Abstract
Reactive thiols of cysteine (cys) residues in proteins play a key role in transforming chemical reactivity into a biological response. The heme oxygenase-2 (HO-2) isozyme contains two cys residues that have been implicated in binding of heme and also the regulation of its activity. In this paper, we address the question of a role for cys residues for the HO-2 inhibitors or activators designed in our laboratory. We tested the activity of full length recombinant human heme oxygenase-2 (FL-hHO-2) and its analog in which cys265 and cys282 were both replaced by alanine to determine the effect on activation by menadione (MD) and inhibition by QC-2350. Similar inhibition by QC-2350 and almost identical activation by MD was observed for both recombinant FL-hHO-2s. Our findings are interpreted to mean that thiols of FL-hHO-2s are not involved in HO-2 activation or inhibition by the compounds that have been designed and identified by us. Activation or inhibition of HO-2 by our compounds should be attributed to a mechanism other than altering binding affinity of HO-2 for heme through cys265 and cys282.
Keywords: Heme degradation, heme oxygenase-2, HO-2 activator, HO-2 inhibitor, thiols, menadione, QC-2350, in vitro
INTRODUCTION
Most proteins contain cysteine (cys) residues, which can play key roles in their function as well as their physical and chemical stability; cys residues may be involved in various actions such metal coordination, catalysis, and tertiary or quaternary protein structure. The thiol moiety is important because of its ability to participate in redox reactions, chelate transition metals, react with various ligand (such as nitric oxide, CH3), and form intra- or inter-peptide disulfide bonds. These thiol-reactions may alter protein function locally or may act allosterically as exemplified in the regulation of enzyme activity. For example, S-nitrosylation of the cys residue in the active site of glutathione peroxidase is likely responsible for its inhibition (Asahi et al., 1995), and a cys residue is involved in allosteric regulation of pyruvate kinase M2 (Ikeda and Noguchi, 1998).
The pharmacology of heme oxygenase (HO) has been an interest of our laboratory for several years; we have designed a number of inhibitors of HO-1 (Kinobe et al., 2006) and HO-2 (Vlahakis et al., 2013; Kong et al., 2015) and have identified activators of HO-2 (Vukomanovic et al., 2014). HO is the membrane-bound enzyme that catalyzes heme degradation into biliverdin, iron and carbon monoxide (CO). HO is considered to be cytoprotective by virtue of the antioxidant action of bilirubin, and the anti-apoptotic, anti-inflammatory and anti-proliferative actions of CO. There are two isoforms of HO, the oxidative stress-inducible HO-1 and the constitutive HO-2. The HO-2 isozyme possesses three cys residues, two of which have been implicated in the binding of heme and also the regulation of HO activity (Ragsdale and Yi, 2011). In this paper, we address the hypothesis that cys residues are important for our inhibitors or activators. For these experiments we have used a benzimidazole derivative, QC-2350, 1-(2-phenylethyl)-2-(pyrrolidin-1-ylmethyl)-1H-benzimidazole dihydrochloride (Vlahakis et al., 2013), as a representative HO-2 inhibitor and menadione (MD, 2-methyl-1,4-naphthoquinone, vitamin K3) as a representative HO-2 activator.
MATERIALS AND METHODS
To examine a potential role of HO-2 thiols in its activation/inhibition we prepared FL-hHO-2 and FL-hHO-2 Cys265,282-Ala, i.e., full length forms of recombinant human HO-2s with and without thiols on N-termini, respectively. Full details of our preparation of FL-hHO-2 were given in a previous publication (Vukomanovic et al., 2014). In brief, a cDNA clone of full-length human HO-2 (FL-hHO-2) in pOTB7 was obtained from Thermo Scientific (Lafayette, CO, USA). The double cys mutant of FL-hHO-2 (FL-hHO-2 Cys265,282-Ala) was obtained as a GST-fusion protein encoded in the pGETX-4T2 (McCoubrey et al., 1997). For each of these proteins, PCR amplification was performed to engineer NdeI and EcoRI sites at the 5’ and 3’ ends, respectively, and the coding region subcloned into the pET28a vector using these restriction sites. The resultant recombinant proteins contained an N-terminal histidine tag to allow purification by metal chelation using a protocol modified from previous publications (Huber and Backes, 2007; Rahman et al., 2008). Recombinant plasmids were transformed into BL21 (DE3) cells for expression and the resultant proteins were purified over Ni-NTA resin and dialyzed overnight against 20 mM potassium phosphate (pH 7.4), 0.1 mM EDTA, 10% glycerol as described previously (Rahman et al., 2008). Heme was conjugated by slow addition of hemin solution to a final molar ratio of 2:1-heme: HO-2 (Rahman et al., 2008). Further purification of the heme-bound enzyme and removal of excess heme was performed by size-exclusion chromatography over an S200 column. Protein concentration was determined by absorbance (Vreman and Stevenson, 2001) and purity was assessed by measurement of the Rz ratio (A405/A280 > 2.1) and by SDS-PAGE analysis.
HO-2 activity was determined as described by Vukomanovic et al. (2014). In brief, a reaction mixture (150 μL) containing 100 mM phosphate buffer (pH 7.4), 50 μM methemalbumin and 0.7 μM FL-hHO-2 and 0.01 μM purified human NADPH-P450 reductase (recombinant Becton Dickinson Canada Inc., Toronto, ON, Canada). The reaction was initiated by adding 1 mM NADPH and was continued for 15 minutes at 37°C. The reaction was stopped by instantly freezing the reaction mixture on dry ice, and the generated CO was determined by gas chromatography. Background CO is removed by a catalytic converter. Controls in which methemalbumin, NADPH or enzymes were omitted have been reported previously (Vukomanovic et al., 2011).
Source of materials. Sigma-Aldrich (Toronto, ON, Canada)- menadione, NADPH, bovine serum albumin, hemin; QC-2350 was synthesized in our laboratory (Vlahakis et al., 2013).
RESULTS AND DISCUSSION
We recently reported (Vukomanovic et al., 2014) in vitro activation of HO-2 by a number of MD (vitamin K3) analogs; we observed up to a 7-fold increase in the activity of rat brain microsomal HO-2 in the presence of MD (Vukomanovic et al., 2014). Similar activation was observed for recombinant hHO-2 isozymes as shown in Figure 1. Addition of 25 μM MD resulted in an 8-fold increase in CO production by FL-hHO-2 and FL-hHO-2 Cys265,282-Ala, respectively.
Figure 1.
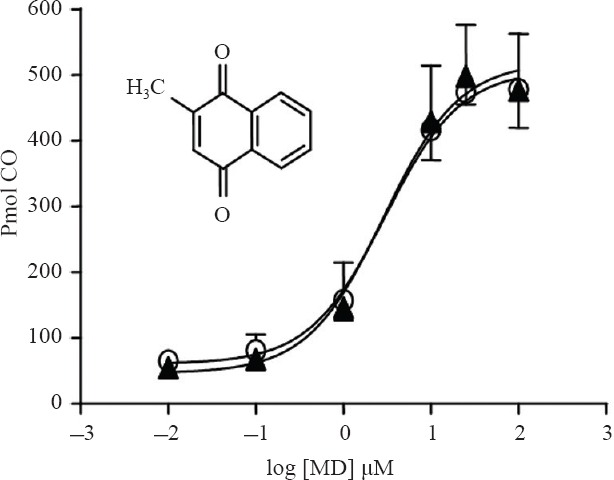
Menadione activation of recombinant FL-hHO-2 (open circles) was similar to the same enzyme without thiols-FL-hHO-2 Cys265,282-Ala (closed triangles).
Note: Heme oxygenase-2 (HO-2) activity was measured as described in Methods. The abscissa shows the log of the drug concentration (μM) and the ordinate shows HO-2 activity as pmol CO formed in 15 minutes by 0.7 μM FL-hHO-2s (the symbols represent the mean ± SD, n = 4). Where the SD bars are missing, the SD fell with the symbols. Inset shows the chemical structure of menadione. FL-hHO-2: Full length recombinant human heme oxygenase-2; FL-hHO-2 Cys265,282Ala: full length recombinant human heme oxygenase-2 in which cys265 and cys 282 were replaced with ala; CO: carbon monoxide.
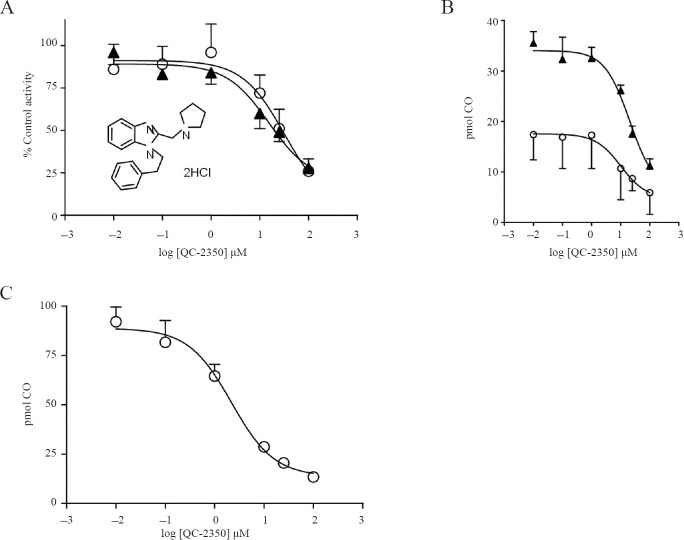
QC-2350 is one of a several novel clemizole analogs we have synthesized (Vlahakis et al., 2013; Kong et al., 2015) that showed potent and selective HO-2 inhibition. The presence of QC-2350, 0.01 to 100 μM, resulted in decreases of activity of both FL-hHO-2 and FL-hHO-2 Cys265,282-Ala almost identically when the data are presented as % control activity, and similarly when the data are presented as pmol CO produced (Figure 2A, B). The IC50 values of 20 and 29 μM for the FL-hHO-2 and FL-hHO-2 Cys265,282-Ala, respectively, were also similar. QC-2350 was found to be somewhat more potent against rat brain, microsomal HO-2 as it yielded an IC50 value of 2.7 μM (Figure 2C).
Figure 2.
QC-2350 inhibition of recombinant and microsomal HO-2.
Note: (A) QC-2350 (HO-2 inhibitor) decreased the activity of both FL-hHO-2 (closed triangles) and FL-hHO-2 Cys265,282-Ala (open circles) almost identically when the data are plotted as % Control Activity. When the data are plotted as pmol CO formed in 15 minutes (B), a similar inhibition of the isozymes was observed. (C) QC-2350 inhibition of rat brain microsomal HO-2 was similar to both recombinant forms of HO-2, but was slightly more potent. HO-2 activities were measured as described in Methods. Preparation of microsomal HO-2 was described in our recent paper (Vlahakis et al., 2013). The abscissa shows the log of drug concentration (μM) and ordinate (B, C) shows HO-2 activity as pmol of CO formed in 15 minutes by 0.7 μM FL-hHO-2 (mean ± SD, n = 4). Inset in A shows the chemical structure of QC-2350. HO-2: Heme oxygenase-2; FL-hHO-2: full length recombinant human heme oxygenase-2; FL-hHO-2 Cys265,282Ala: full length recombinant human heme oxygenase-2 in which cys265 and cys 282 were replaced with ala; CO: carbon monoxide.
Our observations indicate that the activation of HO-2 by MD was unchanged by the substitution of cys residues 265 and 282 with ala, and the inhibition of HO-2 by QC-2350 was not affected. We interpret these results to mean that these cys residues are not necessary for either activation of HO-2 by menadione or inhibition of HO-2 by QC-2350. Moreover, the similar results observed in the rat brain microsomal fraction (Vukomanovic et al., 2011; Vlahakis et al., 2013) suggests that this interpretation may apply to mammalian HO-2 generally and are not a peculiarity of human or recombinant HO-2 forms. We anticipate that this finding is applicable to all the drugs that we have developed as inhibitors (Vlahakis et al., 2013; Vukomanovic et al., 2014) or identified as activators (Kong et al., 2015) of HO-2. We cannot however extend this to the first generation porphyrin-based HO inhibitors as we have not tested the present HO forms in the presence of metalloporphyrin HO inhibitors. Nevertheless, they are known to act at the active site as competitive inhibitors so one would anticipate that the cys residues in question would not be essential for their inhibitor action.
While the present observations deny a role for Cys265 and Cys282 in activation of HO-2 by MD or its inhibition by QC-2350, they do not bear on other different functions that have been suggested previously by other laboratories. Accordingly Ragsdale and Yi (2011) reported that in the C-terminal Heme Regulatory Motifs (HRMs), a reversible thiol/disulfide redox switch modulates affinity of HO-2 for ferric heme. Varfaj et al. (2012) confirmed this difference in heme affinity between oxidized and reduced states of HO-2, but considered the difference to be too small to be physiologically significant. Regardless of the physiological importance of the putative redox switch, it seems unimportant for QC-2350 inhibition. Furthermore, it was reported (McCoubrey et al., 1997) that even though HO-2 binds heme through HRMs, HRMs are not involved in heme catalysis, and that is in concert with our findings about activation and inhibition of HO-2 with our compounds. Previously we reported that MD selectively activates HO-2 compared to HO-1 (Vukomanovic et al., 2011), and that QC-2350 selectively inhibits HO-2. As the major difference between the isozymes is the presence of cys in HO-2, these cys residues would be candidates to explain the observed selectivity. The present results do not support such an interpretation, which are consistent with the notion that HRM cys residues do not have a role in the selectivity demonstrated by our activators or inhibitors.
Abbreviations
HO: heme oxygenase; HO-2: heme oxygenase-2; FLhHO-2: full length recombinant human heme oxygenase-2; FL-hHO-2 Cys265,282Ala: full length recombinant human heme oxygenase-2 in which cys265 and cys 282 were replaced with ala; MD: menadione.
Footnotes
Conflicts of interest
None declared.
REFERENCES
- Asahi M, Fujii J, Suzuki K, Seo HG, Kuzuya T, Hori M, Tada M, Fujii S, Taniguchi N. Inactivation of glutathione peroxidase by nitric oxide. Implication for cytotoxicity. J Biol Chem. 1995;270:21035–21039. doi: 10.1074/jbc.270.36.21035. [DOI] [PubMed] [Google Scholar]
- Huber WJ, 3rd, Backes WL. Expression and characterization of full-length human heme oxygenase-1: the presence of intact membrane-binding region leads to increased binding affinity for NADPH cytochrome P450 reductase. Biochemistry. 2007;46:12212–12219. doi: 10.1021/bi701496z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y, Noguchi T. Allosteric regulation of pyruvate kinase M2 isozyme involves a cysteine residue in the intersubunit contact. J Biol Chem. 1998;273:12227–12233. doi: 10.1074/jbc.273.20.12227. [DOI] [PubMed] [Google Scholar]
- Kinobe RT, Vlahakis JZ, Vreman HJ, Stevenson DK, Brien JF, Szarek WA, Nakatsu K. Selectivity of imidazole-dioxolane compounds for in vitro inhibition of microsomal haem oxygenase isoforms. Br J Pharmacol. 2006;147:307–315. doi: 10.1038/sj.bjp.0706555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong X, Vukomanovic D, Nakatsu K, Szarek WA. Structureactivity relationships of 1,2-disubstituted benzimidazoles: selective inhibition of heme oxygenase-2 activity. ChemMedChem. 10:1435–1441. doi: 10.1002/cmdc.201500128. [DOI] [PubMed] [Google Scholar]
- McCoubrey WK, Jr, Huang TJ, Maines MD. Heme oxygenase-2 is a hemoprotein and binds heme through heme regulatory motifs that are not involved in heme catalysis. J Biol Chem. 1997;272:12568–12574. doi: 10.1074/jbc.272.19.12568. [DOI] [PubMed] [Google Scholar]
- Ragsdale SW, Yi L. Thiol/Disulfide redox switches in the regulation of heme binding to proteins. Antioxid Redox Signal. 2011;14:1039–1047. doi: 10.1089/ars.2010.3436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MN, Vlahakis JZ, Szarek WA, Nakatsu K, Jia Z. X-ray crystal structure of human heme oxygenase-1 in complex with 1-(adamantan-1-yl)-2-(1H-imidazol-1-yl)ethanone: a common binding mode for imidazole-based heme oxygenase-1 inhibitors. J Med Chem. 2008;51:5943–5952. doi: 10.1021/jm800505m. [DOI] [PubMed] [Google Scholar]
- Varfaj F, Lampe JN, Ortiz de Montellano PR. Role of cysteine residues in heme binding to human heme oxygenase-2 elucidated by two-dimensional NMR spectroscopy. J Biol Chem. 2012;287:35181–35191. doi: 10.1074/jbc.M112.378042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlahakis JZ, Vukomanovic D, Nakatsu K, Szarek WA. Selective inhibition of heme oxygenase-2 activity by analogs of 1-(4-chlorobenzyl)-2-(pyrrolidin-1-ylmethyl)-1H-benzimidazole (clemizole): Exploration of the effects of substituents at the N-1 position. Bioorg Med Chem. 2013;21:6788–6795. doi: 10.1016/j.bmc.2013.07.050. [DOI] [PubMed] [Google Scholar]
- Vreman HJ, Stevenson DK. Detection of heme oxygenase activity by measurement of CO. Curr Protoc Toxicol Chapter 9. 2001 doi: 10.1002/0471140856.tx0902s00. Unit 9.2. [DOI] [PubMed] [Google Scholar]
- Vukomanovic D, McLaughlin BE, Rahman MN, Szarek WA, Brien JF, Jia Z, Nakatsu K. Selective activation of heme oxygenase-2 by. Can J Physiol Pharmacol. 2011;89:861–864. doi: 10.1139/y11-091. [DOI] [PubMed] [Google Scholar]
- Vukomanovic D, Rahman MN, Bilokin Y, Golub AG, Brien JF, Szarek WA, Jia Z, Nakatsu K. In vitro Activation of heme oxygenase-2 by menadione and its analogs. Med Gas Res. 2014;4:4. doi: 10.1186/2045-9912-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]