Abstract
AIM
To compare the surgical outcomes of trabeculectomy with Ex-PRESS implant and Ahmed glaucoma valve (AGV) implantation.
METHODS
Patients who underwent trabeculectomy with Ex-PRESS implants or AGV implantation separately were included in this retrospective chart review. Main outcome measures were surgical failure and complications. Failure was defined as intraocular pressure (IOP) >21 mm Hg or <5 mm Hg on two consecutive visits after 3mo, reoperation for glaucoma, or loss of light perception. Eyes that had not failed were considered as complete success if they did not required supplemental medical therapy.
RESULTS
A total of 64 eyes from 57 patients were included: 31 eyes in the Ex-PRESS group and 33 eyes in the AGV group. The mean follow-up time was 2.6±1.1y and 3.3±1.6y, respectively. Patients in the AGV group had significantly higher baseline mean IOP (P=0.005), lower baseline mean visual acuity (VA) (P=0.02), and higher proportion of patients with history of previous trabeculectomy (P<0.0001). Crude failure rates were 16.1%, n=5/31 in the Ex-PRESS group and 24.2%, n=8/33 in the AGV group. The cumulative proportion of failure was similar between the groups, P=0.696. The proportion of eyes that experienced postoperative complications was 32.3% in the Ex-PRESS group and 60.1% in the AGV group (P=0.0229).
CONCLUSION
Trabeculectomy with Ex-PRESS implant and AGV implantation had comparable failure rates. The AGV group had more post-operative complications, but also included more complex cases with higher baseline mean IOP, worse baseline mean VA, and more previous glaucoma surgeries. Therefore, the results are limited to the cohort included in this study.
Keywords: Ex-PRESS glaucoma implant, trabeculectomy, Ahmed glaucoma valve implantation
INTRODUCTION
Trabeculectomy with Ex-PRESS glaucoma implant and Ahmed glaucoma valve (AGV) implantations are surgical procedures that have been gaining in popularity over the past decade[1]–[11]. The Ex-PRESS implant is thought by some authors to reduce the rate of postoperative complications compared with the standard trabeculectomy, and to possibly reduce the number of adjunctive postoperative glaucoma medications[1]–[5]. However more recent studies suggest that the Ex-PRESS implant does not offer real advantage over standard trabeculectomy[6]–[10]. An AGV is often used in cases where the risk for filtration failure is high[11]. The goal of this study was to compare surgical outcomes of trabeculectomy with Ex-PRESS implant to AGV implantation. Broad inclusion criteria were used to reflect “real world” setting of the population undergoing these procedures.
SUBJECTS AND METHODS
This retrospective chart review was approved by the Institutional Review Board of the Tel-Aviv Medical Center and conducted according to the tenets of the Declaration of Helsinki. The surgical outcomes of all Ex-PRESS and AGV surgeries performed between the years 2006-2011were reviewed.
Trabeculectomy with Ex-PRESS implant (Alcon Inc., Forth Worth, TX, USA) was performed by two surgeons (Shemesh G and Kurtz S). Following a fornix-based peritomy, mitomycin C (MMC) was applied for 2-3min. A rectangular or triangular scleral flap was developed and the Ex-PRESS was introduced into the anterior chamber at the base of the scleral flap. The flap was then sutured using 10-0 Nylon sutures, and the conjunctiva was approximated and sutured to the limbus using 10-0 Nylon and Mersilene sutures. Following surgery, gonioscopy was performed to confirm the location of the implant if needed, at the physicians' discretion. AGV (New World Medical, Inc., Rancho Cucamonga, CA, USA) implantation, the most commonly performed tube-shunt surgery in our hospital, was performed by a single surgeon (Rachmiel R). Following a fornix-based peritomy, the AGV was primed and its plate was secured to the sclera approximately 8-10 mm posterior to the limbus using 8-0 Nylon sutures. The tube was then cut to the appropriate length, introduced into the eye through a scleral track, and secured using a 10-0 Nylon suture. Partial thickness corneal graft was used to cover the tube and the conjunctiva was closed using 8-0 Vicryl and 10-0 Nylon sutures.
Post-operatively, antibiotic drops were prescribed for a period of 1wk four times daily and topical steroids were prescribed for 4wk four times daily and tapered-down over a period of additional 4wk. Intraocular-lowering medications were prescribed at the physicians' discretion.
All patients over 18 years of age, with at least one year of follow-up post-operatively were included. The following data was recorded: age, gender, glaucoma type, history of prior diabetes mellitus and hypertension, previous ocular surgeries, and lens status. Clinical measures including intraocular pressure (IOP), visual acuity (VA) and number of glaucoma medications were recorded at baseline and post-operatively after 1d, 1wk, 1, 3, 6mo and every 6mo until the final follow-up visit. Intra-operative and postoperative complications, as well as postoperative procedures were documented.
Definitions of success and failure were based on the Tube versus Trabeculectomy (TVT) study[12]–[13]. Failure was defined as an IOP >21 mm Hg or ≤5 mm Hg on two consecutive postoperative follow-up visits 3mo following surgery. Other failure criteria were re-operation for glaucoma, or loss of light perception. Eyes were censored once they met failure criteria. The eyes that had not failed and were not using supplemental medical therapy were considered as complete successes. Eyes that had not failed but required supplemental medical therapy were defined as qualified successes. Early complications were defined as those occurring within 1mo after surgery, and late complications were defined as those occurring after more than 1mo postoperatively.
The groups were compared by Student's t-test and one-way ANOVA with ad-hoc Tukey correction for continuous variables. A P-value correction (Tukey) was used for multiple comparisons. The Chi square test and Fisher exact test were applied for categorical variables. Snellen VA measurements were converted to a logarithm of the minimal angle of resolution (logMAR) equivalents for the purpose of data analysis. The time to failure was defined either as the time from surgical treatment to reoperation for glaucoma, or as the time from surgical treatment to the first of two consecutive follow-up visits in which the patient had persistent hypotony (IOP ≤5 mm Hg) or inadequately reduced IOP (IOP>21 mm Hg). Treatment comparisons of time to failure were assessed with the Kaplan-Meier survival analysis log-rank test. A P value of ≤0.05 was considered statistically significant.
RESULTS
A total of 64 eyes from 57 patients were included in this study: 31 eyes from 28 patients in the Ex-PRESS group and 33 eyes from 29 patients in the AGV group. The mean follow-up time was 2.6±1.1y in the Ex-PRESS group and 3.3±1.6y in the AGV group (P=0.041). In the Ex-PRESS group all subjects who underwent prior trabeculectomy have had a standard trabeculectomy surgery. In the AGV group, of the total (n=23) subjects who underwent previous trabeculectomy, two have had a prior trabeculectomy with Ex-PRESS implant. Patients' demographic and baseline characteristics are presented in Table 1. The AGV group had significantly higher baseline mean IOP (30.1±10.4 mm Hg versus 23.5±7.5 mm Hg, P=0.005), more pseudophakic eyes (72.7% vs 41.9%, P=0.02), and more eyes with previous ocular surgery, including trabeculectomy, vitrectomy or keratoplasty (P=0.012). The Ex-PRESS group had significantly better baseline vision compared with the AGV group (logMAR 0.67±0.82 vs 1.23±1.03, P=0.02).
Table 1. Baseline demographic and clinical characteristics of the study patients.
| Parameters | Ex-PRESS group (n=31) | AGV group (n=33) | P |
| Age | 73.0±9.5 | 72.4±9.9 | 0.815 |
| Gender, n (%) | 0.209 | ||
| M | 14 (45.2) | 21 (63.6) | |
| F | 17 (54.8) | 12 (36.4) | |
| Total follow up (a) | 2.6±1.1 | 3.3±1.6 | 0.041 |
| Snellen visual acuity | |||
| Snellen (range) | 20/25 - CF | 20/40 - LP | |
| LogMAR | 0.67±0.82 | 1.23±1.03 | 0.02 |
| IOP (mm Hg) | 23.5±7.5 | 30.1±10.4 | 0.005 |
| Glaucoma meds (n) | 3.6±0.9 | 3.9±0.9 | 0.115 |
| Diagnosis, n (%) | 0.136 | ||
| POAG | 7 (22.6) | 7 (21.1) | |
| PXFG | 14 (45.2) | 8 (24.2) | |
| NVG | 5 (16.1) | 8 (24.2) | |
| Uveitic glaucoma | 5 (16.1) | 4 (12.1) | |
| PKP glaucoma | 0 | 3 (9.1) | |
| CACG | 0 | 3 (9.1) | |
| Lens status, n (%) | 0.022 | ||
| Phakic | 18 (58.1) | 9 (27.3) | |
| Pseudophakic | 13 (41.9) | 24 (72.7) | |
| Previous trabeculectomy | 7 (22.6) | 23 (69.7) | <0.0001 |
| Other intraocular surgery (vitrectomy or keratoplasty) | 1 (3.2) | 8 (24.3) | 0.012 |
| Previous diode CPC | 1 (3.2) | 3 (9.1) | 0.614 |
| Diabetes, n (%) | 10 (32.3) | 7 (21.2) | 0.40 |
| Hypertension, n (%) | 22 (71.0) | 14 (42.4) | 0.026 |
AGV: Ahmed glaucoma valve; IOP: Intraocular pressure; POAG: Primary open angle glaucoma; PXFG: Pseudoexfoliation glaucoma; NVG: Neovascular glaucoma; PKP: Penetrating keratoplasty; CACG: Chronic angle closure glaucoma; LogMAR: Logarithm of the minimal angle of resolution; CF: Count finger; LP: Light perception; CPC: Cyclophotocoagulation.
x±s
Baseline and follow-up IOP measurements and number of glaucoma medication in each group are presented in Table 2. Both surgical procedures resulted in a significant and sustained reduction in IOP. The Ex-PRESS group had a mean IOP reduction of 9.1±11.1 mm Hg and the AGV group had a mean IOP reduction of 14.1±11.4 mm Hg, P=0.078. When the results were stratified into patients undergoing Ex-PRESS alone and Ex-PRESS combined with cataract surgery, IOP reduction was only 4.0 mm Hg for the combined group, compared with 11.9 mm Hg for the Ex-PRESS alone group and 14.1 mm Hg for the AGV group (P=0.037). However, the combined surgery group also had a significantly lower baseline IOP (combined surgery 18.7 mm Hg; Ex-PRESS alone 26.2 mm Hg; AGV 30.1 mm Hg, P=0.002). None of the patients in the AGV group underwent concomitant cataract surgery. There was a significant reduction in the number of glaucoma medications in both the Ex-PRESS and AGV groups from baseline to final visit (P<0.001 for both groups). This decrease was similar between the groups (P=0.693). At the 2-year follow up visit, the AGV group received more IOP-lowering medications compared with the Ex-PRESS group (P=0.032).
Table 2. IOP and medical therapy at baseline and follow-up.
| IOP/No. of Meds | Ex-PRESS group | AGV group | P |
| Baseline | |||
| IOP (mm Hg) | 23.5±7.5 (31) | 30.1±10.4 (33) | 0.005 |
| Glaucoma Meds | 3.5±0.9 (31) | 3.9 ±0.9 (33) | 0.115 |
| 1d | |||
| IOP (mm Hg); % reduction from baseline | 13.1±6.4 (29); 44.3 | 10.5±4.0 (31); 66.0 | 0.068 |
| Glaucoma Meds | 0.0±0.25 (27) | 0.0±0.0 (32) | 0.280 |
| 1wk | |||
| IOP (mm Hg); % reduction from baseline | 14.3±7.7 (28); 39.1 | 8.9±4.2 (29); 70.5 | 0.001 |
| Glaucoma Meds | 0.0±0.0 (28) | 0.1±0.7 (30) | 0.338 |
| 1mo | |||
| IOP (mm Hg); % reduction from baseline | 13.0±5.3 (28); 44.7 | 12.4±4.0 (29); 58.7 | 0.633 |
| Glaucoma Meds | 0.4±1.0 (28) | 0.5±1.1 (31 | 0.478 |
| 3mo | |||
| IOP (mm Hg); % reduction from baseline | 13.6±6.3 (20); 42.2 | 13.6±5.5 (27); 54.8 | 0.997 |
| Glaucoma Meds | 0.7±1.1 (21) | 1.1±1.3 (29) | 0.325 |
| 6mo | |||
| IOP (mm Hg); % reduction from baseline | 14.4±4.2 (16); 38.6 | 12.5±3.2 (20); 58.5 | 0.122 |
| Glaucoma Meds | 1.2±1.3 (14) | 1.3±1.5 (22) | 0.830 |
| 12mo | |||
| IOP (mm Hg); % reduction from baseline | 12.3±3.5 (16); 47.7 | 14.2±4.3 (24); 52.8 | 0.153 |
| Glaucoma Meds | 0.7±1.1 (15) | 1.5±1.4 (24) | 0.083 |
| 1.5a | |||
| IOP (mm Hg); % reduction from baseline | 14.6±5.2 (7); 37.7 | 14.2±4.3 (19); 53.8 | 0.153 |
| Glaucoma Meds | 1.0±1.5 (7) | 1.3±1.5 (20) | 0.681 |
| 2a | |||
| IOP (mm Hg); % reduction from baseline | 18.4±11.7 (8); 21.9 | 12.3±3.2 (19); 59.1 | 0.042 |
| Glaucoma Meds | 0.8±1.3 (9) | 2.1±1.5 (19) | 0.032 |
| 2.5a | |||
| IOP (mm Hg); % reduction from baseline | 14.7±5.8 (10); 37.5 | 14.0±6.1 (13); 53.3 | 0.798 |
| Glaucoma Meds | 1.0±1.6 (11) | 1.9±1.6 (14) | 0.162 |
| 3a | |||
| IOP (mm Hg); % reduction from baseline | 10.6±3.3 (10); 55.1 | 13.3±4.2 (10); 55.7 | 0.120 |
| Glaucoma Meds | 0.7±1.4 (8) | 1.6±1.7 (8) | 0.261 |
| 3.5a | |||
| IOP (mm Hg); % reduction from baseline | 14.3±1.5 (4); 39.4 | 13.8±1.8 (5); 54.0 | 0.726 |
| Glaucoma Meds | 1.7±2.1 (3) | 1.3±1.5 (4) | 0.769 |
| 4a | |||
| IOP (mm Hg); % reduction from baseline | 14.3±1.8 (2); 39.4 | 13.9±2.3 (6); 53.6 | 0.870 |
| Glaucoma Meds | 1.5±0.7 (2) | 1.7±1.4 (6) | 0.879 |
| 4.5a | |||
| IOP (mm Hg); % reduction from baseline | 12.0 (1); 49.0 | 15.8±3.6 (6); 47.3 | 0.372 |
| Glaucoma Meds | 2.5±0.7 (2) | 1.5±1.5 (6) | 0.420 |
| Difference from baseline | |||
| IOP improvement (mm Hg); % reduction from baseline | 9.1±11.1 (31); 38.7 | 14.1±11.4 (32); 46.8 | 0.078 |
| Reduction of Meds | 2.7±1.6 (31) | 2.5±1.4 (33) | 0.693 |
AGV: Ahmed glaucoma valve; LogMAR: Logarithm of the minimal angle; Meds: Medications; IOP: Intraocular pressure.
x±s (n)
The reasons for treatment failure are listed in Table 3. The most common reason for failure in both study groups was inadequate IOP reduction (IOP>21 mm Hg on two consecutive follow-up visits with failure after 3mo) with 3/31 (9.7%) for the Ex-PRESS group versus 4/33 (12.1%) for the AGV group (P=0.478). One patient in the AGV group failed because he required reoperation for glaucoma as opposed to none in the Ex-PRESS group.
Table 3. Reasons for surgical failure.
| Variables | Ex-PRESS group (n=5) | AGV group (n=8) |
| Inadequate IOP reduction | 3 (60.0) | 5 (62.5) |
| Re-operation for glaucoma | 0 (0.0) | 1 (12.5) |
| Persistent hypotony | 1 (20.0) | 1 (12.5) |
| Loss of light perception | 1 (20.0) | 1 (12.5) |
IOP: Intraocular pressure; AGV: Ahmed glaucoma valve.
n (%)
Failure was defined as IOP >21 mm Hg or an IOP ≤5 mm Hg that were measured on two consecutive follow-up visits after 3mo, or a need for re-operation for glaucoma, or loss of light perception.
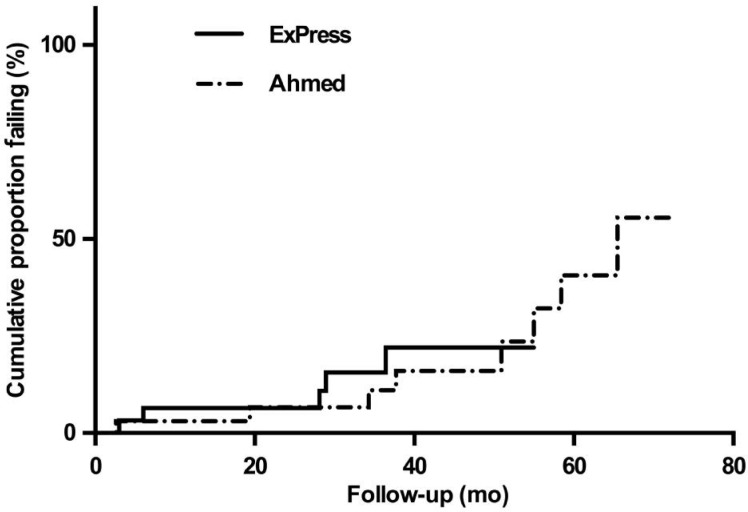
Figure 1 shows the probability of failure over time (Kaplan-Meier curve) for the two groups. There was no significant difference in failure over time between the groups (P=0.696). For the Ex-PRESS group, overall 5/31 (16.1%) met failure criteria, 15/31 (48.3%) had complete success and 11/31 (35.5%) had qualified success. For the AGV group, 8/33 (24.2%) failed, 13/33 (39.4%) had complete success, and 12/33 (36.4%) had qualified success. There was no significant difference between the groups (P=0.845). When failure was defines as inadequate intraocular pressure reduction >18 mm Hg on two consecutive follow up-visits after 3mo, persistent hypotony, reoperation for glaucoma, or loss of light perception, there was a trend toward a significant lower cumulative failure rates for the Ex-PRESS group compared with AGV (P=0.073)
Figure 1. Kaplan-Meier plots of the probability of failure for trabeculectomy with the Ex-PRESS implant versus the AGV.
Failure was defined as inadequate intraocular pressure reduction >21 mm Hg on two consecutive follow up-visits after 3mo, persistent hypotony, reoperation for glaucoma, or loss of light perception.
The postoperative VA results are shown in Table 4. The mean logMAR VA worsened, with a mean change of logMAR -0.02±0.86 in the Ex-PRESS group and a mean logMAR reduction of -0.55±1.22 in the AGV group (P=0.037).
Table 4. Visual acuity at baseline and follow-up.
| Visual acuity (logMAR ) | Ex-PRESS group | AGV group | P |
| Baseline | 0.67±0.82 (31) | 1.23±1.0 (33) | 0.02 |
| 1mo | 0.66±0.66 (26) | 1.38±0.88 (29) | 0.001 |
| 3mo | 0.7±0.77 (22) | 1.43±0.92 (27) | 0.005 |
| 6mo | 0.79±0.86 (14) | 1.36±1.03(20) | 0.101 |
| 12mo | 0.73±0.86 (14) | 1.61±1.08 (22) | 0.015 |
| 1.5a | 0.14±0.08 (6) | 1.92±1.27 (19) | 0.002 |
| 2a | 0.52±0.70 (9) | 1.63±1.10 (19) | 0.011 |
| 2.5a | 0.31±0.22 (12) | 1.06±1.19 (15) | 0.001 |
| 3a | 1.25±1.78 (8) | 1.50±1.20 (10) | 0.730 |
| 3.5a | 0.18±0.20 (3) | 1.62±1.31 (5) | 0.118 |
| 4a | 0.18±0.05 (2) | 1.79±1.27 (7) | 0.132 |
| 4.5a | 0.46±0.09 (2) | 2.13±1.65 (6) | 0.225 |
| Difference from baseline | -0.02±0.86 (31) | -0.55±1.22 (33) | 0.037 |
AGV: Ahmed glaucoma valve; LogMAR: Logarithm of the minimal angle of resolution.
x±s (n)
Table 5 lists the postoperative complications. There were 10 complications (5 early and 5 late) in 10 eyes (32.3%) for the Ex-PRESS group. This compared with 19 complications (6 early and 19 late) in 20 patients (60.1%) in the AGV group (P=0.0229). The AGV group had significantly more corneal edema than the Ex-PRESS group (P=0.001). Overall 4 patients had undergone prior keratoplasty, all in the AGV group. Two of these subjects developed corneal edema following surgery.
Table 5. Postoperative complications.
| Variables | Ex-PRESS group (n=31) | AGV group (n=33) |
| Early postoperative complications1 | ||
| Choroidal detachment | 1 (3.2) | 0 |
| TASS | 1 (3.2) | 0 |
| Hyphema | 1 (3.2) | 1 (3.0) |
| Choroidal effusion/ hemorrhage | 0 | 1 (3.0) |
| Iris expulsion | 1 (3.2) | 0 |
| Exposure of Ahmed tube | 0 | 1 (3.0) |
| Malpositioned tube | 0 | 1 (3.0) |
| Hypotony | 0 | 2 (6.1) |
| Corneal erosion | 1 (3.2) | 0 |
| Total early postoperative complications | 5 (16.1) | 6 (18.2) |
| Late postoperative complications2 | ||
| Anterior uveitis | 0 | 1 (3.0) |
| Malignant glaucoma | 1 (3.2) | 0 |
| Exposure of Ahmed tube | 0 | 2 (6.1) |
| Cystic/encapsulated bleb3 | 3 (9.1) | 4 (12.1) |
| Corneal edema | 0 | 8 (24.2) |
| Corneal graft rejection | 0 | 2 (6.1) |
| Hypotony | 1 (3.0) | 1 (3.0) |
| Hypopion | 0 | 1 (3.0) |
| Total late postoperative complications | 5 (16.1) | 19 (57.6) |
| Total number of eyes with post-operative complications (early and late)4 | 10 (32.3) | 20 (60.1) |
AGV: Ahmed glaucoma valve; TASS: Toxic anterior segment syndrome. 1Early complications were defined as complications occurring within 1mo after surgery; 2Late complications were defined as occurring after more than 1mo after surgery; 3Bleb morphology was based on clinical examination by the surgeon; 4Some patients had more than one complication.
n (%)
DISCUSSION
This study compared the outcomes of a trabeculectomy with an Ex-PRESS glaucoma implant and AGV implantation. Overall there was no significant difference in failure rates between the groups. The AGV group had more late complications, in particular corneal edema, compare with the Ex-PRESS group. However, the AGV group also had worse baseline characteristics with worse baseline mean VA, higher baseline mean IOP and more previous ocular surgeries.
The TVT study compared tube-shunt surgery using the 350-mm2 Baerveldt glaucoma implant to trabeculectomy with MMC in eyes with previous cataract and/or failed glaucoma surgery, and found a higher rate of surgical success with the tube-shunt approach at 5y[12]. Christakis et al[14] compared Ahmed vs Baerveldt glaucoma drainage devices over 3y for refractory glaucoma. Both devices were effective in reducing IOP and had similar complication rates. The Baerveldt group had lower failure rate and required fewer medications than the Ahmed group but there were also higher hypotony related complications in the Baerveldt group.
Budenz et al[15] recently published 5-year treatment outcomes in the Ahmed-Baerveldt Comparison Study. They found similar rates of surgical success with both implants, however the Baerveldt produced greater IOP reduction and a lower rate of glaucoma reoperation than the AGV, but it was also associated with twice as many failures because of safety issues.
To the best of our knowledge, this is the first study comparing directly between trabeculectomy with Ex-PRESS implant and glaucoma drainage implant. Previous studies showed conflicting results when standard trabeculecomy was compared to AGV implantation. Tran et al[16] found a significantly higher 5-year cumulative probability of success with standard trabeculectomy when greater IOP reduction was necessary. Wilson et al also compared the two surgeries and concluded that the trabeculectomy group achieved lower IOPs during the first year, and that the IOPs and the cumulative probabilities of success were comparable after longer follow-up[17]. Shen et al[18] found similar results when comparing trabeculectomy with MMC and AGV implantation in eyes with neovascular glaucoma. Notably these studies did not compare trabeculectomy with Ex-PRESS implant and had different population characteristics compared to our study. While the TVT study included comparison between Baerveldt glaucoma implant with standard trabeculectomy, our study included only patients who received AGV, and compared them with trabeculectomy with Ex-PRESS.
Skaat et al[19] compared gold-micro shunt implants versus AGV over 5y. Success rates were similar for both devices. However their study, similar to the TVT protocol, excluded complicated glaucoma cases including NVG and uveitic glaucoma. The TVT study also excluded patients with history of previous diode cyclophotocoagulation (CPC) and combined surgery[12]. Our study aimed to reflect the “real world” population setting, and therefore these patient populations were not excluded.
The rate of mean IOP reduction in our study was similar between groups and comparable to other studies[3],[20]. The time-adjusted cumulative rate of success was also similar between groups. VA was better in the Ex-PRESS group compared to the AVG group over the first 2.5y but afterwards this significant difference was lost. The Ex-PRESS group could have been expected to have better VA than the AGV group as the former included procedures that were combined with cataract surgery, and the AGV group typically had prior glaucoma surgery or included more complex cases at baseline. The AGV group had more corneal-related complications. This could in part be due to tube proximity to the endothelium, but this group also had more patients with history of previous ocular surgeries, which could have compromised their endothelial cell count prior to the AGV implantation.
The current study has several limitations: the data were collected retrospectively, there were differences between the baseline characteristics of the two groups and different follow-up periods, and the sample size was relatively small. In the AGV group, 70% underwent prior trabeculectomy compared with only 23% in the Ex-PRESS group. Higher rates of prior vitrectomy/keratoplasty (24% vs 3%) and prior diod CPC (9% vs 3%) were also noted in the AGV group, and therefore selection bias likely affected our results.
In conclusion, our study findings demonstrated similar success rates among the AGV patients compared with the Ex-PRESS implant patients in terms of IOP reduction. Late complications, in particular corneal decompensation, were significantly higher for the AGV group but this group had more complex glaucoma cases with history of more glaucoma and other ocular surgeries at baseline. This study suggests a place for the use of both Ex-PRESS and AGV in glaucoma treatment. Further studies are needed to define optimal glaucoma characteristics for the use of each device.
Acknowledgments
Ilanah Gelernter and Deborah Fischer PhD are thanked for their assistance with the statistical analysis used in this study.
Conflicts of Interest: Waisbourd M, None; Fischer N, None; Shalev H, None; Spierer O, None; Ben Artsi E, None; Rachmiel R, None; Shemesh G, None; Kurtz S, None.
REFERENCES
- 1.Dahan E, Ben Simon GJ, Lafuma A. Comparison of trabeculectomy and Ex-PRESS implantation in fellow eyes of the same patient: a prospective, randomised study. Eye (Lond) 2012;26(5):703–710. doi: 10.1038/eye.2012.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.de Jong L, Lafuma A, Aguade AS, Berdeaux G. Five-year extension of a clinical trial comparing the EX-PRESS glaucoma filtration device and trabeculectomy in primary open-angle glaucoma. Clin Ophthalmol. 2011;5:527–533. doi: 10.2147/OPTH.S18565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Jong LA. The Ex-PRESS glaucoma shunt versus trabeculectomy in open-angle glaucoma: a prospective randomized study. Adv Ther. 2009;26(3):336–345. doi: 10.1007/s12325-009-0017-6. [DOI] [PubMed] [Google Scholar]
- 4.Good TJ, Kahook MY. Assessment of bleb morphologic features and postoperative outcomes after Ex-PRESS drainage device implantation versus trabeculectomy. Am J Ophthalmol. 2011;151(3):507–513.e1. doi: 10.1016/j.ajo.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 5.Maris PJ, Jr, Ishida K, Netland PA. Comparison of trabeculectomy with Ex-PRESS miniature glaucoma device implanted under scleral flap. J Glaucoma. 2007;16(1):14–19. doi: 10.1097/01.ijg.0000243479.90403.cd. [DOI] [PubMed] [Google Scholar]
- 6.Buys YM. Trabeculectomy with ExPRESS: weighing the benefits and cost. Curr Opin Ophthalmol. 2013;24(2):111–118. doi: 10.1097/ICU.0b013e32835907a6. [DOI] [PubMed] [Google Scholar]
- 7.Wagschal LD, Trope GE, Jinapriya D, Jin YP, Buys YM. Prospective randomized study comparing Ex-PRESS to trabeculectomy: 1-year results. J Glaucoma. 2015;24(8):624–629. doi: 10.1097/IJG.0000000000000029. [DOI] [PubMed] [Google Scholar]
- 8.Angmo D, Sharma R, Temkar S, Dada T. Evaluation of ExPress glaucoma filtration device in Indian patients with advanced glaucoma. Indian J Ophthalmol. 2015;63(5):459–462. doi: 10.4103/0301-4738.159894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buys YM. Trabeculectomy versus ExPRESS shunt surgery in residency training. Surv Ophthalmol. 2012;57(4):375–378. doi: 10.1016/j.survophthal.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Rodriguez JM, Trope GE, Drori-Wagschal L, Jinapriya D, Buys YM. Comparison of trabeculectomy versus Ex-PRESS: 3-year follow-up. Br J Ophthalmol. 2016;100(9):1269–1273. doi: 10.1136/bjophthalmol-2015-307161. [DOI] [PubMed] [Google Scholar]
- 11.Minckler DS, Francis BA, Hodapp EA, Jampel HD, Lin SC, Samples JR, Smith SD, Singh K. Aqueous shunts in glaucoma: a report by the American Academy of Ophthalmology. Ophthalmology. 2008;115(6):1089–1098. doi: 10.1016/j.ophtha.2008.03.031. [DOI] [PubMed] [Google Scholar]
- 12.Gedde SJ, Herndon LW, Brandt JD, Budenz DL, Feuer WJ, Schiffman JC, Tube Versus Trabeculectomy Study G Postoperative complications in the Tube Versus Trabeculectomy (TVT) study during five years of follow-up. Am J Ophthalmol. 2012;153(5):804–814.e1. doi: 10.1016/j.ajo.2011.10.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gedde SJ, Schiffman JC, Feuer WJ, Herndon LW, Brandt JD, Budenz DL, Tube versus Trabeculectomy Study G Treatment outcomes in the Tube Versus Trabeculectomy (TVT) study after five years of follow-up. Am J Ophthalmol. 2012;153(5):789–803.e782. doi: 10.1016/j.ajo.2011.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Christakis PG, Tsai JC, Kalenak JW, Zurakowski D, Cantor LB, Kammer JA, Ahmed II. The Ahmed versus Baerveldt study: three-year treatment outcomes. Ophthalmology. 2013;120(11):2232–2240. doi: 10.1016/j.ophtha.2013.04.018. [DOI] [PubMed] [Google Scholar]
- 15.Budenz DL, Barton K, Gedde SJ, Feuer WJ, Schiffman J, Costa VP, Godfrey DG, Buys YM, the Ahmed Baerveldt Comparison Study Group Five-year treatment outcomes in the ahmed baerveldt comparison study. Ophthalmology. 2015;122(2):308–316. doi: 10.1016/j.ophtha.2014.08.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tran DH, Souza C, Ang MJ, Loman J, Law SK, Coleman AL, Caprioli J. Comparison of long-term surgical success of Ahmed Valve implant versus trabeculectomy in open-angle glaucoma. Br J Ophthalmol. 2009;93(11):1504–1509. doi: 10.1136/bjo.2008.150870. [DOI] [PubMed] [Google Scholar]
- 17.Wilson MR, Mendis U, Paliwal A, Haynatzka V. Long-term follow-up of primary glaucoma surgery with Ahmed glaucoma valve implant versus trabeculectomy. Am J Ophthalmol. 2003;136(3):464–470. doi: 10.1016/s0002-9394(03)00239-3. [DOI] [PubMed] [Google Scholar]
- 18.Shen CC, Salim S, Du H, Netland PA. Trabeculectomy versus Ahmed Glaucoma Valve implantation in neovascular glaucoma. Clin Ophthalmol. 2011;5:281–286. doi: 10.2147/OPTH.S16976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Skaat A, Sagiv O, Kinori M, Ben Simon GJ, Goldenfeld M, Melamed S. Gold micro-shunt implants versus ahmed glaucoma valve: long-term outcomes of a prospective randomized clinical trial. J Glaucoma. 2016;25(2):155–161. doi: 10.1097/IJG.0000000000000175. [DOI] [PubMed] [Google Scholar]
- 20.Budenz DL, Barton K, Feuer WJ, Schiffman J, Costa VP, Godfrey DG, Buys YM. Treatment outcomes in the Ahmed Baerveldt Comparison Study after 1 year of follow-up. Ophthalmology. 2011;118(3):443–452. doi: 10.1016/j.ophtha.2010.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]