Abstract
AIM
To test the therapeutic effects of delayed treatment of mesenchymal stem cells (MSCs) in recurrent experimental autoimmune uveitis (rEAU).
METHODS
The efficacy of different regimens of MSC administration in rEAU were tested by evaluation of clinical and pathological intraocular inflammation, as well as retinal structural and functional integrity using optical coherence tomography (OCT) and electroretinogram (ERG). The retinal sections were also immunostained with antibodies to glial fibrillary acidic protein (GFAP) and rhodopsin (RHO).
RESULTS
Delayed treatment of MSCs effectively alleviated the severity of intraocular inflammation with relative intact of outer retinal structure and function. Moreover, double therapies with longer interval led to an even better clinical evaluation, as well as a trend of decrease in relapse and amelioration of retinal function. MSC therapies also effectively reduced GFAP expression and increased RHO expression in the retina.
CONCLUSION
MSC administration can effectively treat developed diseases of rEAU, and multiple therapies can provide additional therapeutic benefits.
Keywords: mesenchymal stem cells, recurrent experimental autoimmune uveitis, electroretinogram, optical coherence tomography
INTRODUCTION
Without efficient therapy, chronic and recurrent autoimmune uveitis involving the choroid and retina will result in irreversible visual disability and blindness caused by retinal damage and optic nerve atrophy. In a majority of cases, long-term usage of systemic corticosteroids or combination of corticosteroids and immunosuppressants is needed. However, these treatments may lead to a wide range of systemic and ocular complications, which limit their applications in severe cases that require more powerful therapeutic regimens[1]. Biologics, as newly developed steroid-sparing agents, potentially offer a safer profile, but do not necessarily apply to all cases because they target only a single specific mediator, and human uveitis is highly heterogeneous[2]–[4].
The immunosuppressive and anti-inflammatory properties of mesenchymal stem cells (MSCs) are now very well established[5], raising the possibility of new therapies for autoimmune diseases (ADs), including uveitis[6]–[9]. MSC therapy can target multiple pathogenic processes in ADs, and no tissue toxicity has been found in MSCs[5]. Although several preclinical models and pilot clinical studies provide a strong impetus for translating MSC therapy to widespread clinical use, the appropriate protocols for MSC administration still lack rigorous standards. Further data are necessary to evaluate and optimize the therapeutic regimens and establish clear guidelines for MSC-based therapies. Our previous studies on recurrent experimental autoimmune uveitis (rEAU) of rats demonstrated that a single course of MSC treatment administered at the onset of the disease effectively reduced ocular inflammation and frequency of relapses, whereas double courses of therapy failed to enhance the therapeutic effect with the second therapy applied before the second attack[9].
However, the above results cannot completely guide the complicated clinical settings. To further optimize the therapeutic regimens of MSC therapy in chronic and recurrent uveitis, we tested the efficiency of MSC therapy administered at the later phase of the disease in the present study. We also tested double courses of therapy with the second course applied after a longer interval. This study will provide additional referential basis for future clinical trials.
MATERIALS AND METHODS
Materials
Male Wistar rats (4-6wk) and female Lewis rats (6-8wk) were obtained from Vital River (Beijing, China). These rats were fed and maintained in specified pathogen-free conditions under 12h light/12h dark cycles. All procedures involving rats were approved by the Laboratory Animal Care and Use Committee of Tianjin Medical University and conformed to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research.
Induction of Recurrent Experimental Autoimmune Uveitis in Lewis Rats
The induction of rEAU in Lewis rats by transfer of antigen-specific T cells was performed as described previously[5]. The rats were subcutaneously immunized with 200 µg of emulsion containing 500 µg of Mycobacterium tuberculosis H37Ra (Difco, Detroit, MI, USA) and 50 µg of IRBP peptide R16 (residues, 1177-1191, ADGSSWEGVGVVPDV; Sangon, Shanghai, China) in incomplete Freund's adjuvant (Sigma-Aldrich Corp., St. Louis, MO, USA), distributed over six spots on the tail base and flank. A single-cell suspension was obtained from the lymph nodes and spleens of the rats by passage through a nylon wool column at 10d post-immunization. Nonadherent cells were identified as T cells. Adherent cells were then incubated on ice and irradiated with 30 Gy to serve as antigen-presenting cells (APCs). The T cells (1×107) were stimulated by 10 µg/mL R16 with 1×107 irradiated APCs in 2 mL of RPMI-1640 medium supplemented with 15% interleukin-2 in a six-well plate at 37°C and 5% CO2 for 48h. The T cells were then isolated in Lymphoprep (Robbins Scientific, Mountain View, CA, USA). To induce recurrent uveitis, 1×107 stimulated T cells were injected intravenously into one Lewis rat.
Isolation and Characterization of Mesenchymal Stem Cells
Bone marrow MSCs were isolated from the Wistar rats as described previously [8]. Bone marrow samples from tibiae and femurs were collected by flushing the diaphysis with Dulbecco's modifiled Eagle's medium (DMEM). Collected cells were incubated in complete media containing DMEM/ F12 (Invitrogen, Carlsbad, CA, USA) and 10% fetal bovine serum (Invitrogen), supplemented with 100 U/mL penicillin and 100 µg/ml streptomycin (Invitrogen) with 5% CO2 at 37°C for at least 24h. The medium was then changed and the adherent cells were incubated for 8-12d until they reached approximately 80% confluency. These cells were then passaged 3-5 times before use.
MSCs are defined by their capacity to differentiate into osteocytes, chondrocytes, and adipocytes under appropriate in vitro conditions. The expression of CD90 and CD29, and the lack of hematopoietic markers CD34 and CD45, was detected to further identify MSCs as described previously[8].
Treatment with Mesenchymal Stem Cells
To investigate the therapeutic effects of different protocols of MSC administration on rEAU, the immunized rats were intravenously treated once daily with 5×106 allogeneic MSCs suspended in 1 mL of PBS or with an equal volume of PBS in control groups for three consecutive days. MSCs were administrated on day 4-6 for onset therapy, on day 20-22 for later therapy, and on day 4-6 and 20-22 for double therapy.
Clinical and Histological Assessment of Recurrent Experimental Autoimmune Uveitis
Slit-lamp microscopy was used to clinically examine the EAU activity, and the rats were followed up for 40d after transfer. The incidence and severity of inflammation were scored in a masked manner on a scale of 0 to 4, in accordance with the standard of Caspi et al[10]: score 0: no disease; 1: engorged blood vessels in the iris and an abnormal pupil configuration; 2: a hazy anterior chamber; 3: moderately opaque anterior chamber, with the pupil still visible; and 4: opaque anterior chamber, obscured pupil, and frequent proptosis.
For histological examination, eyes were enucleated on day 40 after transfer. The eyes were firstly fixed in 4% phosphate-buffered glutaraldehyde for 1h at room temperature, and then transferred to 10% phosphate-buffered glutaraldehyde overnight until processed. Fixed and dehydrated tissues were embedded in paraffin wax. And 4 µm thick sections were cut through the papillary optic nerve plane and stained with hematoxylin and eosin. The sections were scored blindly on a scale of 0 (no disease) to 4 (maximum disease) in half-point increments based on the presence of inflammatory cell infiltration in the eye according to the criteria used by Shao et al[11]. Photographs of the superior and inferior hemispheres at six defined points were obtained using a camera attached to a light microscope (Olympus BX51; Olympus, Tokyo, Japan). The degree of retinal damage was also assessed by measuring the thickness of both the retina and the outer nuclear layer (ONL) as previously described[9].
Electroretinogram Recordings
The retinal function of rats was evaluated by recording dark-adapted electroretinogram (ERG) (RetiMINER-C System, IRC Technologies, Chongqing, China) at 10, 15, 20, 25, 30, 35, and 40d after T cell transfer. The rats were dark-adapted overnight before ERG recording, and all procedures were performed under dim red light. The rats were anesthetized by intraperitoneal injection of 10% chloral hydrate, and the pupils were dilated using 5% tropicamide. One drop of 4% oxybuprocaine was administered for ocular surface anesthesia.
For recording, a golden-ring electrode was placed at the center of the cornea, a needle ground electrode was placed in the tail, and a reference needle electrode was placed at the back region of the neck. A-wave amplitudes were measured from baseline to trough, and b-wave amplitudes were measured from the a-wave trough or baseline to the peak of b-wave. Four oscillatory potentials (OPs) were subsequently measured using an algorithm written to identify the times and determine the amplitudes of each OP. The last OP value, which is the sum of previous OPs, was recorded as the final measurement. Each experimental group consisted of six rats.
Optical Coherence Tomography Imaging
The rats were systemically anesthetized, and the pupils were dilated as described above. Optical coherence tomography (OCT) images were acquired using Heidelberg Spectralis HRA+OCT system (Heidelberg Engineering, Heidelberg, Germany) on day 40 after transfer. Image acquisition was controlled with Heidelberg Eye Explore (LabVIEW-based software), which can display the OCT images in real time. The OCT images were displayed in grayscale mode, in which the low-backscattering regions appeared dark, and the high-backscattering layers provided bright readings.
Semi-quantitative scoring of the OCT was evaluated on a scale of 0-4, modified according to the grading system of Gadjanski et al[12](Table 1).
Table 1. Semi-quantitative scoring of the OCT.
| OCT score | Description |
| 0 | Strong OCT signal, normal retinal morphology, no cells in vitreous |
| 1 | Strong OCT signal, mild infiltration in the vitreous and retina |
| 2 | Low OCT signal, moderate infiltration in the vitreous, blurred retinal architecture |
| 3 | Very low OCT signal, massive infiltration in the vitreous, further blurred and disorganized retinal architecture |
| 4 | Blocked OCT signal |
Immunohistochemistry
For further immunohistological studies, 4 µm-thick serial paraffin wax sections were prepared and mounted on a glass slide for antigen retrieval immunohistochemistry (IHC). After deparaffinization and dehydration, the sections were washed at room temperature using 0.1 mol/L sodium PBS (pH 7.2) with 0.1% Triton X-100 (PBS/Triton). The sections were then incubated overnight at 4°C with antibodies against the following antigens: rhodopsin (RHO; rod photoreceptors) (Rho4D2, Abcam, SwissProt, Shanghai, China, diluted 1:100) and glial fibrillary acidic protein (GFAP, activated Müller cells) (clone G-A-5; Millipore, Sundbyberg, Sweden; diluted 1:200 with PBS/Triton with 1% bovine serum albumin). Immunopositive reaction was developed using a polymer 3′3′-diaminobenzidine tetrachloride (Sigma-Aldrich) with methenamine silver color intensification. The sections were counterstained in hematoxylin and treated with 1% freshly prepared acid alcohol to reduce the counter stain intensity.
Statistical Analysis
Experiments were repeated at least thrice. Statistical analyses were performed using two-tailed Student's t-test for two sets of data, one-way ANOVA Dunnett test for three or more means at one time, or repeated ANOVA for clinical score of uveitis. Data were expressed as means±standard deviation (SD). A P<0.05 was considered significant.
RESULTS
Therapeutic Benefits of Different Mesenchymal Stem Cells Therapeutic Regimens
In contrast to our previous study, we changed the time point of MSC administration in the current experiment to identify the therapeutic effects of a single MSC therapy given during the later phase of the disease (20-22d post-transfer). For the double-therapy protocol, we postponed the second therapy until after the second attack (20-22d post-transfer) to further define the efficiency of double-course MSC administration. Inflammation was observed until 40d after transfer. The incidence and mean clinical scores during the first 20d and the last 20d were used respectively to evaluate the therapeutic effects of different MSC transfusion manners. Compared to the control group, the incidence and severity of the intraocular inflammation was significantly decreased in both double therapy and onset therapy groups during the entire 40d observation (Figure 1A-1D); whereas later therapy significantly alleviated the clinical severity but slightly affected the frequency of relapse (Figure 1C, 1D, 1E4) for the last 20d. Double therapies led to better clinical evaluation during the last 20d than single therapy given at the start of the disease (Figure 1C, 1E3).
Figure 1. Clinical evaluation of different MSC therapeutic protocols.
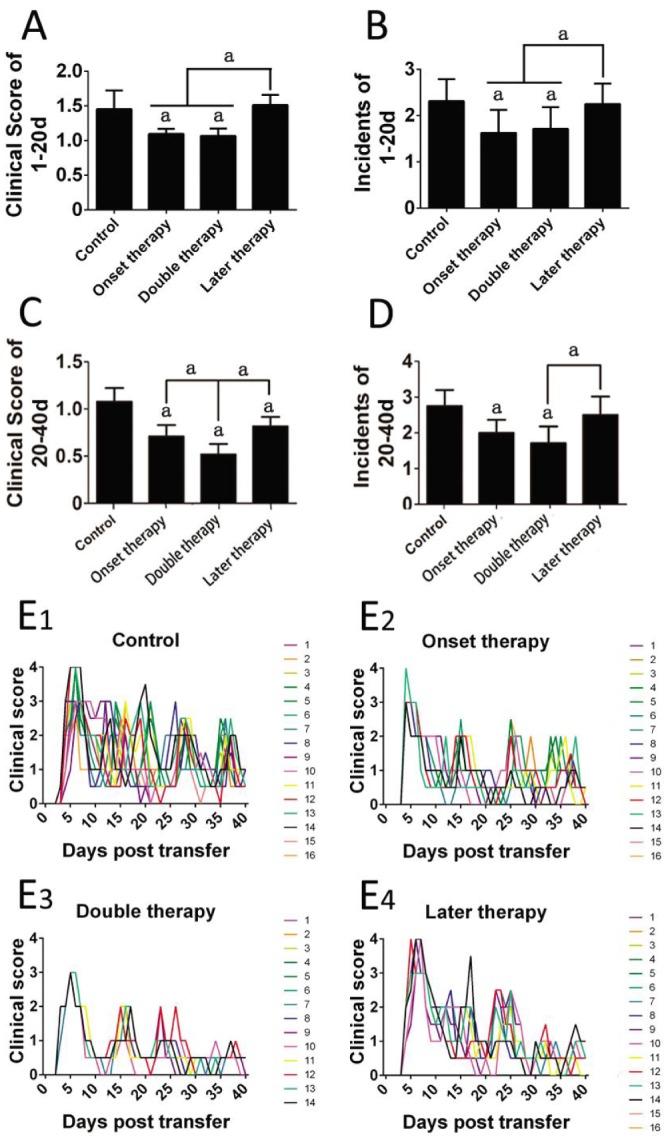
The mean clinical scores and incidence for the first 20d (A, B) and the last 20d (C, D) were compared among different groups. The clinical score curves of all the eyes in different groups were presented as overlays. Eight rats (16 eyes) were used in each group of control, onset therapy and later therapy, and 7 rats (14 eyes) in double therapy; aP<0.05.
It was previously reported that the progression of posterior disease is not always associated with the severity of anterior inflammation, and inflammatory changes in the posterior chamber and retina degeneration are less readily detected by slim-lamp biomicroscopy[13]. Therefore, histological examination was necessary to confirm the efficiency of MSC therapy. Severe retinal structure damage and inflammatory cell infiltration in the control group was found by histological observation performed at day 40 post-transfer. Histological evaluations showed that all the MSC therapeutic regimens significantly ameliorated the histological scores (Figure 2A, 2B) and improved the thickness of both the retina and ONL (Figure 2C-2F). However, no significant difference was found between the double therapy and onset therapy groups. Compared with later MSC administration, early MSC therapy (including both double and onset therapies) exhibited no significant advantages with regard to histological scores emphasizing the infiltration of inflammatory cells but provided more benefits in the maintenance of both retinal and ONL thicknesses (Figure 2C, 2D). Although in later therapy group the retina and ONL were thinner with decreased nuclear numbers than those in both double and onset therapy groups, the ONL was relatively organized compared to the non-treatment control, indicating that late therapy slows down the damage of photoreceptor cells.
Figure 2. Histological changes of the retina on day 40.
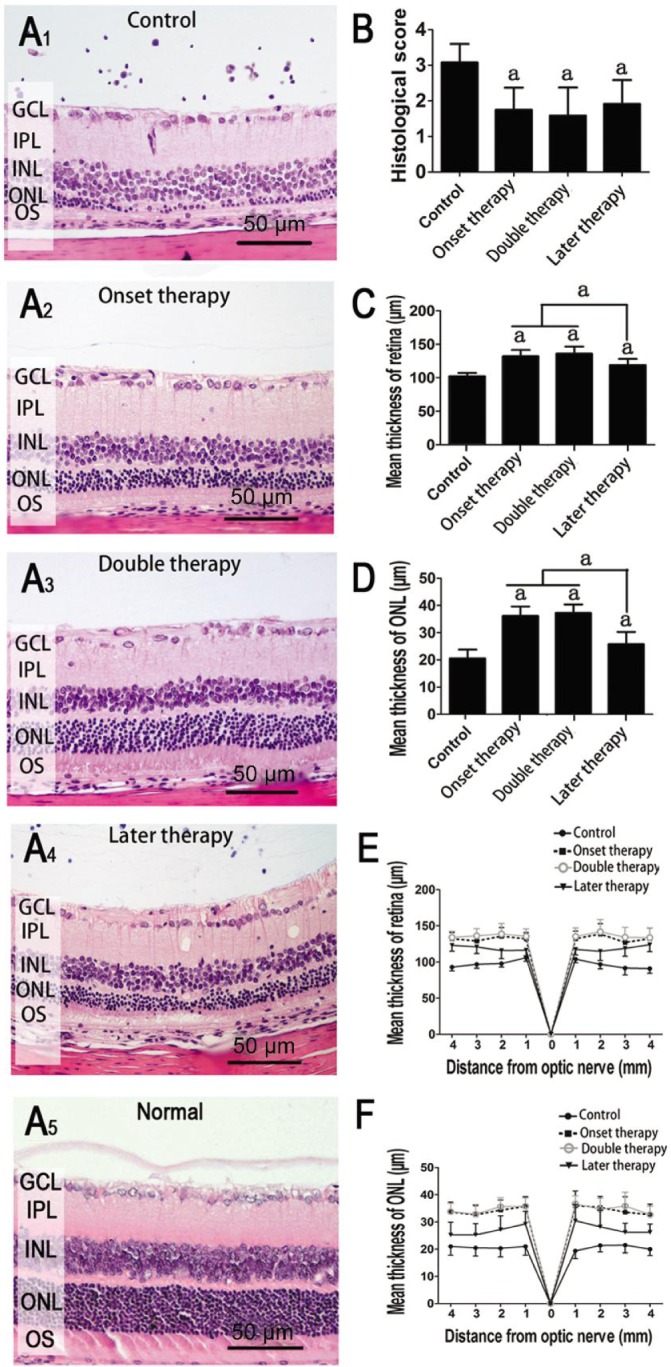
A: Representative retinal histological features; B: Histological evaluation of different groups; C, D: Average thicknesses of retina and ONL; E, F: Thicknesses of retina and ONL were measured at 8 retinal locations at 1 mm intervals. Values are expressed as mean±SD. Eight rats (16 eyes) were used in each group of control, onset therapy and later therapy, and 7 rats (14 eyes) in double therapy; aP<0.05 compared to uveitis without treatment group.
Assessment of Retinal Function by Electroretinogram
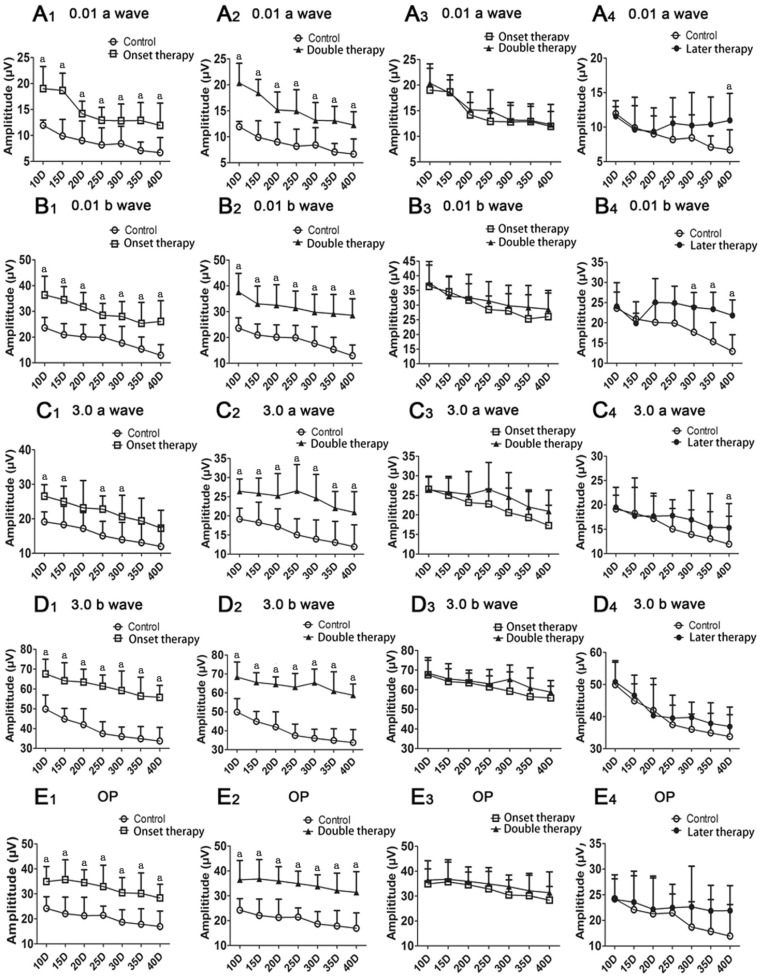
We employed an ERG recording to evaluate the functional properties in rats with rEAU. The ERG was recorded from the same eye at 10, 15, 20, 25, 30, 35, and 40d after transfer, and both scotopic (dark-adaptation) and OP responses were followed. The a-wave amplitude, which is measured from baseline to trough, represents the physiological function of rods and cones. By contrast, the b-wave amplitude, which is measured from a-wave trough or baseline to the peak of b-wave, expresses the ability of bipolar nerve cells (secondary sensory neurons), and OP shows microcirculation of the retina. Consistent with the progressive deterioration of retinal structures, the five ERG components (0.01 and 3.0 a-waves, 0.01 and 3.0 b-waves, and OPs) dropped continuously over time in amplitude in the control group (Figure 3). Early MSC treatment significantly ameliorated the OP responses, as well as the a-wave and b-wave amplitudes at all intensities throughout the entire follow-up period (Figure 3A1-3E1, 3A2-3E2). Most of the EGR components showed an amelioration trend after the second therapy, but the results were not significant (Figure 3A3-3E3). An obvious increase occurred in the 0.01 a/b- and 3.0 a-wave amplitudes of rats treated at later stage in the MSC-treated group compared with those in the control group (P<0.05) (Figure 3A4-3C4); an improving tendency can also be found in other components (Figure 3D4, 3E4).
Figure 3. Comparison of ERG amplitudes between different groups.
ERG responses were determined over time. Values are expressed as mean±SD. Eight rats (16 eyes) were used in each group of control, onset therapy and later therapy, and 7 rats (14 eyes) in double therapy; aP<0.05.
Optical Coherence Tomography Imaging
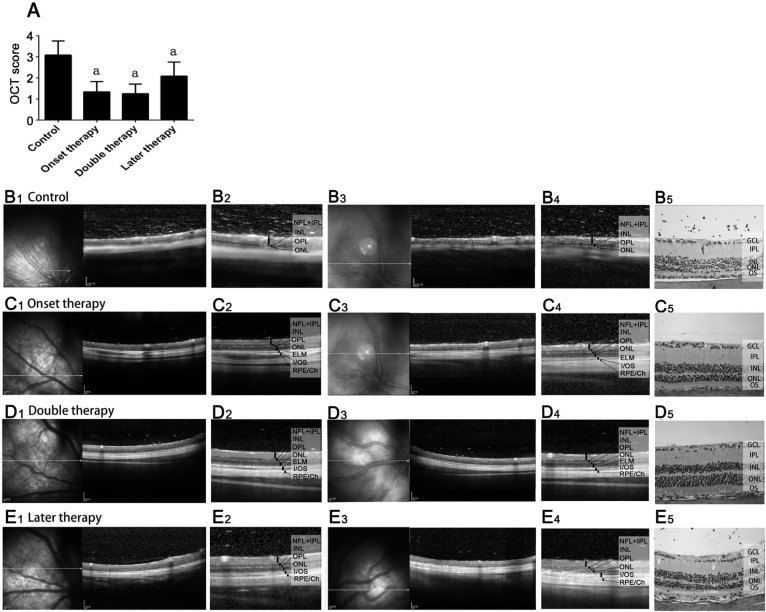
To assess the in vivo rEAU-induced changes in the retina, all the rats underwent OCT scanning at day 40 post-induction, and the OCT image changes were assessed by our grading system indicated in Table 1. The OCT image evaluation was in accord with those of histological assessment (Figure 4A). In the control group, OCT revealed dense and highly reflective dots in the vitreous and multiple highly reflective masses in the inner retina due to massive infiltration, damaged retinal structure and decreased retinal thickness (Figure 4B1-4B4). This image accurately corresponded with the infiltration of inflammatory cells in the vitreous and retina, as well as the atrophy of ONL exhibited in histological sections (Figure 4B5). By contrast, OCT images in all the three MSC therapy groups demonstrated better outer retinal structure, strikingly reduced highly reflective dots in the vitreous and highly reflective masses in the retina (Figure 4B-4D).
Figure 4. OCT evaluations.
A: OCT image evaluation of different groups; B-E: Typical OCT changes of two eyes from different groups (B1-E1 and B3-E3 covered a range of 4 mm of the retina, whereas B2-E2 and B4-E4 covered a range of 2 mm). Matched histology demonstrated similar changes as in OCT scan (B5-E5). Twelve eyes of 7-8 rats in each group; aP<0.05.
Immunohistochemistry of Glial Fibrillary Acidic Protein and Rhodopsin
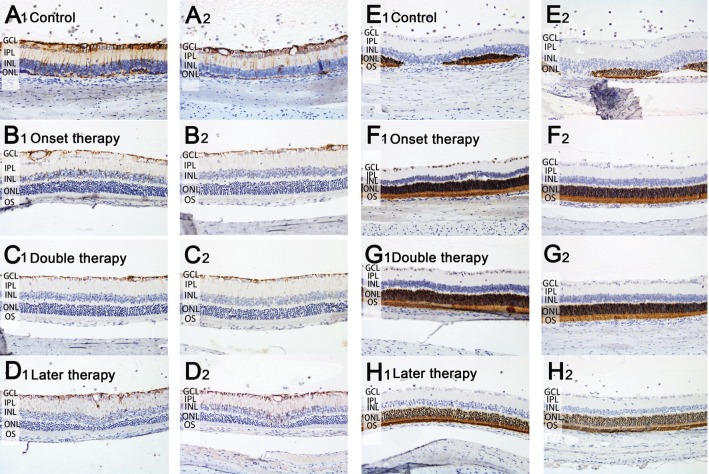
In the retina, GFAP is mainly present in Müller cell processes. GFAP immunoreactivity can indicate the proliferation state of Müller cells, which mainly exist in the inner layers of the retina. Our results showed that the over-expression of GFAP among untreated rats was found in all retinal layers, suggesting hypertrophy and proliferation of Müller cells (Figure 5A). All MSC therapies, including later therapy, significantly reduced the GFAP expression, which restricted Müller cell processes from extending to the outer layers of the retina (Figure 5B-5D). RHO was expressed in the ONL of all rods. In the untreated rats, the expression of RHO remained in small amounts in the collapsed ONL and outer plexiform layer (Figure 5E). Early therapy resulted in relatively preserved RHO immunoreactivity in terms of thickness and intensity, whereas later therapy contributed to retain the RHO expression compared with the control group (Figure 5F-5H).
Figure 5. IHC of retinal sections from rats of different groups.
A-D: Typical expressions of GFAP (brown) of two eyes from different groups; E-H: Rhodopsin (brown) of two eyes from different groups. Eight rats (16 eyes) were used in each group of control, onset therapy and later therapy, and 7 rats (14 eyes) in double therapy; P<0.05.
DISCUSSION
The rEAU rat model used in this study can exhibit resolution of acute episode with spontaneous recurrences and evidence of chronic posterior intraocular inflammation. Persistent inflammation results in progressive deterioration of retinal structures, which mimics the situation observed in human posterior uveitis[13]. By using this model, we demonstrated that a single course of MSC treatment administered during the onset of the disease sufficed to inhibit the progress and relapse of the disease[9]. However, in clinical settings where situations are usually more complicated, not all patients can receive timely and sufficient treatment at the beginning of the disease. Therefore, despite the observation that early treatment with MSCs is more effective, it is of therapeutic importance that MSCs were also effective when administered in developed diseases.
In this experiment, we found that although early treatment is advantageous over later treatment in terms of reducing the relapse of the disease and protecting retinal structure and function, later treatment can also effectively alleviate clinical severity, decrease inflammation, interrupt disruption of outer retinal layers, and improve retinal function. In chronic experimental autoimmune encephalomyelitis (EAE) model of mice, MSCs administered after the peak of disease severity significantly ameliorated the clinical course[14]. In recent successful clinical trials of other ADs, MSC therapies were only applied in patients with refractory inflammation, which is difficult to be controlled by currently available therapies. Therefore, our results do not only underline the importance of early MSC treatment for recurrent and chronic uveitis, but also further support the practicability of clinical trials on MSC therapy for chronic refractory autoimmune uveitis.
The optimal dosage and frequency of MSC administration remain obscure despite many related clinical and animal experiments. Our previous studies demonstrated that, for rats, one course of intravenous injection of rat MSCs for three consecutive days was needed to control the disease[7]–[9], whereas one injection of both mouse- and human-derived MSCs was sufficient for a long-term inflammation suppression in mice (unpublished results). Furthermore, we demonstrated that single course of MSC treatment, started at the onset of the disease, sufficed to inhibit the inflammation, while double therapies, with the second therapy applied before the second attack (11d after the first therapy), did not improve the therapeutic benefit in rEAU in rats[9]. Although other studies reported a similar lasting effect of one MSC therapy and the failure of second therapy to provide additional benefits [15]–[16], an enhanced clinical efficacy by multiple therapies was also found in lupus mice [17] and refractory systemic lupus erythematosus and graft-versus-host disease patients[18]–[19]. In the current experiment, we postponed the second therapy until after the second attack. Interestingly, we found that double therapies with longer interval led to a better clinical evaluation, as well as a trend of decrease in relapse and amelioration of retinal function.
These opposing results might be attributed to the differences in dose, time, and intervals of MSC administrations, as well as the different clinical conditions of the animals and patients. Previous in vitro studies revealed that T cells were arrested at the G1 phase in the presence of MSCs, and the proliferation potential could not recover after MSCs removal[20], which may explain the long-term immunosuppressive effect of MSCs in vivo. However, the arrested T cells might eventually restore their proliferation ability in vivo after a certain time, and the second MSC therapy applied at this time may suppress their restored effector ability. Anyway, our data suggest that different regimens should be planned in clinical trials to explore the therapeutic potential of MSCs.
Although the pattern of clinical symptoms varied from case to case, in rEAU, the persistent chronic intraocular inflammation leads to progressive retinal damage, especially photoreceptor loss, which becomes increasingly prominent with the time[13]. In our study, histological examination revealed that both early and later MSC therapies slowed down the loss of photoreceptor cells. The deterioration of retinal structures in rEAU and the therapeutic effect of MSCs were also reflected in ERG responses. Consistent with histological assessment, ERG results demonstrated that both early and later MSC therapy effectively rescued the retinal function, which further confirmed the therapeutic efficacy of MSCs in rEAU.
It is believed that MSCs exert comprehensive immune modulation in a multifactorial manner[5]. Our previous results[7]–[9] revealed that, in rEAU, MSCs could inhibit both Th1 and Th17 responses, suppress functions of APCs, and up-regulate Treg cells by inhibitory cytokine production, CD73 expression (unpublished results), and the secretion of immunoregulatory extracellular vesicles (unpublished results). In addition, MSCs demonstrate neuroprotective properties by reducing oxidative stress[21] and secreting neurotrophic factors[22]. MSCs were proven to produce a beneficial effect via bimodal mechanism in chronic EAE by inhibiting the immune response in the early phases of the disease, as well as by inducing local neuroregeneration in stabilized diseases[14]. In the present study, we demonstrated that MSC therapy protected the retinal structure, preserved photoreceptors, improved the retinal function, and reduced the GFAP expression. All the benefits of MSC therapy in EAE might also imply to rEAU, with immunomodulation in early stage, and both immunosuppressive and neuroprotective effects in later phase.
In conclusion, our results showed that although MSC treatment during the early phase of rEAU produced more satisfactory clinical efficacy, later administration during the recurrent phase can also prevent structural and functional damage of the retina. Furthermore, double therapies with longer interval demonstrated additional therapeutic advantage. In future clinical research, numerous prospective trials in autoimmune uveitis are required to evaluate the safety and efficacy of MSC therapy, given the lack of standard therapeutic regimens.
Acknowledgments
Foundations: Supported by the National Natural Science Foundation of China (No.81371005; No.81428012); Tianjin Municipal Science and Technology Commission (No.15JCZDJC35600).
Conflicts of Interest: Zhao PT, None; Zhang LJ, None; Shao H, None; Bai LL, None; Yu B, None; Su C, None; Dong LJ, None; Liu X, None; Li XR, None; Zhang XM, None.
REFERENCES
- 1.Castiblanco C, Foster CS. Review of systemic immunosuppression for autoimmune uveitis. Ophthalmol Ther. 2014;3(1–2):17–36. doi: 10.1007/s40123-014-0023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou S, Kijlstra A, Yang P. Molecular genetic advances in uveitis. Prog Mol Biol Transl Sci. 2015;134:283–298. doi: 10.1016/bs.pmbts.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 3.Levy-Clark G, Jabs DA, Read RW, Rosenbaum JT, Vitale A, Van Gelder RN. Expert panel recommendations for the use of anti-tumor necrosis factor biologic agents in patients with ocular inflammatory disorders. Ophthalmology. 2014;121(3):785–796. doi: 10.1016/j.ophtha.2013.09.048. [DOI] [PubMed] [Google Scholar]
- 4.Yang P. Editorial: uveitis: pathology, molecular mechanism and therapy. Curr Mol Med. 2015;15(6):510. doi: 10.2174/1566524015999150804104144. [DOI] [PubMed] [Google Scholar]
- 5.Castro-Manrreza ME, Montesinos JJ. Immunoregulation by mesenchymal stem cells: biological aspects and clinical applications. J Immunol Res. 2015;2015:394917. doi: 10.1155/2015/394917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tasso R, Ilengo C, Quarto R, Cancedda R, Caspi RR, Pennesi G. Mesenchymal stem cells induce functionally active T regulatory lymphocytes in a paracrine fashion and ameliorate experimental autoimmune uveitis. Invest Ophthalmol Vis Sci. 2012;53(2):786–793. doi: 10.1167/iovs.11-8211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Li G, Yuan L, Ren X, Nian H, Zhang L, Han ZC, Zhang X. The effect of mesenchymal stem cells on dynamic changes of T cell subsets in experimental autoimmune uveoretinitis. Clin Exp Immunol. 2013;173(1):28–37. doi: 10.1111/cei.12080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang XM, Jiao CN, Jia Z, Ren XJ, Li XR, Zhao SZ. Investigation of the role of mesenchymal stem cells in keratoplasty rejection. Zhonghua Yan Ke Za Zhi. 2012;48(8):733–738. [PubMed] [Google Scholar]
- 9.Zhang L, Zheng H, Shao H, Nian H, Zhang Y, Bai L, Su C, Liu X, Dong L, Li X, Zhang X. Long-term therapeutic effects of mesenchymal stem cells compared to dexamethasone on recurrent experimental autoimmune uveitis of rats. Invest. Ophthalmol Vis Sci. 2014;55(9):5561–5571. doi: 10.1167/iovs.14-14788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caspi RR, Grubbs BG, Chan CC, Chader GJ, Wiggert B. Genetic control of susceptibility to experimental autoimmune uveoretinitis in the mouse model. Concomitant regulation by MHC and non MHC genes. J Immunol. 1992;148(8):2384–2389. [PubMed] [Google Scholar]
- 11.Shao H, Fu Y, Song L, Sun S, Kaplan HJ, Sun D. Lymphotoxin β receptor-Ig fusion protein treatment blocks actively induced, but not adoptively transferred, uveitis in Lewis rats. Eur J Immunol. 2003;33(6):1736–1743. doi: 10.1002/eji.200323745. [DOI] [PubMed] [Google Scholar]
- 12.Gadjanski I, Williams SK, Hein K, Sattler MB, Bahr M, Diem R. Correlation of optical coherence tomography with clinical and histopathological findings in experimental autoimmune uveoretinitis. Exp Eye Res. 2011;93(1):82–90. doi: 10.1016/j.exer.2011.04.012. [DOI] [PubMed] [Google Scholar]
- 13.Shao H, Shi H, Kaplan HJ, Sun D. Chronic recurrent autoimmune uveitis with progressive photoreceptor damage induced in rats by transfer of IRBP-specific T cells. J Neuroimmunol. 2005;163(1–2):102–109. doi: 10.1016/j.jneuroim.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 14.Constantin G, Marconi S, Rossi B, Angiari S, Calderan L, Anghileri E, Gini B, Bach SD, Martinello M, Bifari F, Galiè M, Turano E, Budui S, Sbarbati A, Krampera M, Bonetti B. Adipose-derived mesenchymal stem cells ameliorate chronic experimental autoimmune encephalomyelitis. Stem Cells. 2009;27(10):2624–2635. doi: 10.1002/stem.194. [DOI] [PubMed] [Google Scholar]
- 15.Ciccocioppo R, Bernardo ME, Sgarella A, Maccario R, Avanzini MA, Ubezio C, Minelli A, Alvisi C, Vanoli A, Calliada F, Dionigi P, Perotti C, Locatelli F, Corazza GR. Autologous bone marrow-derived mesenchymal stromal cells in the treatment of fistulising Crohn's disease. Gut. 2011;60(6):788–798. doi: 10.1136/gut.2010.214841. [DOI] [PubMed] [Google Scholar]
- 16.Wang D, Akiyama K, Zhang H, Yamaza T, Li X, Feng X, Wang H, Hua B, Liu B, Xu H, Chen W, Shi S, Sun L. Double allogenic mesenchymal stem cells transplantations could not enhance therapeutic effect compared with single transplantation in systemic lupus erythematosus. Clin Dev Immunol. 2012;2012:273291. doi: 10.1155/2012/273291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gu Z, Akiyama K, Ma X, Zhang H, Feng X, Yao G, Hou Y, Lu L, Gilkeson GS, Silver RM, Zeng X, Shi S, Sun L. Transplantation of umbilical cord mesenchymal stem cells alleviates lupus nephritis in MRL/lpr mice. Lupus. 2010;19(13):1502–1514. doi: 10.1177/0961203310373782. [DOI] [PubMed] [Google Scholar]
- 18.Glennie S, Soeiro I, Dyson PJ, Lam EW, Dazzi F. Bone marrow mesenchymal stem cells induce division arrest anergy of activated T cells. Blood. 2005;105(7):2821–2827. doi: 10.1182/blood-2004-09-3696. [DOI] [PubMed] [Google Scholar]
- 19.Sun L, Wang D, Liang J, Zhang H, Feng X, Wang H, Hua B, Liu B, Ye S, Hu X, Xu W, Zeng X, Hou Y, Gilkeson GS, Silver RM, Lu L, Shi S. Umbilical cord mesenchymal stem cell transplantation in severe and refractory systemic lupus erythematosus. Arthritis Rheum. 2010;62(8):2467–2475. doi: 10.1002/art.27548. [DOI] [PubMed] [Google Scholar]
- 20.Al Jumah MA, Abumaree MH. The immunomodulatory and neuroprotective effects of mesenchymal stem cells (MSCs) in experimental autoimmune encephalomyelitis (EAE): a mode of multiple sclerosis (MS) Int J Mol Sci. 2012;13(7):9298–9331. doi: 10.3390/ijms13079298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lanza C, Morando S, Voci A, Canesi L, Principato MC, Serpero LD, Mancardi G, Uccelli A, Vergani L. Neuroprotective mesenchymal stem cells are endowed with a potent antioxidant effect in vivo. J Neurochen. 2009;110(5):1674–1684. doi: 10.1111/j.1471-4159.2009.06268.x. [DOI] [PubMed] [Google Scholar]
- 22.Maltman DJ, Hardy SA, Przyborski SA. Role of mesenchymal stem cells in neurogenesis and nervous system repair. Neurochem Int. 2011;59(3):347–356. doi: 10.1016/j.neuint.2011.06.008. [DOI] [PubMed] [Google Scholar]