0
Grade B (Very good): B, B, B
Grade C (Good): 0
Grade D (Fair): 0
Grade E (Poor): 0
Institutional review board statement: The study was reviewed and approved by the Institutional Review Board of Shandong Academy of Medical Sciences.
Informed consent statement: All participants gave their written informed consent.
Conflict-of-interest statement: There are no conflicts of interest to declare for all authors of the manuscript.
Data sharing statement: No additional data are available.
Peer-review started: June 20, 2016
First decision: July 29, 2016
Article in press: September 14, 2016
P- Reviewer: Garg P, Kuribayashi S, La Mazza A S- Editor: Gong ZM L- Editor: Webster JR E- Editor: Wang CH
References
- 1.Taylor PR, Abnet CC, Dawsey SM. Squamous dysplasia--the precursor lesion for esophageal squamous cell carcinoma. Cancer Epidemiol Biomarkers Prev. 2013;22:540–552. doi: 10.1158/1055-9965.EPI-12-1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shimada H, Nabeya Y, Okazumi S, Matsubara H, Shiratori T, Gunji Y, Kobayashi S, Hayashi H, Ochiai T. Prediction of survival with squamous cell carcinoma antigen in patients with resectable esophageal squamous cell carcinoma. Surgery. 2003;133:486–494. doi: 10.1067/msy.2003.139. [DOI] [PubMed] [Google Scholar]
- 3.Roshandel G, Khoshnia M, Sotoudeh M, Merat S, Etemadi A, Nickmanesh A, Norouzi A, Pourshams A, Poustchi H, Semnani S, et al. Endoscopic screening for precancerous lesions of the esophagus in a high risk area in Northern Iran. Arch Iran Med. 2014;17:246–252. [PMC free article] [PubMed] [Google Scholar]
- 4.Chen JW, Xie JD, Ling YH, Li P, Yan SM, Xi SY, Luo RZ, Yun JP, Xie D, Cai MY. The prognostic effect of perineural invasion in esophageal squamous cell carcinoma. BMC Cancer. 2014;14:313. doi: 10.1186/1471-2407-14-313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Li LD, Lu FZ, Zhang SW. Analysis of cancer mortality rates and distribution in China, 1990-92. Zhonghua Zhongliu Zazhi. 1996;18:403–407. [PubMed] [Google Scholar]
- 6.Daly JM, Karnell LH, Menck HR. National Cancer Data Base report on esophageal carcinoma. Cancer. 1996;78:1820–1828. doi: 10.1002/(sici)1097-0142(19961015)78:8<1820::aid-cncr25>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu M, Ban S, Odze RD. Squamous dysplasia and other precursor lesions related to esophageal squamous cell carcinoma. Gastroenterol Clin North Am. 2007;36:797–811, v-vi. doi: 10.1016/j.gtc.2007.08.005. [DOI] [PubMed] [Google Scholar]
- 8.Kuwano H, Watanabe M, Sadanaga N, Ikebe M, Mori M, Sugimachi K. Squamous epithelial dysplasia associated with squamous cell carcinoma of the esophagus. Cancer Lett. 1993;72:141–147. doi: 10.1016/0304-3835(93)90120-x. [DOI] [PubMed] [Google Scholar]
- 9.Schlemper RJ, Dawsey SM, Itabashi M, Iwashita A, Kato Y, Koike M, Lewin KJ, Riddell RH, Shimoda T, Sipponen P, et al. Differences in diagnostic criteria for esophageal squamous cell carcinoma between Japanese and Western pathologists. Cancer. 2000;88:996–1006. doi: 10.1002/(sici)1097-0142(20000301)88:5<996::aid-cncr8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Rubio CA, Liu FS, Zhao HZ. Histological classification of intraepithelial neoplasias and microinvasive squamous carcinoma of the esophagus. Am J Surg Pathol. 1989;13:685–690. doi: 10.1097/00000478-198908000-00007. [DOI] [PubMed] [Google Scholar]
- 11.Li QD, Li H, Wang MS, Diao TY, Zhou ZY, Fang QX, Yang FY, Li QH. Multi-susceptibility genes associated with the risk of the development stages of esophageal squamous cell cancer in Feicheng County. BMC Gastroenterol. 2011;11:74. doi: 10.1186/1471-230X-11-74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Qiu SL, Yang GR. Precursor lesions of esophageal cancer in high-risk populations in Henan Province, China. Cancer. 1988;62:551–557. doi: 10.1002/1097-0142(19880801)62:3<551::aid-cncr2820620319>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 13.Wang LD, Yang HH, Fan ZM, Lü XD, Wang JK, Liu XL, Sun Z, Jiang YN, He X, Zhou Q. Cytological screening and 15 years’ follow-up (1986-2001) for early esophageal squamous cell carcinoma and precancerous lesions in a high-risk population in Anyang County, Henan Province, Northern China. Cancer Detect Prev. 2005;29:317–322. doi: 10.1016/j.cdp.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 14.Dawsey SM, Lewin KJ, Wang GQ, Liu FS, Nieberg RK, Yu Y, Li JY, Blot WJ, Li B, Taylor PR. Squamous esophageal histology and subsequent risk of squamous cell carcinoma of the esophagus. A prospective follow-up study from Linxian, China. Cancer. 1994;74:1686–1692. doi: 10.1002/1097-0142(19940915)74:6<1686::aid-cncr2820740608>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 15.Wang GQ. Clinical preventive strategies to decrease incidence and death rates of esophageal cancer in high-risk areas. Linchuang Zhongliuxue Zazhi. 1999;21:223 (in Chinese). [Google Scholar]
- 16.Wang LD, Zhou Q, Feng CW, Liu B, Qi YJ, Zhang YR, Gao SS, Fan ZM, Zhou Y, Yang CS, et al. Intervention and follow-up on human esophageal precancerous lesions in Henan, northern China, a high-incidence area for esophageal cancer. Gan To Kagaku Ryoho. 2002;29 Suppl 1:159–172. [PubMed] [Google Scholar]
- 17.Ishihara R, Takeuchi Y, Chatani R, Kidu T, Inoue T, Hanaoka N, Yamamoto S, Higashino K, Uedo N, Iishi H, et al. Prospective evaluation of narrow-band imaging endoscopy for screening of esophageal squamous mucosal high-grade neoplasia in experienced and less experienced endoscopists. Dis Esophagus. 2010;23:480–486. doi: 10.1111/j.1442-2050.2009.01039.x. [DOI] [PubMed] [Google Scholar]
- 18.Domper Arnal MJ, Ferrández Arenas Á, Lanas Arbeloa Á. Esophageal cancer: Risk factors, screening and endoscopic treatment in Western and Eastern countries. World J Gastroenterol. 2015;21:7933–7943. doi: 10.3748/wjg.v21.i26.7933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang J, Wei WQ, Niu J, Liu ZC, Yang CX, Qiao YL. Cost-benefit analysis of esophageal cancer endoscopic screening in high-risk areas of China. World J Gastroenterol. 2012;18:2493–2501. doi: 10.3748/wjg.v18.i20.2493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roshandel G, Nourouzi A, Pourshams A, Semnani S, Merat S, Khoshnia M. Endoscopic screening for esophageal squamous cell carcinoma. Arch Iran Med. 2013;16:351–357. [PubMed] [Google Scholar]
- 21.Wang GQ, Liu YY, Hao CQ, Lai SQ, Wang GQ, Lu N, Yang L. A comparative study of endoscopic image stained by iodine and histopathology in early esophageal cancer and precancerous lesions (dysplasia) Zhonghua Zhongliu Zazhi. 2004;26:342–344. [PubMed] [Google Scholar]
- 22.Bennett WP, Hollstein MC, He A, Zhu SM, Resau JH, Trump BF, Metcalf RA, Welsh JA, Midgley C, Lane DP. Archival analysis of p53 genetic and protein alterations in Chinese esophageal cancer. Oncogene. 1991;6:1779–1784. [PubMed] [Google Scholar]
- 23.Kim SG, Hong SJ, Kwon KW, Jung SW, Kim WY, Jung IS, Ko BM, Ryu CB, Kim YS, Moon JH, et al. The expression of p53, p16, cyclin D1 in esophageal squamous cell carcinoma and esophageal dysplasia. Korean J Gastroenterol. 2006;48:269–276. [PubMed] [Google Scholar]
- 24.Lin DC, Du XL, Wang MR. Protein alterations in ESCC and clinical implications: a review. Dis Esophagus. 2009;22:9–20. doi: 10.1111/j.1442-2050.2008.00845.x. [DOI] [PubMed] [Google Scholar]
- 25.Kerkhof M, Steyerberg EW, Kusters JG, van Dekken H, van Vuuren AJ, Kuipers EJ, Siersema PD. Aneuploidy and high expression of p53 and Ki67 is associated with neoplastic progression in Barrett esophagus. Cancer Biomark. 2008;4:1–10. doi: 10.3233/cbm-2008-4101. [DOI] [PubMed] [Google Scholar]
- 26.Bellini MF, Leite KR, Cury PM, Silva AE. p53, p16 and Fhit proteins expressions in chronic esophagitis and Chagas disease. Anticancer Res. 2008;28:3793–3799. [PubMed] [Google Scholar]
- 27.Alcolea MP, Greulich P, Wabik A, Frede J, Simons BD, Jones PH. Differentiation imbalance in single oesophageal progenitor cells causes clonal immortalization and field change. Nat Cell Biol. 2014;16:615–622. doi: 10.1038/ncb2963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lukaszewicz-Zając M, Mroczko B, Kozłowski M, Nikliński J, Laudański J, Siewko M, Szmitkowski M. Comparative evaluation of serum C-reactive protein (CRP) levels in the different histological subtypes of esophageal cancer (squamous cell carcinoma and adenocarcinoma of esophagus) J Clin Lab Anal. 2012;26:73–81. doi: 10.1002/jcla.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sanders DS, Kerr MA. Lewis blood group and CEA related antigens; coexpressed cell-cell adhesion molecules with roles in the biological progression and dissemination of tumours. Mol Pathol. 1999;52:174–178. doi: 10.1136/mp.52.4.174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.O’Brien DP, Sandanayake NS, Jenkinson C, Gentry-Maharaj A, Apostolidou S, Fourkala EO, Camuzeaux S, Blyuss O, Gunu R, Dawnay A, et al. Serum CA19-9 is significantly upregulated up to 2 years before diagnosis with pancreatic cancer: implications for early disease detection. Clin Cancer Res. 2015;21:622–631. doi: 10.1158/1078-0432.CCR-14-0365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yu J, Zhang S, Zhao B. Differences and correlation of serum CEA, CA19-9 and CA72-4 in gastric cancer. Mol Clin Oncol. 2016;4:441–449. doi: 10.3892/mco.2015.712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grotowski M. Antigens (CEA and CA 19-9) in diagnosis and prognosis colorectal cancer. Pol Merkur Lekarski. 2002;12:77–80. [PubMed] [Google Scholar]
- 33.Kannagi R. Carbohydrate antigen sialyl Lewis a--its pathophysiological significance and induction mechanism in cancer progression. Chang Gung Med J. 2007;30:189–209. [PubMed] [Google Scholar]
- 34.Indinnimeo M, Reale MG, Cicchini C, Stazi A, Fiori E, Izzo P. CEA, TPA, CA 19-9, SCC and CYFRA at diagnosis and in the follow-up of anal canal tumors. Int Surg. 1997;82:275–279. [PubMed] [Google Scholar]
- 35.Qian Y, Chen X. Senescence regulation by the p53 protein family. Methods Mol Biol. 2013;965:37–61. doi: 10.1007/978-1-62703-239-1_3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kandioler D, Schoppmann SF, Zwrtek R, Kappel S, Wolf B, Mittlböck M, Kührer I, Hejna M, Pluschnig U, Ba-Ssalamah A, et al. The biomarker TP53 divides patients with neoadjuvantly treated esophageal cancer into 2 subgroups with markedly different outcomes. A p53 Research Group study. J Thorac Cardiovasc Surg. 2014;148:2280–2286. doi: 10.1016/j.jtcvs.2014.06.079. [DOI] [PubMed] [Google Scholar]
- 37.Yokoyama A, Tanaka Y, Yokoyama T, Mizukami T, Matsui T, Maruyama K, Omori T. p53 protein accumulation, iodine-unstained lesions, and alcohol dehydrogenase-1B and aldehyde dehydrogenase-2 genotypes in Japanese alcoholic men with esophageal dysplasia. Cancer Lett. 2011;308:112–117. doi: 10.1016/j.canlet.2011.04.020. [DOI] [PubMed] [Google Scholar]
- 38.Nicolopoulou-Stamati P, Tsipis A, Chelidonis G, Patsouris E, Athanassiadou P, Gonidi M, Athanassiadou AM. Prognostic value of COX-2, P53, and EZH-2 evaluated by quantitative image analysis in premalignant and malignant breast lesions. Diagn Cytopathol. 2015;43:294–300. doi: 10.1002/dc.23217. [DOI] [PubMed] [Google Scholar]
- 39.Apostolou G, Apostolou N, Biteli M, Kavantzas N, Patsouris E, Athanassiadou P. Utility of Ki-67, p53, Bcl-2, and Cox-2 biomarkers for low-grade endometrial cancer and disordered proliferative/benign hyperplastic endometrium by imprint cytology. Diagn Cytopathol. 2014;42:134–142. doi: 10.1002/dc.23010. [DOI] [PubMed] [Google Scholar]
- 40.van Dekken H, Hop WC, Tilanus HW, Haringsma J, van der Valk H, Wink JC, Vissers KJ. Immunohistochemical evaluation of a panel of tumor cell markers during malignant progression in Barrett esophagus. Am J Clin Pathol. 2008;130:745–753. doi: 10.1309/AJCPO31THGVEUIDH. [DOI] [PubMed] [Google Scholar]
- 41.Sato H, Usuda N, Kuroda M, Hashimoto S, Maruta M, Maeda K. Significance of serum concentrations of E-selectin and CA19-9 in the prognosis of colorectal cancer. Jpn J Clin Oncol. 2010;40:1073–1080. doi: 10.1093/jjco/hyq095. [DOI] [PubMed] [Google Scholar]
- 42.Bagaria B, Sood S, Sharma R, Lalwani S. Comparative study of CEA and CA19-9 in esophageal, gastric and colon cancers individually and in combination (ROC curve analysis) Cancer Biol Med. 2013;10:148–157. doi: 10.7497/j.issn.2095-3941.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scarpa M, Noaro G, Saadeh L, Cavallin F, Cagol M, Alfieri R, Plebani M, Castoro C. Esophageal cancer management: preoperative CA19.9 and CEA serum levels may identify occult advanced adenocarcinoma. World J Surg. 2015;39:424–432. doi: 10.1007/s00268-014-2835-1. [DOI] [PubMed] [Google Scholar]
- 44.Setoyama T, Natsugoe S, Okumura H, Matsumoto M, Uchikado Y, Ishigami S, Owaki T, Takao S, Aikou T. Carcinoembryonic antigen messenger RNA expression in blood predicts recurrence in esophageal cancer. Clin Cancer Res. 2006;12:5972–5977. doi: 10.1158/1078-0432.CCR-06-0637. [DOI] [PubMed] [Google Scholar]
- 45.Mataki Y, Takao S, Maemura K, Mori S, Shinchi H, Natsugoe S, Aikou T. Carcinoembryonic antigen messenger RNA expression using nested reverse transcription-PCR in the peripheral blood during follow-up period of patients who underwent curative surgery for biliary-pancreatic cancer: longitudinal analyses. Clin Cancer Res. 2004;10:3807–3814. doi: 10.1158/1078-0432.CCR-03-0130. [DOI] [PubMed] [Google Scholar]
- 46.Liu J, Hu Y, Hu W, Xie X, Ela Bella A, Fu J, Rao D. Expression and prognostic relevance of p21WAF1 in stage III esophageal squamous cell carcinoma. Dis Esophagus. 2012;25:67–71. doi: 10.1111/j.1442-2050.2011.01217.x. [DOI] [PubMed] [Google Scholar]
- 47.Wang WC, Wu TT, Chandan VS, Lohse CM, Zhang L. Ki-67 and ProExC are useful immunohistochemical markers in esophageal squamous intraepithelial neoplasia. Hum Pathol. 2011;42:1430–1437. doi: 10.1016/j.humpath.2010.12.009. [DOI] [PubMed] [Google Scholar]
- 48.Hussain S, M Y, Thakur N, Salam I, Singh N, Mir MM, Bhat MA, Siddiqi MA, Das BC, Bharadwaj M. Association of cyclin D1 gene polymorphisms with risk of esophageal squamous cell carcinoma in Kashmir Valley: a high risk area. Mol Carcinog. 2011;50:487–498. doi: 10.1002/mc.20732. [DOI] [PubMed] [Google Scholar]


