Abstract
AIM
To assess the safety, efficacy, predictability and stability of implantable collamer lens (ICL) for residual refractive error after corneal refractive surgery.
METHODS
This study evaluated 19 eyes of 12 patients who underwent ICL implantation after corneal refractive surgeries. They were followed up for 1y to 5y of uncorrected distance visual acuity (UDVA), corrected distance visual acuity (CDVA), manifest refractive error, flat and steep K value, axial length, intraocular pressure, corneal endothelial cell density, adverse events after ICL surgery.
RESULTS
The mean follow-up period was 39.05±19.22 mo (range, 1-5y). Spherical equivalent refractive error changed from -7.45±3.02 D preoperatively to -0.85±1.10 D 1wk to 1mo after ICL implantation, with the safety and efficacy indices being 1.12 and 1.15, respectively. A total of 52.63% of eyes were within ±0.5 D of the predicted spherical equivalents, 73.68% were within ±1.0 D. A trend of mild regression towards myopia with axial elongation after 5y was observed. One eye with mild anterior capsule opacity and retinal detachment 1y after surgery were observed.
CONCLUSION
ICL implantation is safe and effective for the correction of residual refractive error after corneal refractive surgeries, especially in moderate to high residual myopia.
Keywords: implantable collamer lens, radial keratotomy, photorefractive keratectomy, laser-assisted in situ keratomileusis, laser-assisted subepithelial keratomileusis
INTRODUCTION
Refractive surgeries have been performed in the recent decades to improve life quality of patients with significant refractive errors. Corneal refractive surgeries have gained widespread popularity as a safe and effective surgical method for low to moderate myopia and implantable collamer lens (ICL) for the correction of moderate to high myopia. However, corneal refractive surgeries are the only choices for the correction of high myopia before the commencement of ICL implantation. However, the cornea is relatively too thin to fully correct the high myopia therefore a postoperative undercorrection or refractive regression resultes. Several modalities have been described to manage residual refractive error after corneal refractive surgeries. Nonsurgical options include spectacles and contact lenses, while surgical options include photorefractive keratectomy (PRK), laser-assisted in situ keratomileusis (LASIK), laser-assisted subepithelial keratomileusis (LASEK), and automated lamellar keratoplasty (ALK). Corneal enhancement surgeries can be used to correct lower amount of residual refractive error when corneal thickness is still in the safe range. In some studies, PRK and LASEK were reported to cause postoperative haze without the use of mitomycin[1], ALK to introduce irregular astigmatism that could decrease corrected distance visual acuity (CDVA)[2], and LASIK to be associated with flap related complications[3]. In the case of higher residual refractive error, on the other hand, the corneal thickness may not meet the need of secondary corneal ablation, because the ablation of stroma may further impair the biomechanical stability of the cornea that has already been weakened by the previous corneal surgeries. In these cases, a phakic intraocular lens (pIOL) implantation surgery is an alternative option as it leaves the cornea intact and avoids secondary damage.
To the best of our knowledge, numerous studies[4]–[12] have reported the safety and efficacy of corneal refractive surgeries for the correction of residual refractive error after ICL implantation, but a few case reports[8],[13]–[14] have reported the short-term safety and efficacy of ICL for the correction of residual hyperopia after corneal refractive surgeries. Therefore, the current study was performed to assess the long-term safety, efficacy, predictability and stability of ICL surgeries for residual myopia after corneal refractive surgeries.
SUBJECTS AND METHODS
This study adhered to the Declaration of Helsinki and was approved by the Ethical Committee Review Board of Fudan University Eye and ENT Hospital. Written informed consents were signed by all patients after the nature and possible consequences of the study were explained.
Total 19 eyes of 12 patients (4 men and 8 women) who underwent implantation of phakic posterior chamber ICL for residual refractive error after corneal refractive surgeries from 18 November 2008 to 17 May 2013 at Fudan University Eye and ENT Hospital were included in this study.
Preoperative Evaluation
A comprehensive ophthalmic examination was performed preoperatively. The examinations included uncorrected distance visual acuity (UDVA) and corrected distance visual acuity (CDVA) using a Snellen chart, manifest spherical and cycloplegic refractive error, slit-lamp biomicroscopic and funduscopic examinations, intraocular pressure (IOP; non-contact tonometer), corneal topography (Pentacam, Oculus, Germany), flat and steep K value (Pentacam), central corneal thickness (Pentacam), horizontal visible iris diameter (HVID; IOL-Master, Carl Zeiss, Germany), axial length (A-scan ultrasound), anterior chamber depth (ACD; Pentacam, measured from the corneal endothelium to the anterior lens), corneal endothelial cell counting (ECC; noncontact specular microscopy, SP-2000P, Topcon Corporation, Japan), optical coherence tomography (OCT; Optovue, USA), and ultrasound biomicroscopy (UBM; Quantel medical, France). Inclusion criteria for this surgery were age between 21 and 48 years, myopia between -2.00 and -18.00 D, astigmatism between -0.50 and -5.00 D, ACD of 2.80 mm or deeper, and endothelial cell counting greater than 2000 cells/mm2, reasonable expectation for outcomes, no preexisting ocular pathology, no previous keratoconus, no cataract or glaucoma, no systemic disease.
The Posterior Chamber Phakic Intraocular Lens
ICL, a plate-haptic single-piece lens, is made of collamer which is a flexible, hydrophilic material consists of HEMA hydrogel, water and porcine collagen. It can be folded, implanted in posterior chamber through a 2.8-3.2 mm corneal incision, with a high degree of biocompatibility, good permeability of gases and metabolites, absorption of ultraviolet. It is 6.0 mm wide and comes in five sizes (11.0, 11.5, 12.0, 12.5 and 13.0 mm). The lens has a central convex-concave optic zone with a diameter of 4.5-6.0 mm, and a power of -3.00 D to -23.00 D. The ICL design has been modified many times. In this study, all phakic intraocular lens patients implanted were the ICL V4 models.
ICL power calculation was performed by a modified vertex formula provided by the manufacturer (STAAR Surgical). The variables in the formula were preoperative manifest spherical and cycloplegic refractive error, keratometric power, corneal thickness, HVID and central ACD. The size of the ICL to be implanted was determined based on the patient's HVID and ACD.
Implantable Collamer Lens Surgical Procedure
Peripheral iridotomy was done by YAG laser at least 1wk before ICL implantation. On the day of surgery, the patients were administered dilating and cycloplegic agents (2.5% phenylephrine and 1% tropicamide, Alcon, China). After topical anaesthesia (0.4% oxybuprocaine hydrochloride, Santen, Japan) and a placement of visco surgical device (1.7% sodium hyaluronate; Bausch & Lomb, China) into the anterior chamber, ICL V4 was inserted through a 2.8-3.2 mm temporal clear corneal incision with the use of an injector cartridge (STAAR Surgical). After the ICL was placed in the posterior chamber, the surgeon then completely removed the visco surgical device with balanced salt solution, and instilled a miotic agent (0.005% carbachol, Bausch & Lomb, China). All surgeries were uneventful and no intraoperative complication was observed. After surgery, patients were given steroidal medications (0.1% tobramycin dexamethasone, Alcon, China) four times daily for 3d followed by fluorometholone four times daily which tapered off in 2wk, antibiotic medications (the left ofloxacin, Santen, Japan) four times daily for 1wk, non-steroidal anti-inflammatory drug (NSAID; pranoprofen, Senju, Japan) four times daily for 2wk, and artificial tears four times daily for 1mo.
Postoperative Evaluation and Follow-up
UDVA, CDVA, IOP, endothelial cell density (ECD), refractive error, corneal topography were evaluated postoperatively at every follow-up visit from 1d through to 5y. The mean follow-up time was 39.05±19.22mo (range, 1-5y) for all subjects.
Statistical Analysis
All statistical analyses were performed using SPSS version 20.0 (SPSS Inc., IBM, USA), the results are expressed as mean±SD. The P value was determined using the multivariate analysis of variance of repeated measures with Bonferroni-adjusted post-hoc comparisons. A P value less than 0.05 was considered statistically significant.
RESULTS
The pre- and post-operative characteristics and IOL parameter for all the study eyes were showed in Table 1.The mean age of subjects was 37.54±7.59 (range, 24 to 48)y at the time of surgery. Among these patients, 2 eyes of 1 patients had done radial keratotomy (RK), 5 eyes of 4 patients had done PRK, 9 eyes of 5 patients had done LASIK, 1 eye of 1 patient had done topography-guided customized ablation (TOGCA), and 2 eyes of 1 patient had done LASEK. The preoperative spherical refractive error was -6.88±2.84 (range, -2.50 to -11.75) D with cylinder being -1.13±0.84 (range, 0.00 to -3.00) D. The preoperative manifest spherical equivalent refractive error was -7.45±3.02 (range, -2.75 to -13.00) D. The preoperative mean K value and central corneal thickness was 38.41±1.07 (range, 35.7 to 40.2) D and 465.37±54.12 (range, 376 to 575) µm, respectively. The preoperative axial length was 30.19±1.82 (range, 27.76 to 35.27) mm.
Table 1. Preoperative, postoperative characteristics and IOL parameter.
| No. | Age (a) | Sex | eye | ST | IT (a) | Preop. UDVA | Preop. R | Preop. CDVA | Mean K (D) | CCT (µm) | Preop. IOP (mm Hg) | Preop. ECD (cells/mm2) |
| 1 | 48 | F | R | RK | 19 | 0.05 | -10.25/-1.25×180 | 0.7 | 38.70 | 575 | 16.2 | 3352 |
| L | 0.05 | -10.00/-1.25×160 | 0.7 | 39.30 | 568 | 14.7 | 3630 | |||||
| 2 | 41 | F | R | PRK | 15 | 0.1 | -6.00/-0.50×105 | 0.7 | 39.75 | 498 | 14.5 | 2902 |
| L | 0.1 | -5.75/-0.75×80 | 0.7 | 40.20 | 511 | 14.5 | 3003 | |||||
| 3 | 42 | F | R | 15 | 0.05 | -7.50/-0.50×175 | 0.7 | 38.60 | 431 | 7.9 | 2951 | |
| 4 | 39 | M | R | 14 | 0.05 | -7.00/-2.00×100 | 1.0 | 38.35 | 518 | 14.6 | 2796 | |
| 5 | 32 | M | R | 12 | 0.08 | -11.50/-2.50×100 | 0.8 | 35.70 | 412 | 12.5 | 2265 | |
| 6 | 26 | F | R | LASIK | 6 | 0.1 | -3.75/-1.50×25 | 0.8 | 37.05 | 445 | 8.8 | 3025 |
| L | 0.1 | -4.75/-1.50×145 | 0.8 | 36.90 | 442 | 11.1 | 2979 | |||||
| 7 | 27 | M | R | 7 | 0.25 | -2.50/-0.50×150 | 1.0 | 38.00 | 390 | 9.0 | 3042 | |
| L | 0.1 | -3.00/-0.50×60 | 1.0 | 38.10 | 376 | 9.0 | 2886 | |||||
| 8 | 40 | F | R | 13 | 0.05 | -6.75/-0.50×55 | 0.9 | 38.80 | 478 | 13.2 | 3081 | |
| L | 0.1 | -5.75/-0.75×150 | 1.0 | 38.65 | 452 | 12.8 | 3190 | |||||
| 9 | 45 | F | R | 5 | 0.1 | -4.00/-0.50×10 | 1.2 | 38.75 | 454 | 8.0 | 3758 | |
| L | 0.1 | -5.25/-0.50×175 | 1.0 | 37.95 | 451 | 8.0 | 3500 | |||||
| 10 | 39 | F | R | 16 | 0.1 | -6.25/-3.00×100 | 0.5 | 37.90 | 402 | 9.6 | 3192 | |
| 11 | 24 | F | R | TOGCA | 1 | 0.05 | -10.50 | 0.3 | 38.80 | 466 | 11.7 | 3128 |
| 12 | 40 | M | R | LASEK | 6 | 0.05 | -11.75/-2.50×80 | 0.2 | 39.15 | 485 | 9.8 | 3795 |
| L | 0.05 | -8.5/-1×150 | 0.4 | 39.50 | 488 | 7.7 | 3025 |
Table 1. Preoperative, postoperative characteristics and IOL parameter (continued).
| No. | Eye | ICL Model | ICL power (D) | Postop. UDVA | Postop. R | Postop. CDVA | Postop. IOP (mm Hg) | Postop. ECD (cells/mm2) | Follow-up (a) |
| 1 | R | 125 ICLv4 | -11.5 | 0.6 | -0.50/-1.50×170 | 0.7 | 17.4 | 3567 | 5 |
| L | 125 ICLv4 | -12.0 | 0.6 | -1.50/-1.00×160 | 0.8 | 19.0 | 3225 | ||
| 2 | R | 125 ICLv4 | -8.0 | 1.0 | 0.00/-0.75×90 | 1.0 | 9.5 | 3117 | 3 |
| L | 125 ICLv4 | -9.0 | 1.0 | 0.00/-0.50×85 | 1.0 | 10.1 | 3086 | ||
| 3 | R | 120 ICLv4 | -9.0 | 0.8 | -0.50/-0.50×170 | 1.0 | 7.1 | 3164 | 5 |
| 4 | R | 120 TICLv4 | -11.5/+2*9 | 1.5 | -0.75DS | 1.0 | 11.3 | 2386 | 3 |
| 5 | R | 120 ICLv4 | -15.0 | 0.8 | -0.25/-2.00×100 | 0.8 | 10.0 | 3081 | 1 |
| 6 | R | 125 ICLv4 | -5.5 | 1.0 | -0.50/-1.50×40 | 0.9 | 9.1 | 3373 | 1 |
| L | 125 ICLv4 | -7.0 | 1.0 | -0.25/-1.00×155 | 0.9 | 18.4 | 3987 | ||
| 7 | R | 125 ICLv4 | -4.0 | 1.0 | 0.25/-0.75×170 | 1.0 | 8.4 | 3125 | 1 |
| L | 125 ICLv4 | -5.0 | 1.0 | 0.50/-0.50×10 | 1.0 | 8.3 | 3120 | ||
| 8 | R | 125 ICLv4 | -9.0 | 1.2 | 0.25DS | 1.2 | 13.0 | 2651 | 5 |
| L | 125 ICLv4 | -8.5 | 1.2 | 0.25/-0.50×10 | 1.0 | 12.9 | 2572 | ||
| 9 | R | 115 ICLv4 | -4.0 | 0.8 | -1.00/-1.00×180 | 1.0 | 7.0 | 3001 | 5 |
| L | 115 ICLv4 | -7.5 | 1.2 | -0.25DS | 1.0 | 9.7 | 3321 | ||
| 10 | R | 120 ICLv4 | -11.5/+3.5*17 | 0.6 | 0.75/-1.75×145 | 0.9 | 10.0 | 3758 | 5 |
| 11 | R | 125 TICLv4 | -13.5 | 0.5 | -2.50/-3.50×65 | 0.4 | 8.6 | 3142 | 3 |
| 12 | R | 120 ICLv4 | -15.5 | 0.2 | -0.50/-1.00×65 | 0.3 | 8.3 | 3207 | 3 |
| L | 120 ICLv4 | -11.5 | 0.5 | -1.00/-2.00×180 | 0.5 | 7.9 | 3217 |
No: Patients number; ST: Surgery type before ICL implantation; IT: Internal time between two surgeries; Preop. UDVA: Preoperative UDVA (Snellen lines); Preop. R: Preoperative manifest spherical and cycloplegic refractive error; Preop. CDVA: Preoperative CDVA (Snellen lines); CCT: Central corneal thickness; Preop. IOP: Preoperative intraocular pressure; Preop. ECD: Corneal endothelial cell density; Postop. UDVA: Postoperative UDV A(Snellen lines); Postop. R: Postoperative manifest spherical and cycloplegic refractive error; Postop. CDVA: Postoperative CDVA(Snellen lines); Postop. IOP: Intraocular pressure 1d postoperatively; Postop. ECD: Postoperative corneal endothelial cell density at most recent follow-up.
Safety
The pre-operative, post-operative (1wk to 1mo) and last CDVA was 0.76±0.26, 0.85±0.23 and 0.79±0.24, with the safety index (mean postoperative CDVA/mean preoperative CDVA) being 1.12 and 1.04, respectively. At 1wk to 1mo after surgery, 31.58% of eyes gained 1 line, 21.05% of eyes gained 1 more line of CDVA, and 47.37% of eyes did not change compared to preoperation; no eye had CDVA lost. At their most recent postoperative visit, 31.58% of eyes gained 1 line, 26.32% of eyes gained 1 more line of CDVA, 5.26% of eyes lost 1 line, 15.79% of eyes lost 1 more line of CDVA, and 21.05% of eyes did not change compared to preoperation (Figure 1).
Figure 1. Changes in CDVA after implantation of ICL.
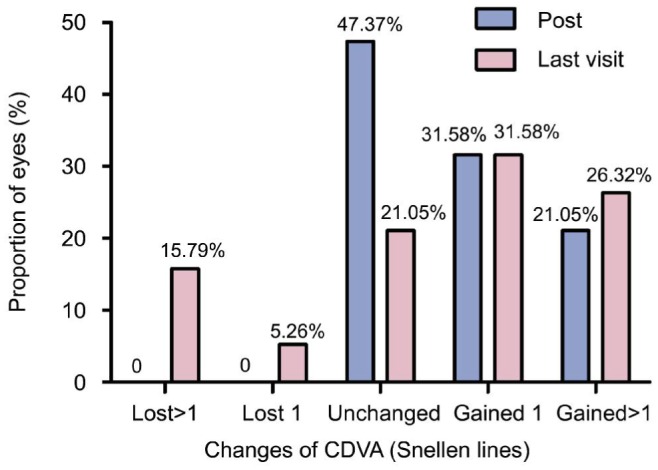
Post means 1wk to 1mo after ICL implantation, last visit means their most recent postoperative visit.
Efficacy
Figure 2 showed the UDVA before and after implantation of ICL. The pre-operative, post-operative and last UDVA was 0.09±0.05, 0.87±0.31 and 0.64±024. 27. At 1wk to 1mo after surgery, 94.7% eyes were 0.5 or better and 52.6% were 1.0 or better, and 78.9% eyes were 0.5 or better at their most recent postoperative visit and 21.1% were 1.0 or better. The efficacy index (mean postoperative UDVA/mean preoperative CDVA) at 1wk to 1mo after surgery and the most recent postoperative visit was 1.15 and 0.84, respectively.
Figure 2. UDVA before and after implantation of ICL.
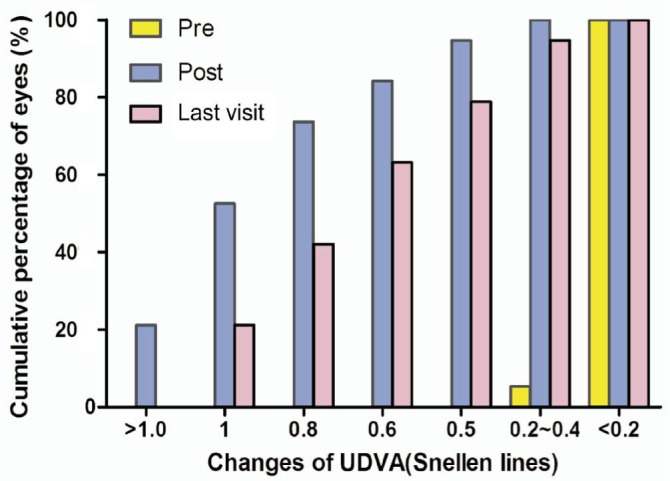
Post means 1wk to 1mo after ICL implantation, last visit means their most recent postoperative visit.
Predictability
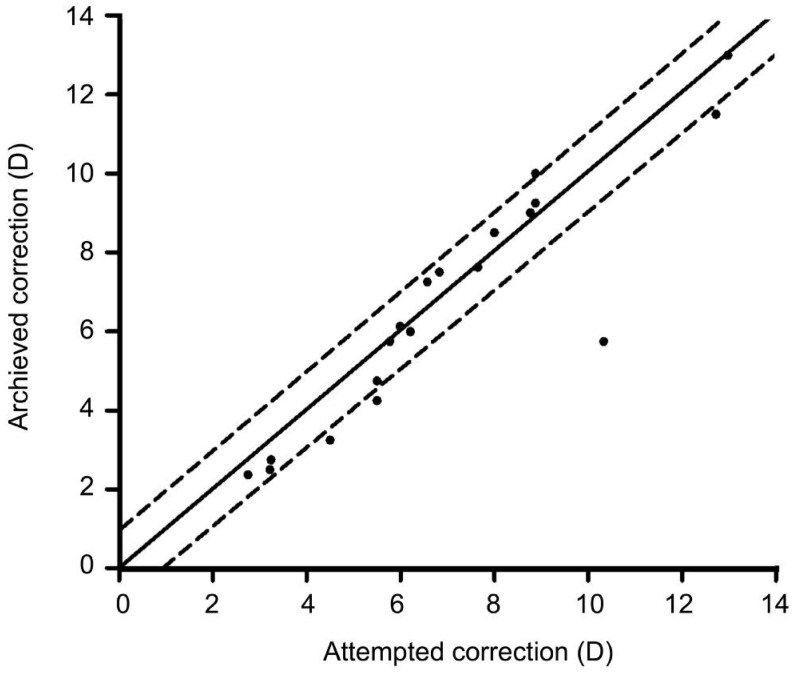
Figure 3 showed the predictability of ICL for residual refractive error after corneal refractive surgeries. Total 52.63% of eyes were within ±0.5 D of the predicted spherical equivalents, 73.68% were within ±1.0 D, and 94.74% were within ±2.0 D.
Figure 3. Scatter plot of attempted versus achieved correction (spherical equivalent) after implantation of ICL.
The solid line represented achieved correction (attempted correction); The dotted line represented achieved correction (attempted correction ±1.0 D).
Stability
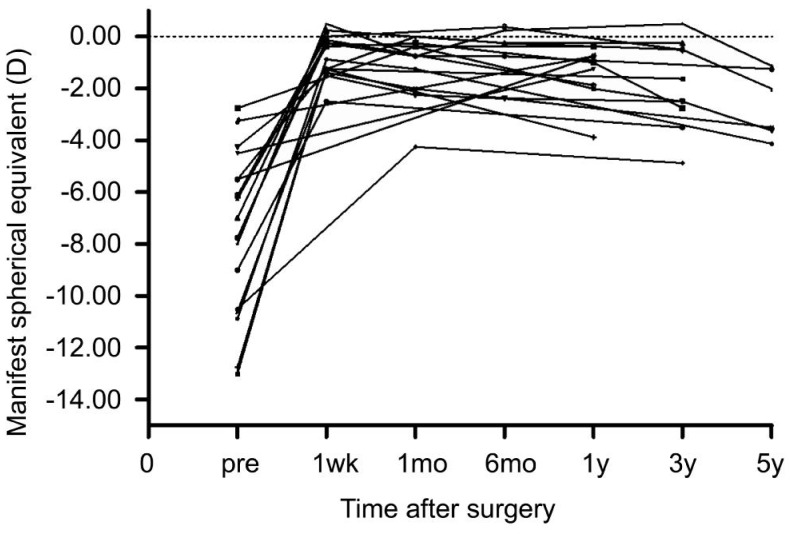
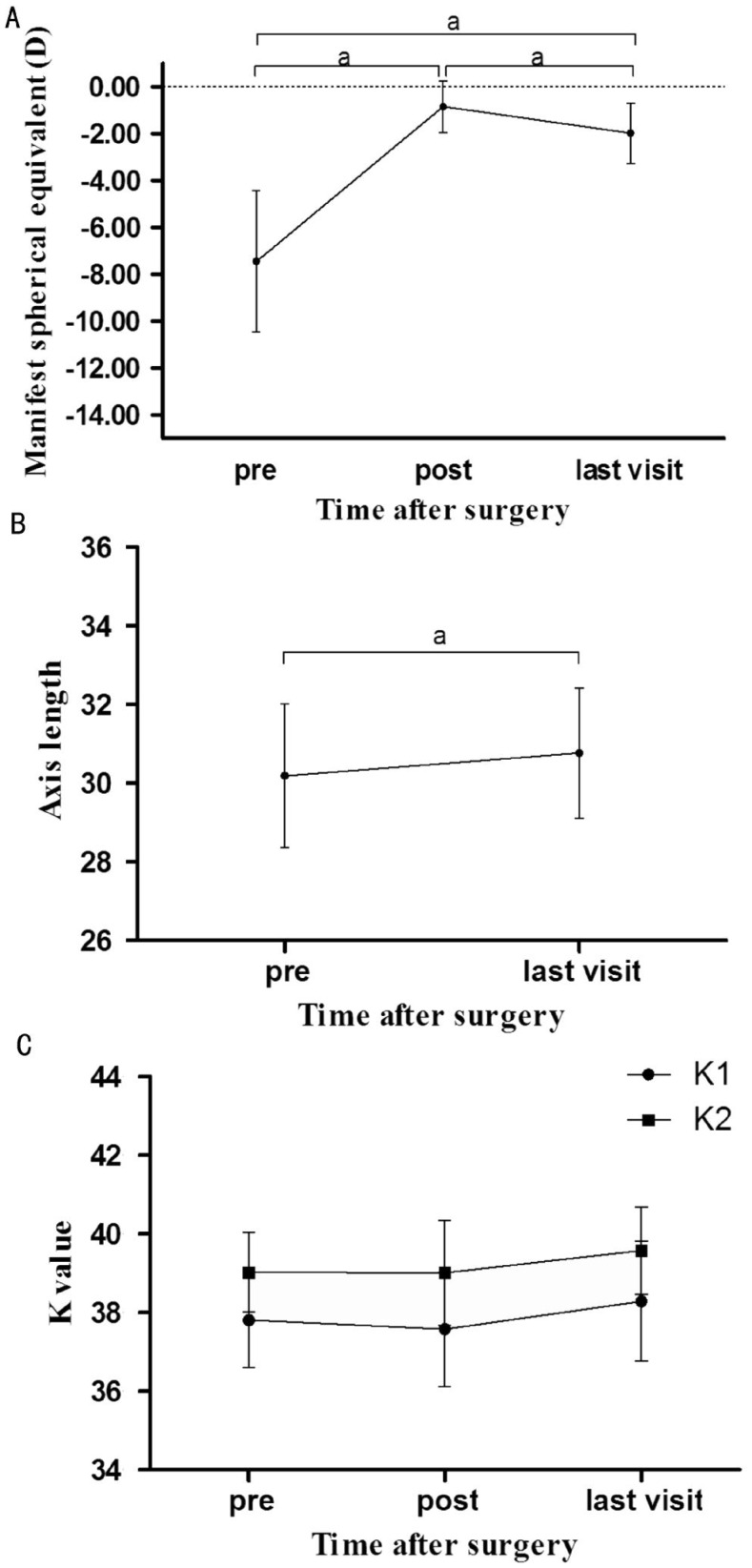
The manifest spherical equivalents (MRSE) over time after ICL surgery of all the patients were presented in Figure 4. The pre-operative, post-operative (1wk to 1mo) and last visit MRSE were -7.45±3.02 D, -0.85±1.10 D and -1.97±1.29 D, respectively; a significant change of -1.13±0.61 (0.00--2.00) D in MRSE was seen from post-operative to last visit (P<0.001). The pre-operative, post-operative and last visit K1 (flat K) values were 37.81±1.21 D, 37.58±1.45 D and 38.29±1.52 D, with K2 (steep K) value being 39.02±1.01 D, 39.01±1.33 D and 39.58±1.11 D, respectively. The mean K [1/2(K1+K2)] value changed from post-operative to last visit were 0.65±1.13 (-0.60-3.95) D. No significant differences in K1 and K2 value were observed over time after ICL implantation. The pre- and post-operative axis length were 30.19±1.82 and 30.77±1.66 mm, respectively; a significant change of 0.58±0.53 (0.12-2.53) mm was seen from pre-operative to last visit (P<0.001)(Figure 5).
Figure 4. Manifest spherical equivalent after implantation of ICL over time for all the subject eyes.
Figure 5. Manifest spherical equivalent (A), axis length (B), K1 (flat K) and K2 (steep K) value (C) after implantation of ICL over time.
Post means 1wk to 1mo after ICL implantation, last visit means their most recent postoperative visit (aP<0.001).
Intraocular Pressure, Endothelial Cell Counting
The IOP of all the patients from 1d to 1mo postoperatively ranged from 7.0 to 19.0 mm Hg with no high IOP observed. The ECD of all the patients at most recent follow-up was 3086.37±364.78 (2386-3987) cell/mm2(Table 1).
Adverse Event and Secondary Surgery
There were no complications during the surgical procedure and all implantations were uneventful. Anterior capsule opacity and retinal detachment was observed in one eye one year after Toric implantable collamer lens (TICL) implantation; the lens was not removed and the eye underwent scleral buckling.
DISCUSSION
In this study, we measured UDVA, CDVA, manifest refractive error, IOP, corneal ECD, adverse events in eyes implanted with ICL after corneal surgeries. We assessed the safety, efficacy, predictability and stability of ICL for residual refractive error after corneal refractive surgeries with a relatively large cohort and long follow-up periods.
Our study demonstrated that ICL for the correction of residual refractive error after corneal refractive surgeries was a safe and effective technique with the safety and efficacy indices being 1.12 and 1.15, respectively, showing consistences with previous case reports[8],[13]–[14]. On angle-supported phakic IOL implantation after corneal surgeries, his personal series about 12 eyes (10 after PRK, 2 after LASIK) in 10 patients showed that safety index was 1.4 and efficacy index was 1.0 in Leccisotti's overview[4]. Besides, Kamiya and Shimizu[8] showed in their one-month case report that ICL implantation might be an effective alternative for the treatment of hyperopia after overcorrected LASIK. Srinivasan et al's[14] study on 4 eyes of 3 patients and Kamiya et al's[13] study on 1 eye of 1 patient indicated that ICL implantation was an effective surgical option for the management of hyperopia after RK, respectively. Our results were comparable with these studies in the effectiveness of ICL implantation as an enhancement for previous corneal refractive surgeries.
The posterior chamber pIOL implantation surgery has been reported to be a predictable surgery for the correction of myopia[15], astigmatism[16], and hyperopia[17]. In contrast, the predictability of ICL for the correction of residual refractive error after corneal refractive surgeries is questionable as it is difficult to measure the corneal refractive power precisely after corneal refractive surgeries, which has been shown to yield less accurate IOL power calculation when performing cataract surgery[8]. However, in the case of pIOL implantation, the calculation is less dependent on corneal refractive power, thus we proposed that the accuracy of pIOL power calculation was higher than that of IOL power calculation in cataract surgery[8]. In our study, predictability of ICL for the correction of residual refractive error after corneal refractive surgeries was good except one eye that had done TOGCA before ICL implantation. We believed that her highly irregular cornea on corneal topography before surgery and the short interval time of just 1y between the two surgeries had contributed to the undesirable predictability in this specific subject. Leccisotti[4] used the van der Heijde formula[18] for the calculation of IOL power, which almost invariably led to undercorrection of myopia in his first case series. He then adjusted the formula by overcorrecting myopia by 10%, thus achieving better predictability. In our study, we did not adjust the formula by overcorrecting myopia by 10% but still achieved good results. This is probably because we used a different formula (a modified vertex formula provided by the manufacturer, STAAR Surgical) for the calculation of IOL power for different types of pIOLs. Also, the location of posterior chamber ICL is closer to the optical node than that of the angle-supported pIOL, which might have yielded better predictability than the latter.
The stability of ICL implantation after corneal refractive surgeries has rarely been reported. In our study, all the patients' preoperative refractive error were relatively stable for at least 2y with refractive power change no greater than 0.5 D per year, which is consistent with Leccisotti's[4] overview. Leccisotti[4] concluded that 100% of the study eyes were within 1.00 D after 1-3y of angle-supported pIOL implantation after corneal surgeries. However, there was a trend of mild progression towards myopia after 5y in our study; we speculated that this might be attributable to axial elongation associated with high myopia on one hand, and to the inflated crystal lens associated with age on the other (Figure 5). Although no significant differences in K value were observed over time after ICL implantation, we could see a steepening trend from short-term postoperative period to the last visit (Figure 5), with the K value changes being 0.65±1.13 D, which might have contributed on the MRSE changes as well. Nevertheless, the regression was less than 2.00 D in all the subjects, yielding a relatively good stability.
The IOP after surgery remained within a safe range, and ECD was relatively stable. Anterior capsule opacity was observed in one eye that underwent TICL implantation one year after surgery, whereas no opacity was observed in the contralateral eye without ICL implantation. We inferred that this could be due to the relatively low vault of about one half of corneal thickness; no measure was taken to this patient, as his visual acuity had not been affected. The eye experiencing retinal detachment received scleral buckling in time without serious vision loss.
The limitations of this study are that the sample size is relatively small from a statistical standpoint and the subjects are heterogeneous as referred to their previous corneal procedure. But the volume of ICL for the correction of residual refractive error after corneal refractive surgeries is very small, so our long term study still added significantly to the scientific literature. Although the subjects involved in this study underwent various kinds of corneal refractive surgeries, they shared the same characteristics of a flattened cornea, a residual myopic refractive error while remaining their intact intraocular structures. Therefore, the results of ICL implantation are independent of the previous corneal procedures.
In summary, our results suggest that the implantation of ICL is a safe and predictable procedure to correct residual myopic refractive error after corneal refractive surgeries. The absence of secondary damage to the cornea indicates that ICL implantation in some circumstances might be superior to corneal enhancement surgeries.
Acknowledgments
Thanks to Professor Baichuan Jiang for the modification of this article.
Part of the article was presented in the 29th ESCRS meeting in 2011 in Vienna, Austria.
Foundations: Supported by the Committee of Science and Technology of Shanghai, China (No.09411962100); the Health and Family Planning Committee of Pudong New District of Shanghai, China (No.PW2014D-1).
Conflicts of Interest: Chen X, None; Wang XY, None; Zhang X, None; Chen Z, None; Zhou XT, None.
REFERENCES
- 1.Sher NA, Golben MP, Bond W, Trattler WB. A retrospective review of photorefractive keratectomy to enhance earlier radial keratotomy. Int Ophthalmol Clin. 2011;51(2):39–49. doi: 10.1097/IIO.0b013e31820f88f0. [DOI] [PubMed] [Google Scholar]
- 2.Manche EE, Judge A, Maloney RK. Lamellar keratoplasty for hyperopia. J Refract Surg. 1996;12(1):42–49. doi: 10.3928/1081-597X-19960101-11. [DOI] [PubMed] [Google Scholar]
- 3.Xiao J, Jiang C, Zhang M, Jiang H, Li S, Zhang Y. When case report became case series: 45 cases of late traumatic flap complications after laser-assisted in situ keratomileusis and review of Chinese literature. Br J Ophthalmol. 2014;98(9):1282–1286. doi: 10.1136/bjophthalmol-2013-304422. [DOI] [PubMed] [Google Scholar]
- 4.Leccisotti A. Bioptics: where do things stand? Curr Opin Ophthalmol. 2006;17(4):399–405. doi: 10.1097/01.icu.0000233962.19004.14. [DOI] [PubMed] [Google Scholar]
- 5.Zaldivar R, Oscherow S, Piezzi V. Bioptics in phakic and pseudophakic intraocular lens with the Nidek EC-5000 excimer laser. J Refract Surg. 2002;18(3 Suppl):S336–S339. doi: 10.3928/1081-597X-20020502-10. [DOI] [PubMed] [Google Scholar]
- 6.Zaldivar R, Davidorf JM, Oscherow S, Ricur G, Piezzi V. Combined posterior chamber phakic intraocular lens and laser in situ keratomileusis: bioptics for extreme myopia. J Refract Surg. 1999;15(3):299–308. doi: 10.3928/1081-597X-19990501-04. [DOI] [PubMed] [Google Scholar]
- 7.Velarde JI, Anton PG, de Valentin-Gamazo L. Intraocular lens implantation and laser in situ keratomileusis (bioptics) to correct high myopia and hyperopia with astigmatism. J Refract Surg. 2001;17(2 Suppl):S234–S237. doi: 10.3928/1081-597X-20010302-19. [DOI] [PubMed] [Google Scholar]
- 8.Kamiya K, Shimizu K. Implantable Collamer lens for hyperopia after radial keratotomy. J Cataract Refract Surg. 2008;34(8):1403–1404. doi: 10.1016/j.jcrs.2008.03.045. [DOI] [PubMed] [Google Scholar]
- 9.Sanchez-Galeana CA, Smith RJ, Rodriguez X, Montes M, Chayet AS. Laser in situ keratomileusis and photorefractive keratectomy for residual refractive error after phakic intraocular lens implantation. J Refract Surg. 2001;17(3):299–304. doi: 10.3928/1081-597X-20010501-02. [DOI] [PubMed] [Google Scholar]
- 10.Macsai MS, Fontes BM. Refractive enhancement following presbyopia-correcting intraocular lens implantation. Curr Opin Ophthalmol. 2008;19(1):18–21. doi: 10.1097/ICU.0b013e3282f14d9f. [DOI] [PubMed] [Google Scholar]
- 11.Alfonso JF, Lisa C, Fernandez-Vega Cueto L, Fernandes P, Gonzalez-Meijome JM, Montes-Mico R. Comparison of visual and refractive results of Toric Implantable Collamer Lens with bioptics for myopic astigmatism. Graefes Arch Clin Exp Ophthalmol. 2013;251(3):967–975. doi: 10.1007/s00417-012-2155-9. [DOI] [PubMed] [Google Scholar]
- 12.Perez-Vives C, Dominguez-Vicent A, Madrid-Costa D, Ferrer-Blasco T, Montes-Mico R. Myopic astigmatism correction: comparison of a Toric Implantable Collamer Lens and a bioptics technique by an adaptive optics visual simulator. Ophthalmic Physiol Opt. 2013;33(2):114–122. doi: 10.1111/opo.12019. [DOI] [PubMed] [Google Scholar]
- 13.Kamiya K, Shimizu K, Komatsu M. Implantable Collamer Lens implantation and limbal relaxing incisions for the correction of hyperopic astigmatism after laser in situ keratomileusis. Cornea. 2010;29(1):99–101. doi: 10.1097/ICO.0b013e31818a7de3. [DOI] [PubMed] [Google Scholar]
- 14.Srinivasan S, Drake A, Herzig S. Early experience with implantable collamer lens in the management of hyperopia after radial keratotomy. Cornea. 2008;27(3):302–304. doi: 10.1097/ICO.0b013e31815ea268. [DOI] [PubMed] [Google Scholar]
- 15.Moya T, Javaloy J, Montés-Micó R, Beltrán J, Muñoz G, Montalbán R. Implantable Collamer Lens for Myopia: Assessment 12 Years After Implantation. J Refract Surg. 2015;31(8):548–556. doi: 10.3928/1081597X-20150727-05. [DOI] [PubMed] [Google Scholar]
- 16.Alfonso JF, Lisa C, Alfonso-Bartolozzi B, Perez-Vives C, Montes-Mico R. Collagen copolymer toric phakic intraocular lens for myopic astigmatism: one-year follow-up. J Cataract Refract Surg. 2014;40(7):1155–1162. doi: 10.1016/j.jcrs.2013.11.034. [DOI] [PubMed] [Google Scholar]
- 17.Pesando PM, Ghiringhello MP, Di Meglio G, Fanton G. Posterior chamber phakic intraocular lens (ICL) for hyperopia: ten-year follow-up. J Cataract Refract Surg. 2007;33(9):1579–1584. doi: 10.1016/j.jcrs.2007.05.030. [DOI] [PubMed] [Google Scholar]
- 18.Van der Heijde GL. Some optical aspects of implantation of an IOL in a myopic eye. Eur J Implant Refract Surg. 1989;1(4):245–248. [Google Scholar]