Dear Editor,
We present a case of dexamethasone (DEX) intravitreal implantation (Ozurdex®; Allergan, Irvine, CA, USA) to treat diabetic macular edema (DME) during pregnancy. According to Pescosolido et al[1], pregnancy may promote the onset of diabetic retinopathy in about 10% of cases and may contribute to its worsening when already present, causing macular edema[1]. Although one report has indicated that DME during pregnancy spontaneously regresses after delivery[1], others have reported that DME can persist and be associated with severe and persistent visual dysfunction[2]. Treatment of diabetic retinopathy during pregnancy is limited. The National Institute for Clinical Excellence guidelines state that evidence supports the use of laser treatment for DME[3]. Intravitreal injections of anti-vascular endothelial growth factor (anti-VEGF) such as bevacizumab to women early in a pregnancy can result in miscarriage, although the exact cause-and-effect relationship is not demonstrated[4]–[5]. Cases of patients who were suffering from preeclampsia and caesarean section with preterm delivery have been reported. The infant also has respiratory distress syndrome, pulmonary hemorrhage, pulmonary stenosis, and intraventricular cerebral hemorrhage[6]. There has been a case report of a single intravitreal injection of triamcinolone acetonomide to treat DME in a woman who was 6mo pregnant. The resolution of DME and the improved vision persisted throughout the pregnancy without further intervention[7].
The Ozurdex®, DEX intravitreal implant is a sustained-release biodegradable implant approved for treating macular edema due to retinal vein occlusion or diabetic retinopathy. The safety and the effectiveness of this implant have been demonstrated in several studies, including a report of a mean of four to five injections over 3y with robust long-term improvement in vision and resolution of macular edema in patients with DME[8]. Here, we present a case of a pregnant patient treated with intravitreal DEX implantation at Konkuk University Medical Center. The patient was examined by slit-lamp biomicroscopy, dilated ophthalmoscopy, and spectral-domain optical coherence tomography (SD-OCT).
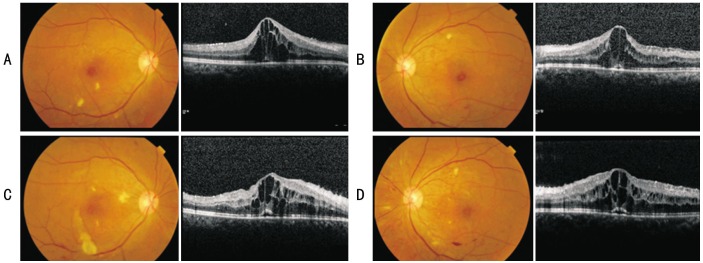
A 30-year-old pregnant female with a 14y history of type 1 diabetes under combined long and rapid acting insulin presented to a tertiary care ophthalmology department with a complaint of decreased vision in both eyes beginning 1wk ago. Her diabetes was well-controlled before pregnancy (hemoglobin A1c, 5.8%) without diabetes-related complications other than non-proliferative diabetic retinopathy with non-perfusion area on fluorescein angiography (FA). During the entire pregnancy period, her diabetes was controlled by long and rapid-acting insulin as before pregnancy. Her hemoglobin A1c during pregnancy was maintained from 5.4% to 5.8%. Her fasting blood sugar was from 90 to 100 mg/dL. She had undergone full pan retinal photocoagulation (PRP) in both eyes for bilateral non-high risk proliferative diabetic retinopathy (PDR) 1mo previously. She was in week 10 of another wise healthy intrauterine pregnancy (IUP). An ophthalmologic examination revealed 20/66 best corrected visual acuity (BCVA) in both eyes. The anterior segment examination was unremarkable without neovasulcarization of iris or angle. Adilated funduscopic examination showed significant intraretinal hemorrhages with cotton wool spots, diffuse macular edema, and scars from previous PRP in both eyes. No prominent retinal neovascularization was observed in both eyes. SD-OCT revealed central retinal thickness (CRT) of 733 µm in the right eye and 694 µm in the left eye with intraretinal cysts and subretinal fluid in both eyes (Figure 1A, 1B). After full discussion with the patient and her obstetrician, we decided to closely observe the DME until the third trimester of pregnancy. We did not consider an intravitreal injection of bevacizumab or ranibizumab to treat the DME because of possible risk of miscarriage or teratogenic effect on the fetus. The DME was persistent. BCVA during the follow-up period (from IUP 10 to 24, fundus examination was done every 2wk) was maintained at 20/66-20/50 (Figure 1C, 1D).
Figure 1. Fundus photographs and SD-OCT images at initial visit and 10wk after.
A: The right eye at initial visit; B: The left eye at initial visit. BCVA was 20/66 in both eyes at initial visit; C: Follow-up fundus photograph and SD-OCT of the right eye; D: Follow-up fundus photograph and SD-OCT of the left eye. After 10wk, vision did not change in either eye.
New onset vitreous hemorrhage (VH) with persistent DME and decrease of vision to 20/500 was noted in her right eye at week 24 of the IUP. Given her bilateral DME with diminishing vision in both eyes, effective treatment was considerable to be imperative at this time. Some literature searches were performed[4]–[7], reaching a conclusion that intravitreal steroid would likely to be safer compared to anti-VEGF. After extensive discussion with the patient and her obstetrician regarding the risk and benefit of the treatment, informed consent was obtained from the patient to treat her DME and VH with a DEX intravitreal implant. The patient received 700 µg DEX implantation in the right eye at week 24.
The patient returned 1wk after implantation. The vision in her right eye was improved to 20/50. A funduscopic examination revealed decreased VH but no adverse reactions such as increased intraocular pressure (IOP) or progression of cataract. Additional PRP was performed in the right eye. The BCVA was 20/40 in her right eye with resolution of the macular edema 4wk after DEX implantation. CRT of the right eye was 288 µm on SD-OCT (Figure 2A). The DME in the left eye sustained at 31wk of pregnancy. Despite of encouraging result in her right eye, the patient refused to treat the left eye during pregnancy. Her vision of the right eye remained stable 3mo after DEX implantation. No increased IOP or significant lenticular changes of the right eye was detected during follow up. However, at week 35 IUP, she visited our clinic due to rapid decrease of vision into light perception in the left eye over one week. Extensive tractional retinal detachment with VH was found in the left eye. Thus, caesarean section was performed at the same week with delivery of a healthy baby boy followed by total vitrectomy and gas tamponade in the left eye 3d after giving birth. The patient did not experience any complications during pregnancy or parturition.
Figure 2. Fundus photographs and SD-OCT images after Ozurdex® implantation.
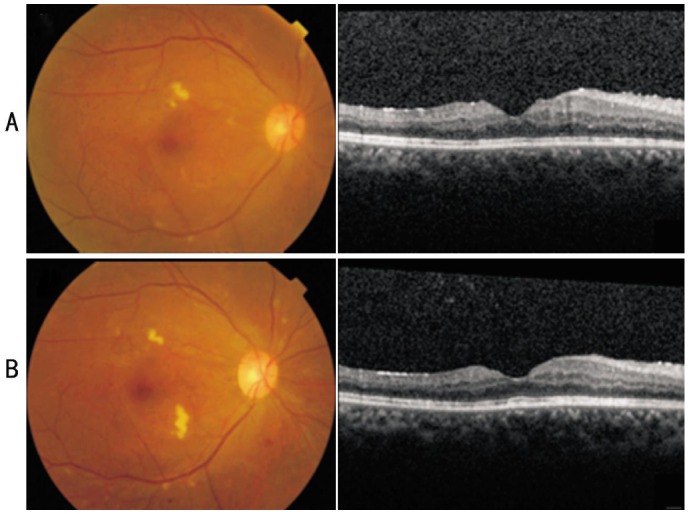
A: The right eye 4wk after Ozurdex® implantation. Vision was improved to 20/40; B: Fundus photograph and SD-OCT of the right eye 5mo after Ozurdex® implantation. Vision was 20/25.
On follow up 5mo after implantation, the patient's BCVA in her right eye was 20/25. Vision in her left eye was 20/200 even after retinal re-attachment with surgery. No recurrence of macular edema or VH occurred in both eye (Figures 2B).
Concentration of VEGF in the maternal serum is increased during the first 10wk of pregnancy. The VEGF concentration is positively correlated with placental volume and birth weight[9]. Vasculogenesis and angiogenesis, the formation of new blood vessels in the developing embryo, occur during the first trimester. During this period, VEGF family and their receptors are important growth factors[10]. Vasculogenesisis also mediated by VEGF-A. Therefore, anti-VEGF agents may have harmful effect both on the mother and the fetus[11]. An intravitreal anti-VEGF injection administered to a woman early in pregnancy has been reported to result in miscarriage[4].
Pre-eclampsia (hypertension and proteinuria during pregnancy, usually in the third trimester) may be a manifestation of inadequate angiogenic growth factor activities. Pharmacological inhibition of VEGF decrease the levels of circulating angiogenic growth factors, thus potentially increasing the risk of pre-eclampsia[12]. A study has suggested that both systolic and diastolic blood pressures may rise 3wk after a single 1.25 mg bevacizumab intravitreal injection[13].
Previous studies have shown that there are several advantages of using intravitreal corticosteroid versus anti-VEGF. In terms of safety, anti-VEGF such as ranibizumab and bevacizumab is designated as a pregnancy category C drug. However, corticosteroid is designated as pregnancy category B drug. In addition, multiple anti-VEGF injections are usually necessary to treat DME. Thus, continuous inhibition of VEGF-A for treating retinal diseases can potentially affect the developing embryo and the mother[12]. However, corticostereoid such as DEX implantation is known to be effective for up to 6mo with a single injection.
According to Degenring and Jonas[14], after an intravitreal high-dose injection of 20 to 25 mg triamcinolone acetonide, serum levels of triamcinolone acetonide did not differ significantly between preoperatively (0 mg/L) and postoperatively (0.065 mg/L). In 90% eyes, triamcinolone acetonide could not be detected in serum samples. A pharmacokinetic study of intravitreally injected DEX in monkey eyes also has shown that DEX is present only at low concentrations in the plasma at any time after implanataion. After 60d of 0.7 mg DEX implantation, DEX is detected 1110 ng/g in retina, 213 ng/mL in vitreous humor and 1.1 ng/mL in serum[15]. Thus, we could expect that DEX could greatly minimize adverse effects associated with systemic exposure to glucocorticoids. In addition, DEX, a synthetic glucocorticoid, has been used to accelerate fetal lung maturation. It is sometimes used clinically during premature labor for this purpose. Therefore, contrary to intravitreal anti-VEGF injections to pregnant women, prenatal steroids appear to improve the survival of infants and limit brain injuries to infants[8]. Also, resolution of DME without harmful effect on pregnant woman and her baby has been described with intravitreal injection of triamcinolone acetonomide[7]. Intravitreal DEX implantation has been approved as treatment of macular edema. Thus, DEX is probably safer than triamcinolone acetonomide, an off-label drug.
Our patient presented a case of DME in both eyes during week 10 of an IUP. No spontaneous resolution or improvement of the DME was detected during close follow-up. Moreover, an abrupt decrease in vision with VH and persistent edema in her right eye at week 24 IUP necessitated treatment. Although there were several case reports about the use of intravitreal anti-VEGF injections during the third trimester without complications[16]–[17], intravitreal DEX was implanted in her right eye, considering the relative safety of intravitreal corticosteroids[18] toward a fetus compared to bevacizumab or ranibizumab[7]. This treatment clearly resolved her macular edema with good visual outcome and long-term safety without causing any apparent adverse reaction on both the patientand fetus.
In conclusion, we propose that intravitreal DEX implantation may be a safe and effective treatment modality for managing severe DME as shown in this case, that causes decreased vision without spontaneous improvement in pregnant women. It should be considered as a treatment option for these patients, but more studies are needed to prove its safety.
Acknowledgments
Foundation: Supported by the Korea Health Technology R&D Project through the Korea Health Industry Development Institute (KHIDI), funded by the Ministry of Health & Welfare (No.HI14C1872).
Conflicts of Interest: Yoo R, None; Kim HC, None; Chung H, None.
REFERENCES
- 1.Pescosolido N, Campagna O, Barbato A. Diabetic retinopathy and pregnancy. Int Ophthalmol. 2014;34(4):989–997. doi: 10.1007/s10792-014-9906-z. [DOI] [PubMed] [Google Scholar]
- 2.Sinclair SH, Nesler C, Foxman B, Nichols CW, Gabbe S. Macular edema and pregnancy in insulin-dependent diabetes. Am J Ophthalmol. 1984;97(2):154–167. doi: 10.1016/s0002-9394(14)76085-4. [DOI] [PubMed] [Google Scholar]
- 3.Walker JD. NICE guidance on diabetes in pregnancy: management of diabetes and its complications from preconception to the postnatal period. NICE clinical guideline 63. London, March 2008. Diabet Med. 2008;25(9):1025–1027. doi: 10.1111/j.1464-5491.2008.02532.x. [DOI] [PubMed] [Google Scholar]
- 4.Petrou P, Georgalas I, Giavaras G, Anastasiou E, Ntana Z, Petrou C. Early loss of pregnancy after intravitreal bevacizumab injection. Acta Ophthalmol. 2010;88(4):e136. doi: 10.1111/j.1755-3768.2009.01572.x. [DOI] [PubMed] [Google Scholar]
- 5.Gomez Ledesma I, de Santiago Rodriguez MA, Follana Neira I, Leon Garrigosa F. Neovascular membrane and pregnancy. Treatment with bevacizumab. Arch Soc Esp Oftalmol. 2012;87(9):297–300. doi: 10.1016/j.oftal.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 6.Sullivan L, Kelly SP, Glenn A, Williams CP, McKibbin M. Intravitreal bevacizumab injection in unrecognised early pregnancy. Eye (Lond) 2014;28(4):492–494. doi: 10.1038/eye.2013.311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fazelat A, Lashkari K. Off-label use of intravitreal triamcinolone acetonide for diabetic macular edema in a pregnant patient. Clin Ophthalmol. 2011;5:439–441. doi: 10.2147/OPTH.S14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Effect of corticosteroids for fetal maturation on perinatal outcomes. NIH Consens Statement. 1994;12(2):1–24. [PubMed] [Google Scholar]
- 9.Wheeler T, Evans PW, Anthony FW, Godfrey KM, Howe DT, Osmond C. Relationship between maternal serum vascular endothelial growth factor concentration in early pregnancy and fetal and placental growth. Hum Reprod. 1999;14(6):1619–1623. doi: 10.1093/humrep/14.6.1619. [DOI] [PubMed] [Google Scholar]
- 10.Demir R, Seval Y, Huppertz B. Vasculogenesis and angiogenesis in the early human placenta. Acta Histochem. 2007;109(4):257–265. doi: 10.1016/j.acthis.2007.02.008. [DOI] [PubMed] [Google Scholar]
- 11.Demir R, Kayisli UA, Cayli S, Huppertz B. Sequential steps during vasculogenesis and angiogenesis in the very early human placenta. Placenta. 2006;27(6–7):535–539. doi: 10.1016/j.placenta.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Wu Z, Huang J, Sadda S. Inadvertent use of bevacizumab to treat choroidal neovascularisation during pregnancy: a case report. Ann Acad Med Singapore. 2010;39(2):143–145. [PubMed] [Google Scholar]
- 13.Rasier R, Artunay O, Yuzbasioglu E, Sengul A, Bahcecioglu H. The effect of intravitreal bevacizumab (avastin) administration on systemic hypertension. Eye (Lond) 2009;23(8):1714–1718. doi: 10.1038/eye.2008.360. [DOI] [PubMed] [Google Scholar]
- 14.Degenring RF, Jonas JB. Serum levels of triamcinolone acetonide after intravitreal injection. Am J Ophthalmol. 2004;137(6):1142–1143. doi: 10.1016/j.ajo.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 15.Chang-Lin JE, Attar M, Acheampong AA, Robinson MR, Whitcup SM, Kuppermann BD, Welty D. Pharmacokinetics and pharmacodynamics of a sustained-release dexamethasone intravitreal implant. Invest Ophthalmol Vis Sci. 2011;52(1):80–86. doi: 10.1167/iovs.10-5285. [DOI] [PubMed] [Google Scholar]
- 16.Tarantola RM, Folk JC, Boldt HC, Mahajan VB. Intravitreal bevacizumab during pregnancy. Retina. 2010;30(9):1405–1411. doi: 10.1097/IAE.0b013e3181f57d58. [DOI] [PubMed] [Google Scholar]
- 17.Sarhianaki A, Katsimpris A, Petropoulos IK, Livieratou A, Theoulakis PE, Katsimpris JM. Intravitreal administration of ranibizumab for idiopathic choroidal neovascularization in a pregnant woman. Klin Monbl Augenheilkd. 2012;229(4):451–453. doi: 10.1055/s-0031-1299207. [DOI] [PubMed] [Google Scholar]
- 18.Sim DA, Sheth HG, Kaines A, Tufail A. Punctate inner choroidopathy-associated choroidal neovascular membranes during pregnancy. Eye (Lond) 2008;22(5):725–727. doi: 10.1038/eye.2008.93. [DOI] [PubMed] [Google Scholar]