Abstract
Brain injury is the leading cause of death and disability worldwide and clinically there is no effective therapy for neuroprotection. Hyperbaric oxygen preconditioning (HBO-PC) has been experimentally demonstrated to be neuroprotective in several models and has shown efficiency in patients undergoing on-pump coronary artery bypass graft (CABG) surgery. Compared with other preconditioning stimuli, HBO is benign and has clinically translational potential. In this review, we will summarize the results in experimental brain injury and clinical studies, elaborate the mechanisms of HBO-PC, and discuss regimes and opinions for future interventions in acute brain injury.
Keywords: hyperbaric oxygen preconditioning, ischemic stroke, traumatic brain injury, neonatal hypoxic-ischemia, neuroprotection
INTRODUCTION
Brain injury is the leading cause of death and disability worldwide. In the United States, brain injury accounts for one million hospital admissions per year. Brain injury can occur at birth, or later from an illness or a trauma, and cause physical, cognitive, and behavioral/emotional impairments--ranging from subtle to severe and may be either temporary or permanent. Despite advances in treatment, clinical management to prevent neurological damage after brain injury remains inefficacious. Although some promising strategies have been reported in animal models, they often fail to work in clinical practice (No authors listed, 1990; Kaste et al., 1994; Majid, 2014), therefore, novel treatment strategies protecting against brain injury should be developed and exploited. Hyperbaric oxygen preconditioning (HBO-PC) has been experimentally demonstrated to be neuroprotective, and has shown efficiency in patients undergoing on-pump coronary artery bypass graft (CABG) surgery (Li et al., 2011). Compared with other preconditioning stimuli, HBO is benign and has clinically translational potential (Camporesi and Bosco, 2014). In this review, we will sort out the studies of HBO-PC in experimental and clinical brain injury, elaborate its mechanisms in neuroprotection, and discuss regimes and opinions for future research.
INTRODUCTION OF PRECONDITIONING AND HBO-PC
Preconditioning is the treatment of a noxious stimulus near the threshold of damage and activates certain advantageous intrinsic mechanisms for protection and repair, creating a tolerance in the organ to the insult and, thus, protecting it from subsequent injury (Dirnagl et al., 2009). The concept of preconditioning was first proposed by Murry and his colleagues in 1986 (Murry et al., 1986). They documented that multiple brief ischemic episodes (four cycles of 5 minutes occlusion/5 minutes reperfusion) protected the heart from a subsequent sustained ischemic insult in dogs. And subsequently, the protective effects of preconditioning were shown in the brain in 1991. Kitagawa et al. (1991) confirmed non-lethal ischemic insult prevented neuronal death in different brain regions after global ischemia in gerbils, and the authors coined this phenomenon ‘ischemic tolerance’. To date, preconditioning has been applied in various organ systems, including liver (Yu et al., 2005), kidney (Islam et al., 1997), lung (Du et al., 1996), pancreas (Dembinski et al., 2003) and skeleton muscles (Mounsey et al., 1992), and the protective effects of preconditioning have been demonstrated in multiple species (Murry et al., 1986; Schott et al., 1990; Osada et al., 1991) and clinical trials (Alkhulaifi et al., 1994; Koneru et al., 2005; Pimentel et al., 2006).
Preconditioning can be induced by various stimuli. Brief ischemia or hypoxia serves as prototypical preconditioning stimuli in stroke (Gidday, 2006; Liu et al., 2014). Ischemic tolerance can also be induced by spreading depression, prolonged hypoperfusion, ischemia of remote skeletal muscle, hyperthermia and physical exercise (Zemke et al., 2004). Molecules known to cause ischemic brain injury — such as glutamate, reactive oxygen species (ROS), inflammatory cytokines and caspases, delivered exogenously at the lower levels could duplicate the protective effects of ischemic tolerance (Gidday, 2006). Moreover, volatile anaesthetics and some metabolic inhibitors could trigger ischemic tolerance pharmacologically (Gidday, 2006).
Hyperbaric oxygen (HBO) refers to 100% oxygen at twice to three times the atmospheric pressure at sea level (Wang et al., 2014). HBO might mimic hypoxia preconditioning (HPC) to attenuate ischemia/reperfusion injury. In 1996, a Japanese group demonstrated that repeated HBO (2 atmosphere absolute [ATA] for 1 hour daily for 5 days) could increase the tolerance of the brain against ischemic neuronal damage in gerbils, in which the induction of heat shock protein (HSP) 72 played an important role (Wada et al., 1996). Four years later, Xiong et al. (2000) found HBO preconditioning (2.5 ATA 1 hour daily for 3 or 5 days) could improve the neurological outcome and decrease infarct volume in transient middle cerebral artery occlusion (MCAO) rats in a “dose-dependent“ manner. Since then, the protective effects of HBO preconditioning have been investigated in various animal models, such as myocardial ischemia (Kim et al., 2001), spinal cord ischemia (Dong et al., 2002), hepatic ischemia (Yu et al., 2005), neonatal hypoxia-ischemia (Li et al., 2008b), intestinal ischemia (Bertoletto et al., 2008), surgical brain injury (Jadhav et al., 2009), intracerebral hemorrhage (Qin et al., 2008), traumatic brain injury (Hu et al., 2010) and renal ischemia (He et al., 2011). Freiberger et al. (2006) showed that HBO preconditioning elicited similar preconditioning efficacy as HPC and defended against oxidative stress in neonatal brain. The results of these laboratory studies suggested that HBO can produce a wide range of protective effects and may be a safer preconditioning stimulus compared with other stimuli such as ischemia/hypoxia and chemical agents. Recently HBO-PC has shown potent efficacy in preventing neuron death and improving neurological functions in many experimental acute brain injury models (Jadhav et al., 2010; Cheng et al., 2011; Soejima et al., 2012; Yan et al., 2013). In clinical practice, HBO-PC exhibited cerebral protective effects in patients undergoing CABG surgery (Li et al., 2011).
In contrast to conventional neuroprotection trials that target intervention post-insult, preconditioning strategies are advantageous in up-regulating mechanisms of innate cytoprotection and attenuating the deleterious cascades at the earliest possible time points, due to the intervention preceding the injury (Bahjat et al., 2013). HBO-PC provides new hope and window for the treatment of brain injury compared to the traditional post-insult neuroprotective agents.
HBO-PC IN BRAIN INJURY MODELS
Neonatal hypoxic-ischemia (HI)
Neonatal hypoxic-ischemic brain damage is a significant cause of acute mortality and chronic neurologic morbidity in infants and children worldwide (Shankaran, 2012). The pathogenesis of perinatal hypoxic-ischemic brain damage is highly complex and involves impaired blood-brain barrier permeability, energy failure, acidosis, excitotoxicity, oxidative stress and activation of inflammatory cascades in the immature brain (Biagas, 1999). Despite extensive research on neonatal HI, no effective strategy for treatment of HI has been developed yet.
The ability of HBO-PC to alleviate brain injury after HI was tested in two studies. Freiberger et al. (2006) demonstrated for the first time that HBO-PC and hypoxic preconditioning elicited similar effects on a neonatal brain and those effects were mediated by activating intrinsic defenses against oxidative stress. Further, it was shown that three days of HBO-PC (2.5 ATA for 2.5 hours each day) generated sufficient oxidative stress in order to suppress the mitochondrial aconitase activity in neonatal rats, resulting in a reduced infarction volume (Freiberger et al., 2006). After one year, Li et al. (2008b) reported that a single session of HBO-PC (2.5 ATA for 2.5 hours) significantly decreased the infarct size and reduced apoptosis via suppressing the activities of caspase-3 and -9 after hypoxia-ischemia brain injury. Although the authors concluded that HBO-PC is an effective, safe and non-invasive therapy for neonatal HI, it is hard to administrate HBO-PC in clinical practice.
Ischemic stroke
Ischemic stroke is a leading cause of disability and is considered now the fourth leading cause of death (Hafez et al., 2014). Ischemic stroke is characterized by the sudden loss of blood circulation to an area of the brain, resulting in the loss of neurologic function. About 85% of strokes are ischemic strokes and there are two major forms of ischemic stroke: focal and global ischemia.
Focal cerebral ischemia
Focal ischemia occurs due to a blockage of a brain blood vessel, usually the middle cerebral artery, and results in sudden brain dysfunction; this represents the majority of human ischemic stroke. Currently, tissue plasminogen activator (tPA) is the only Food and Drug Administration-approved treatment for acute ischemic stroke (No authors listed, 1995). However, tPA has several limitations, first, a short therapeutic time window restricts the treatment use to only about 2–5% of stroke patients (Brainin et al., 2007) and creates a demand for the development of new neuroprotective therapies. While many treatments have been effective in research, but neuroprotectants have failed in clinical trials thus far. Recent trials, with approaches focusing on preconditioning, postconditioning, and hypothermia, have been shown to be neuroprotective and have shifted the development of treatments for brain ischemia.
The first pre-clinical study of HBO-PC in focal brain ischemia, conducted by Xiong et al. (2000), proved that HBO-PC induced ischemic tolerance after transient but not permanent middle cerebral artery occlusion (MCAO) rats in a “dose-dependent” manner; 5 days of HBO-PC was more effective compared with 3 days of HBO-PC. Several years later, Sun's group published three studies investigating the molecular mechanisms underlying the HBO-PC-induced neuroprotection after MCAO. They demonstrated that the HBO-PC-induced neuroprotection is mediated, at least partly, by up-regulation of hypoxia-inducible factor-1α (HIF-1α) and its target gene erythropoietin (EPO) (Gu et al., 2008). Furthermore, HBO-PC increased the activity of antioxidant enzymes, catalase (CAT) and superoxide dismutase (SOD) (Li et al., 2008a), and suppressed mitochondrial apoptotic pathways (Li et al., 2009). On the other hand, Gao-Yu et al. (2011) reported that HBO-PC stabilized the glucose level and decreased both the lactate/pyruvate ratio and glycerol in the peri-infarct area, as well as inhibited the increase of the glutamate level. The enhancement of energy metabolism and decrease of glutamate level are factors contributing to the neuroprotective property of HBO-PC. Recently, Yan et al. (2011) reported two new mechanisms involved in HBO-PC-induced protection after MCAO in rats. They found that HBO-PC increased the protein expression of microtubule-associated protein 1 light chain 3-II (LC3-II) and Beclin 1, as well as both mRNA expression and protein level of sirtuin1 (Sirt1). HBO-PC induced tolerance to cerebral ischemia/reperfusion through promoting autophagy and decreasing of apoptosis after MCAO.
The effects of HBO-PC were also investigated in rat models of forebrain ischemia (Hirata et al., 2007; Yamashita et al., 2009). A previous study demonstrated a dose dependent effect of HBO-PC and they proved that HBO-PC at 3.5 ATA produced stronger neuroprotective effects than at 1 or 2 ATA. The neuroprotective effects were associated with suppression of p38 phosphorylation (Yamashita et al., 2009). In another study, Hirata et al. (2007) investigated the therapeutic window of HBO-PC and demonstrated that HBO induced neuroprotection against ischemic injury if applied at 6, 12 and 24 hours, but it had no beneficial effects at 72 hours forebrain ischemia. The genes/proteins relevant to neurotrophin and inflammatory-immune system were involved in HBO-PC-induced neuroprotection.
Global cerebral ischemia
Global cerebral ischemia occurs when blood flow to the brain is drastically reduced or completely stopped. Global ischemia can be the result of a heart attack, drowning, suffocation, or any sort of blockage that results in lack of blood flow to the head. Thus, it could also be a consequence of cardiac arrest or happen during major surgery. Because of the high risk of neurological complications associated with cardiac surgery, coronary artery bypass grafting and carotid endarterectomy, patients scheduled for these procedures could potentially benefit from therapeutic HBO-PC. The earliest study of HBO-PC on experimental global ischemia began in 2008 in John H. Zhang's lab. The lab demonstrated that in four-vessel occlusion model in rats, five sections of HBO-PC had significantly larger beneficial effects than 3 HBO-PC treatments. HBO-PC improved neurobehavioral scores and reduced the number of early apoptotic cells by increasing the level of brain brain-derived neurotrophic factor (BDNF) and by suppression of p38 activation (Ostrowski et al., 2008). Two years later, the same group published two more papers, investigating the molecular mechanisms underlying HBO-PC-induced neuroprotection in a four-vessel occlusion model of global cerebral ischemia. Ostrowski et al. (2010) found the mechanism of HBO-PC is dependent on the induction of matrix metalloproteinase-9 (MMP-9) in the pre-ischemic phase and is, at least, partly mediated by exhaustion of MMP-9 stores in cerebral tissues. Another study by Cheng et al. (2011) reported that HBO preconditioning reduced the expression of cyclooxygenase-2 (COX-2) and neuronal apoptosis at 1 and 3 days after global ischemia and suggested COX-2 is a mediator of HBO-PC in the ischemic brain. The data of these studies demonstrated HBO-PC is neuroprotective for global ischemia and might be an alternative strategy for the patients, which will undergo major surgeries.
In conclusion, the positive findings in the experimental studies of HBO-PC on brain ischemia indicate that HBO-PC may have neuroprotective potentials in the clinic. Patients who have pre-existing vascular diseases in brain or heart and will undergo procedures resulting in temporary clipping of major intracranial vessels might have a more important role to take HBO-PC.
Hemorrhagic transformation
Hemorrhagic transformation (HT) is a multifactorial phenomenon in which ischemic brain tissue converts into a hemorrhagic lesion with blood-vessel leakage, extravasation, and further brain injury (Wang and Lo, 2003). Approximately 10–40% of all ischemic stroke patients suffer spontaneous HT. HT is the major complication of treatment with the only one FDA-approved medication for acute ischemic stroke, treatment with tPA (Hafez et al., 2015). Many efforts have been made in searching for the effective treatment of HT. Recently, HBO-PC has been shown as a promising strategy. The combination of HBO with tPA treatment decreased incidence of hemorrhagic transformation compared with tPA treatment alone (Kuppers-Tiedt et al., 2011). Molecular mechanisms underlying this effect were not investigated but results clearly have significant clinical relevance. Well in agreement with this finding, several studies from John H. Zhang's lab demonstrated the neuroprotective effects of HBO-PC in HT after MCAO (Soejima et al., 2012, 2013; Bian et al., 2015; Guo et al., 2015). They revealed that molecular mechanisms of HBO-PC were mediated by decreasing the level of HIF-1α (Soejima et al., 2013), increasing 15d-PGJ2 (Bian et al., 2015) and activating reactive oxygen species/thioredoxin-interacting protein/nod-Like receptor protein 3 (NLRP3) pathway (Guo et al., 2015).
In light of the positive results of these studies, HBO-PC appears to be a promising treatment for HT, especially as an adjunctive treatment combined with tPA to prevent HT. Further studies are needed to confirm the effects of HBO-PC during tPA infusion and explore the clinic translation.
Intracerebral hemorrhage (ICH)
Non-traumatic intracerebral hemorrhage results from rupture of blood vessels in the brain. Multiple mechanisms, including early hematoma enlargement, mass effect, and iron-induced neuronal toxicity, contribute to brain damage following ICH (Pandey and Xi, 2014). In animals, ischemic preconditioning can protect the brain by decreasing brain edema through activating p44/42 mitogen-activated protein kinases (MAPKs) and upregulation of HO-1 after ICH (He et al., 2012). HBO-PC has been used in experimental ICH models. Qin et al. (2007, 2008) compared single and multiple HBO-PC in ICH rats and found that five sessions of HBO-PC significantly reduced perihematomal edema 24 and 72 hours after ICH. Recently Fang et al. (2015)showed HBO-PC might down-regulate AQP-4 expression to reduce the intracerebral edema in ICH rats. Ongoing research will continue to investigate the mechanisms of HBO-PC-induced neuroprotection, and possibly expand HBO-PC use for ICH patients.
Surgical brain injury (SBI)
There are over 800,000 cranial and spinal neurosurgical operations performed each year in the United States. Neurosurgical procedures cause inevitable brain damage resulting from the procedure itself. Unavoidable cortical and parenchymal incisions, intraoperative hemorrhage, brain lobe retraction and thermal injuries from electrocautery cause brain injuries attributable exclusively to the neurosurgical operations and collectively referred as SBI (Frontczak-Baniewicz et al., 2011). SBI causes localized neuronal cell death, oxidative stress, inflammation, brain edema and BBB disruption (Di et al., 2008). Presently, there are no therapeutic regimens used to prevent SBI during neurosurgical procedures. However, results of pre-clinical studies on different models of brain injury and the fact that the time of injury by SBI is well predictable make preconditioning very lucrative. Jadhav et al. (2010) showed that HBO-PC at 2.5 ATA for 1 hour for 5 consecutive days attenuated post-operative brain edema and improved neurological outcomes following SBI in mice; they also proved that the COX-2 inhibitor effectively blocked neuroprotection induced by HBO-PC (Jadhav et al., 2009). They suggested HBO preconditioned the brain by increasing COX-2 expression/activation to sub-injurious levels. Ongoing research will continue to investigate the efficacy of HBO-PC in patients who intent to have vascular neurosurgery or surgical procedures with a high risk of cerebral ischemia, and possibly expand HBO-PC not only in neurosurgery but also in other surgery fields.
Subarachnoid hemorrhage (SAH)
SAH, predominantly caused by a ruptured aneurysm, is a devastating neurological disease that has a morbidity and mortality rate higher than 50% (King, 1997). A ruptured aneurysm brings on many physiological derangements such as increased intracranial pressure (ICP), decreased cerebral blood flow (CBF), and global cerebral ischemia. These events initiate secondary injuries such as blood-brain barrier disruption, inflammation, and oxidative cascades that all ultimately lead to cell death (Tso and Macdonald, 2014). Despite advances in diagnosis and surgical treatment of SAH, effective therapeutic interventions are still limited and clinical outcomes remain disappointing. Most studies suggest a beneficial role of HBO treatment in experimental SAH previously (Ostrowski et al., 2005, 2006). However, no experimental studies have been conducted to investigate the neuroprotective effects of HBO-PC on SAH.
Traumatic brain injury (TBI)
TBI is defined as a severe intracranial injury due to external impact force. In the United States it has been estimated that more than 1.7 million individuals suffer a TBI annually (Coronado et al., 2011). TBI is a highly complex disorder that includes varying degrees of contusion, diffuse axonal injury, hemorrhage and hypoxia (Saatman et al., 2008). Collectively, these effects induce biochemical and metabolic changes that lead to progressive tissue damage and associated cell death (McIntosh et al., 1996). Although preclinical studies have suggested many promising pharmacological agents, more than 30 phase III prospective clinical trials have failed to show significance (Schouten, 2007). Emerging non-pharmacological approaches such as pre/post-conditioning or exercise have shown neuroprotective in experimental TBI studies. Hu and his team proved that HBO-PC improved neurological deficits and attenuated pathological injuries by increasing regional cerebral blood flow and brain tissue oxygen pressure in rats at high altitude (Hu et al., 2010). They further proved that suppression of the expression of MMP-9 is another mechanism for HBO-PC in TBI rats (Hu et al., 2008).
Clinical trials of HBO-PC in neuroprotection
Despite the increasing number of pre-clinical studies on HBO-PC in neuroprotection, studies describing HBO-PC in the clinical practice of acute brain injury remain scarce. To date, only a few studies have investigated the preconditioning effects of HBO in the human brain and myocardium. Alex et al. (2015) observed that repetitive pretreatment with 3 sessions of HBO (2.4 ATA, 30 minutes each time) before on-pump CABG surgery reduced neuropsychometric dysfunction and modulated the inflammatory response after cardiopulmonary bypass. Li et al. reported that preconditioning with repeated HBO (2.0 ATA, 35 minutes, twice daily for 5 consecutive days) suppressed the elevation of biomarkers of neurologic injury and improved clinical outcomes in Chinese patients undergoing on-pump CABG surgery by increasing the antioxidant activity of catalase (Bahjat et al., 2013). However, no clinical implications of HBO-PC in human neurological disease have been conducted presently. Further randomized, double-blinded and placebo-controlled studies are needed by other investigators to confirm these findings. Application of HBO-PC before surgery may be a direction in further clinical studies.
Potential mechanisms of HBO-PC
Experimental studies on HBO-PC have clarified the diverse adaptive mechanisms leading to HBO-PC induced neuroprotection. It appears that many of the pathways work in parallel, or together, to induce preconditioning in the brain. These factors include: 1) generation reactive oxygen species (ROS) and inhibiting inflammatory responsiveness; 2) induction of HIF-1α and its target genes; 3) decreasing apoptosis and promoting neuron survival; 4) other mechanisms, for example increasing oxygen pressure in brain tissue, promoting autophagy, and stimulating factors with neuroprotective properties. The identification of intrinsic cell survival pathways should provide more direct opportunities for HBO-PC in translational neuroprotection trials. For the purpose of this review, a brief summary of the recent discoveries in the mechanism of HBO-PC will be discussed. Most recent exciting discoveries in animal models were listed in Table 1, potential mechanisms involved in HBO-PC were summarized in Figure 1.
Table 1.
HBO-PC in animal models of acute brain injury
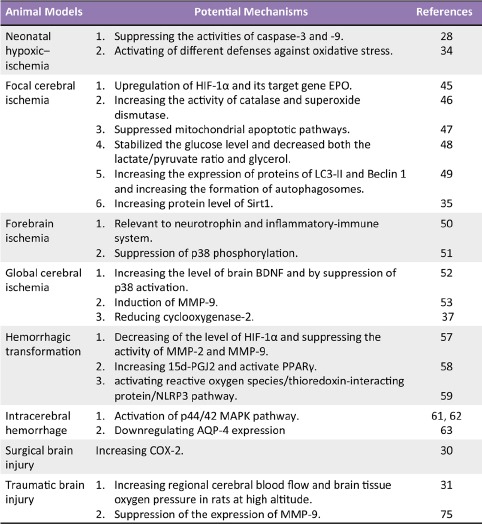
Figure 1.
Potential mechanisms involved in neruoprotection of HBO-PC.
Generation of ROS and inflammation suppression
Oxidative stress is essentially an imbalance between the production of free radicals and the ability of the body to counteract or detoxify their harmful effects through neutralization by antioxidants (Rahal et al., 2014). Increasing evidence suggests that oxidative stress and inflammation are closely linked phenomena in the pathophysiology of both acute brain injury (Chan, 1996; Collino et al., 2006; Wong and Crack, 2008) and neurodegenerative diseases (Smith et al., 1991; Mecocci et al., 1994; Gonfloni et al., 2012; Koppula et al., 2012). Numerous experimental and clinical observations have shown increased free radical formation during all forms of stroke, especially in ischemia/reperfusion injury (Chan, 1996). Several enzymes, including SOD, glutathione peroxidase (GSHPx), glutathione reductase, and CAT, are endogenous antioxidants that process specific free radical scavenging properties. Reoxygenation during reperfusion provides oxygen to sustain neuronal viability and also provides oxygen as a substrate for numerous enzymatic oxidation reactions that produce reactive oxidants. In addition, reflow after occlusion often causes an increase in oxygen to levels that cannot be utilized by mitochondria under normal physiological flow conditions. The overproduction of oxygen radicals, inactivation of detoxification systems, consumption of antioxidants, and failure to adequately replenish antioxidants in the ischemic brain tissue resulted in accumulation of reactive oxidants and damage to brain tissue.
It is well accepted that exposure to HBO will increase production of ROS (Benedetti et al., 2004; Korkmaz et al., 2008; Thom, 2009; Matsunami et al., 2011; Gasier and Fothergill, 2013). ROS are produced intracellularly through multiple mechanisms and their production depends on the cell and tissue types. The major source of ROS is from mitochondria (Adam-Vizi, 2005). Under normal conditions, mitochondria reduce O2 completely to H2O through oxidative phosphorylation. When exposed to HBO, oxygen is instead prematurely and incompletely reduced to give the superoxide anion (•O2–). Superoxide anion is not particularly reactive by itself, but can inactivate specific enzymes or initiate lipid peroxidation in its protonated form, hydroperoxyl HO2•. Superoxide anion also rapidly dismutates to hydrogen peroxide (H2O2) either spontaneously or enzymatically via manganese SOD. HBO has been shown to elevate O2 partial pressure and increase mitochondrial generation of H2 O2 in pigeon hearts (Boveris and Chance, 1973). In rats, HBO has been shown to increase oxidative stress (Zhang et al., 2010; Guo et al., 2015). In vitro, Conconi et al. (2003) showed that exposure to HBO at 2.5 ATA enhanced ROS production in cultured fibroblasts.
Another important reason for ROS accumulation after HBO exposure is the dysfunction of antioxidant defense system. Many studies have reported the alteration of enzymatic antioxidant activity after HBO exposure (Camporesi and Bosco, 2014). Gregorevic et al. (2001) observed that acute HBO exposure (60 mm at 3 atmospheres absolute) reduced CAT activity by approximately 51% in rat skeletal muscles, and that repeated HBO (twice daily for 28 days) increased SOD activity by approximately 241%. Harabin et al. (1990) in their experimental study regarding the effects of HBO on oxidative parameters in both rats and guinea pigs, used continuous and intermittent (10 minutes of O2 followed by 2.5 minutes of ambient air) exposure to 2.8 ATA HBO. They reported a slight increase in SOD and decrease in GSHPx and CAT in both the lung and the brain that was more marked in guinea pigs.
The increased ROS can serve as signaling molecules in transduction cascades, or pathways, for a variety of inflammatory cytokines. The pre-increased inflammatory cytokines by HBO-PC will exhaust the reservation of innate inflammatory cytokines and generate tolerance against subsequent brain injury. HBO-PC involves the induction of COX-2 in cerebral tissues before ischemia, which leads to a suppression of COX-2 and its downstream targets after global ischemic insult (Cheng et al., 2011). Qi et al. (2013) reported HBO-PC reduced interleukin-1, tumor necrosis factor-α, and interleukin-6 levels and increased skin flap survival in rats. HBO-PC could protect rat spinal neurons in vitro against oxidative injury and oxygen-glucose deprivation (OGD) by up-regulating the expression of heat shock protein-32 (HSP-32) (Huang et al., 2014), and by increasing expression of HO-1 (Li et al., 2007).
HIF-1α and its target genes
HIF-1α is a transcription factor specifically activated by hypoxia. Under normoxic conditions, HIF-1α is constitutively transcribed and translated. However, the stability of the protein is drastically reduced by the hydroxylation of HIF-1α at prolines 402 and 564 by HIF-1α prolyl hydroxylases. The prolyl hydroxylases, which are involved in regulating HIF-1α stability, are oxygen-dependent (Maxwell, 2004). Hydroxylated HIF-1α recruits the E3-ubiquitin ligase Von Hippel Lindau protein, which tags HIF-1α with ubiquitin groups and targets it for degradation by the proteasome. Under hypoxic conditions, prolyl hydroxylases function with low efficiency, resulting in HIF-1α that is not hydroxylated and cannot interact with Von Hippel–Lindau (VHL) protein. The stable HIF-1α can then bind to its heterodimeric partner HIF-1β, and together these proteins can act in the nucleus to transactivate genes involved in adaptation to hypoxic-ischemic stress.
HBO has been demonstrated to increase the expression HIF-1α (Salhanick et al., 2006; Duan et al., 2015; Sunkari et al., 2015). However, the mechanisms of HBO-PC-induced HIF-1α accumulation remain to be established. There are, however, some hypotheses about it. First, exposure to HBO increased arterial oxygen tension and arterial O2 pressure (PaO2) in tissues (Tibbles and Edelsberg, 1996; Bai et al., 2009). After HBO-PC, brain tissues will experience a relative hypoxia because the oxygen level is reduced to normal level at 21%. Repeating HBO-PC may produce a cycle of hyperoxia and then hypoxia and lead to HIF-1α accumulation. Another possible mechanism for HIF-1α accumulation is the generation of ROS by HBO-PC. HBO induced HIF-1α accumulation may involve the formation of ROS that are produced during HBO exposure. ROS could regulate HIF-1α by activation of PI3-K/PKB (Gao et al., 2004) and MAPK (Wang et al., 2004). ROS may also have the potential to inhibit the activity of prolyl hydroxylases (Metzen et al., 2003).
HIF-1α induces the expression of hundreds of gene products in response to hypoxia or ischemia, and plays an important role in neuroprotection after brain injury. HIF-1α activates the transcription of several genes involved in angiogenesis, glycolysis, inflammation, proliferation and growth, which collectively initiate cell survival mechanisms under ischemic/hypoxic injury. Semenza et al. (1991) reported that EPO was the first target gene for HIF-1α. Gu et al. (2008) showed HBO-PC induced a marked increase of HIF-1α and EPO after focal cerebral ischemia. Vascular endothelial growth factor (VEGF) is the most potent angiogenic factor and one of the downstream target genes of HIF-1α. Upregulation of HIF-1α and VEGF promote endothelia cells proliferation and remediation of brain injury after stroke (Mu et al., 2003). In addition, HIF-1α regulates genes involved in governing inflammation such as HO-1 (Dawn and Bolli, 2005), which has immunomodulatory and anti-inflammatory properties (Paine et al., 2010). Moreover, HIF-1α induces genes involved in growth such as insulin-like growth factor-2 (IGF-2) (Feldser et al., 1999), transforming growth factor-α (TGF-α) (Krishnamachary et al., 2003) and BDNF (Avramovich-Tirosh et al., 2010). Binding of such growth factors to their cognate receptors activates signal transduction pathways that lead to cell survival and stimulates the expression of HIF-1α itself (Sullivan et al., 2008).
Inhibition of apoptosis
Apoptosis is the term given to programmed cell death, which has been proved to play an important role in neuron loss after brain injury. There are two general pathways of apoptosis: the intrinsic pathway which originates from mitochondrial release of cytochrome c and associated stimulation of caspase-3, and the extrinsic pathway which originates from the activation of cell surface death receptors and subsequent stimulation of caspase-8 (Broughton et al., 2009). Several studies have shown that HBO-PC prevents apoptosis in experimental ischemic models (Wang et al., 2010; Lu et al., 2013). Lou et al. (2006) found that HBO therapy prevented apoptosis and promoted neurological functional recovery after focal cerebral ischemia by opening mitochondrial ATP-sensitive potassium channel. Simultaneously, Li et al. (2009) showed that HBO-PC reduced cytochrome c and decreased caspase-9 and caspase-3 in the hippocampus and ischemic penumbra in an MCAO models. They concluded HBO-PC protected brain tissues from ischemia/reperfusion injury by suppressing mitochondrial apoptotic pathways. Ostrowski et al. (2009) reported HBO-PC both inhibited early apoptosis in a transient global cerebral ischemia rat model through decreasing phosphorylated p38 and increased the expression of BDNF. Additionally, HSP-70 can also be involved in anti-apoptosis mechanism of HBO-PC. HSP-70 overexpression reduced ischemic injury and protected both neurons and glial cells, which attributed to the prevention of protein aggregation, refolding of partially denatured proteins, reduction of inflammatory responses, and inhibition of cell death pathways (Brown, 2007). Vince et al. (2011) recently noted two circles of 20 minutes of HBO-PC did not induce the expression of HSP-70 in circulating blood cells. That could be, however, because the chosen regime of HBO was not potent enough to induce the stress response.
Other mechanisms
The neuroprotective mechanisms of HBO-PC are complicated. There are many other mechanisms underlying HBO-PC in neuroprotection. In focal brain ischemia models, HBO-PC was demonstrated to be beneficial by stabilizing the glucose level and decreasing both the lactate/pyruvate ratio and glycerol (Gao-Yu et al., 2011). HBO-PC increased the formation of autophagosomes by increasing the expression of proteins of LC3-II and Beclin-1 (Yan et al., 2011), and inhibited apoptosis through up-regulating the protein level of Sirt1 (Yan et al., 2013). In hyperglycemia-induced HT, HBO-PC reduced bleeding volume via increase of 15d-PGJ2 and activation of PPARγ (Bian et al., 2015). HBO-PC also was found to increase regional cerebral blood flow and brain tissue oxygen pressure after traumatic brain injury (Hu et al., 2010). To date, the mechanisms of neuroprotection involved in HBO-PC are still elusive and therefore worthy of further study.
Regime of HBO-PC
Pressure, frequency, and period of time of HBO are critical factors that decide the efficacy of preconditioning. Yamashita et al. (2009) tested HBO-PC at 1, 2, and 3.5 ATA and found 3.5 ATA produced more pronounced protective results compared with 1 or 2 ATA. Because 3 ATA is the upper limit in clinical practice for the oxygen toxicity (Wolfe and DeVries, 1975), the most used pressure is 2–3 ATA. The duration of each HBO exposure ranges from 60 to 90 minutes and the sessions for HBO-PC vary from 1 to 6 in different studies. Prolonged HBO exposure is unacceptable due to the central nervous system oxygen toxicity and impracticality (Hampson and Atik, 2003).
The time window between the last HBO-PC and the subsequent brain insult is another critical factor that affects the efficiency. The therapeutic effects of preconditioning wane within hours and days after the stimulus. Hirata et al. (2007) investigated the therapeutic time window of HBO-PC against ischemic brain injury and found that HBO-PC at 3.5 ATA for 1 hour for 5 consecutive days is neuroprotective at 6, 12 and 24 but not at 72 hours after last HBO session. In present, the interval of 24 hours is the most commonly applied in HBO-PC.
Another concern is the efficiency of different HBO-PC paradigms. A short-lasting protective phenotype can be induced within minutes of exposure to HBO and requires long-time and multiple exposure to become fully manifest. Longer-term HBO-PC paradigms appear to be more effective than acute paradigms in establishing of brain tolerance following injury. Ostrowski et al. (2008) compared 3 HBO-PC (2.5 ATA for 1 hour at 24, 12 and 4 hours before ischemic insult) with 5 HBO-PC (2.5 ATA for 1 hour for 5 consecutive days) and showed preconditioning with 5 HBO treatments proved more beneficial than with 3 HBO. Xiong et al. (2000) also reported HBO-PC induced ischemic tolerance in transient MCAO rats in a “dose-dependent” manner and showed that 1 hour of HBO at 2.5 ATA every day for 5 days was more effective than for 3 days. Currently, the commonly used regime of HBO-PC in rodents is at 2.5 ATA for 1 hour for 5 consecutive days (Xiong et al., 2000; Jadhav et al., 2009; Li et al., 2009; Cheng et al., 2011; Soejima et al., 2013) or at 2.5 ATA for 1 hour twice every day for 2 days (Li et al., 2008a, 2009). In clinical application, preconditioning for 5 days might be problematic for the practical reasons. Recently, 3 HBO treatments applied within 24 hours before anticipated brain insult established a clinically effective preconditioning regimen (Alex et al., 2005).
Current issues and future directions
Despite the promising pre-clinical studies outlined above, the translation of evidentiary experimental results of HBO-PC into clinical implementation remains difficult. Several issues need to be addressed before routinely recommending HBO-PC as additional therapy in clinical practice.
First, in clinical practice, HBO-PC is mainly applied in aged subjects with high risk for neurological diseases; pre-clinical studies, however, typically use healthy and young adult rodents to mimic these diseases in animal models. These models do not mimic common comorbidities such as hypertension, and diabetes, which could elicit endogenous neuroprotection and reduce the effects of preconditioning (Wang et al., 2002; Purcell et al., 2003). Indeed aged animals do not respond to preconditioning as well as younger animals do, suggesting that age is an important limitation to translate results of pre-clinical HBO-PC studies into clinical situations (He et al., 2005). Therefore, the applicability of experimental findings should be re-evaluated before HBO-PC is widely used in the clinic.
Second, tailoring HBO-PC to the patient population will be critical. Although hyperbaric oxygen therapy is usually well tolerated and has few side effects, subpopulations of patients may respond differently to HBO-PC stimuli. Such factors as gender, aging, smoking, diabetes and hypertension need to be taken into consideration before use of HBO-PC on patients. Some patients with diabetes experience a rapid drop in blood glucose during HBO exposure (Plafki et al., 2000). Prolonged exposure to HBO can be detrimental to the lungs (Clark and Lambertsen, 1971; You et al., 2014). In addition, HBO can be toxic to the central nervous system and result in seizure activity (Hampson and Atik, 2003). Thus, the optimal pressure, duration as well as numbers of HBO sessions need to be specified to avoid undesirable effects (Hu et al., 2015).
Third, one of the inherent weaknesses of preconditioning is that the preconditioning response usually fades within a week or two and has a very narrow therapeutic window. Preconditioning is best suited to clinical situations with predictable brain damage such as coronary artery bypass grafting, carotid endarterectomyor neurosurgery. Application of HBO-PC during tPA infusion or before surgery may be a direction in further clinical studies.
CONCLUSIONS
Though much progress has been made in medicine and technology, there is no effective therapy for neuroprotection after brain injury in clinic. However, as a promising nondrug and noninvasive treatment, HBO-PC represents safe, well-tolerated and feasible therapy for brain injury. HBO-PC shows great potential in patients who intent to have vascular neurosurgery or surgical procedures with a high risk of cerebral ischemia. Furthermore, HBO-PC combined with thrombolysis seems promising in reducing secondary hemorrhage of ischemic stroke patients, which is worthy of further studies. It is important to conduct clinical trials, based on solid and rigorous preclinical testing, and to design trials using optimized HBO-PC protocols. Further preclinical testing is needed to make HBO-PC a reliable option for brain injury and other neurology diseases.
Abbreviations
HBO-PC: hyperbaric oxygen preconditioning; CABG: coronary artery bypass graft; HBO: hyperbaric oxygen; HI: hypoxic-ischemia; ATA: atmospheres absolute; tPA: tissue plasminogen activator; MCAO: middle cerebral artery occlusion; MAPKs: mitogen-activated protein kinases; HIF-1α: hypoxia-inducible factor-1α; EPO: erythropoietin; CAT: catalase; SOD: superoxide dismutase; LC3-II: microtubuleassociated protein 1 light chain 3-II; Sirt1: sirtuin1; siRNA: short interfering RNA; BDNF: brain-derived neurotrophic factor; MMP-9: matrix metalloproteinase-9; COX-2: cyclooxygenase-2; HT: hemorrhagic transformation; NLRP3: nod-Like receptor protein 3; HO-1: heme oxygenase-1; PPARγ: peroxisome proliferator-activated receptor γ; ICH: intracerebral hemorrhage; SBI: surgical brain injury; SAH: subarachnoid hemorrhage; ICP: intracranial pressure; CBF: decreased cerebral blood flow; TBI: traumatic brain injury; ROS: reactive oxygen species; GSHPx: glutathione peroxidase; OGD: oxygen-glucose deprivation; HSP-32: heat shock protein-32; PaO2: arterial O2 pressure; VEGF: vascular endothelial growth factor; IGF-2: insulin-like growth factor-2; TGF-α: transforming growth factor-α.
Footnotes
Conflicts of interest
The authors declared that there is no conflict of interests regarding the publication of this paper.
REFERENCES
- Randomised, double-blind, placebo-controlled trial of nimodipine in acute stroke. Trust Study Group. Lancet. 1990;336:1205–1209. [PubMed] [Google Scholar]
- Tissue plasminogen activator for acute ischemic stroke. The National Institute of Neurological Disorders and Stroke rt-PA Stroke Study Group. N Engl J Med. 1995;333:1581–1587. doi: 10.1056/NEJM199512143332401. [DOI] [PubMed] [Google Scholar]
- Adam-Vizi V. Production of reactive oxygen species in brain mitochondria: contribution by electron transport chain and non-electron transport chain sources. Antioxid Redox Signal. 2005;7:1140–1149. doi: 10.1089/ars.2005.7.1140. [DOI] [PubMed] [Google Scholar]
- Alex J, Laden G, Cale AR, Bennett S, Flowers K, Madden L, Gardiner E, McCollum PT, Griffin SC. Pretreatment with hyperbaric oxygen and its effect on neuropsychometric dysfunction and systemic inflammatory response after cardiopulmonary bypass: a prospective randomized double-blind trial. J Thorac Cardiovasc Surg. 2005;130:1623–1630. doi: 10.1016/j.jtcvs.2005.08.018. [DOI] [PubMed] [Google Scholar]
- Alkhulaifi AM, Yellon DM, Pugsley WB. Preconditioning the human heart during aorto-coronary bypass surgery. Eur J Cardiothorac Surg. 1994;8:270–275. doi: 10.1016/1010-7940(94)90159-7. [DOI] [PubMed] [Google Scholar]
- Avramovich-Tirosh Y, Bar-Am O, Amit T, Youdim MB, Weinreb O. Up-regulation of hypoxia-inducible factor (HIF)-1alpha and HIF-target genes in cortical neurons by the novel multifunctional iron chelator anti-Alzheimer drug, M30. Curr Alzheimer Res. 2010;7:300–306. doi: 10.2174/156720510791162403. [DOI] [PubMed] [Google Scholar]
- Bahjat FR, Gesuete R, Stenzel-Poore MP. Steps to translate preconditioning from basic research to the clinic. Transl Stroke Res. 2013;4:89–103. doi: 10.1007/s12975-012-0223-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai X, Sun B, Pan S, Jiang H, Wang F, Krissansen GW, Sun X. Down-regulation of hypoxia-inducible factor-1alpha by hyperbaric oxygen attenuates the severity of acute pancreatitis in rats. Pancreas. 2009;38:515–522. doi: 10.1097/MPA.0b013e31819cac24. [DOI] [PubMed] [Google Scholar]
- Benedetti S, Lamorgese A, Piersantelli M, Pagliarani S, Benvenuti F, Canestrari F. Oxidative stress and antioxidant status in patients undergoing prolonged exposure to hyperbaric oxygen. Clin Biochem. 2004;37:312–317. doi: 10.1016/j.clinbiochem.2003.12.001. [DOI] [PubMed] [Google Scholar]
- Bertoletto PR, Chaves JC, Fagundes AT, Simoes RS, Oshima CT, Simoes Mde J, Fagundes DJ. Effect of different periods of hyperbaric oxygen on ischemia-reperfusion injury of rat small bowel. Acta Cir Bras. 2008;23:11–15. doi: 10.1590/s0102-86502008000100003. [DOI] [PubMed] [Google Scholar]
- Biagas K. Hypoxic-ischemic brain injury: advancements in the understanding of mechanisms and potential avenues for therapy. Curr Opin Pediatr. 1999;11:223–228. doi: 10.1097/00008480-199906000-00009. [DOI] [PubMed] [Google Scholar]
- Bian H, Hu Q, Liang X, Chen D, Li B, Tang J, Zhang JH. Hyperbaric oxygen preconditioning attenuates hemorrhagic transformation through increasing PPARgamma in hyperglycemic MCAO rats. Exp Neurol. 2015;265:22–29. doi: 10.1016/j.expneurol.2014.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brainin M, Teuschl Y, Kalra L. Acute treatment and long-term management of stroke in developing countries. Lancet Neurol. 2007;6:553–561. doi: 10.1016/S1474-4422(07)70005-4. [DOI] [PubMed] [Google Scholar]
- Broughton BR, Reutens DC, Sobey CG. Apoptotic mechanisms after cerebral ischemia. Stroke. 2009;40:e331–339. doi: 10.1161/STROKEAHA.108.531632. [DOI] [PubMed] [Google Scholar]
- Brown IR. Heat shock proteins and protection of the nervous system. Ann N Y Acad Sci. 2007;1113:147–158. doi: 10.1196/annals.1391.032. [DOI] [PubMed] [Google Scholar]
- Camporesi EM, Bosco G. Hyperbaric oxygen pretreatment and preconditioning. Undersea Hyperb Med. 2014;41:259–263. [PubMed] [Google Scholar]
- Chan PH. Role of oxidants in ischemic brain damage. Stroke. 1996;27:1124–1129. doi: 10.1161/01.str.27.6.1124. [DOI] [PubMed] [Google Scholar]
- Cheng O, Ostrowski RP, Wu B, Liu W, Chen C, Zhang JH. Cyclooxygenase-2 mediates hyperbaric oxygen preconditioning in the rat model of transient global cerebral ischemia. Stroke. 2011;42:484–490. doi: 10.1161/STROKEAHA.110.604421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark JM, Lambertsen CJ. Pulmonary oxygen toxicity: a review. Pharmacol Rev. 1971;23:37–133. [PubMed] [Google Scholar]
- Collino M, Aragno M, Mastrocola R, Benetti E, Gallicchio M, Dianzani C, Danni O, Thiemermann C, Fantozzi R. Oxidative stress and inflammatory response evoked by transient cerebral ischemia/reperfusion: effects of the PPAR-alpha agonist WY14643. Free Radic Biol Med. 2006;41:579–589. doi: 10.1016/j.freeradbiomed.2006.04.030. [DOI] [PubMed] [Google Scholar]
- Conconi MT, Baiguera S, Guidolin D, Furlan C, Menti AM, Vigolo S, Belloni AS, Parnigotto PP, Nussdorfer GG. Effects of hyperbaric oxygen on proliferative and apoptotic activities and reactive oxygen species generation in mouse fibroblast 3T3/J2 cell line. J Investig Med. 2003;51:227–232. doi: 10.1136/jim-51-04-24. [DOI] [PubMed] [Google Scholar]
- Coronado VG, Xu L, Basavaraju SV, McGuire LC, Wald MM, Faul MD, Guzman BR, Hemphill JD. Centers for Disease Control and Prevention (CDC). Surveillance for traumatic brain injury-related deaths--United States, 1997-2007. MMWR Surveill Summ. 2011;60:1–32. [PubMed] [Google Scholar]
- Dawn B, Bolli R. HO-1 induction by HIF-1: a new mechanism for delayed cardioprotection? Am J Physiol Heart Circ Physiol. 2005;289:H522–524. doi: 10.1152/ajpheart.00274.2005. [DOI] [PubMed] [Google Scholar]
- Dembinski A, Warzecha Z, Ceranowicz P, Tomaszewska R, Dembinski M, Pabianczyk M, Stachura J, Konturek SJ. Ischemic preconditioning reduces the severity of ischemia/reperfusion-induced pancreatitis. Eur J Pharmacol. 2003;473:207–216. doi: 10.1016/s0014-2999(03)01994-0. [DOI] [PubMed] [Google Scholar]
- Di F, Yan-Ting G, Hui L, Tao T, Zai-Hua X, Xue-Ying S, Hong-Li X, Yun-Jie W. Role of aminoguanidine in brain protection in surgical brain injury in rat. Neurosci Lett. 2008;448:204–207. doi: 10.1016/j.neulet.2008.10.038. [DOI] [PubMed] [Google Scholar]
- Dirnagl U, Becker K, Meisel A. Preconditioning and tolerance against cerebral ischaemia: from experimental strategies to clinical use. Lancet Neurol. 2009;8:398–412. doi: 10.1016/S1474-4422(09)70054-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong H, Xiong L, Zhu Z, Chen S, Hou L, Sakabe T. Preconditioning with hyperbaric oxygen and hyperoxia induces tolerance against spinal cord ischemia in rabbits. Anesthesiology. 2002;96:907–912. doi: 10.1097/00000542-200204000-00018. [DOI] [PubMed] [Google Scholar]
- Du ZY, Hicks M, Winlaw D, Spratt P, Macdonald P. Ischemic preconditioning enhances donor lung preservation in the rat. J Heart Lung Transplant. 1996;15:1258–1267. [PubMed] [Google Scholar]
- Duan S, Shao G, Yu L, Ren C. Angiogenesis contributes to the neuroprotection induced by hyperbaric oxygen preconditioning against focal cerebral ischemia in rats. Int J Neurosci. 2015;125:625–634. doi: 10.3109/00207454.2014.956101. [DOI] [PubMed] [Google Scholar]
- Fang J, Li H, Li G, Wang L. Effect of hyperbaric oxygen preconditioning on peri-hemorrhagic focal edema and aquaporin-4 expression. Exp Ther Med. 2015;10:699–704. doi: 10.3892/etm.2015.2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldser D, Agani F, Iyer NV, Pak B, Ferreira G, Semenza GL. Reciprocal positive regulation of hypoxia-inducible factor 1alpha and insulin-like growth factor 2. Cancer Res. 1999;59:3915–3918. [PubMed] [Google Scholar]
- Freiberger JJ, Suliman HB, Sheng H, McAdoo J, Piantadosi CA, Warner DS. A comparison of hyperbaric oxygen versus hypoxic cerebral preconditioning in neonatal rats. Brain Res. 2006;1075:213–222. doi: 10.1016/j.brainres.2005.12.088. [DOI] [PubMed] [Google Scholar]
- Frontczak-Baniewicz M, Chrapusta SJ, Sulejczak D. Long-term consequences of surgical brain injury - characteristics of the neurovascular unit and formation and demise of the glial scar in a rat model. Folia Neuropathol. 2011;49:204–218. [PubMed] [Google Scholar]
- Gao N, Shen L, Zhang Z, Leonard SS, He H, Zhang XG, Shi X, Jiang BH. Arsenite induces HIF-1alpha and VEGF through PI3K, Akt and reactive oxygen species in DU145 human prostate carcinoma cells. Mol Cell Biochem. 2004;255:33–45. doi: 10.1023/b:mcbi.0000007259.65742.16. [DOI] [PubMed] [Google Scholar]
- Gao-Yu C, Cong-Yina D, Li-Jun Z, Fei L, Hua F. Effects of hyperbaric oxygen preconditioning on energy metabolism and glutamate level in the peri-infarct area following permanent MCAO. Undersea Hyperb Med. 2011;38:91–99. [PubMed] [Google Scholar]
- Gasier HG, Fothergill DM. Oxidative stress, antioxidant defenses and nitric oxide production following hyperoxic exposures. Undersea Hyperb Med. 2013;40:125–134. [PubMed] [Google Scholar]
- Gidday JM. Cerebral preconditioning and ischaemic tolerance. Nat Rev Neurosci. 2006;7:437–448. doi: 10.1038/nrn1927. [DOI] [PubMed] [Google Scholar]
- Gonfloni S, Maiani E, Di Bartolomeo C, Diederich M, Cesareni G. Oxidative Stress, DNA Damage, and c-Abl Signaling: At the Crossroad in Neurodegenerative Diseases? Int J Cell Biol. 2012:683097. doi: 10.1155/2012/683097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregorevic P, Lynch GS, Williams DA. Hyperbaric oxygen modulates antioxidant enzyme activity in rat skeletal muscles. Eur J Appl Physiol. 2001;86:24–27. doi: 10.1007/s004210100503. [DOI] [PubMed] [Google Scholar]
- Gu GJ, Li YP, Peng ZY, Xu JJ, Kang ZM, Xu WG, Tao HY, Ostrowski RP, Zhang JH, Sun XJ. Mechanism of ischemic tolerance induced by hyperbaric oxygen preconditioning involves upregulation of hypoxia-inducible factor-1alpha and erythropoietin in rats. J Appl Physiol (1985) 2008;104:1185–1191. doi: 10.1152/japplphysiol.00323.2007. [DOI] [PubMed] [Google Scholar]
- Guo ZN, Xu L, Hu Q, Matei N, Yang P, Tong LS, He Y, Guo Z, Tang J, Yang Y, Zhang JH. Hyperbaric oxygen preconditioning attenuates hemorrhagic transformation through reactive oxygen species/thioredoxin-interacting protein/Nod-like receptor protein 3 pathway in hyperglycemic middle cerebral artery occlusion rats. Crit Care Med. 2015 doi: 10.1097/CCM.0000000000001468. [DOI] [PubMed] [Google Scholar]
- Hafez S, Coucha M, Bruno A, Fagan SC, Ergul A. Hyperglycemiaacute ischemic stroke, and thrombolytic therapy. Transl Stroke Res. 2014;5:442–453. doi: 10.1007/s12975-014-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafez S, Hoda MN, Guo X, Johnson MH, Fagan SC, Ergul A. Comparative Analysis of Different Methods of Ischemia/Reperfusion in Hyperglycemic Stroke Outcomes: Interaction with tPA. Transl Stroke Res. 2015;6:171–180. doi: 10.1007/s12975-015-0391-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampson N, Atik D. Central nervous system oxygen toxicity during routine hyperbaric oxygen therapy. Undersea Hyperb Med. 2003;30:147–153. [PubMed] [Google Scholar]
- Harabin AL, Braisted JC, Flynn ET. Response of antioxidant enzymes to intermittent and continuous hyperbaric oxygen. J Appl Physiol (1985) 1990;69:328–335. doi: 10.1152/jappl.1990.69.1.328. [DOI] [PubMed] [Google Scholar]
- He X, Xu X, Fan M, Chen X, Sun X, Luo G, Chen L, Mu Q, Feng Y, Mao Q, Chao Z. Preconditioning with hyperbaric oxygen induces tolerance against renal ischemia-reperfusion injury via increased expression of heme oxygenase-1. J Surg Res. 2011;170:e271–277. doi: 10.1016/j.jss.2011.06.008. [DOI] [PubMed] [Google Scholar]
- He Y, Karabiyikoglu M, Hua Y, Keep RF, Xi G. Ischemic preconditioning attenuates brain edema after experimental intracerebral hemorrhage. Transl Stroke Res. 2012;3:180–187. doi: 10.1007/s12975-012-0171-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Z, Crook JE, Meschia JF, Brott TG, Dickson DW, McKinney M. Aging blunts ischemic-preconditioning-induced neuroprotection following transient global ischemia in rats. Curr Neurovasc Res. 2005;2:365–374. doi: 10.2174/156720205774962674. [DOI] [PubMed] [Google Scholar]
- Hirata T, Cui YJ, Funakoshi T, Mizukami Y, Ishikawa Y, Shibasaki F, Matsumoto M, Sakabe T. The temporal profile of genomic responses and protein synthesis in ischemic tolerance of the rat brain induced by repeated hyperbaric oxygen. Brain Res. 2007;1130:214–222. doi: 10.1016/j.brainres.2006.10.077. [DOI] [PubMed] [Google Scholar]
- Hu Q, Manaenko A, Guo Z, Huang L, Tang J, Zhang JH. Hyperbaric oxygen therapy for post concussion symptoms: issues may affect the results. Med Gas Res. 2015;5:10. doi: 10.1186/s13618-015-0033-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu S, Li F, Luo H, Xia Y, Zhang J, Hu R, Cui G, Meng H, Feng H. Amelioration of rCBF and PbtO2 following TBI at high altitude by hyperbaric oxygen pre-conditioning. Neurol Res. 2010;32:173–178. doi: 10.1179/174313209X414524. [DOI] [PubMed] [Google Scholar]
- Hu SL, Hu R, Li F, Liu Z, Xia YZ, Cui GY, Feng H. Hyperbaric oxygen preconditioning protects against traumatic brain injury at high altitude. Acta Neurochir Suppl. 2008;105:191–196. doi: 10.1007/978-3-211-09469-3_37. [DOI] [PubMed] [Google Scholar]
- Huang G, Xu J, Xu L, Wang S, Li R, Liu K, Zheng J, Cai Z, Zhang K, Luo Y, Xu W. Hyperbaric oxygen preconditioning induces tolerance against oxidative injury and oxygen-glucose deprivation by up-regulating heat shock protein 32 in rat spinal neurons. PLoS One. 2014;9:e85967. doi: 10.1371/journal.pone.0085967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Islam CF, Mathie RT, Dinneen MD, Kiely EA, Peters AM, Grace PA. Ischaemia-reperfusion injury in the rat kidney: the effect of preconditioning. Br J Urol. 1997;79:842–847. doi: 10.1046/j.1464-410x.1997.00209.x. [DOI] [PubMed] [Google Scholar]
- Jadhav V, Ostrowski RP, Tong W, Matus B, Jesunathadas R, Zhang JH. Cyclo-oxygenase-2 mediates hyperbaric oxygen preconditioning-induced neuroprotection in the mouse model of surgical brain injury. Stroke. 2009;40:3139–3142. doi: 10.1161/STROKEAHA.109.549774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav V, Ostrowski RP, Tong W, Matus B, Chang C, Zhang JH. Hyperbaric oxygen preconditioning reduces postoperative brain edema and improves neurological outcomes after surgical brain injury. Acta Neurochir Suppl. 2010;106:217–220. doi: 10.1007/978-3-211-98811-4_40. [DOI] [PubMed] [Google Scholar]
- Kaste M, Fogelholm R, Erila T, Palomaki H, Murros K, Rissanen A, Sarna S. A randomized, double-blind, placebo-controlled trial of nimodipine in acute ischemic hemispheric stroke. Stroke. 1994;25:1348–1353. doi: 10.1161/01.str.25.7.1348. [DOI] [PubMed] [Google Scholar]
- Kim CH, Choi H, Chun YS, Kim GT, Park JW, Kim MS. Hyperbaric oxygenation pretreatment induces catalase and reduces infarct size in ischemic rat myocardium. Pflugers Arch. 2001;442:519–525. doi: 10.1007/s004240100571. [DOI] [PubMed] [Google Scholar]
- King JT., Jr Epidemiology of aneurysmal subarachnoid hemorrhage. Neuroimaging Clin N Am. 1997;7:659–668. [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Kuwabara K, Tagaya M, Ohtsuki T, Hata R, Ueda H, Handa N, Kimura K, Kamada T. ‘Ischemic tolerance’ phenomenon detected in various brain regions. Brain Res. 1991;561:203–211. doi: 10.1016/0006-8993(91)91596-s. [DOI] [PubMed] [Google Scholar]
- Koneru B, Fisher A, He Y, Klein KM, Skurnick J, Wilson DJ, de la Torre AN, Merchant A, Arora R, Samanta AK. Ischemic preconditioning in deceased donor liver transplantation: a prospective randomized clinical trial of safety and efficacy. Liver Transpl. 2005;11:196–202. doi: 10.1002/lt.20315. [DOI] [PubMed] [Google Scholar]
- Koppula S, Kumar H, Kim IS, Choi DK. Reactive oxygen species and inhibitors of inflammatory enzymes, NADPH oxidase, and iNOS in experimental models of Parkinson's disease. Mediators Inflamm. 2012:823902. doi: 10.1155/2012/823902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korkmaz A, Oter S, Sadir S, Topal T, Uysal B, Ozler M, Ay H, Akin A. Exposure time related oxidative action of hyperbaric oxygen in rat brain. Neurochem Res. 2008;33:160–166. doi: 10.1007/s11064-007-9428-4. [DOI] [PubMed] [Google Scholar]
- Krishnamachary B, Berg-Dixon S, Kelly B, Agani F, Feldser D, Ferreira G, Iyer N, LaRusch J, Pak B, Taghavi P, Semenza GL. Regulation of colon carcinoma cell invasion by hypoxia-inducible factor 1. Cancer Res. 2003;63:1138–1143. [PubMed] [Google Scholar]
- Kuppers-Tiedt L, Manaenko A, Michalski D, Guenther A, Hobohm C, Wagner A, Zhang JH, Schneider D. Combined systemic thrombolysis with alteplase and early hyperbaric oxygen therapy in experimental embolic stroke in rats: relationship to functional outcome and reduction of structural damage. Acta Neurochir Suppl. 2011;111:167–172. doi: 10.1007/978-3-7091-0693-8_28. [DOI] [PubMed] [Google Scholar]
- Li J, Liu W, Ding S, Xu W, Guan Y, Zhang JH, Sun X. Hyperbaric oxygen preconditioning induces tolerance against brain ischemia-reperfusion injury by upregulation of antioxidant enzymes in rats. Brain Res. 2008a;1210:223–229. doi: 10.1016/j.brainres.2008.03.007. [DOI] [PubMed] [Google Scholar]
- Li JS, Zhang W, Kang ZM, Ding SJ, Liu WW, Zhang JH, Guan YT, Sun XJ. Hyperbaric oxygen preconditioning reduces ischemia-reperfusion injury by inhibition of apoptosis via mitochondrial pathway in rat brain. Neuroscience. 2009;159:1309–1315. doi: 10.1016/j.neuroscience.2009.01.011. [DOI] [PubMed] [Google Scholar]
- Li Q, Li J, Zhang L, Wang B, Xiong L. Preconditioning with hyperbaric oxygen induces tolerance against oxidative injury via increased expression of heme oxygenase-1 in primary cultured spinal cord neurons. Life Sci. 2007;80:1087–1093. doi: 10.1016/j.lfs.2006.11.043. [DOI] [PubMed] [Google Scholar]
- Li Y, Dong H, Chen M, Liu J, Yang L, Chen S, Xiong L. Preconditioning with repeated hyperbaric oxygen induces myocardial and cerebral protection in patients undergoing coronary artery bypass graft surgery: a prospective, randomized, controlled clinical trial. J Cardiothorac Vasc Anesth. 2011;25:908–916. doi: 10.1053/j.jvca.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Li Z, Liu W, Kang Z, Lv S, Han C, Yun L, Sun X, Zhang JH. Mechanism of hyperbaric oxygen preconditioning in neonatal hypoxia-ischemia rat model. Brain Res. 2008b;1196:151–156. doi: 10.1016/j.brainres.2007.12.039. [DOI] [PubMed] [Google Scholar]
- Liu Y, Zhu S, Wang Y, Hu J, Xu L, Ding L, Liu G. Neuroprotective effect of ischemic preconditioning in focal cerebral infarction: relationship with upregulation of vascular endothelial growth factor. Neural Regen Res. 2014;9:1117–1121. doi: 10.4103/1673-5374.135313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lou M, Chen Y, Ding M, Eschenfelder CC, Deuschl G. Involvement of the mitochondrial ATP-sensitive potassium channel in the neuroprotective effect of hyperbaric oxygenation after cerebral ischemia. Brain Res Bull. 2006;69:109–116. doi: 10.1016/j.brainresbull.2005.11.009. [DOI] [PubMed] [Google Scholar]
- Lu PG, Feng H, Yuan SJ, Zhang RW, Li M, Hu R, Liu ZS, Yin J. Effect of preconditioning with hyperbaric oxygen on neural cell apoptosis after spinal cord injury in rats. J Neurosurg Sci. 2013;57:253–258. [PubMed] [Google Scholar]
- Majid A. Neuroprotection in stroke: past, present, and future. ISRN Neurol. 2014:515716. doi: 10.1155/2014/515716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsunami T, Sato Y, Hasegawa Y, Ariga S, Kashimura H, Sato T, Yukawa M. Enhancement of reactive oxygen species and induction of apoptosis in streptozotocin-induced diabetic rats under hyperbaric oxygen exposure. Int J Clin Exp Pathol. 2011;4:255–266. [PMC free article] [PubMed] [Google Scholar]
- Maxwell PH. HIF-1's relationship to oxygen: simple yet sophisticated. Cell Cycle. 2004;3:156–159. [PubMed] [Google Scholar]
- McIntosh TK, Smith DH, Meaney DF, Kotapka MJ, Gennarelli TA, Graham DI. Neuropathological sequelae of traumatic brain injury: relationship to neurochemical and biomechanical mechanisms. Lab Invest. 1996;74:315–342. [PubMed] [Google Scholar]
- Mecocci P, MacGarvey U, Beal MF. Oxidative damage to mitochondrial DNA is increased in Alzheimer's disease. Ann Neurol. 1994;36:747–751. doi: 10.1002/ana.410360510. [DOI] [PubMed] [Google Scholar]
- Metzen E, Zhou J, Jelkmann W, Fandrey J, Brune B. Nitric oxide impairs normoxic degradation of HIF-1alpha by inhibition of prolyl hydroxylases. Mol Biol Cell. 2003;14:3470–3481. doi: 10.1091/mbc.E02-12-0791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mounsey RA, Pang CY, Forrest C. Preconditioning: a new technique for improved muscle flap survival. Head Neck Surg. 1992;107:549–552. doi: 10.1177/019459989210700406. [DOI] [PubMed] [Google Scholar]
- Mu D, Jiang X, Sheldon RA, Fox CK, Hamrick SE, Vexler ZS, Ferriero DM. Regulation of hypoxia-inducible factor 1alpha and induction of vascular endothelial growth factor in a rat neonatal stroke model. Neurobiol Dis. 2003;14:524–534. doi: 10.1016/j.nbd.2003.08.020. [DOI] [PubMed] [Google Scholar]
- Murry CE, Jennings RB, Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- Osada M, Sato T, Komori S, Tamura K. Protective effect of preconditioning on reperfusion induced ventricular arrhythmias of isolated rat hearts. Cardiovasc Res. 1991;25:441–444. doi: 10.1093/cvr/25.6.441. [DOI] [PubMed] [Google Scholar]
- Ostrowski RP, Colohan AR, Zhang JH. Mechanisms of hyperbaric oxygen-induced neuroprotection in a rat model of subarachnoid hemorrhage. J Cereb Blood Flow Metab. 2005;25:554–571. doi: 10.1038/sj.jcbfm.9600048. [DOI] [PubMed] [Google Scholar]
- Ostrowski RP, Colohan AR, Zhang JH. Neuroprotective effect of hyperbaric oxygen in a rat model of subarachnoid hemorrhage. Acta Neurochir Suppl. 2006;96:188–193. doi: 10.1007/3-211-30714-1_41. [DOI] [PubMed] [Google Scholar]
- Ostrowski RP, Jadhav V, Chen W, Zhang JH. Reduced matrix metalloproteinase-9 activity and cell death after global ischemia in the brain preconditioned with hyperbaric oxygen. Acta Neurochir Suppl. 2010;106:47–49. doi: 10.1007/978-3-211-98811-4_7. [DOI] [PubMed] [Google Scholar]
- Ostrowski RP, Graupner G, Titova E, Zhang J, Chiu J, Dach N, Corleone D, Tang J, Zhang JH. The hyperbaric oxygen preconditioning-induced brain protection is mediated by a reduction of early apoptosis after transient global cerebral ischemia. Neurobiol Dis. 2008;29:1–13. doi: 10.1016/j.nbd.2007.07.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paine A, Eiz-Vesper B, Blasczyk R, Immenschuh S. Signaling to heme oxygenase-1 and its anti-inflammatory therapeutic potential. Biochem Pharmacol. 2010;80:1895–1903. doi: 10.1016/j.bcp.2010.07.014. [DOI] [PubMed] [Google Scholar]
- Pandey AS, Xi G. Intracerebral hemorrhage: a multimodality approach to improving outcome. Transl Stroke Res. 2014;5:313–315. doi: 10.1007/s12975-014-0344-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pimentel WA, Martinez EE, Ambrose JA, Mathias W, Arruda A, Horta PE, Ribeiro EE, Esteves A, Lemos PA, Ramires JA. Human myocardium preconditioning during successive balloon inflations: irrelevant influence of both collateral recruitment and clinical pre-intervention interference. EuroIntervention. 2006;2:345–350. [PubMed] [Google Scholar]
- Plafki C, Peters P, Almeling M, Welslau W, Busch R. Complications and side effects of hyperbaric oxygen therapy. Aviat Space Environ Med. 2000;71:119–124. [PubMed] [Google Scholar]
- Purcell JE, Lenhard SC, White RF, Schaeffer T, Barone FC, Chandra S. Strain-dependent response to cerebral ischemic preconditioning: differences between spontaneously hypertensive and stroke prone spontaneously hypertensive rats. Neurosci Lett. 2003;339:151–155. doi: 10.1016/s0304-3940(02)01476-3. [DOI] [PubMed] [Google Scholar]
- Qi Z, Gao CJ, Wang YB, Ma XM, Zhao L, Liu FJ, Liu XH, Sun XJ, Wang XJ. Effects of hyperbaric oxygen preconditioning on ischemia-reperfusion inflammation and skin flap survival. Chin Med J (Engl) 2013;126:3904–3909. [PubMed] [Google Scholar]
- Qin Z, Song S, Xi G, Silbergleit R, Keep RF, Hoff JT, Hua Y. Preconditioning with hyperbaric oxygen attenuates brain edema after experimental intracerebral hemorrhage. Neurosurg Focus. 2007;22:E13. doi: 10.3171/foc.2007.22.5.14. [DOI] [PubMed] [Google Scholar]
- Qin Z, Hua Y, Liu W, Silbergleit R, He Y, Keep RF, Hoff JT, Xi G. Hyperbaric oxygen preconditioning activates ribosomal protein S6 kinases and reduces brain swelling after intracerebral hemorrhage. Acta Neurochir Suppl. 2008;102:317–320. doi: 10.1007/978-3-211-85578-2_60. [DOI] [PubMed] [Google Scholar]
- Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, Dhama K. Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int. 2014:761264. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saatman KE, Duhaime AC, Bullock R, Maas AI, Valadka A, Manley GT. Classification of traumatic brain injury for targeted therapies. J Neurotrauma. 2008;25:719–738. doi: 10.1089/neu.2008.0586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salhanick SD, Belikoff B, Orlow D, Holt D, Reenstra W, Buras JA. Hyperbaric oxygen reduces acetaminophen toxicity and increases HIF-1alpha expression. Acad Emerg Med. 2006;13:707–714. doi: 10.1197/j.aem.2006.01.029. [DOI] [PubMed] [Google Scholar]
- Schott RJ, Rohmann S, Braun ER, Schaper W. Ischemic preconditioning reduces infarct size in swine myocardium. Circ Res. 1990;66:1133–1142. doi: 10.1161/01.res.66.4.1133. [DOI] [PubMed] [Google Scholar]
- Schouten JW. Neuroprotection in traumatic brain injury: a complex struggle against the biology of nature. Curr Opin Crit Care. 2007;13:134–142. doi: 10.1097/MCC.0b013e3280895d5c. [DOI] [PubMed] [Google Scholar]
- Semenza GL, Nejfelt MK, Chi SM, Antonarakis SE. Hypoxia-inducible nuclear factors bind to an enhancer element located 3’ to the human erythropoietin gene. Proc Natl Acad Sci U S A. 1991;88:5680–5684. doi: 10.1073/pnas.88.13.5680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankaran S. Hypoxic-ischemic encephalopathy and novel strategies for neuroprotection. Clin Perinatol. 2012;39:919–929. doi: 10.1016/j.clp.2012.09.008. [DOI] [PubMed] [Google Scholar]
- Smith CD, Carney JM, Starke-Reed PE, Oliver CN, Stadtman ER, Floyd RA, Markesbery WR. Excess brain protein oxidation and enzyme dysfunction in normal aging and in Alzheimer disease. Proc Natl Acad Sci U S A. 1991;88:10540–10543. doi: 10.1073/pnas.88.23.10540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soejima Y, Ostrowski RP, Manaenko A, Fujii M, Tang J, Zhang JH. Hyperbaric oxygen preconditioning attenuates hyperglycemia enhanced hemorrhagic transformation after transient MCAO in rats. Med Gas Res. 2012;2:9. doi: 10.1186/2045-9912-2-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soejima Y, Hu Q, Krafft PR, Fujii M, Tang J, Zhang JH. Hyperbaric oxygen preconditioning attenuates hyperglycemia-enhanced hemorrhagic transformation by inhibiting matrix metalloproteinases in focal cerebral ischemia in rats. Exp Neurol. 2013;247:737–743. doi: 10.1016/j.expneurol.2013.03.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan R, Pare GC, Frederiksen LJ, Semenza GL, Graham CH. Hypoxia-induced resistance to anticancer drugs is associated with decreased senescence and requires hypoxia-inducible factor-1 activity. Mol Cancer Ther. 2008;7:1961–1973. doi: 10.1158/1535-7163.MCT-08-0198. [DOI] [PubMed] [Google Scholar]
- Sunkari VG, Lind F, Botusan IR, Kashif A, Liu ZJ, Yla-Herttuala S, Brismar K, Velazquez O, Catrina SB. Hyperbaric oxygen therapy activates hypoxia-inducible factor 1 (HIF-1), which contributes to improved wound healing in diabetic mice. Wound Repair Regen. 2015;23:98–103. doi: 10.1111/wrr.12253. [DOI] [PubMed] [Google Scholar]
- Thom SR. Oxidative stress is fundamental to hyperbaric oxygen therapy. J Appl Physiol (1985) 2009;106:988–995. doi: 10.1152/japplphysiol.91004.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tibbles PM, Edelsberg JS. Hyperbaric-oxygen therapy. N Engl J Med. 1996;334:1642–1648. doi: 10.1056/NEJM199606203342506. [DOI] [PubMed] [Google Scholar]
- Tso MK, Macdonald RL. Subarachnoid hemorrhage: a review of experimental studies on the microcirculation and the neurovascular unit. Transl Stroke Res. 2014;5:174–189. doi: 10.1007/s12975-014-0323-4. [DOI] [PubMed] [Google Scholar]
- Vince RV, Midgley AW, Laden G, Madden LA. The effect of hyperbaric oxygen preconditioning on heat shock protein 72 expression following in vitro stress in human monocytes. Cell Stress Chaperones. 2011;16:339–343. doi: 10.1007/s12192-010-0246-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada K, Ito M, Miyazawa T, Katoh H, Nawashiro H, Shima K, Chigasaki H. Repeated hyperbaric oxygen induces ischemic tolerance in gerbil hippocampus. Brain Res. 1996;740:15–20. doi: 10.1016/s0006-8993(96)00831-1. [DOI] [PubMed] [Google Scholar]
- Wang FS, Wang CJ, Chen YJ, Chang PR, Huang YT, Sun YC, Huang HC, Yang YJ, Yang KD. Ras induction of superoxide activates ERK-dependent angiogenic transcription factor HIF-1alpha and VEGF-A expression in shock wave-stimulated osteoblasts. J Biol Chem. 2004;279:10331–10337. doi: 10.1074/jbc.M308013200. [DOI] [PubMed] [Google Scholar]
- Wang R, Xu J, Xie J, Kang Z, Sun X, Chen N, Liu L, Xu J. Hyperbaric oxygen preconditioning promotes survival of retinal ganglion cells in a rat model of optic nerve crush. J Neurotrauma. 2010;27:763–770. doi: 10.1089/neu.2009.1005. [DOI] [PubMed] [Google Scholar]
- Wang WZ, Jones S, Stepheson LL, Khiabani KT, Zamboni WA. Microvascular protection induced by late preconditioning was abolished in STZ-induced acute diabetic rats. J Reconstr Microsurg. 2002;18:689–696. doi: 10.1055/s-2002-36501. [DOI] [PubMed] [Google Scholar]
- Wang X, Lo EH. Triggers and mediators of hemorrhagic transformation in cerebral ischemia. Mol Neurobiol. 2003;28:229–244. doi: 10.1385/MN:28:3:229. Mol Neurobiol 28:229-244. [DOI] [PubMed] [Google Scholar]
- Wang Y, Zhang S, Luo M, Li Y. Hyperbaric oxygen therapy improves local microenvironment after spinal cord injury. Neural Regen Res. 2014;9:2182–2188. doi: 10.4103/1673-5374.147951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfe WG, DeVries WC. Oxygen toxicity. Annu Rev Med. 1975;26:203–217. doi: 10.1146/annurev.me.26.020175.001223. [DOI] [PubMed] [Google Scholar]
- Wong CH, Crack PJ. Modulation of neuro-inflammation and vascular response by oxidative stress following cerebral ischemia-reperfusion injury. Curr Med Chem. 2008;15:1–14. doi: 10.2174/092986708783330665. [DOI] [PubMed] [Google Scholar]
- Xiong L, Zhu Z, Dong H, Hu W, Hou L, Chen S. Hyperbaric oxygen preconditioning induces neuroprotection against ischemia in transient not permanent middle cerebral artery occlusion rat model. Chin Med J (Engl) 2000;113:836–839. [PubMed] [Google Scholar]
- Yamashita S, Hirata T, Mizukami Y, Cui YJ, Fukuda S, Ishida K, Matsumoto M, Sakabe T. Repeated preconditioning with hyperbaric oxygen induces neuroprotection against forebrain ischemia via suppression of p38 mitogen activated protein kinase. Brain Res. 2009;1301:171–179. doi: 10.1016/j.brainres.2009.08.096. [DOI] [PubMed] [Google Scholar]
- Yan W, Zhang H, Bai X, Lu Y, Dong H, Xiong L. Autophagy activation is involved in neuroprotection induced by hyperbaric oxygen preconditioning against focal cerebral ischemia in rats. Brain Res. 2011;1402:109–121. doi: 10.1016/j.brainres.2011.05.049. [DOI] [PubMed] [Google Scholar]
- Yan W, Fang Z, Yang Q, Dong H, Lu Y, Lei C, Xiong L. SirT1 mediates hyperbaric oxygen preconditioning-induced ischemic tolerance in rat brain. J Cereb Blood Flow Metab. 2013;33:396–406. doi: 10.1038/jcbfm.2012.179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- You P, Fang Y, Bao X, Ma J, Zhang S, Yao J. Effects of hyperbaric oxygen on the expression of endogenous matrix metalloproteinase 9 in rat lung tissue. Undersea Hyperb Med. 2014;41:1–7. [PubMed] [Google Scholar]
- Yu SY, Chiu JH, Yang SD, Yu HY, Hsieh CC, Chen PJ, Lui WY, Wu CW. Preconditioned hyperbaric oxygenation protects the liver against ischemia-reperfusion injury in rats. J Surg Res. 2005;128:28–36. doi: 10.1016/j.jss.2005.04.025. [DOI] [PubMed] [Google Scholar]
- Zemke D, Smith JL, Reeves MJ, Majid A. Ischemia and ischemic tolerance in the brain: an overview. Neurotoxicology. 2004;25:895–904. doi: 10.1016/j.neuro.2004.03.009. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Ouyang S, Zhang L, Tang X, Song Z, Liu P. Oxygen-induced changes in mitochondrial DNA and DNA repair enzymes in aging rat lens. Mech Ageing Dev. 2010;131:666–673. doi: 10.1016/j.mad.2010.09.003. [DOI] [PubMed] [Google Scholar]