Abstract
The medicinal value of hydrogen (H2) was ignored prior to research illustrating that inhalation of 2% H2 can significantly decrease the damage of cerebral ischemia/reperfusion caused by oxidative stress via selective elimination of hydroxyl freebase (OH) and peroxynitrite anion (ONOOˉ). Subsequently, there have been numerous experiments on H2. Most research and trials involving the mechanisms underlying H2 therapy show the effects of antioxygenation, anti-inflammation, and anti-apoptosis. Among quantities of diseases related with H2 therapy, the brain disease is a hotspot as brain tissue and cell damage are easier to be induced by oxidative stress and other stimulations. In this review, emphasis is on stroke, traumatic brain injuries, and degenerative diseases, such as Alzheimer's disease and Parkinson's disease. Taking into account the blood-brain barrier, penetrability, possible side effects, and the molecular properties of H2 within a single comprehensive review should contribute to advancing both clinical and non-clinical research and therapies. A systematic introduction of H2 therapy with regards to mechanisms and cerebral diseases both in animal and human subjects can make it easier to comprehend H2 therapy and therefore provide the basis for further clinical strategy.
Keywords: hydrogen therapy, ingestion, oxidative stress, inflammation, apoptosis, cerebral diseases
INTRODUCTION
Hydrogen (H2) therapy has been an intense subject of interest following the discovery of selective antioxygenation by Ohsawa in 2007 (Ohsawa et al., 2007). Currently, there are only a minority of antioxidants used in the clinical treatment of nervous system diseases and they are not very effective (Jang et al., 2009; Munakata et al., 2011). Combined with special natures and multiple manners of ingestion, H2 is a strong prospect for clinical application (Ichihara et al., 2015). In our review, we discuss the advantages and disadvantages of H2 therapy, its underlying mechanisms, and ingestion characteristics. Furthermore, we focus heavily on H2 therapy's relative role in treating brain diseases, since cerebral cells and tissues are sensitive to oxidative stress and other relevant stimulation (Allen and Bayraktutan, 2009).
INGESTION CHARACTERISTICS
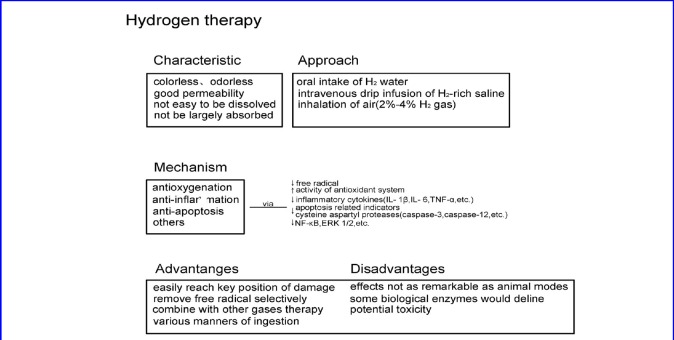
Under normal temperature and pressure, the solubility of H2 is low and it cannot be largely absorbed by the body. Although humans and most mammals do not have endogenous cells that produce H2, a large number of anaerobic bacteria in the large intestine can produce H2 by decomposing plant fibers and carbohydrates from polysaccharide fragments. Additionally, H2 can be expelled by anus exhaust, intestinal flora metabolism, and the respiratory tract (Levitt, 1969; Sahakian et al., 2010).
Clinically, methods for ingestion of H2 include oral intake of H2 water, intravenous drip infusion of H2-rich saline, and inhalation of air containing 2–4% H2 gas (Ono et al., 2011; Ishibashi et al., 2012, 2014, 2015; Sakai et al., 2014). Side effects related to the concentration of H2 are often neglected, occasionally resulting in a lack or excess of H2 administered to patients, and, potentially, toxic effects (Nakao et al., 2010a). Research reveals that concentrations of H2 in tissue corresponds to its concentration in the administered water or gas, suggesting that it is important to consider the disease of interest when selecting the most efficient route of H2 administration.
MECHANISMS
Antioxygenation
Free radicals are generated during metabolic processes. By breathing 2% H2, the free radical can be effectively removed, decreasing cerebral ischemia/reperfusion injury (Ohsawa et al., 2007). It was demonstrated at the cellular level that H2 can selectively neutralize •OH and ONOOˉ, and therefore concluded that this selective antioxidant effect is the basis of H2 therapy for cerebral ischemia/reperfusion injury. Additionally, a variety of animal experiments confirmed that H2 has the capacity to improve the activity of antioxidant system, reducing damage to cells and tissues induced by oxidative stress (Xie et al., 2010; Wang et al., 2015). Other experiments showed improvement in the activity of antioxidant enzymes under H2 inducement (Kawamura et al., 2010).
Anti-inflammation effect
Inflammation is a common pathological process accompanying most diseases, whereby activation of immunocytes and the release inflammatory cytokines are involved. This includes interleukin 1 beta (IL- 1β), IL- 6 and tumor necrosis factor-alpha (TNF-α). Animal experiments confirmed that ingestion of H2 can decrease both the amount of inflammatory cytokines and immunocyte stimulation (Kawamura et al., 2010; (Kajiya et al., 2009; Liu et al., 2010; Wang et al., 2011b; Zhang et al., 2011). As a result, the degree of inflammation was alleviated through H2 therapy.
Anti-apoptosis effect
According to recent studies, apoptosis can be triggered by intrinsic stimulation through the mitochondrial signaling pathway, or by extrinsic stimulation through cell surface death receptors (DR), such as TNFα, TNF-related apoptosis-inducing ligand (TRAIL) receptors, and Fas (CD95/APO1) (Adams, 2003; Green, 2005). In either case, activation of cysteine aspartyl proteases (caspases) is necessary, and therefore, we define apoptosis as a caspase-dependent manner of cell death. Research showed that the apoptosis of neurons in newborn rats induced by hypoxia and ischemia is inhibited if inhaling H2, as the ratio of Terminal-deoxynucleotidyl Transferase Mediated Nick End Labeling (TUNEL) staining positive cells and the activity of caspase-3 and caspase-12 of the hippocampus and cortex is decreased (Cai et al., 2008). Other animal studies discovered the anti-apoptosis effect of H2. For instance, in the case of spinal cord injury, apoptosis-related indicators decline if an intraperitoneal injection of H2-saturated saline is administered within a certain time period (Chen et al., 2010a).
Additional mechanisms
Nuclear factor-kappaB (NF-κB) is quick and widespread transcription factors in the cytoplasm that regulate expression of target genes, such as cytokines, chemokines, adhesion molecules, and oxidative stress-related enzymes. Studies based on specific animal models have demonstrated the inhibition of NF-κB with the introduction of H2 (Wang et al., 2011a). H2 can also control the activity of extracellular signal-regulated kinase, such as with the inhibition of phosphorylation of extracellular signal-regulated kinase1/2 (ERK 1/2) (Liu et al., 2010).
ADVANTAGES AND DISADVANTAGES
Easy preparation, low cost, non-toxicity, powerful permeability, absence of residue, are characteristics that make H2 a strong prospect for clinical application (Ichihara et al., 2015). H2‘s powerful permeability grants it accessibility to secondary organelles, such as mitochondria and nuclei, which are primary locations of deoxyribonucleic acid (DNA) and reactive oxygen species (ROS) damage (Ohsawa et al., 2007; Nakata et al., 2015). Additionally, H2 can selectively remove hydroxyl free radicals and nitrous acid anions. Hypoergia between H2 and other gases when in therapeutic concentration makes H2 gas capable of combining with other gas therapies, such as anesthesia inhalation (Nakao et al., 2010b). Furthermore, there are many methods for ingestion of H2, such as oral intake of H2 water, intravenous drip of H2 -rich saline, and inhalation of air containing 2–4% H2 gas (Zheng et al., 2009; Qian et al., 2010; Lin et al., 2011; Kurokawa et al., 2015). Having various means of ingestion produces multiplicity when faced with different diseases. Unfortunately, until 2011, clinical outcomes were poor, and the effects of H2 on human subjects are often less remarkable than those seen in animal models (Ichihara et al., 2015).
The prominent effects of H2 play an important role in a variety of clinical diseases and animal models, but unclear and unsolved conundrums remain (Katz et al., 2015). Research reported that levels of some biological enzymes, such as aspartate aminotransferase, alanine aminotransferase, γ-glutathione transferase and total bilirubin, declined upon ingestion of a certain amount of H2 (Nakao et al., 2010a; Saitoh et al., 2010). These variations and interactions were also observed in clinical trials and do not exceed standard ranges.
Intuitive understanding of H2 therapy was reached through the summary and visual representation of the above content (Figure 1).
Figure 1.
Relevant features and mechanisms of hydrogen therapy.
Role in cerebral diseases: Stroke
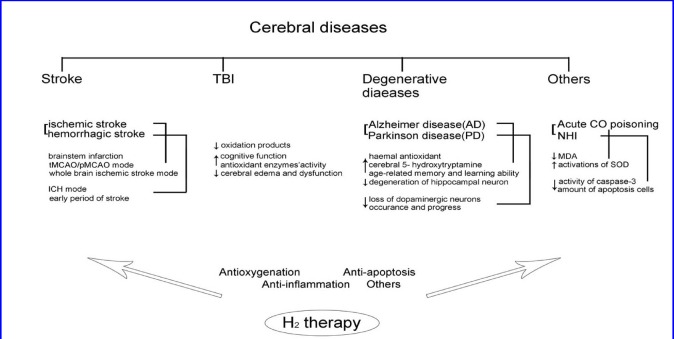
Stroke is a devastating illness second only to cardiac ischemia as a cause of death worldwide. Its main causes include cerebral vasospasm, obstacles in cerebral blood circulation, and the rupture of cerebral vessels. Oxidative stress and immunity are key elements of stroke pathobiology and are present from the stroke's early damaging stages, to the late post-ischemic tissue repair (Iadecola and Anrather, 2011; Behrouz, 2016; Sukumari-Ramesh et al., 2016). Strokes are divided into two categories: ischemic stroke and hemorrhagic stroke.
An ischemic stroke refers to local brain tissue damage caused by external or intracranial artery stenosis, or occlusion lesions sustained as a result of insufficient collateral circulation within the brain (Lyden and Zivin, 1993; Li et al., 2015). Studies show that cerebral ischemia and ischemia-reperfusion injuries contain more impact factors and mechanisms, and oxidative stress plays an important role (Allen and Bayraktutan, 2009; Hafez et al., 2014; Seifert and Pennypacker, 2014; Weaver and Liu, 2015).
One study on rats revealed that H2 provides nerve protection in the transient middle cerebral artery occlusion (tMCAO) (Wardlaw et al., 2012). The volume of infarctus, malondialdehyde (a product of lipid oxidation) and 8-hydroxy-2-deoxyguanosine (8-OHdG, a product of DNA oxidation) declined after ingesting 2% H2. In the same experiment, researchers also confirmed that H2 can act as an antioxidant and selectively remove •OH and ONOOˉ. Furthermore, its curative effects on cerebral ischemia/reperfusion injury were more significant than those of edaravone. H2 also demonstrated an anti-inflammatory and anti-apoptotic effect. In addition, TNF-α and caspase-3 is inhibited after intraperitoneal injection of H2 saline.
In clinical studies, upon ischemic stroke onset, 8.5-30% of patients suffer a hemorrhagic stroke (Wardlaw et al., 2012). Among patients in both the high sugar and tMCAO groups, due to its suppressive effects, this risk of brain hemorrhaging was decreased upon H2 administration. After persistent inhalation of 2.9% H2 for 2 hours, oxydic products and matrix metalloproteinases-9 (MMP-9) decreased, illustrating protection of the BBB (Chen et al., 2010b). Researchers speculated this effect contributed to the lower occurrence of hemorrhage accompanying cerebral infarction. Following intraperitoneal injection of H2 saline, persistent middle cerebral artery occlusion (pMCAO) conformed to continuous ischemia of vessels, activity of antioxidant enzymes increased, and infarction areas were effectively reduced (Nagatani et al., 2012). In animals, small doses of H2 can significantly reduce mortality in cases of ischemic strokes that target the entire brain. Another clinical trial on brain stem infarction revealed that cooperation of H2 and edaravone can cut down recovery time significantly better than using edaravone alone.
A hemorrhagic stroke is defined as a cerebral hemorrhage following compression and necrosis of brain tissue (Chen et al., 2015). It is also known as a hemorrhagic cerebrovascular accident, which is generally divided into two categories: subarachnoid hemorrhage (SAH) and intracerebral hemorrhage (ICH). Hemorrhagic strokes are typically more dangerous than ischemic strokes (Engelhardt and Sorokin, 2009). Microglia and inflammatory cells are activated upon hemorrhage, producing free radicals. A series of changes, like the formation of hematoma, decomposition of hemoglobin (Hb), and Feton reactions can aggravate oxidative stress. In an ICH model for mice, inhalation of 2% H2 for 1 hour reduced the degree of cerebral edema and improved neural function significantly, though only for 72 hours, suggesting that H2 demonstrates only acute protection from ICH. This was speculated to be a due to neutrophil infiltration and microglial activation not peaking until after 72 hours, and antioxygenation of H2 not persistent or sufficient at that time (Manaenko et al., 2011). Lastly, infiltration and activation of mastocytes play an important role in inflammatory responses during the initial stages of stroke. H2 was shown to protect BBB and decrease cerebral edema by preventing activation of mastocytes.
Traumatic brain injury
Traumatic brain injury (TBI) is one of the leading causes of death and disability of young people, and recovering patients usually experience impairment in learning and memory (Chua et al., 2007). Experimentation on rats showed that TBI led to brain edema, dysfunction of the nervous system, and delays in remodeling (Zhang et al., 2014). Furthermore, oxidative stress was determined an important factor in pathological changes (Zhang et al., 2014). When TBI occurred in rats, in-drawing of 2% H2 decreased oxidation products, increased antioxidant enzyme activity, improved cerebral edema, and decreased nervous system dysfunction (Ji et al., 2010). Other studies revealed the protective effects of H2 -rich saline on hydraulic coup injury: malondialdehyde (an important antioxidant adjustment factor) declined, silent information regulators and brain-derived neurotrophic factors elevated (factors which mediate the synaptic plasticity associated with learning and memory). Additionally, Morris water maze tests also confirmed improved cognitive function following H2 therapy (Hou et al., 2012).
Degenerative diseases
A neurodegenerative disease is characterized as the loss of cells in the brain and spinal cord, which are generally not renewable. As time progresses, neural deterioration is aggravated, leading to devastating and irreversible neural dysfunction. Neurodegenerative diseases are divided into two types: One impacting movement, such as cerebellar ataxia, and the other affecting memory and is related to dementia.
Alzheimer's disease (AD) is the most common neurological degenerative disease, in which glial cells and inflammation are activated and free radicals are produced, damaging neurons (Cupino and Zabel, 2014). One study found that drinking H2 saline deterred the decline of age-related memory and learning ability (Gu et al., 2010). In that study, activation of cerebral 5-hydroxytryptamine and haemal antioxidant increased, resulting in a reduction of hippocampal neuron degeneration and improved scores on the Morris water maze test (Gu et al., 2010). In other AD models, NF-κB was combined with H2 therapy (Guo et al., 2015).
Parkinson's disease (PD) is an age-related neurodegenerative disease characterized by neural degeneration in the substantia nigra and striatum. Administration of H2-rich saline for 6-hydroxydopamine-induced Parkinson's syndrome resulted in neural protection effects, and drinking H2 saline yielded similar effects in 1-methyl-4-phenolic group-1,2,3,6-4 hydrogen purineinduced PD (Gu et al., 2010).
Additional cerebral diseases
Acute carbon monoxide poisoning is a systemic disease mainly involving damage of the central nervous system and delayed encephalopathy. Investigations concluded that acute brain injury and delayed encephalopathy of carbon monoxide poisoning have a close relationship with oxidative stress, cell apoptosis and immune injuries (Kao and Nanagas, 2005). Related research showed that H2 -rich saline improved activation of superoxide dismutase (SOD) in brain tissues and serum, and decreased malondialdehyde (MDA), therefore improving memory, learning, and environmental adaptation during acute carbon monoxide poisoning(Sun et al., 2011; Shen et al., 2013).
In recent years, mortality rate in premature births have increased, but effective treatments for neonatal hypoxia-ischemia (NHI) remain few. Antioxidant systems in newborns are immature and are more sensitive to free radical damage. H2 therapy was shown useful in NHI treatment, as activity of caspase-3 and degree of cell apoptosis decreased, suggesting that H2 offers neural protection by inhibiting apoptosis (Cai et al., 2008).
In order to facilitate simple comprehension, the relationships between H2 therapy and the brain diseases discussed above is summarized through illustration (Figure 2).
Figure 2.
Summary of the relationship between hydrogen therapy and brain diseases.
DISCUSSION
Due to the feasibility and effectiveness of H2 therapy, H2 is a strong prospect for clinical application. Until now, studies and clinical trials of H2 were carried out internationally and involved the investigation of a variety of diseases. In this review, we simplify comprehension of H2 therapy and provide the basis for further clinical strategy. It is important to note that underlying mechanisms, optimal concentration, and biological safety of H2 are worthy of deeper investigation. Additionally, the interrelationships between effects such as antioxygenation, anti-inflammation, and anti-apoptosis, remain unclear. In conclusion, we recommend more research in both the individual and molecular levels in order to drive H2 to become a more effective therapy in clinical settings.
Abbreviations
H2: hydrogen; •OH: hydroxyl freebase; ONOOˉ: peroxynitrite anion; BBB: blood brain barrier; IL-1β: interleukin 1 beta; TNF-α: tumor necrosis factor-alpha; DR: death receptors; TRAIL: TNF related apoptosis inducing ligand; TUNEL: Terminal-deoxynucleotidyl Transferase Mediated Nick End Labeling; NF-κB: Nuclear factor-κB; ERK 1/2: extracellular-regulated kinase1/2; DNA: deoxyribonucleic acid; ROS: reactive oxygen species; tMCAO: transient middle cerebral artery occlusion; pMCAO: persistent middle cerebral artery occlusion; 8-OhdG: 8-hydroxy-2-deoxyguanosine; MMP-9: matrix metalloproteinases-9; SAH: subarachnoid hemorrhage; ICH: intracerebral hemorrhage; Hb: hemoglobin; TBI: Traumatic brain injury; AD: Alzheimer's disease; PD: Parkinson's disease; SOD: superoxide dismutase; MDA: malondialdehyde; NHI: neonatal hypoxia-ischemia.
Footnotes
Funding: This work was supported by a grant from Suzhou Key Medical Center of China (No. Szzx201501), grants from the National Natural Science Foundation of China (No. 81571115, 81422013, and 81471196), a grant from the Scientific Department of Jiangsu Province of China (No. BL2014045), a grant from Suzhou Government of China (No. LCZX201301, SZS201413, and SYS201332), and A Project Funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Conflicts of interest
The authors declare no competing interests.
REFERENCES
- Adams JM. Ways of dying: multiple pathways to apoptosis. Genes Dev. 2003;17:2481–2495. doi: 10.1101/gad.1126903. [DOI] [PubMed] [Google Scholar]
- Allen CL, Bayraktutan U. Oxidative stress and its role in the pathogenesis of ischaemic stroke. Int J Stroke. 2009;4:461–470. doi: 10.1111/j.1747-4949.2009.00387.x. [DOI] [PubMed] [Google Scholar]
- Behrouz R. Re-exploring tumor necrosis factor alpha as a target for therapy in intracerebral hemorrhage. Transl Stroke Res. 2016;7:93–96. doi: 10.1007/s12975-016-0446-x. [DOI] [PubMed] [Google Scholar]
- Cai J, Kang Z, Liu WW, Luo X, Qiang S, Zhang JH, Ohta S, Sun X, Xu W, Tao H, Li R. Hydrogen therapy reduces apoptosis in neonatal hypoxia-ischemia rat model. Neurosci Lett. 2008;441:167–172. doi: 10.1016/j.neulet.2008.05.077. [DOI] [PubMed] [Google Scholar]
- Chen C, Chen Q, Mao Y, Xu S, Xia C, Shi X, Zhang JH, Yuan H, Sun X. Hydrogen-rich saline protects against spinal cord injury in rats. Neurochem Res. 2010a;35:1111–1118. doi: 10.1007/s11064-010-0162-y. [DOI] [PubMed] [Google Scholar]
- Chen CH, Manaenko A, Zhan Y, Liu WW, Ostrowki RP, Tang J, Zhang JH. Hydrogen gas reduced acute hyperglycemia-enhanced hemorrhagic transformation in a focal ischemia rat model. Neuroscience. 2010b;169:402–414. doi: 10.1016/j.neuroscience.2010.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen S, Yang Q, Chen G, Zhang JH. An update on inflammation in the acute phase of intracerebral hemorrhage. Transl Stroke Res. 2015;6:4–8. doi: 10.1007/s12975-014-0384-4. [DOI] [PubMed] [Google Scholar]
- Chua KS, Ng YS, Yap SG, Bok CW. A brief review of traumatic brain injury rehabilitation. Ann Acad Med Singapore. 2007;36:31–42. [PubMed] [Google Scholar]
- Cupino TL, Zabel MK. Alzheimer's silent partner: cerebral amyloid angiopathy. Transl Stroke Res. 2014;5:330–337. doi: 10.1007/s12975-013-0309-7. [DOI] [PubMed] [Google Scholar]
- Engelhardt B, Sorokin L. The blood-brain and the blood-cerebrospinal fluid barriers: function and dysfunction. Semin Immunopathol. 2009;31:497–511. doi: 10.1007/s00281-009-0177-0. [DOI] [PubMed] [Google Scholar]
- Green DR. Apoptotic pathways: ten minutes to dead. Cell. 2005;121:671–674. doi: 10.1016/j.cell.2005.05.019. [DOI] [PubMed] [Google Scholar]
- Gu Y, Huang CS, Inoue T, Yamashita T, Ishida T, Kang KM, Nakao A. Drinking hydrogen water ameliorated cognitive impairment in senescence-accelerated mice. J Clin Biochem Nutr. 2010;46:269–276. doi: 10.3164/jcbn.10-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo SX, Fang Q, You CG, Jin YY, Wang XG, Hu XL, Han CM. Effects of hydrogen-rich saline on early acute kidney injury in severely burned rats by suppressing oxidative stress induced apoptosis and inflammation. J Transl Med. 2015;13:183. doi: 10.1186/s12967-015-0548-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hafez S, Coucha M, Bruno A, Fagan SC, Ergul A. Hyperglycemia, acute ischemic stroke, and thrombolytic therapy. Transl Stroke Res. 2014;5:442–453. doi: 10.1007/s12975-014-0336-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou Z, Luo W, Sun X, Hao S, Zhang Y, Xu F, Wang Z, Liu B. Hydrogen-rich saline protects against oxidative damage and cognitive deficits after mild traumatic brain injury. Brain Res Bull. 2012;88:560–565. doi: 10.1016/j.brainresbull.2012.06.006. [DOI] [PubMed] [Google Scholar]
- Iadecola C, Anrather J. The immunology of stroke: from mechanisms to translation. Nat Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichihara M, Sobue S, Ito M, Ito M, Hirayama M, Ohno K. Beneficial biological effects and the underlying mechanisms of molecular hydrogen - comprehensive review of 321 original articles. Med Gas Res. 2015;5:12. doi: 10.1186/s13618-015-0035-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T, Sato B, Rikitake M, Seo T, Kurokawa R, Hara Y, Naritomi Y, Hara H, Nagao T. Consumption of water containing a high concentration of molecular hydrogen reduces oxidative stress and disease activity in patients with rheumatoid arthritis: an open-label pilot study. Med Gas Res. 2012;2:27. doi: 10.1186/2045-9912-2-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishibashi T, Sato B, Shibata S, Sakai T, Hara Y, Naritomi Y, Koyanagi S, Hara H, Nagao T. Therapeutic efficacy of infused molecular hydrogen in saline on rheumatoid arthritis: a randomized, double-blind, placebo-controlled pilot study. Int Immunopharmacol. 2014;21:468–473. doi: 10.1016/j.intimp.2014.06.001. [DOI] [PubMed] [Google Scholar]
- Ishibashi T, Ichikawa M, Sato B, Shibata S, Hara Y, Naritomi Y, Okazaki K, Nakashima Y, Iwamoto Y, Koyanagi S, Hara H, Nagao T. Improvement of psoriasis-associated arthritis and skin lesions by treatment with molecular hydrogen: A report of three cases. Mol Med Rep. 2015;12:2757–2764. doi: 10.3892/mmr.2015.3707. [DOI] [PubMed] [Google Scholar]
- Jang YG, Ilodigwe D, Macdonald RL. Metaanalysis of tirilazad mesylate in patients with aneurysmal subarachnoid hemorrhage. Neurocrit Care. 2009;10:141–147. doi: 10.1007/s12028-008-9147-y. [DOI] [PubMed] [Google Scholar]
- Ji X, Liu W, Xie K, Liu W, Qu Y, Chao X, Chen T, Zhou J, Fei Z. Beneficial effects of hydrogen gas in a rat model of traumatic brain injury via reducing oxidative stress. Brain Res. 2010;1354:196–205. doi: 10.1016/j.brainres.2010.07.038. [DOI] [PubMed] [Google Scholar]
- Kajiya M, Silva MJ, Sato K, Ouhara K, Kawai T. Hydrogen mediates suppression of colon inflammation induced by dextran sodium sulfate. Biochem Biophys Res Commun. 2009;386:11–15. doi: 10.1016/j.bbrc.2009.05.117. [DOI] [PubMed] [Google Scholar]
- Kao LW, Nanagas KA. Carbon monoxide poisoning. Med Clin North Am. 2005;89:1161–1194. doi: 10.1016/j.mcna.2005.06.007. [DOI] [PubMed] [Google Scholar]
- Katz I, Murdock J, Palgen M, Pype J, Caillibotte G. Pharmacokinetic analysis of the chronic administration of the inert gases Xe and Ar using a physiological based model. Med Gas Res. 2015;5:8. doi: 10.1186/s13618-015-0029-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura T, Huang CS, Tochigi N, Lee S, Shigemura N, Billiar TR, Okumura M, Nakao A, Toyoda Y. Inhaled hydrogen gas therapy for prevention of lung transplant-induced ischemia/reperfusion injury in rats. Transplantation. 2010;90:1344–1351. doi: 10.1097/TP.0b013e3181fe1357. [DOI] [PubMed] [Google Scholar]
- Kurokawa R, Seo T, Sato B, Hirano S, Sato F. Convenient methods for ingestion of molecular hydrogen: drinking, injection, and inhalation. Med Gas Res. 2015;5:13. doi: 10.1186/s13618-015-0034-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt MD. Production and excretion of hydrogen gas in man. N Engl J Med. 1969;281:122–127. doi: 10.1056/NEJM196907172810303. [DOI] [PubMed] [Google Scholar]
- Li XJ, Li CK, Wei LY, Lu N, Wang GH, Zhao HG, Li DL. Hydrogen sulfide intervention in focal cerebral ischemia/reperfusion injury in rats. Neural Regen Res. 2015;10:932–937. doi: 10.4103/1673-5374.158353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin Y, Kashio A, Sakamoto T, Suzukawa K, Kakigi A, Yamasoba T. Hydrogen in drinking water attenuates noise-induced hearing loss in guinea pigs. Neurosci Lett. 2011;487:12–16. doi: 10.1016/j.neulet.2010.09.064. [DOI] [PubMed] [Google Scholar]
- Liu Q, Shen WF, Sun HY, Fan DF, Nakao A, Cai JM, Yan G, Zhou WP, Shen RX, Yang JM, Sun XJ. Hydrogen-rich saline protects against liver injury in rats with obstructive jaundice. Liver Int. 2010;30:958–968. doi: 10.1111/j.1478-3231.2010.02254.x. [DOI] [PubMed] [Google Scholar]
- Lyden PD, Zivin JA. Hemorrhagic transformation after cerebral ischemia: mechanisms and incidence. Cerebrovasc Brain Metab Rev. 1993;5:1–16. [PubMed] [Google Scholar]
- Manaenko A, Lekic T, Ma Q, Ostrowski RP, Zhang JH, Tang J. Hydrogen inhalation is neuroprotective and improves functional outcomes in mice after intracerebral hemorrhage. Acta Neurochir Suppl. 2011;111:179–183. doi: 10.1007/978-3-7091-0693-8_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munakata A, Ohkuma H, Shimamura N. Effect of a free radical scavenger, edaravone, on free radical reactions: related signal transduction and cerebral vasospasm in the rabbit subarachnoid hemorrhage model. Acta Neurochir Suppl. 2011;110:17–22. doi: 10.1007/978-3-7091-0356-2_4. [DOI] [PubMed] [Google Scholar]
- Nagatani K, Wada K, Takeuchi S, Kobayashi H, Uozumi Y, Otani N, Fujita M, Tachibana S, Nawashiro H. Effect of hydrogen gas on the survival rate of mice following global cerebral ischemia. Shock (Augusta, Ga) 2012;37:645–652. doi: 10.1097/SHK.0b013e31824ed57c. [DOI] [PubMed] [Google Scholar]
- Nakao A, Toyoda Y, Sharma P, Evans M, Guthrie N. Effectiveness of hydrogen rich water on antioxidant status of subjects with potential metabolic syndrome-an open label pilot study. J Clin Biochem Nutr. 2010a;46:140–149. doi: 10.3164/jcbn.09-100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao A, Kaczorowski DJ, Wang Y, Cardinal JS, Buchholz BM, Sugimoto R, Tobita K, Lee S, Toyoda Y, Billiar TR, McCurry KR. Amelioration of rat cardiac cold ischemia/reperfusion injury with inhaled hydrogen or carbon monoxide, or both. J Heart Lung Transplant. 2010b;29:544–553. doi: 10.1016/j.healun.2009.10.011. [DOI] [PubMed] [Google Scholar]
- Nakata K, Yamashita N, Noda Y, Ohsawa I. Stimulation of human damaged sperm motility with hydrogen molecule. Med Gas Res. 2015;5:2. doi: 10.1186/s13618-014-0023-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohsawa I, Ishikawa M, Takahashi K, Watanabe M, Nishimaki K, Yamagata K, Katsura K, Katayama Y, Asoh S, Ohta S. Hydrogen acts as a therapeutic antioxidant by selectively reducing cytotoxic oxygen radicals. Nat Med. 2007;13:688–694. doi: 10.1038/nm1577. [DOI] [PubMed] [Google Scholar]
- Ono H, Nishijima Y, Adachi N, Tachibana S, Chitoku S, Mukaihara S, Sakamoto M, Kudo Y, Nakazawa J, Kaneko K, Nawashiro H. Improved brain MRI indices in the acute brain stem infarct sites treated with hydroxyl radical scavengers, Edaravone and hydrogen, as compared to Edaravone alone. A non-controlled study. Med Gas Res. 2011;1:12. doi: 10.1186/2045-9912-1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qian L, Cao F, Cui J, Huang Y, Zhou X, Liu S, Cai J. Radioprotective effect of hydrogen in cultured cells and mice. Free Radic Res. 2010;44:275–282. doi: 10.3109/10715760903468758. [DOI] [PubMed] [Google Scholar]
- Sahakian AB, Jee SR, Pimentel M. Methane and the gastrointestinal tract. Dig Dis Sci. 2010;55:2135–2143. doi: 10.1007/s10620-009-1012-0. [DOI] [PubMed] [Google Scholar]
- Saitoh Y, Harata Y, Mizuhashi F, Nakajima M, Miwa N. Biological safety of neutral-pH hydrogen-enriched electrolyzed water upon mutagenicity, genotoxicity and subchronic oral toxicity. Toxicol Ind Health. 2010;26:203–216. doi: 10.1177/0748233710362989. [DOI] [PubMed] [Google Scholar]
- Sakai T, Sato B, Hara K, Hara Y, Naritomi Y, Koyanagi S, Hara H, Nagao T, Ishibashi T. Consumption of water containing over 3.5 mg of dissolved hydrogen could improve vascular endothelial function. Vasc Health Risk Manag. 2014;10:591–597. doi: 10.2147/VHRM.S68844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seifert HA, Pennypacker KR. Molecular and cellular immune responses to ischemic brain injury. Transl Stroke Res. 2014;5:543–553. doi: 10.1007/s12975-014-0349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen MH, Cai JM, Sun Q, Zhang DW, Huo ZL, He J, Sun XJ. Neuroprotective effect of hydrogen-rich saline in acute carbon monoxide poisoning. CNS Neurosci. 2013;19:361–363. doi: 10.1111/cns.12094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sukumari-Ramesh S, Alleyne CH, Jr, Dhandapani KM. The histone deacetylase inhibitor suberoylanilide hydroxamic acid (saha) confers acute neuroprotection after intracerebral hemorrhage in mice. Transl Stroke Res. 2016;7:141–148. doi: 10.1007/s12975-015-0421-y. [DOI] [PubMed] [Google Scholar]
- Sun Q, Cai J, Zhou J, Tao H, Zhang JH, Zhang W, Sun XJ. Hydrogen-rich saline reduces delayed neurologic sequelae in experimental carbon monoxide toxicity. Crit Care Med. 2011;39:765–769. doi: 10.1097/CCM.0b013e318206bf44. [DOI] [PubMed] [Google Scholar]
- Wang C, Li J, Liu Q, Yang R, Zhang JH, Cao YP, Sun XJ. Hydrogen-rich saline reduces oxidative stress and inflammation by inhibit of JNK and NF-kappaB activation in a rat model of amyloid-beta-induced Alzheimer's disease. Neurosci Lett. 2011a;491:127–132. doi: 10.1016/j.neulet.2011.01.022. [DOI] [PubMed] [Google Scholar]
- Wang JL, Zhang QS, Zhu KD, Sun JF, Zhang ZP, Sun JW, Zhang KX. Hydrogen-rich saline injection into the subarachnoid cavity within 2 weeks promotes recovery after acute spinal cord injury. Neural Regen Res. 2015;10:958–964. doi: 10.4103/1673-5374.158361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y, Jing L, Zhao XM, Han JJ, Xia ZL, Qin SC, Wu YP, Sun XJ. Protective effects of hydrogen-rich saline on monocrotaline-induced pulmonary hypertension in a rat model. Respir Res. 2011b;12:26. doi: 10.1186/1465-9921-12-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wardlaw JM, Murray V, Berge E, del Zoppo G, Sandercock P, Lindley RL, Cohen G. Recombinant tissue plasminogen activator for acute ischaemic stroke: an updated systematic review and meta-analysis. Lancet. 2012;379:2364–2372. doi: 10.1016/S0140-6736(12)60738-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weaver J, Liu KJ. Does normobaric hyperoxia increase oxidative stress in acute ischemic stroke? A critical review of the literature. Med Gas Res. 2015;5:11. doi: 10.1186/s13618-015-0032-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie K, Yu Y, Pei Y, Hou L, Chen S, Xiong L, Wang G. Protective effects of hydrogen gas on murine polymicrobial sepsis via reducing oxidative stress and HMGB1 release. Shock (Augusta, Ga) 2010;34:90–97. doi: 10.1097/SHK.0b013e3181cdc4ae. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Sun Q, He B, Xiao J, Wang Z, Sun X. Anti-inflammatory effect of hydrogen-rich saline in a rat model of regional myocardial ischemia and reperfusion. Int J Cardiol. 2011;148:91–95. doi: 10.1016/j.ijcard.2010.08.058. [DOI] [PubMed] [Google Scholar]
- Zhang YP, Cai J, Shields LB, Liu N, Xu XM, Shields CB. Traumatic brain injury using mouse models. Transl Stroke Res. 2014;5:454–471. doi: 10.1007/s12975-014-0327-0. [DOI] [PubMed] [Google Scholar]
- Zheng X, Mao Y, Cai J, Li Y, Liu W, Sun P, Zhang JH, Sun X, Yuan H. Hydrogen-rich saline protects against intestinal ischemia/reperfusion injury in rats. Free Radic Res. 2009;43:478–484. doi: 10.1080/10715760902870603. [DOI] [PubMed] [Google Scholar]