Abstract
Objectives: To investigate the effect of pubertal timing, assessed in adolescence, on bone size, strength and density in men and women in early old age.
Design: A British birth cohort study with prospective indicators of pubertal timing based on age at menarche, clinical assessment of pubertal stage, and growth tempo from serial height measures, and bone measures derived from peripheral quantitative computed tomography (pQCT) and dual-energy X-ray absorptiometry (DXA) at 60-64 years of age among 866 women and 792 men.
Methods: A first set of regression models investigated the relationships between pubertal timing and bone size, strength and density, adjusting for current height and weight, smoking and adult socioeconomic position. To make an equivalent comparison between men and women, the percentage difference in bone outcomes was calculated for a 5-year difference in age at menarche, and in men a comparison between those who were fully mature or pre-adolescent at 14.5 years. A second set of models investigated the percentage difference in bone outcomes for a 5-year difference in timing of peak height velocity (height tempo) derived from longitudinal growth modelling (Superimposition by Translation and Rotation model; SITAR).
Results: After adjustment for current height and weight, a 5-year increase in age at menarche was associated with an 8% [95% confidence interval (CI) -17%, 0.5%, P = 0.07) lower trabecular volumetric bone mineral density (vBMD); men who were pre-adolescent at 14.5 years had a 9%, (95% CI -14%, -4%; P = 0.001) lower trabecular vBMD compared with those who had been fully mature. Other confounders did not attenuate these estimates further. Patterns of association were similar but somewhat weaker for lumbar spine and total hip areal BMD. Age at peak height velocity was associated with even larger differences in BMD in men and women, and was negatively associated with bone size and strength.
Conclusions: The association between later puberty and lower BMD persists into early old age. The 9-10% lower trabecular vBMD in later compared with earlier maturers could be clinically important given a rate of bone loss from midlife of 1-2% a year and the negative association between BMD and fracture.
Keywords: Puberty, bone, birth cohort, life course
Introduction
Puberty is an important period for longitudinal and appositional bone growth and mineral accrual: 20-30% of an individual’s total body bone mineral is accrued during the pubertal growth spurt.1,2 It follows that ensuring optimal growth during this period will be important for future bone health and fracture risk.3,4 The extent to which pubertal timing is related to later bone phenotype and fracture risk has been investigated in a number of studies of different types.5 Most recently, a large genomic analysis revealed genetic correlations between timing of puberty in men and women and a range of health outcomes, including an inverse correlation with areal bone mineral density (aBMD) of the lumbar spine in 33 000 individuals.6 Such studies have the added benefit of avoiding any problems of bias due to retrospective recall of pubertal timing.7 This study supports the findings of some (for example 8–10), but not all11 retrospective epidemiological studies of premenopausal and postmenopausal women that have shown that later age at menarche is associated with reduced aBMD and increased fracture risk. The most recent of the retrospective epidemiological studies is based on over 250 000 women from UK Biobank and reported a reduced risk of self-reported doctor-diagnosed osteoporosis for those with an early menarche;12 however, in men from the same study, recalled timing of voice breaking was not strongly associated with osteoporosis risk.12
Prospective longitudinal studies with gold-standard bone phenotyping are required to fully understand relationships between pubertal timing, the development of peak bone mass, and bone architecture and fracture risk. There are a number of highly informative longitudinal studies,2,13–17.but none have follow-up beyond the third decade of life. Findings from studies using peripheral quantitative computed tomography (pQCT) or high-resolution pQCT have reported small but significant, negative associations between cortical thickness, medullary area and bone failure load in young women and age at menarche.13,14 Pubertal timing in younger cohorts, and especially in men, has more often been assessed using measures of peak height velocity (PHV), as clinical assessment of the Tanner stages of physical development is rare.2,15,16,18 Later age at PHV was associated with lower trabecular and cortical volumetric BMD (vBMD) and with total body and radius aBMD, in Swedish men aged 19 years;16,18 but by 24 years, there had been substantial catch-up and only deficits in aBMD and vBMD of the radius remained. These findings suggest that any differences in bone due to timing of puberty may be attenuated once catch-up growth has occurred.17
The MRC National Survey of Health and Development (NSHD), the oldest British birth cohort, initiated in 1946, has markers of pubertal maturation based on clinical assessments in adolescence, serial growth measures, and bone measures at 60-64 years derived from pQCT, as well as dual-energy X-ray absorptiometry (DXA), in a large sample of men and women. We have previously described in this cohort associations between height and weight gain at different stages of growth, and bone phenotype at 60-64 years.19 Some of those differences described may have been due to pubertal timing. Therefore the first aim of the current study was to investigate the effect of age at menarche and pubertal stage, acquired during adolescence, on pQCT and DXA-derived bone outcomes in early old age. The second aim was to compare the these relationships with those between pubertal growth markers of tempo, derived using an instrument for longitudinal growth curve analysis called the Superimposition by Translation and Rotation (SITAR) model,20,21 and the bone parameters.
Methods
Sample
The NSHD is cohort study of 2815 men and 2547 women followed up since their birth in a week in March 1946 in England, Scotland and Wales. At the 24th follow-up, when study members were aged between 60 and 64 years, 2856 were still alive and had a known current address in mainland Britain. Participants were invited for assessment at one of six clinical research facilities (CRFs); those unable or unwilling to travel were offered a home visit by a research nurse.22 A total 2229 participants out of the 2856 invited (78%) underwent assessment: 1690 attended a CRF and the remaining 539 were seen in their homes.23 A total of 778 participants had died. Of the remaining participants, 570 were living abroad, 594 had previously withdrawn from the study and 564 were lost to follow-up.
Bone health assessment at 60-64 years
Of those attending a CRF, 792 men and 866 women underwent a DXA and 658 men and 697 women had a pQCT scan of the radius (non-dominant side). DXA scans were acquired in all six CRFs using the QDR 4500 Discovery (Hologic Inc, Bedford, MA), and in five CRFs pQCT data using a XCT 2000 (Stratec, Pforzheim, Germany) scanner were additionally collected. Details of scan acquisition and cross-calibration have been previously described.19 Standard manufacturer protocols were followed for data acquisition. Machine variability between centres was monitored using the European Spine Phantom and the pQCT scanners using the European Forearm phantom and, where necessary, cross-calibration was performed. Standard manufacturer procedures were followed for daily Quality Assurance/Quality Control and all phantom and scan analyses were centralized to one centre (JEA) for grading, analysis and collation of a harmonized database. Repeat precision was determined in one centre and was < 1% for DXA measurements, and for pQCT ranged between 1% and 3%.
The bone outcomes for this analysis were pQCT-derived measures at the radius distal 4% site of trabecular and total vBMD and distal cross-sectional area (CSA), and at the radius 50% site of CSA of the diaphysis and the medullary cavity (medullary CSA), cortical vBMD and polar strength strain index (SSI), an in vivo estimate of bone strength.24 DXA-derived measurements of areal BMD for lumbar spine (L1 L4) and total hip were also obtained.
Pubertal timing
Reports of pubertal timing were obtained in 1961 when study members were aged 14-15 years (mean 14.5, range 14.3-15.2 years), when they underwent a medical examination and interview by a school doctor.25 Age at menarche was obtained from mothers’ reports at the examination. For 94 of the 188 girls who had not reached menarche by the time of the examination, retrospective reports from woman study members were later obtained from a postal questionnaire at age 48. Age at menarche in years was used for descriptive analyses, and modelled as months since birth. In boys, the school doctor assessed: the development of genitalia (advanced or complete, early or infantile); voice breaking (completely broken, starting to break, not yet started); visible pubic hair (profuse, sparse, none); and visible axillary hair (yes or no). Based on these observations, boys were classified as fully mature (advanced development of genitalia, profuse pubic hair and axillary hair, and voice broken), advanced puberty (advanced development of genitalia, but at least one other indicator not fully mature), early puberty (early development of genitalia, and some pubic or axillary hair or voice starting to break) and pre-adolescent (infantile genitalia or early adolescent genitalia, no pubic or axillary hair and voice not broken).26
Individual patterns of height growth during puberty were estimated using the SITAR model of growth curve analysis.20,21 Data were collected using standardized protocols at ages 2, 4, 6, 7, 11 and 15, and self-reported at ages 20 and 26. To provide additional information at intermediate ages, the NSHD data were augmented by height data between 5 and 19 years from the ALSPAC cohort,27 as described by Cole et al.21 The SITAR model summarizes each individual’s growth curve in terms of three parameters: size, tempo and velocity, each expressed relative to the mean curve. The model is estimated as a mixed effects growth model with a cubic B-spline mean curve, including both fixed and random (subject-specific) effects for size, tempo and velocity. For the purposes of this paper, we present only height tempo data, as these indicate the timing of puberty which is the focus of the current paper. A negative height tempo indicates earlier puberty, positive indicates later puberty. Details of the other SITAR variables are given in Cole et al.21
Covariables
Current body size was assessed by height (m) and weight (kg), according to a standard protocol. Smoking was split into two categories, cigarette smokers versus non-smokers at 60-64 years. According to the Registrar General’s social class classification, social class was categorized based on the participant’s occupation at age 53 years (or at other ages if missing, n = 4) to split those who were in the manual social classes from those in the non-manual social classes in adulthood. Other potential confounders or mediators that were investigated in additional analyses were: leisure-time physical activity (distinguishing those who were most active (reporting vigorous leisure time activity more than fibe times a month) from those less active (one to four times a month) or inactive;28 and certain health conditions assessed at age 60-64 years and detailed elsewhere.29 We included a set of cardiometabolic health conditions (cardiovascular disease, hypertension, raised cholesterol and diabetes) and a second set of conditions (liver disease, thyroid disease and psychiatric problems) that may be relevant for bone.
In women, age when periods ceased naturally or because of hysterectomy or bilateral oophorectomy was obtained from information on menstrual irregularity and date of last menstrual period or any operation to remove the uterus or ovaries. Information was collected in annual postal questionnaires from age 47 to 54 years and at 57 years, and from face-to-face interviews with trained research nurses at 43, 53 and 60-64 years of age.30
Statistical analysis
Of those who had a DXA or pQCT scan, 75% also had reports of pubertal timing. R version 3.2 (www.R-project.org) was used to fit the SITAR model and generate the SITAR random effects. For all other analyses, Stata v10.1 was used. Regression models used natural logarithms for all bone variables for comparative purposes. The coefficients from these models are presented as the percentage difference in the bone outcome by category, or per unit increase.
Age at menarche and pubertal stage
Initial adjustments were for current body size (height and weight at 60-64 years), and then for current smoking and adult social class. In women, additional adjustments were made for age at period cessation in a subset of women where those data were available. In a set of further analyses, we additionally adjusted for physical activity, cardiometabolic conditions, and liver disease, thyroid disease and psychiatric problems. We also re-ran the analyses excluding 11% with osteoporosis, based on bone density T-score from the DXA scan ≤ 2.5 at spine, femoral neck or hip.29
To make an equivalent comparison between men and women that compared those with the earliest and latest pubertal timing, we calculated the percentage difference in the bone outcomes for a 5-year difference in age at menarche and the percentage difference between men who were fully mature and pre-adolescent at age 14.5 years.
SITAR analysis of height tempo
Using the same participants with reported pubertal timing, the percentage difference in each of the bone outcomes by the derived SITAR parameter of height tempo was derived. The models first included height tempo unadjusted, and then additionally included current height and weight. To compare the estimates for tempo with those based on reported pubertal timing, we calculated the percentage difference for a 5-year difference in timing of puberty for women (10.5 to 15.5 years) and men (11.5 to 16.5 years).
Results
In the sample of 704 women and 655 men with at least one bone outcome and reported pubertal timing, mean age at menarche was 13.0 years (standard deviation (SD) 1 year, 2 months); and by 14.5 years, 26% of the boys were fully mature, 30% were advanced, 34% were at an early stage and 10% were still pre-adolescent. Descriptions of the bone outcomes, puberty indicators and covariables are shown in Table 1.
Table 1.
Characteristics of 655 men and 704 women from the MRC National Survey of Health and Development with at least one bone measure and information on age at menarche or pubertal stage
| Measures | Men |
Women |
||
|---|---|---|---|---|
| No. | Mean (SD) | No. | Mean (SD) | |
| pQCT measures | ||||
| pQCT-cortical sites | ||||
| 50% radius | ||||
| Diaphysis CSA (mm2) | 547 | 155 (23) | 571 | 113 (16) |
| Medullary CSA (mm2) | 546 | 43 (14) | 569 | 35 (12) |
| Polar SSI (mm3) | 543 | 348 (70) | 572 | 211 (43) |
| pQCT-trabecular sites | ||||
| Distal radius (4%) | ||||
| Distal CSA (mm2) | 547 | 171 (34) | 566 | 133 (24) |
| pQCT-50% radius | ||||
| Cortical vBMD (mg/cm3) | 547 | 1159 (35) | 572 | 1148.2 (39) |
| pQCT-distal radius (4%) | ||||
| Trabecular vBMD (mg/cm3) | 546 | 205 (41) | 565 | 173 (42) |
| Total density vBMD (mg/cm3) | 547 | 391 (67) | 566 | 332 (71) |
| DXA measures | ||||
| DXA aBMD (g/cm2) | ||||
| Spine L1-L4 aBMD | 652 | 1.05 (0.18) | 699 | 0.95 (0.16) |
| Total hip aBMD | 645 | 1.00 (0.14) | 695 | 0.87 (0.13) |
| Current body size | ||||
| Height (m) at 60-64 years | 655 | 1.75 (0.06) | 704 | 1.62 (0.06) |
| Weight (kg) at 60-64 years | 655 | 85 (13.1) | 704 | 72 (14.2) |
| Pubertal timing indicators | ||||
| Age at menarche (years months) | n/a | 704 | 13years 0 months (1year 2 months) | |
| % | % | % | ||
| Age at menarche | n/a | |||
| 9-10 | 24 | 3.4 | ||
| 11 | 91 | 12.9 | ||
| 12 | 214 | 30.4 | ||
| 13 | 243 | 34.5 | ||
| 14 | 98 | 13.9 | ||
| 15-19 | 34 | 4.8 | ||
| Total (= 100%) | 704 | |||
| Pubertal stage (14.5 year) | ||||
| Fully mature | 168 | 25.6 | ||
| Advanced | 200 | 30.5 | ||
| Early | 222 | 33.9 | ||
| Pre-adolescent | 65 | 9.9 | ||
| Total (= 100%) | 655 | |||
| Development of genitalia | ||||
| Advanced or complete | 368 | 56.2 | ||
| Early | 263 | 40.1 | ||
| Pre-adolescent | 24 | 3.7 | ||
| Total (= 100%) | 655 | |||
| Broken voice | ||||
| Completely broken | 245 | 37.6 | ||
| Starting to break | 235 | 36.0 | ||
| Not yet broken | 172 | 26.4 | ||
| Total (=100%) | 652 | |||
| Pubic hair | ||||
| Profuse | 313 | 47.9 | ||
| Sparse | 263 | 40.3 | ||
| None | 77 | 11.8 | ||
| Total (= 100%) | 653 | |||
| Axillary hair | ||||
| Yes | 368 | 56.5 | ||
| No | 283 | 43.5 | ||
| Total (= 100%) | 651 | |||
| Covariables | ||||
| Smoking | ||||
| No | 584 | 89.9 | 626 | 89.4 |
| Yes | 66 | 10.1 | 74 | 10.6 |
| Total (=100%) | 650 | 700 | ||
| Own adult social class | ||||
| Non-manual | 473 | 72.2 | 562 | 79.8 |
| Manual | 182 | 27.8 | 142 | 20.2 |
| Total (= 100%) | 655 | 704 | ||
| Age at period cessation (years) | ||||
| 27-39 | 40 | 7.0 | ||
| 40-44 | 57 | 9.9 | ||
| 45-49 | 131 | 22.9 | ||
| 50-52 | 159 | 27.7 | ||
| 53-55 | 126 | 22.0 | ||
| 56-62 | 60 | 10.5 | ||
| Total (= 100%) | 573 | |||
Mean differences in bone size, density and strength by pubertal timing
In women, age at menarche was not associated with any measures of CSA (diaphysis, medullary or distal radius); nor was it associated with polar SSI (Table 2a). However, later age at menarche was associated with lower total and trabecular vBMD and lumbar spine and total hip aBMD (Table 2a).
Table 2a.
Mean and standard deviation (SD) for pQCT-derived outcomes at 60-64 years by age at menarche, women
| Age at menarche |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| 9-10 | 11 | 12 | 13 | 14 | 15-19 | Totalsample | P-value * | ||
| No. | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| pQCT | |||||||||
| Distal CSA mm2 (4%) | 566 | 132 (23) | 132 (24) | 130 (24) | 134 (25) | 136 (24) | 129 (24) | 133 (24) | 0.3 |
| Diaphysis CSA mm2 (50%) | 571 | 111 (12) | 113 (17) | 115 (17) | 111 (16) | 112 (14) | 112 (18) | 113 (16) | 0.3 |
| Medullary CSA mm2 (50%) | 570 | 31 (9) | 34 (13) | 37 (13) | 36 (12) | 35 (12) | 35 (13) | 35 (12) | 0.7 |
| Polar SSI mm3 (50%) | 572 | 212 (34) | 217 (45) | 214 (45) | 207 (42) | 208 (39) | 211 (39) | 211 (43) | 0.1 |
| Total vBMD mg/cm3 (4%) | 566 | 351 (82) | 339 (69) | 340 (74) | 322 (70) | 318 (64) | 337 (69) | 332 (71) | 0.01 |
| Trabecular vBMD mg/cm3 (4%) | 565 | 178 (40) | 177 (41) | 179 (46) | 169 (41) | 168 (35) | 158 (40) | 173 (42) | 0.004 |
| Cortical vBMD mg/cm3 (50%) | 572 | 1157 (33) | 1146 (44) | 1146 (40) | 1149 (40) | 1152 (33) | 1151 (40) | 1148 (39) | 0.5 |
| DXA | |||||||||
| Lumbar spine aBMD g/cm2 | 699 | 0.98 (0.14) | 0.98 (0.18) | 0.95 (0.16) | 0.95 (0.16) | 0.92 (0.15) | 0.91 (0.16) | 0.95 (0.16) | 0.006 |
| Total hip aBMD g/cm2 | 695 | 0.90 (0.14) | 0.90 (0.13) | 0.87 (0.12) | 0.86 (0.13) | 0.85 (0.12) | 0.84 (0.12) | 0.87 (0.13) | 0.001 |
*P-values from regression models with logged bone outcomes and including age at menarche in months since birth as a continuous variable.
In men, early puberty at 14.5 years was associated with smaller diaphysis and medullary CSA, but not distal CSA, and with lower polar SSI (Table 2b). Later pubertal maturation was associated with lower mean values of trabecular vBMD and lumbar spine and total hip aBMD, and higher mean values of cortical vBMD; but there were no differences in total vBMD. All the indicators of pubertal maturation (axillary hair, pubic hair and voice breaking) showed similar patterns with these bone parameters (Supplementary Data, available as Supplementary Data at IJE online).
Table 2b.
Mean and standard deviation (SD) for pQCT-derived and DXA-derived outcomes at 60-64 years by pubertal stage at 14.5 years, men
| Pubertal stage |
|||||||
|---|---|---|---|---|---|---|---|
| Fully mature | Advanced | Early | Pre-adolescent | Total sample | P-value | ||
| No. | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | Mean (SD) | ||
| pQCT | |||||||
| Distal CSA mm2 (4%) | 547 | 172 (38) | 174 (31) | 170 (33) | 167 (32) | 171 (34) | 0.3 |
| Diaphysis CSA mm2 (50%) | 547 | 159 (25) | 155 (21) | 152 (22) | 153 (22) | 154 (23) | 0.01 |
| Medullary CSA mm2 (50%) | 546 | 45 (16) | 44 (14) | 41 (12) | 41 (14) | 43 (14) | 0.02 |
| Polar SSI mm3 (50%) | 543 | 358 (77) | 349 (64) | 342 (72) | 340 (61) | 348 (70) | 0.03 |
| Total vBMD mg/cm3 (4%) | 547 | 403 (71) | 384 (67) | 388 (64) | 397 (71) | 391 (67) | 0.3 |
| Trabecular vBMD mg/cm3 (4%) | 546 | 216 (43) | 205 (41) | 201 (40) | 197 (41) | 205 (41) | < 0.001 |
| Cortical vBMD mg/cm3 (50%) | 547 | 1153 (37) | 1159 (35) | 1161 (33) | 1162 (31) | 1159 (35) | 0.03 |
| DXA | |||||||
| Lumbar spine aBMD g/cm2 | 652 | 1.08 (0.18) | 1.04 (0.19) | 1.05 (0.17) | 1.01 (0.15) | 1.05 (0.18) | 0.02 |
| Total hip aBMD g/cm2 | 645 | 1.02 (0.14) | 1.00 (0.16) | 0.99 (0.14) | 0.97 (0.15) | 1.00 (0.14) | 0.01 |
*P-values from regression models with logged bone outcomes and including pubertal stage as a continuous variable.
Regression models for age at menarche and pubertal stage
In women with complete data (n = 573 for pQCT-derived measures and 704 for DXA-derived measures), a 5-year increase in age at menarche was associated with an 11% (95% CI -19%, -3%, P = 0.01) lower trabecular vBMD (Table 3a, model 1). Adjusting for current height and weight attenuated this estimate somewhat (-8%, 95% CI -17%, 0.5%; P = 0.07) (Table 3a, model 2). There was no further attenuation after adjusting for smoking and social class (Table 3a, model 3). The associations for total vBMD and aBMD in lumbar spine and total hip were in the same direction but slightly weaker; no other associations were observed. In the subset of women with age at period cessation, the association between age at menarche and BMD was attenuated after adjustment for body size, particularly for trabecular vBMD, but not on further adjustment for age at period cessation or other confounders (Supplementary Data, available as Supplementary Data at IJE online).
Table 3a.
Percentage difference in DXA-derived and pQCT-derived outcomes for a 5-year difference (diff) in age at menarche , additionally adjusted for current height and weight, smoking and adult social class, women
| Unadjusted |
Adjusted for height & weight |
Additionally adjusted for smoking and adult social class |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % diff | 95% CI | P-value | % diff | 95% CI | P-value | % diff | 95% CI | P-value | |
| pQCT measures | ||||||||||
| pQCT-cortical sites | ||||||||||
| 50% radius | ||||||||||
| Diaphysis CSA (mm2) | 567 | −2.5 | −7.1, 2.12 | 0.3 | −3.3 | −7.5, 1.0 | 0.1 | −3.3 | −7.6, 0.9 | 0.1 |
| Medullary CSA (mm2) | 565 | 0.8 | −10.4, 12 | 0.9 | −2.2 | −13.4, 8.9 | 0.7 | −2.5 | −13.7, 8.7 | 0.7 |
| Polar stress strain index (mm3) | 568 | −4.1 | −10.6, 2.5 | 0.2 | −4.9 | −11.1, 1.2 | 0.1 | −4.9 | −11.1 to1.3 | 0.1 |
| pQCT-trabecular sites | ||||||||||
| Distal radius (4%) | ||||||||||
| Distal CSA (mm2) | 562 | 3.6 | −2.7, 9.9 | 0.3 | 2.0 | −4.1, 8.0 | 0.5 | 2.1 | −3.9, 8.2 | 0.5 |
| pQCT-50% radius | ||||||||||
| Cortical vBMD (mg/cm3) | 568 | 0.2 | −0.9, 1.4 | 0.7 | 0.4 | −0.8, 1.5 | 0.5 | 0.4 | −0.7, 1.6 | 0.5 |
| pQCT-distal radius (4%) | ||||||||||
| Trabecular vBMD (mg/cm3) | 561 | −11 | −19.3, -2.5 | 0.01 | −8.0 | −16.6, 0.5 | 0.07 | −8.1 | −16.7, 0.5 | 0.07 |
| Total density vBMD (mg/cm3) | 562 | −8 | −15.1, -0.9 | 0.03 | −5.5 | −12.7, 1.7 | 0.1 | −5.7 | −12.9, 1.5 | 0.1 |
| DXA measures | ||||||||||
| DXA aBMD (g/cm2) | ||||||||||
| Spine L1-L4 aBMD | 695 | −7.2 | −12.4, -2.1 | 0.006 | −4.8 | −9.9, 0.3 | 0.06 | −4.8 | −9.9, 0.3 | 0.06 |
| Total hip aBMD | 691 | −8.2 | −12.6, -3.8 | < 0.001 | −4.3 | −8.3, −0.3 | 0.04 | −4.2 | −8.1 to−0.1 | 0.04 |
In men (n = 550 for pQCT-derived measures and 655 for the DXA-derived measures), the differences in CSA between those assessed as pre-adolescent compared with those fully mature was greatest for medullary CSA (-10%, 95% CI -19%, -1%; P = 0.02); adjusting for height and weight attenuated these differences, but additionally adjusting for smoking and social class did not (Table 3b, models 1-3). The corresponding difference in trabecular vBMD was -9% (95% CI -15%, -4%; P = 0.001), with almost no attenuation after adjustment for current height and weight (-9%, 95% CI -14%, -4%; P = 0.001) or for smoking and social class (Table 3b models 1-3). In contrast, those assessed as pre-adolescent had higher cortical vBMD (0.9%, 95% CI 0.1, 1.7, p = 0.02); this was not attenuated after further adjustment. Negative associations were also seen for aBMD in lumbar spine and total hip, but these were attenuated after adjustment for confounders. The difference in polar SSI was -6% (95% CI -11%, -0.8%; P = 0.02), and this was also reduced after adjustment for body size (-4%, 95% CI -9%, 0.5%; P = 0.08) but not after adjusting for smoking and social class (Table 3b, models 1-3). Regression models substituting pubertal stage for the separate pubertal indicators in turn (axillary hair, public hair, genitalia and broken voice) all showed similar patterns. The results remained the same upon additional adjustments for physical activity and certain health conditions (cardiometabolic disorders, liver disease, thyroid disease or psychiatric problems). Excluding those with osteoporosis had only a minor effect on the estimates.
Table 3b.
Percentage difference in DXA-derived and pQCT-derived outcomes comparing pre-adolescent with fully mature males at 14.5 years, additionally adjusted for current height and weight, smoking and adult social class, men
| Unadjusted |
Adjusted for height & weight |
Additionally adjusted for smoking and adult social class |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. | % diff | 95% CI | P-value | % diff | 95% CI | P-value | % diff | 95% CI | P-value | |
| pQCT measures | ||||||||||
| pQCT-cortical sites | ||||||||||
| 50% radius | ||||||||||
| Diaphysis CSA (mm2) | 542 | −5.0 | −8.7, -1.2 | 0.009 | −3.8 | −7.1, −0.4 | 0.03 | −3.8 | −7.1, −0.5 | 0.03 |
| Medullary CSA (mm2) | 541 | −9.9 | −18.6, -1.3 | 0.02 | −8.4 | −16.8, −0.02 | 0.05 | −8.5 | .03 | 0.046 |
| Polar stress strain index (mm3) | 538 | −6.1 | −11.4, -0.8 | 0.02 | −4.3 | −9.0, 0.5 | 0.08 | −4.4 | −9.0, 0.3 | 0.07 |
| pQCT-trabecular sites | ||||||||||
| Distal radius (4%) | ||||||||||
| Distal CSA (mm2) | 542 | −2.7 | −8.1, 2.7 | 0.3 | −1.8 | −7.1, 3.5 | 0.5 | −1.7 | −6.9, 3.6 | 0.5 |
| pQCT-50% radius | ||||||||||
| Cortical vBMD (mg/cm3) | 542 | 0.9 | 0.1, 1.7 | 0.02 | 0.8 | 0.1, 1.6 | 0.04 | 0.8 | 0.02, 1.6 | 0.04 |
| pQCT-distal radius (4%) | ||||||||||
| Trabecular vBMD (mg/cm3) | 541 | −9.2 | −14.7, -3.8 | 0.001 | −8.9 | −14.4, −3.5 | 0.001 | −9.2 | −14.6 to−3.7 | 0.001 |
| Total density vBMD (mg/cm3) | 542 | −1.5 | −6.0, 3.1 | 0.5 | −1.3 | −5.9, 3.2 | 0.6 | −1.6 | −6.1, 3.0 | 0.5 |
| DXA measures | ||||||||||
| DXA aBMD (g/cm2) | ||||||||||
| Spine L1-L4 aBMD | 647 | −4.5 | −8.5, -0.4 | 0.03 | −2.7 | −6.4, 1.1 | 0.2 | −2.6 | −6.4, 1.2 | 0.2 |
| Total hip aBMD | 640 | −4.5 | −8.0, -1.0 | 0.01 | −2.5 | −5.6, 0.7 | 0.1 | −2.6 | −5.7, 0.5 | 0.1 |
Regression models for SITAR growth parameters
The negative coefficients for height tempo in men and women are consistent with our findings of later pubertal timing, being associated with lower trabecular vBMD, and spine and total hip aBMD (Table 4). Height tempo was also associated with total and trabecular vBMD, and with spine and total hip aBMD in men. In most cases the height tempo effects were larger than those based on reported pubertal timing, though the confidence intervals were also wider and the significance levels broadly similar (Figure 1). Height tempo in men and women was inversely associated with diaphysis CSA and polar SSI; additionally, in men it was negatively associated with medullary CSA. These associations were attenuated by adjustment for current body size in men but not women.
Table 4.
Percentage difference (diff) in DXA-derived and pQCT-derived outcomes for a 5-year difference in height tempo
| Women |
Men |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Height tempo |
Height tempo |
|||||||||||
| Unadjusted |
Adjusted for height & weight |
Unadjusted |
Adjusted for height & weight |
|||||||||
| % diff | 95%CI | P-value | % diff | 95%CI | P-value | % diff | 95% CI | P-value | %diff | 95% CI | P-value | |
| pQCT measures | ||||||||||||
| pQCT-cortical sites | ||||||||||||
| 50% radius | ||||||||||||
| Diaphysis CSA (mm2) | −4.9 | −10.6, 0.7 | 0.09 | −6.9 | −12, −1.8 | 0.009 | −6.9 | −13.5, -0.3 | 0.04 | −8.8 | −14.8, -2.8 | 0.004 |
| Medullary CSA (mm2) | −1.7 | −15.9, 12.5 | 0.8 | −6.4 | −20.5, 7.7 | 0.4 | −16.5 | −31.8, -1.2 | 0.04 | −19.3 | −34.2, -4.4 | 0.01 |
| Polar stress strain index (mm3) | −7.5 | −15.6, 0.7 | 0.07 | −9.8 | −17.3, -2.3 | 0.01 | −8.3 | −17.5, 1 | 0.08 | −10.9 | −19.3, -2.4 | 0.01 |
| pQCT-trabecular sites | ||||||||||||
| Distal radius (4%) | ||||||||||||
| Distal CSA (mm2) | 1.6 | −6, 9.2 | 0.7 | −1.7 | −8.9, 5.5 | 0.7 | 3.2 | −6.5, 12.9 | 0.5 | 1.9 | −7.6, 11.4 | 0.7 |
| pQCT-50% radius | ||||||||||||
| Cortical vBMD (mg/cm3) | −0.6 | −2, 0.8 | 0.4 | −0.5 | −1.9, 0.9 | 00.5 | 0.7 | −0.7, 2.1 | 0.3 | 0.6 | −0.8, 2 | 0.4 |
| pQCT-distal radius (4%) | ||||||||||||
| Trabecular vBMD (mg/cm3) | −22.2 | −32.7, −11.8 | < 0.001 | −18.4 | −28.9, −7.9 | 0.001 | −21.4 | −31.2, 11.6 | < 0.001 | −20.6 | −30.4, −10.8 | < 0.001 |
| Total density vBMD (mg/cm3) | −11.0 | −19.7, −2.4 | 0.01 | −7.5 | −16.2, 1.1 | 0.09 | −9.6 | −17.5, -1.7 | 0.02 | −9.2 | −17.1, -1.2 | 0.02 |
| DXA measures | ||||||||||||
| DXA aBMD (g/cm2) | ||||||||||||
| Spine L1-L4 aBMD | −13.9 | −20.4, −7.5 | < 0.001 | −11.8 | −18, −5.5 | < 0.001 | −9.7 | −16.9, −2.4 | 0.009 | −9.7 | −16.6, −2.9 | 0.006 |
| Total hip aBMD | −9.9 | −15.5, −4.2 | 0.001 | −5.9 | −10.9, −0.9 | 00.02 | −7.0 | −13.2, −0.9 | .03 | −6.4 | −11.9, −0.8 | 0.02 |
Figure 1.
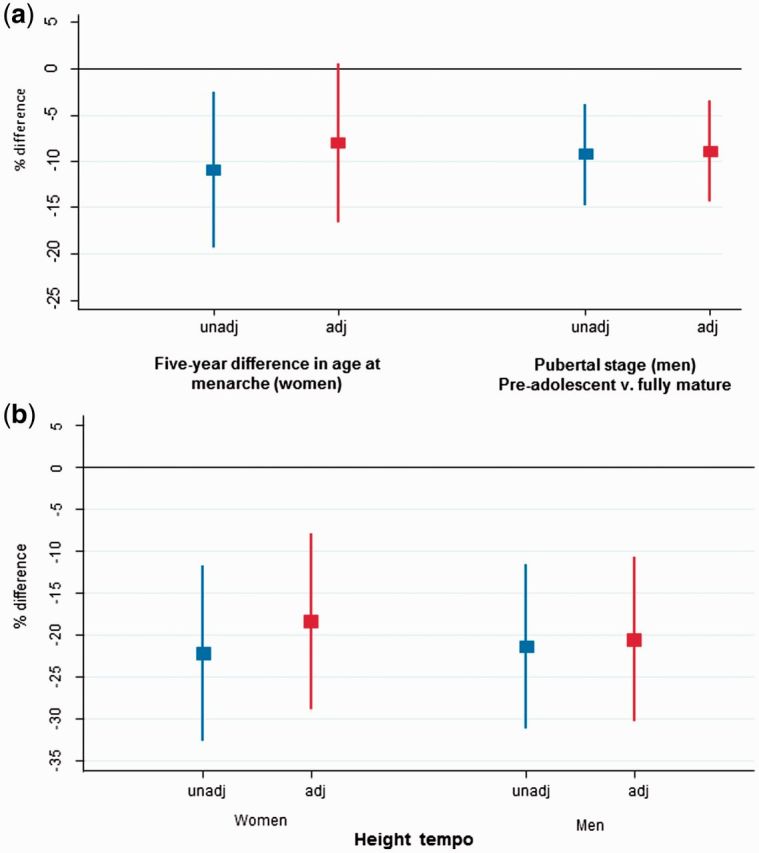
Percentage difference in peripheral trabecular vBMD by a) pubertal timing and b) height tempo for women and men unadjusted and adjusted for height and weight.
Discussion
In a large British birth cohort, later puberty, determined at clinical assessments in adolescence, was associated with lower trabecular vBMD at the radius and lower aBMD at the lumbar spine and hip in early old age. The effect was seen across the range of pubertal maturation and was of a similar size in men and women, explaining 9-10% of the variation in vBMD between the earliest and latest maturers. In men but not women, later puberty was also associated with reduced bone size and strength, although these associations were weaker than for vBMD. Replacing these pubertal indicators with SITAR-derived height tempo gave similar results and the associations were, on the whole, stronger. Later puberty was again negatively associated with trabecular vBMD and aBMD at both sites. In contrast to the analysis with reported age at menarche, bone area and strength were also lower in women with later pubertal growth. Similar relationships for bone size and strength were found in men.
Of the variance in BMD, 60-80% is explained by genetics,5,6,31,32 which could explain the close relationships between vBMD and tempo or pubertal timing. During growth, the cross-section of a bone will also be determined by allometric scaling to height growth and to weight gain.21 Using SITAR in both men and women, later puberty was associated at the diaphysis, with a smaller, less strong, bone, presumably because of the extended limb-growth period in these individuals. Medullary CSA was also smaller in later-maturing men, indicating proportional growth of the periosteal and endosteal surfaces. In women, no associations with medullary CSA existed. There are two possible explanations for this. First, the lack of expansion of the medullary cavity in females during pubertal growth is often attributed as preparation for pregnancy, being less driven by biomechanical adaptation to height growth as is the case in men. Second, at the time of measurement this group of women were all post-menopausal. Bone loss with ageing occurs at the endosteal surface, and so any relationships between puberty and medullary area may have been attenuated by bone loss that had already occurred.
These are important findings as, once skeletal maturity is reached and longitudinal growth ceases, bones grow up to 2 years more in width and 4 years more in mineral content,2and thereafter the skeletal reservoir is set for adult life. As such, the amount of bone an individual has at the end of growth is a determinant of future fracture risk.3,4 Further to this, the vBMD-tempo association is interesting in that vBMD was ∼ 20% less in those with late compared with early puberty. Given that a 1-SD reduction in BMD results in a doubling of fracture risk,33 the differences in the current study may represent a significant increased risk of fracture between the two extremes. There are fewer data relating SSI to fracture risk, though in men with fractures compared with those without, SSI was associated with incident fracture (hazard ratio 2.3, 95% CI 1.3, 4.1).34
The main difference between the two methods of assessment for pubertal timing is that we used a single event to mark puberty, whereas the SITAR analysis used longitudinal growth data. This difference in characterization of pubertal growth may explain why more associations were found between height tempo and bone size and strength than for measures of reported pubertal timing. Because the tempo parameter modelled pubertal timing based on the whole of adolescent growth, and because longitudinal growth is a more proximal indicator of growth in bone cross-section and mineral apposition than the development of secondary sexual characteristics, these differences may have a biological basis and reflect the differential impact of sex hormones and other endocrine factors, rather than simply being due to errors of recall or measurement. Being able to compare these two methods of assessment and relationships with bone is a unique feature of the NSHD. A second advantage of this modelling technique is that men and women were aligned on the same developmental scale to determine timing, which may have improved accuracy of relationships described.
As in the current study, later menarche is associated with lower aBMD in some studies.8,9,13,14 Since then there have been some prospective studies that have used pQCT and followed women into late adolescence or early-mid adulthood.35 Again, results were not conclusive. The most consistent finding was that later age of menarche was associated with greater endosteal circumference (i.e. medullary area) and thinner cortices. A study using high-resolution pQCT reported a negative association between total and cortical vBMD. This is in contrast to our findings which found no association in older women between timing of puberty and cortical bone outcomes.
The UK Biobank study is the largest to date to report the relationship between pubertal timing and osteoporosis risk. In women, self-reported puberty was categorized into early, normal and late. In the late puberty group, incidence of self-reported osteoporosis was increased, and in the early group it was reduced. This confirms previous epidemiological studies showing late menarche to be a risk factor for fracture.36 The associations found in our study would be consistent with these findings of increased risk of fractures and osteoporosis.
In males in the GOOD study, with the exception of cortical vBMD, later age at PHV was not associated with any DXA or pQCT outcome at age 23-25 years (around the age of peak bone mass).17 This is in contrast to the current study, which showed associations particularly between pubertal timing and bone size and distribution at the cortical site. In the UK Biobank, no associations were found between reported time of voice breaking and self-reported osteoporosis.12
Strengths and limitations
The main strength of this study is that it is the only one to have collected growth and pubertal information prospectively and to have been able to relate those measures to gold-standard bone outcomes in early old age. Second, pQCT as well as DXA measurements were taken and results were consistent at both weight-bearing sites (lumbar spine and total hip by DXA) and non weight-bearing sites (radius by pQCT). Peripheral QCT has advantages over DXA in that measures are volumetric and not size dependent, and separate measures of trabecular and cortical compartments allow assessment of the size and distribution of bone, which are important predictors of bone strength. Third, this relatively large British sample included men and women, and the narrow age range of the sample at assessment limits potential confounding by age.
One limitation is that bone was only measured in early old age, so we do not know whether the associations with various bone outcomes were the result of peak bone mass or adult bone loss, although we (and others5) would hypothesize that the former would be more likely. A second limitation is that we cannot translate our findings of pubertal timing and future bone phenotype to fracture risk until sufficient events have accrued; however, it is known that lower BMD is predictive of future osteoporotic fracture.33,37,38 A third limitation of the study was that these analyses were limited to participants who attended a clinic visit. The clinic group were taller and the women were lighter than those who had only a home visit, and have also been found to have fewer health conditions.23 However there is no reason to suspect that the associations between pubertal markers and the bone outcomes should differ between the two groups at this stage of early ageing. A fourth limitation is that the sample were all born in the early post-war period; therefore our findings may not be generalizable to later-born cohorts. The decline in the age at menarche slowed from 2 months a decade to reach 12.8 years in the 1950s, and has been relatively stable since;39 however, we do see a secular decline in pubertal timing between NSHD participants born in 1946 and ALSPAC participants born 1991-92.21
Conclusions
The association between the timing of puberty and BMD persists into early old age. The 9-10% lower peripheral trabecular vBMD in later compared with earlier maturers could be clinically important, given a rate of bone loss from midlife of 1-2% a year and the negative association between BMD and fracture.
Funding
This work was supported by the UK Medical Research Council which provides core funding for the MRC National Survey of Health and Development and supports D.K., S.M. and R.H. by [MC_UU_12019/1, MC_UU_12019/4], K.W. by [U105960371] and T.J.C. by [MR/M012069/1]. The UK Medical Research Council and the Wellcome Trust (grant ref: 092731) and the University of Bristol provide core support for ALSPAC.
Key Messages
Puberty is an important period for bone growth and mineral accrual, but evidence from previous studies on pubertal timing and adult bone health is inconclusive due to a lack of very long-term follow-up in studies with prospectively acquired pubertal indicators and gold-standard bone outcomes.
This birth cohort study is unique in having prospective data acquired in adolescence on puberty, and bone outcomes in early old age derived from peripheral quantitative computed tomography and dual-energy X-ray absorptiometry, on 1359 men and women.
Later puberty, based on reported menarche, on clinically assessed pubertal stage or on growth tempo based on growth curve analysis, was consistently associated with lower trabecular volumetric bone mineral density at the radius and lower areal bone mineral density of lumbar spine and total hip in participants aged 60-64 years.
The 9-10% lower trabecular volumetric bone mineral density in later compared with earlier maturers may be clinically important, given midlife rates of bone loss and a negative association between bone mineral density and fracture.
Supplementary Material
Acknowledgements
The authors would like to thank the two anonymous reviewers for their insightful comments that strengthened this paper. The authors are grateful to NSHD study members who took part in the clinic data collection for their continuing support. We thank members of the NSHD scientific and data collection teams at the following centres: MRC Unit for Lifelong Health and Ageing; Wellcome Trust (WT) Clinical Research Facility (CRF) Manchester; WTCRF and Medical Physics at the Western General Hospital in Edinburgh; WTCRF and Department of Nuclear Medicine at University Hospital Birmingham; WTCRF and the Department of Nuclear Medicine at University College London Hospital; CRF and the Department of Medical Physics at the University Hospital of Wales; and CRF and Twin Research Unit at St Thomas’ Hospital London. We are also extremely grateful to all the families who took part in ALSPAC, the midwives for their help in recruiting them and to the whole ALSPAC team, which includes interviewers, computer and laboratory technicians, clerical workers, research scientists, volunteers, managers, receptionists and nurses. Data used in this publication are available upon request to the MRC National Survey of Health and Development Data Sharing Committee. Further details can be found at http://www.nshd.mrc.ac.uk/data. doi: 10.5522/NSHD/Q101; doi: 10.5522/NSHD/Q102; doi: 10.5522/NSHD/S102A.
Conflict of interest: C.C. has received consultancy and lecture fees and honoraria from Alliance for Better Bone Health, Amgen, Eli Lilly, GSK, Medtronic, Merck, Novartis, Pfizer, Roche, Servier, Takeda and UCB.
References
- 1. Bailey DA, Heather ADM, McKay HA, Whiting S, Mirwald R. Calcium accretion in girls and boys during puberty: A longitudinal analysis. J Bone Miner Res 2000;15:2245-50. [DOI] [PubMed] [Google Scholar]
- 2. Baxter-Jones AD, Faulkner RA, Forwood MR, Mirwald RL, Bailey DA. Bone mineral accrual from 8 to 30 years of age: an estimation of peak bone mass. J Bone Miner Res 2011;26:1729-39. [DOI] [PubMed] [Google Scholar]
- 3. Cooper C, Eriksson JG, Forsen T, Osmond C, Tuomilehto J, Barker DJP. Maternal height, childhood growth and risk of hip fracture in later life: a longitudinal study. Osteoporos Int 2001;12:623. [DOI] [PubMed] [Google Scholar]
- 4. Javaid MK, Eriksson JG, Kajantie E. et al. Growth in childhood predicts hip fracture risk in later life. Osteoporos Int 2011;22:69-73. [DOI] [PubMed] [Google Scholar]
- 5. Bonjour JP, Chevalley T. Pubertal timing, bone acquisition, and risk of fracture throughout life. Endocr Rev 2014;35:820-47. [DOI] [PubMed] [Google Scholar]
- 6. Day FR, Bulik-Sullivan B, Hinds DA. et al. Shared genetic aetiology of puberty timing between sexes and with health-related outcomes. Nat Commun 2015;6:8842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cooper R, Blell M, Hardy R. et al. Validity of age at menarche self-reported in adulthood. J Epidemiol Community Health 2006;60:993-97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ito M, Yamada M, Hayashi K, Ohki M, Uetani M, Nakamura T. Relation of early menarche to high bone mineral density. Calcif Tissue Int 1995;57:11-14. [DOI] [PubMed] [Google Scholar]
- 9. Fox KM, Magaziner J, Sherwin R. et al. Reproductive correlates of bone mass in elderly women. Study of Osteoporotic Fractures Research Group. J Bone Miner Res 1993;8:901-08. [DOI] [PubMed] [Google Scholar]
- 10. Parker SE, Troisi R, Wise LA. et al. Menarche, menopause, years of menstruation, and the incidence of osteoporosis: the influence of prenatal exposure to diethylstilbestrol. J Clin Endocrinol Metab 2014;99:594-601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Waugh EJ, Lam MA, Hawker GA. et al. Risk factors for low bone mass in healthy 40-60 year old women: a systematic review of the literature. Osteoporos Int 2009;20:1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Day FR, Elks CE, Murray A, Ong KK, Perry JR. Puberty timing associated with diabetes, cardiovascular disease and also diverse health outcomes in men and women: the UK Biobank study. Sci Rep 2015;5:11208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Chevalley T, Bonjour JP, Ferrari S, Rizzoli R. Deleterious effect of late menarche on distal tibia microstructure in healthy 20-year-old and premenopausal middle-aged women. J Bone Miner Res 2009;24:144-52. [DOI] [PubMed] [Google Scholar]
- 14. Chevalley T,, Bonjour JP,, Ferrari S,, Rizzoli R. Pubertal timing and body mass index gain from birth to maturity in relation with femoral neck BMD and distal tibia microstructure in healthy female subjects. Osteoporos Int 2011;22:2689-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Gilsanz V, Chalfant J, Kalkwarf H. et al. Age at onset of puberty predicts bone mass in young adulthood. J Pediatr 2011;158:100-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Darelid A, Ohlsson C, Rudang R, Kindblom JM, Mellstrom D, Lorentzon M. Trabecular volumetric bone mineral density is associated with previous fracture during childhood and adolescence in males: the GOOD study. J Bone Miner Res 2010;25:537-44. [DOI] [PubMed] [Google Scholar]
- 17. Darelid A, Ohlsson C, Nilsson M, Kindblom JM, Mellstrom D, Lorentzon M. Catch up in bone acquisition in young adult men with late normal puberty. J Bone Miner Res 2012;27:2198-207. [DOI] [PubMed] [Google Scholar]
- 18. Kindblom JM, Lorentzon M, Norjavaara E. et al . Pubertal timing predicts previous fractures and BMD in young adult men: The GOOD study. J Bone Miner Res 2006;21:790-95. [DOI] [PubMed] [Google Scholar]
- 19. Kuh D, Wills AK, Shah I. et al. Growth from birth to adulthood and bone phenotype in early old age: A British birth cohort study. J Bone Miner Res 2014;29:123-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Cole TJ, Donaldson MD, Ben-Shlomo Y. SITAR—a useful instrument for growth curve analysis. Int J Epidemiol 2010;39:1558-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Cole TJ, Kuh D, Johnson W. et al. Using SITAR to relate pubertal growth to bone health in later life: the MRC National Survey of Health and Development. Int J Epidemiol 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kuh D, Pierce M, Adams J. et al. Cohort Profile: Updating the cohort profile for the MRC National Survey of Health and Development: a new clinic-based data collection for ageing research. Int J Epidemiol 2011;40:e1-e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Stafford M, Black S, Shah I. et al. Using a birth cohort to study ageing: representativeness and response rates in the National Survey of Health and Development. Eur J Ageing 2013;10:145-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ferretti JL, Capozza RF, Zanchetta JR. Mechanical validation of a tomographic (pQCT) index for noninvasive estimation of rat femur bending strength. Bone 1996;18:97-102. [DOI] [PubMed] [Google Scholar]
- 25. Ong KK, Bann D, Wills AK. et al. Timing of voice breaking in males associated with growth and weight gain across the life course. J Clin Endocrinol Metab 2012;97:2844-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Hardy R, Kuh D, Whincup PH, Wadsworth ME. Age at puberty and adult blood pressure and body size in a British birth cohort study. J Hypertens 2006;24:59-66. [DOI] [PubMed] [Google Scholar]
- 27. Boyd A, Golding J, Macleod J. et al . Cohort Profile: The 'children of the 90s'—the index offspring of the Avon Longitudinal Study of Parents and Children. Int J Epidemiol 2013;42:111-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Bann D, Kuh D, Wills AK, Adams J, Brage S, Cooper R. Physical activity across adulthood in relation to fat and lean body mass in early old age: findings from the Medical Research Council National Survey of Health and Development, 1946-2010. Am J Epidemiol 2014;179:1197-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Pierce MB, Silverwood RJ, Nitsch D. et al . Clinical disorders in a post war british cohort reaching retirement: evidence from the first National Birth Cohort Study. PLoS One 2012;7:e44857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mishra G, Hardy R, Kuh D. Are the effects of risk factors for timing of menopause modified by age? Results from a British birth cohort study. Menopause 2007;14:717-24. [DOI] [PubMed] [Google Scholar]
- 31. Seeman E,, Hopper JL,, Young NR,, Formica C,, Goss P,, Tsalamandris C. Do genetic factors explain associations between muscle strength, lean mass, and bone density? A twin study. Am J Physiol 1996;270(2 Pt 1):E320-27. [DOI] [PubMed] [Google Scholar]
- 32. Styrkarsdottir U, Thorleifsson G, Sulem P. et al. Nonsense mutation in the LGR4 gene is associated with several human diseases and other traits. Nature 2013;497:517-20. [DOI] [PubMed] [Google Scholar]
- 33. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996;312:1254-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dennison EM, Jameson KA, Edwards MH, Denison HJ, Aihie SA, Cooper C. Peripheral quantitative computed tomography measures are associated with adult fracture risk: the Hertfordshire Cohort Study. Bone 2014;64:13-17. [DOI] [PubMed] [Google Scholar]
- 35. Chevalley T, Bonjour JP, Ferrari S, Rizzoli R. Influence of age at menarche on forearm bone microstructure in healthy young women. J Clin Endocrinol Metab 2008;93:2594-601. [DOI] [PubMed] [Google Scholar]
- 36. Johnell O, Gullberg B, Kanis JA. et al. Risk factors for hip fracture in European women: the MEDOS Study. Mediterranean Osteoporosis Study. J Bone Miner Res 1995;10:1802-15. [DOI] [PubMed] [Google Scholar]
- 37. Engelke K, Adams JE, Armbrecht G. et al. Clinical use of quantitative computed tomography and peripheral quantitative computed tomography in the management of osteoporosis in adults: the 2007 ISCD Official Positions. J Clin Densitom 2008;11:123-62. [DOI] [PubMed] [Google Scholar]
- 38. Museyko O, Bousson V, Adams J, Laredo J, Engelke K. QCT of the proximal femur-which parameters should be measured to discriminate hip fracture? Osteoporos Int 2016;27:1137-47. [DOI] [PubMed] [Google Scholar]
- 39. Swerdlow A, dos Santos Silva I, Doll R. Cancer Incidence and Mortality in England And Wales: Trends and Risk Factors . Oxford, UK: Oxford University Press, 2001. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


