Abstract
AIM
To evaluate the peripapillary choroidal thickness (PPCT) in Chinese children, and to analyze the influencing factors.
METHODS
PPCT was measured with enhanced depth imaging optical coherence tomography (EDI-OCT) in 70 children (53 myopes and 17 non-myopes) aged 7 to 18y, with spherical equivalent refractive errors between 0.50 and −5.87 diopters (D). Peripapillary choroidal imaging was performed using circular scans of a diameter of 3.4 mm around the optic disc. PPCT was measured by EDI-OCT in six sectors: nasal (N), superonasal (SN), superotemporal (ST), temporal (T), inferotemporal (IT) and inferonasal (IN), as well as global RNFL thickness (G).
RESULTS
The mean global PPCT was 165.49±33.76 µm. The temporal, inferonasal, inferotemporal PPCT were significantly thinner than the nasal, superonasal, superotemporal segments PPCT were significantly thinner in the myopic group at temporal, superotemporal and inferotemporal segments. The axial length was significantly associated with the average global (β=−0.419, P=0.014), superonasal (β=−2.009, P=0.049) and inferonasal (β= −2.000, P=0.049) PPCT. The other factors (gender, age, SE) were not significantly associated with PPCT.
CONCLUSION
PPCT was thinner in the myopic group at temporal, superotemporal and inferotemporal segments. The axial length was found to be negatively correlated to PPCT. We need more further studies about the relationship between PPCT and myopia.
Keywords: peripapillary choroidal thickness, enhanced depth imaging optical coherence tomography, Chinese children
INTRODUCTION
The vascular choroid lining the posterior sector of the eye, plays several anatomic and physiologic roles[1], including supplying the outer retina with oxygen and nutrients[2], regulating the ocular temperature[3] and the intraocular pressure (IOP) [4], and absorbing the light[5]. Some study of animal models [6]indicates that the choroid plays an important role in the modulation of refractive status, and that it may be associated with the development of refractive errors.
Different techniques can be used to measure choroidal thickness (CT), such as ultrasonography and histology. However, the location of the choroid behind the pigmented cells of the retina attenuates incident light and reduces the reliability of these methods. Optical coherence tomography (OCT) is an equipment designed to finish the operation of non-invasive structural imaging or “optical biopsy” of the eye[7]. Now, the enhanced depth imaging optical coherence tomography (EDI-OCT) can be used to acquire the vivo images of the choroid as a modified version of spectral-domain OCT (SD-OCT)[8].
Current choroidal investigations primarily focus on macular CT in both healthy adults and children[9]–[12]. Only a few studies have measured peripapillary choroidal thickness (PPCT)[13]–[18]. These studies may have primary or associated pathology located in the peripapillary choroidal region in adults, including glaucoma[13]–[15] and high myopia[16]–[17]. Previous studies[18]–[22] have reported a range of PPCT in a healthy adult population. There is some difference between the eyes of healthy children and those of adults, and the influence of a normal developmental process on the choroidal structure can be evaluated by determining CT in children of various ages. To the best of our knowledge, there was no research measuring the PPCT in healthy Chinese children. The objective of this study is to evaluate the thickness of the normal peripapillary choroid in healthy Chinese children and to analyze the possible factors influencing the measurements obtained by EDI-OCT.
SUBJECTS AND METHODS
Subjects and Procedures
This prospective study was approved by the Institutional Medical Ethics Committee and adhered to the tents of the Declaration of Helsinki. Informed consent was provided by the parents of the children.
A total of 70 Chinese children aged between 7 and 18 years of age visiting our clinic for refractive error examinations participated this study. Before enrollment in the study, we ensured that all of the children had no history of ocular disease, ocular surgery, or injury, and were reported to be in a healthy status. Also, normal visual acuity in both eyes had to be 0.1 logMAR or better. The children with refractive errors of greater than ±6.0 diopters (D) were excluded. In addition, children with unclear OCT image were excluded. We also excluded the children who could not completethe OCT examination well. All children recruited in our study completed a thorough ophthalmic examination, which included the non-cycloplegic refraction, best-corrected visual acuity (BCVA), IOP, slit lamp biomicroscopy, fundus examination, as well as the axial length measured by partial optical coherence inferometry (IOLMaster; Carl Zeiss Meditec, Inc.).
Subjects were classified according tothe spherical equivalent refractive error (SE) of their selected eye into two groups including myopic group (SE of −0.75 or less) or non-myopic group (SE between 0.50 and −0.50). Seventy subjects were were divided into the myopic group (n=53, mean SE: −2.60±1.32 D) and the non-myopic group (n=17, mean SE: −0.01±0.39 D). The age (average age of the myopic) (mean age of the myop and non-myopes was 11.6±2.4y and 10.7±2.5y, respectively) and sex (the myopes and non-myopes consisted of 41% and 36% female children, respectively) were well matched between the two groups of subjects.
Optical Coherence Tomography Imaging
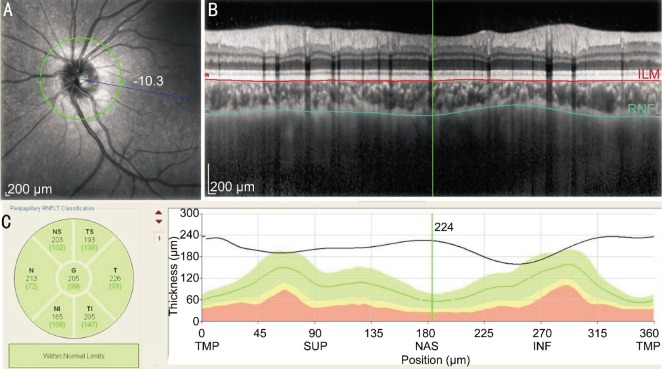
In this study, the SD-OCT device (Spectralis HRA+OCT; Heidelberg Engineering, Heidelberg, Germany) was used to measure all of the children with undilated pupils. The wavelength of the OCT equipment was 870 nm. The enhanced depth imaging system was used to acquire the peripapillary choroidal images of the subjects. The image saved for analysis was averaged after 20 scans for each subject using the automatic averaging and eye tracking features of this OCT device. The circular scans of a semidiameter of 1.7 mm around the optic disc were performed to acquire the peripapillary choroida images. The optic nerve head was just in the center of the scans. CT was measured manually by an expert operator in masked fashion (Wu XS) with the manual tools provided by the Spectral OCT analysis software (version 1.9.10.0; Heidelberg Engineering). We measured the PPCT from the outer part of the hyperreflective line corresponding to the base of retinal pigment epithelium (RPE) to the inner margin of the sclera corresponding to the sclerochoroidal interface (Figure 1). We also measured the retinal nerve fiber layer (RNFL) thickness using the same peripapillary circle scan. OCT included RNFL and PPCT in six parts: nasal (N), superonasal (SN), superotemporal (ST), temporal (T), inferotemporal (IT) and inferonasal (IN), and global RNFL thickness (G).
Figure 1. Example of PPCT measurements (left eye).
A: The green circle was the circular scan of a semidiameter of 1.7 mm around the optic nerve head; B: CT was measured manually from the outer part of the hyperreflective line corresponding to the base of RPE (line coloured red) to the inn*er margin of the sclera (line coloured green); C: Using the the calipers supplied by the Spectral OCT analysis software provided by Heidelberg Eye Explorer software, OCT measured the PPCT in six sectors and the globalthickness.
Statistical Analysis
SPSS (Statistical Package for Social Science, version 20.0; SPSS Inc., Chicago, IL, USA) was used for all statistical analysis of the data. We compared PPCT at different sectors using one-way analysis of variance. A 2-way ANOVA was used to examine the influence of refractive error and sex upon PPCT. The variations in global PPCT relative to axial length, SE, and age was calculated with stepwise multiple linear regression. Also, variations in each sector of PPCT relative to axial length, SE, and age was calculated with stepwise multiple linear regression. The correlation between the global indices of visual field and the RNFL thickness wasevaluated using spearman's correlation coefficient. All statistical tests were two-sided with a 0.05 level of significance. Results are represented as mean±SD.
RESULTS
Of the 70 children enrolled, 44 were boys and 26 were girls enrolled in this study. The average age of the children was 11.40±2.50y (range, 7–18y). The average SE was −1.97±1.62 D (range, −5.87 D to +0.50 D). The average axial length was 24.46±1.06 mm (range, 21.95–26.57 mm). The mean RNFL thickness was 106.57±9.92 µm (range, 86.00–134.00 µm). Table 1 shows the characteristics of the children.
Table 1. Clinical characteristics of subjects.
| Parameters | Average | SD |
| No. of subjects (No. of eyes) | 70 (70) | |
| Age (a) | 11.40 | 2.50 |
| Gender (boys/girls) | 44/26 | |
| Spherical equivalent (D) | −1.97 | 1.62 |
| Axial length (mm) | 24.46 | 1.06 |
SD: Standard deviation.
Peripapillary Choroidal Thickness Measurements
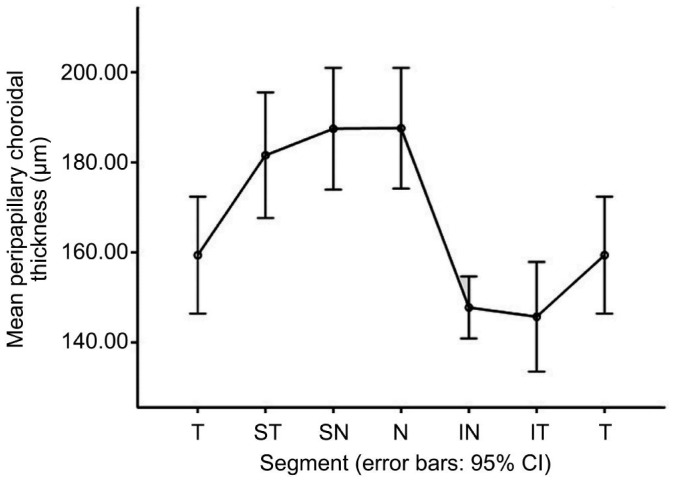
The mean global PPCT was 165.49±33.76 µm. Table 2 shows the PPCT at different sectors. The nasal (187.57±56.19 µm), superonasal (187.46±56.62 µm), superotemporal (181.59±58.53 µm) sectors had thicker CT. The temporal (159.39±54.36 µm), inferonasal (147.76±28.90 µm), had inferotemporal (145.71±51.09 µm) sectors had thinner CT. Post hoc analysis utilizing least significant difference (LSD) t-test demonstrated that the inferotemporal, inferonasal, temporal sectors were thinner than the nasal, superonasal, superotemporal sectors. However, there was no statistically significant difference between the CT of the temporal, inferonasal and inferotemporal sectors. Figure 2 shows the variation trend of PPCT.
Table 2. Average CT at different sectors.
| Sectors | Average CT (µm) | SD |
| G | 165.49 | 33.76 |
| T sector | 159.39 | 54.36 |
| ST sector | 181.59 | 58.53 |
| SN sector | 187.46 | 56.62 |
| N sector | 187.57 | 56.19 |
| IN sector | 147.76 | 28.9 |
| IT sector | 145.71 | 51.09 |
CT: Choroidal thickness; G: Global; T: Temporal; ST: Superotemporal; SN: Superonasal; N: Nasal; IN: Inferonasal; IT: Inferotemporal.
Figure 2. Variation trend of PPCT.
The mean global PPCT was 163.09±32.23 µm in the myopic group and 172.94±38.24 µm in the non-myopic group. Table 3 shows the PPCT at different sectors. Although the difference in the global PPCT between the myopic group and the non-myopic group is not significantgroups, the difference at the temporal, superotemporal and inferotemporal sectors were statistically significant (P<0.05). The difference in the PPCT between the male group and the female group is not significant.
Table 3. PPCT, ouclar parameters and refractive error in the myopic and non-myopic groups.
| Parameters | Myopic subjects | Non-myopic subjects |
| Spherical equivalent (D) | −2.60±1.32 | −0.01±0.39 |
| Axial length (mm) | 24.77±0.97 | 23.52±0.68 |
| G | 163.09±32.23 | 172.94±38.24 |
| T sector | 148.06±35.75 | 194.71±82.71 |
| ST sector | 172.40±33.38 | 210.24±100.01 |
| SN sector | 181.43±38.32 | 206.24±92.52 |
| N sector | 184.06±44.18 | 198.53±84.30 |
| IN sector | 146.62±29.86 | 151.29±26.24 |
| IT sector | 138.68±32.63 | 167.65±84.39 |
PPCT: Peripapillary choroidal thickness; G: Global; T: Temporal; ST: Superotemporal; SN: Superonasal; N: Nasal; IN: Inferonasal; IT: Inferotemporal.
Factors Correlated to Peripapillary Choroidal Thickness
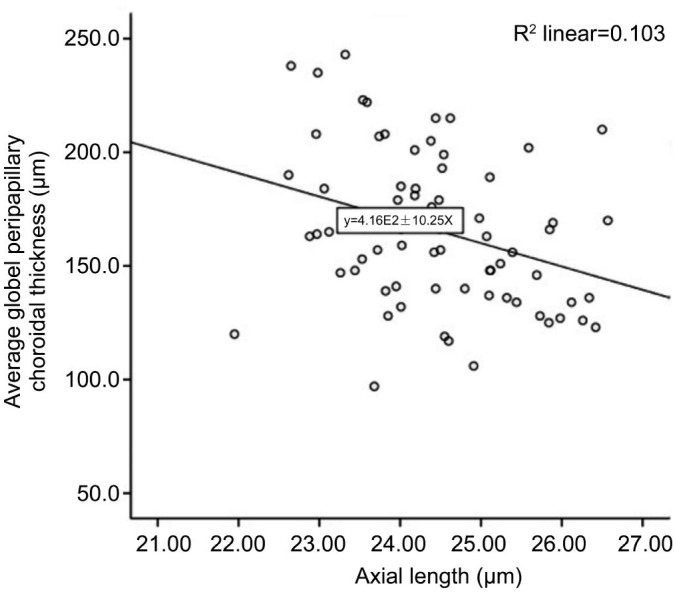
The axial length was significantly correlated to the average thickness of the global peripapillary choroid (β=−0.419, P=0.014), as well as to the peripapillary choroid in the superonasal (β=−2.009, P=0.049) and inferonasal (β=−2.000, P=0.049) parts (Table 4). Figure 3 shows the linear relationship between the global PPCT and the axial length. The other factors (gender, age, SE) were not significantly correlated to the PPCT.
Table 4. Average PPCT and some influencing factors.
| Parameters | G | T | ST | SN | N | IN | IT |
| Age (a) | NS | NS | NS | NS | NS | NS | NS |
| Axial length (mm) | β=−0.419 | NS | NS | β=−2.009 | NS | β=−2.000 | NS |
| P=0.014 | P=0.049 | P=0.049 | |||||
| Spherical equivalent (D) | NS | NS | NS | NS | NS | NS | NS |
PPCT: Peripapillary choroidal thickness; G: Global; T: Temporal; ST: Superotemporal; SN: Superonasal; N: Nasal; IN: Inferonasal; IT: Inferotemporal; NS: Non-significant association, P>0.05.
Figure 3. Scatterplot of average PPCT and axial length in healthy children.
According to Spearman's correlation analysis the PPCT and the corresponding peripapillary RNFL thickness were not correlated. The only exception was a significantly negative association between the temporal PPCT and the corresponding peripapillary RNFL (r=−0.279, P=0.019).
DISCUSSION
The peripapillary choroidal has an important role in the moduulation of the optic nerve head and may be correlated to different diseases such as glaucoma. Few studies have evaluated the PPCT in the pediatric population. To our best of knowledge, the current study is the first to evaluate the PPCT in the healthy Chinese pediatric population using the EDI-OCT. In the present study, the value and influencing factors of the PPCT are evaluated.
In this study, the mean global PPCT was 165.49±33.76 µm, consistent with previous studies in adults[18],[23]. Read et al [24] examined 93 healthy children (the range of the age is from 11 to 16 years old) with EDI-OCT. Every child was examined with a volumetric scanning protocol. Read et al[24] performed the OCT to acquire the images of a 15° by 15° region centered at the optic nerve head, and reported an average PPCT of 191±52 µm in the outermost annulus, which was relatively thicker than the value found in the current study. The outermost annulus was 1.25 mm away from the edge of the optic nerve head, which was roughly 2.0 mm away from the center of the optic nerve head further than our circular scans of a semidiameter of 1.7 mm around the optic nerve head, since the radius of the optic nerve head is about 0.75 mm. The disparity between Read et al's[24] measurements and ours may be arise from differences in measuring method and location of the PPCT, differences in ethnicity, differences in patient profiles.
The previous studies[18]–[22] in normal adults had evaluated the distribution of the PPCT, and the observation of our present study is similar to theirs. In the present study, we found the inferotemporal, inferonasal, temporal sectors were thinner than the nasal, superonasal, superotemporal sectors. Huang et al[18] examined 76 healthy adults by means of an EDI-OCT (wave length: 870 nm; scan pattern: enhanced depth imaging; Spectralis HRA+OCT; Heidelberg Engineering, Heidelberg, Germany), and reported the inferior choroid was the thinnest one in the four quadrants. Read et al[24] found that the inferior sector was thinnest and was significantly different compared to the superior one.
It is unclear why the inferior quadrant of the peripapillary choroid is thinner than the other quadrants. This regional difference may be attributable to the developmental pattern of the eye. The optic fissure is located in the inferior aspect of the optic cup, which is the last part of the globe to close during the ocular development [25]. The thinner choroid may be more susceptible to the hypoxia and elevated IOP, because of the increased vascular resistance and decreased blood flow in the choriochoriocapillary. Some studies [26]–[27] found the superior hemifield is affected more often and more severely than the inferior hemifield in glaucomatous eyes. Paraoptic branches of posterior ciliary arteries which supply peripapillary choroid and lamina cribrosa, may play an important role in blood flow in the pathogenesis of normal tension glaucoma.
We found the thinner PPCT at the temporal, superotemporal and inferotemporal sectors in the myopic group. Read et al[24] also found a thinner CT at temporal sector in the myopic group. These observations indicate the choroidal growth across the posterior eye is inhomogeneous in myopia. Kim et al[28] found a few of changes including optic disc tilting and β-parapapillary atrophy (where choroidal vessels and sclera are visible in the region bordering the optic disc) appear to accompany to myopia and its progression in the childhood. It has been proposed that these optic disc changes in childhood myopia are consistent with axial elongation in myopia being correlated to a dragging of the optic disc in the temporal region. We hypothesise that maybe a temporal dragging of the optic disc with increasing axial length result in a temporal thinning of peripappillary choroid. The low tension of oxygen hypothesis in the pathogenesis of myopia should be also achieved.
Previous studies[14],[29]–[32] found that a negative correlation between the axial length and the macular CT negatively correlated to. Similarly, we found that the axial length was negatively correlated to the PPCT. Read et al[24] also found that the axial length was negatively correlated to the PPCT. Previous studies[18],[20]–[21],[23],[33] reported a negative correlation between the age and the PPCT in adults. Regression analysis suggested an approximated 11 µm decrease in PPCT per decade[23]. But our study found that the age was not correlated to thePPCT. The difference of our finding may be attribute to the narrow range of the age (7-18) and the small sample size. Large sample size is needed to further analysis the correlation between the age and the PPCT.
There are some limitations in our study. First, the CT was manually measured. Ehrilich et al[34] reported that Lin's concordance correlation coefficient (CCC) for PPCT measurement was 0.93 (P<0.001) in adults, which were extremely reproducible. We should further analyze the reproducibility of these measurements in the pediatric population. Second, Tan et al[35] reported the diurnal variance in CT. We didn't examine the subjects at the afternoon constantly. Third, the sample size was not large.
In conclusion, to the best of our knowledge, this is the first study to evaluate the PPCT in Chinese healthy children with EDI-OCT. We found the inferotemporal, inferonasal, temporal sectors were significantly thinner than the nasal, superonasal, superotemporal sectors. The axial length was found to be negatively correlated to the PPCT.
Acknowledgments
Foundations: Supported by Major Scientific and Technological Projects in Zhejiang Province, China (No.2013c03048-3); the Science and Technology Plan Project of Wenzhou Science and Technology Bureau (No.Y20150284); Medical Scientific Research Foundation of Zhejiang Province (No.2016ZDA016).
Conflicts of Interest: Wu XS, None; Shen LJ, None; Chen RR, None; Lyu Z, None.
REFERENCES
- 1.Nickla DL, Wallman J. The multifunctional choroid. Prog Retin Eye Res. 2010;29(2):144–168. doi: 10.1016/j.preteyeres.2009.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bill A, Sperber G, Ujiie K. Physiology of the choroidal vascular bed. Int Ophthalmol. 1983;6(2):101–107. doi: 10.1007/BF00127638. [DOI] [PubMed] [Google Scholar]
- 3.Parver LM. Temperature modulating action of choroidal blood flow. Eye (Lond) 1991;5(Pt 2):181–185. doi: 10.1038/eye.1991.32. [DOI] [PubMed] [Google Scholar]
- 4.Alm A, Nilsson SF. Uveoscleral outflow: a review. Exp Eye Res. 2009;88:760–768. doi: 10.1016/j.exer.2008.12.012. [DOI] [PubMed] [Google Scholar]
- 5.Van Norren D, Tiemeijer LF. Spectral reflectance of the human eye. Vision Res. 1986;26(2):313–320. doi: 10.1016/0042-6989(86)90028-3. [DOI] [PubMed] [Google Scholar]
- 6.Troilo D, Nickla DL, Wildsoet CF. Choroidal thickness changes during altered eye growth and refractive state in a primate. Invest Ophthalmol Vis Sci. 2000;41(6):1249–1258. [PubMed] [Google Scholar]
- 7.Huang D, Swanson EA, Lin CP, Schuman JS, Stinson WG, Chang W, Hee MR, Flotte T, Gregory K, Puliafito CA. Optical coherence tomography. Science. 1991;254(5035):1178–1181. doi: 10.1126/science.1957169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Spaide RF, Koizumi H, Pozonni MC. Enhanced depth imaging spectral-domain optical coherence tomography. Am J Ophthalmol. 2008;146(4):496–500. doi: 10.1016/j.ajo.2008.05.032. [DOI] [PubMed] [Google Scholar]
- 9.Shin JW, Shin YU, Cho HY, Lee BR. Measurement of choroidal thickness in normal eyes using 3D OCT-1000 spectral domain optical coherence tomography. Korean J Ophthalmol. 2012;26(4):255–259. doi: 10.3341/kjo.2012.26.4.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Park KA, Oh SY. Analysis of spectral-domain optical coherence tomography in preterm children: retinal layer thickness and choroidal thickness profiles. Invest Ophthalmol Vis Sci. 2012;53(11):7201–7207. doi: 10.1167/iovs.12-10599. [DOI] [PubMed] [Google Scholar]
- 11.Ruiz-Moreno JM, Flores-Moreno I, Lugo F, Ruiz-Medrano J, Montero JA, Akiba M. Akiba M. Macular choroidal thickness in normal pediatric population measured by swept-source optical coherence tomography. Invest Ophthalmol Vis Sci. 2013;54(1):353–359. doi: 10.1167/iovs.12-10863. [DOI] [PubMed] [Google Scholar]
- 12.Read SA, Collins MJ, Vincent SJ, Alonso-Caneiro D. Choroidal thickness in childhood. Invest Ophthalmol Vis Sci. 2013;54(5):3586–3593. doi: 10.1167/iovs.13-11732. [DOI] [PubMed] [Google Scholar]
- 13.Spraul CW, Lang GE, Lang GK, Grossniklaus HE. Morphometric changes of the choriocapillaris and the choroidal vasculature in eyes with advanced glaucomatous changes. Vision Res. 2002;42(7):923–932. doi: 10.1016/s0042-6989(02)00022-6. [DOI] [PubMed] [Google Scholar]
- 14.Park HY, Lee NY, Shin HY, Park CK. Analysis of macular and peripapillary choroidal thickness in glaucoma patients by enhanced depth imaging opitical coherence tomography. J Glaucoma. 2014;23(4):225–231. doi: 10.1097/IJG.0000000000000045. [DOI] [PubMed] [Google Scholar]
- 15.Lamparter J, Schulze A, Riedel J, Wasielica-Poslednik J, König J, Pfeiffer N, Hoffmann EM. Peripapillary choroidal thickness and choroidal area in glaucoma, ocular hypertension and healthy subjects by SD-OCT. Klin Monbl Augenheilkd. 2015;232(4):390–394. doi: 10.1055/s-0035-1545819. [DOI] [PubMed] [Google Scholar]
- 16.Gupta P, Cheung CY, Saw SM, Bhargava M, Tan CS, Tan M, Yang A, Tey F, Nah G, Zhao P, Wong TY, Cheng CY. Peripapillary choroidal thickness in young Asians with high myopia. Invest Ophthalmol Vis Sci. 2015;56(3):1475–1481. doi: 10.1167/iovs.14-15742. [DOI] [PubMed] [Google Scholar]
- 17.Verkicharla PK, Ohno-Matsui K, Saw SM. Current and predicted demographics of high myopia and an update of its associated pathological changes. Ophthalmic Physiol Opt. 2015;35(5):465–475. doi: 10.1111/opo.12238. [DOI] [PubMed] [Google Scholar]
- 18.Huang W, Wang W, Zhou M, Chen S, Gao X, Fan Q, Ding X, Zhang X. Peripapillary choroidal thickness in healthy Chinese subjects. BMC Ophthalmol. 2013;13:23. doi: 10.1186/1471-2415-13-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ho J, Branchini L, Regatieri C, Krishnan C, Fujimoto JG, Duker JS. Analysis of normal peripapillary choroidal thickness via spectral domain optical coherence tomography. Ophthalmology. 2011;118(10):2001–2007. doi: 10.1016/j.ophtha.2011.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang R, Wang YX, Wei WB, Xu L, Jonas JB. Peripapillary choroidal thickness in adult Chinese: the Beijing eye study. Invest Ophthalmol Vis Sci. 2015;56(6):4045–4052. doi: 10.1167/iovs.15-16521. [DOI] [PubMed] [Google Scholar]
- 21.Gupta P, Jing T, Marziliano P, Baskaran M, Cheung GC, Lamoureux EL, Cheung CY, Wong TY, Aung T, Cheng CY. Peripapillary choroidal thickness assessed using automated choroidal segmentation software in an Asian population. Br J Ophthalmol. 2015;99(7):920–926. doi: 10.1136/bjophthalmol-2014-306152. [DOI] [PubMed] [Google Scholar]
- 22.Erbagci H, Oren B, Okumus S, Kenan S, Celemler P, Erbagci Peripapillary choroidal thickness in healthy Turkish subjects. Clin Ophthalmol. 2015;9:1393–1397. doi: 10.2147/OPTH.S79919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roberts KF, Artes PH, O'Leary N, Reis AS, Sharpe GP, Hutchison DM, Chauhan BC, Nicolela MT. Peripapillary choroidal thickness in healthy controls and patients with focal, diffuse, and sclerotic glaucomatous optic disc damage. Arch Ophthalmol. 2012;130(8):980–986. doi: 10.1001/archophthalmol.2012.371. [DOI] [PubMed] [Google Scholar]
- 24.Read SA, Alonso-Caneiro D, Vincent SJ, Collins MJ. Peripapillary choroidal thickness in childhood. Exp Eye Res. 2015;135:164–173. doi: 10.1016/j.exer.2015.03.002. [DOI] [PubMed] [Google Scholar]
- 25.Schoenwolf G, Bleyl S, Brauer P, et al. Larsen's human embryology. Philadelphia: Elsevier; 2009. pp. 602–616. [Google Scholar]
- 26.Tanabe H, Ito Y, Terasaki H. Choroidal is thinner in the inferior region of optic disks of normal eyes. Retina. 2012;32(1):134–139. doi: 10.1097/IAE.0b013e318217ff87. [DOI] [PubMed] [Google Scholar]
- 27.Hirooka K, Tenkumo K, Fujiwara A, Baba T, Sato S, Shiraga F. Evaluation of peripapillary choroidal thickness in patients with normal-tension glaucoma. BMC Ophthalmol. 2012;12:29. doi: 10.1186/1471-2415-12-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kim TW, Kim M, Weinreb RN, Woo SJ, Park KH, Hwang JM. Optic disc change with incipient myopia of childhood. Ophthalmology. 2012;119(1):21–26. doi: 10.1016/j.ophtha.2011.07.051. [DOI] [PubMed] [Google Scholar]
- 29.Mwanza JC, Hochberg JT, Banitt MR, Feuer WJ, Budenz DL. Lack of association between glaucoma and macular choroidal thickness measured with enhanced depth-imaging optical coherence tomography. Invest Ophthalmol Vis Sci. 2011;52(6):3430–3435. doi: 10.1167/iovs.10-6600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tan CS, Cheong KX. Macular choroidal thicknesses in healthy adults-relationship with ocular and demographic factors. Invest Ophthalmol Vis Sci. 2014;55(10):6452–6458. doi: 10.1167/iovs.13-13771. [DOI] [PubMed] [Google Scholar]
- 31.Bidaut-Garnier M, Schwartz C, Puyraveau M, Montard M, Delbosc B, Saleh M. Choroidal thickness measurement in children using optical coherence tomography. Retina. 2014;34(4):768–774. doi: 10.1097/IAE.0b013e3182a487a4. [DOI] [PubMed] [Google Scholar]
- 32.Zhang JM, Wu JF, Chen JH, Wang L, Lu TL, Sun W, Hu YY, Jiang WJ, Guo da D, Wang XR, Bi HS, Jonas JB. Macular choroidal thickness in children: the Shandong children eye study. Invest Ophthalmol Vis Sci. 2015;56(13):7646–7652. doi: 10.1167/iovs.15-17137. [DOI] [PubMed] [Google Scholar]
- 33.Rhodes LA, Huisingh C, Johnstone J, Fazio MA, Smith B, Wang L, Clark M, Downs JC, Owsley C, Girard MJ, Mari JM, Girkin CA. Peripapillary choroidal thickness variation with age and race in normal eyes. Invest Ophthalmol Vis Sci. 2015;56(3):1872–1879. doi: 10.1167/iovs.14-16179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ehrlich JR, Peterson J, Parlitsis G, Kay KY, Kiss S, Radcliffe NM. Peripapillary choroidal thickness in glaucoma measured with optical coherence tomography. Exp Eye Res. 2011;92(3):189–194. doi: 10.1016/j.exer.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 35.Tan CS, Ouyang Y, Ruiz H, Sadda SR. Diurnal variation of choroidal thickness in normal, healthy subjects measured by spectral domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2012;53(1):261–266. doi: 10.1167/iovs.11-8782. [DOI] [PubMed] [Google Scholar]