Abstract
Climate change will significantly affect plant distribution as well as the quality of medicinal plants. Although numerous studies have analyzed the effect of climate change on future habitats of plants through species distribution models (SDMs), few of them have incorporated the change of effective content of medicinal plants. Schisandra sphenanthera Rehd. et Wils. is an endangered traditional Chinese medical plant which is mainly located in the Qinling Mountains. Combining fuzzy theory and a maximum entropy model, we obtained current spatial distribution of quality assessment for S. spenanthera. Moreover, the future quality and distribution of S. spenanthera were also projected for the periods 2020s, 2050s and 2080s under three different climate change scenarios (SRES-A1B, SRES-A2 and SRES-B1 emission scenarios) described in the Special Report on Emissions Scenarios (SRES) of IPCC (Intergovernmental Panel on Climate Change). The results showed that the moderately suitable habitat of S. sphenanthera under all climate change scenarios remained relatively stable in the study area. The highly suitable habitat of S. sphenanthera would gradually decrease in the future and a higher decline rate of the highly suitable habitat area would occur under climate change scenarios SRES-A1B and SRES-A2. The result suggested that in the study area, there would be no more highly suitable habitat areas for S. sphenanthera when the annual mean temperature exceeds 20 °C or its annual precipitation exceeds 1,200 mm. Our results will be influential in the future ecological conservation and management of S. sphenanthera and can be taken as a reference for habitat suitability assessment research for other medicinal plants.
Keywords: Climate change, GIS, Medicinal plants, Fuzzy membership function, Schisandra sphenanthera, Species distribution modeling
Introduction
Climate is the main determining factor in the distribution of species, and the change of species distribution patterns can be reflected by climate change definitely and directly (Root et al., 2003; Parmesan & Yohe, 2003; Lenoir et al., 2008; Bertrand et al., 2011; Bystriakova, Peregrym & Dragicevic, 2015). Global warming is likely to change the structure and function of essential terrestrial ecosystems (Wu et al., 2010; Euskirchen, Carman & McGuire, 2014) by changing the range of habitats and the distribution area, which has strong effects on herb spatial patterns and can increase the risk of extinction among endangered plants (Bartholomeus et al., 2011; Li et al., 2013). Recent studies have focused on the potential impact of climate change on vegetation distribution and nature reserves (Araújo et al., 2011; Zhang et al., 2014) and have raised great concerns about the future of a range of animal and plant species (Araújo & Rahbek, 2006; Fan et al., 2014; Guo et al., 2014). Although there are an increasing number of studies on the effect of climate change on the spatial distribution of plant species, only a few of them refer to endangered geo-herbs, and studies about the impact of climate change on herb quality are even fewer.
Medicinal plants play a very important role in health care, particularly in developing countries (Madaleno, 2010; Williamson et al., 2013). At present, many medicinal plant species are severely threatened by over exploitation, destructive harvesting, deforestation and habitat deterioration (Guo et al., 2014). The production and quality of medicinal plants are not only influenced by plant genetic characteristics but are also closely related to various environmental factors. Specific geographic spatial information about species is an important part of species conservation (Lu et al., 2012; Guo et al., 2013). This information is essential to addressing the many challenges facing species conservation, such as those induced by climate change, as well as other ecological or biological factors. It is also important for the domestication of wild species.
Schisandra sphenanthera (S. sphenanthera) is an important Chinese herbal medicine (Smith, 1947; Committee of Flora of China, 1996). Currently, it has been included in the Chinese Key Protected Wild Medicinal Species List, and it is considered to be a severely reduced resource among the major common species of wild medicinal herbs (Lu et al., 2012; Guo et al., 2013). Without enough knowledge about the habitat requirements of this species, species resource protection is improbable.
Models are essential tools to assess the potential response of vegetation to climate change, particularly if large spatial and temporal scales are considered (Araújo & Luoto, 2007; Austin & Van Niel, 2011; Adhikari, Barik & Upadhaya, 2012; Bean et al., 2014). SDMs are increasingly used to forecast the potential changes in species distributions under climate change scenarios. However, traditional SDMs can neither predict the change of plants’ medicinal component contents in different geographical conditions nor the potential response of plants’ medicinal component contents to climate change (Lu et al., 2012; Guo et al., 2013).
In this study, researchers collected S. sphenanthera samples at 307 sampling points in 19 sampling sites and extracted schisantherin A using high performance liquid chromatography (HPLC). Meanwhile, in order to test applicability of our model for other habitats besides the Qinling Mountains, 60 samples from four locations have been collected. We used a fuzzy set to determine the association of schisantherin A content with the 19 climatic variables. Then, the maximum entropy model was used to determine the weight of each variable. Finally, we estimated the spatial distribution of S. sphenanthera in the Qinling Mountains using the weighted average method and geographical information system (GIS) spatial analysis. Researchers obtained the suitable range of each variable affecting plant growth and spatial distribution. By using this habitat-suitability assessment model and the same climatic data from three general circulation models (GCM) for three different climate change scenarios (Special Report On Emissions Scenarios, SRES) (SRES-A1B, SRES-A2 and SRES-B1 emission scenarios), the researchers predicted the potential geographic distribution of S. sphenanthera in the Qinling Mountains for the years 2020–2029 (2020s), 2050–2059 (2050s) and 2080–2089 (2080s). This information will supply construction and advice for the protection and sustainable utilization of resources, and provide some measures and proposals for the prediction of the distribution and quality of medicinal plants under climate change conditions.
Materials and Methods
Study area and species data
In this study, we selected the Qinling Mountains as our study area. The Qinling Mountains are one of the main producing areas of medicinal materials and plants. And this area also contains a large number of endangered geoherbs (Dang et al., 2010; Lu et al., 2012). Climate change will certainly affect the Qinling Mountains climate, which will inevitably affect the habitable environment and moisture and temperature conditions in the Qinling Mountains (Fan et al., 2014), thus affecting herb spatial patterns and quality in the region.
According to the literature record of the distribution of S. sphenanthera and studies on the range of the Qinling Mountains (Liu & Chen, 1986; Kang & Zhu, 2007), we defined the whole study area to encompass the Qinling Mountains (Fig. 1). Latitude in this area ranges from N30 °30′ to N35 °30′ and longitude from E103 °45′ to E113 °45′. It runs across six provinces (or municipalities) from the west to the east, including Gansu, Sichuan, Shaanxi, Chongqing, Hubei, and Henan, and it covers nearly 190,000 km2.
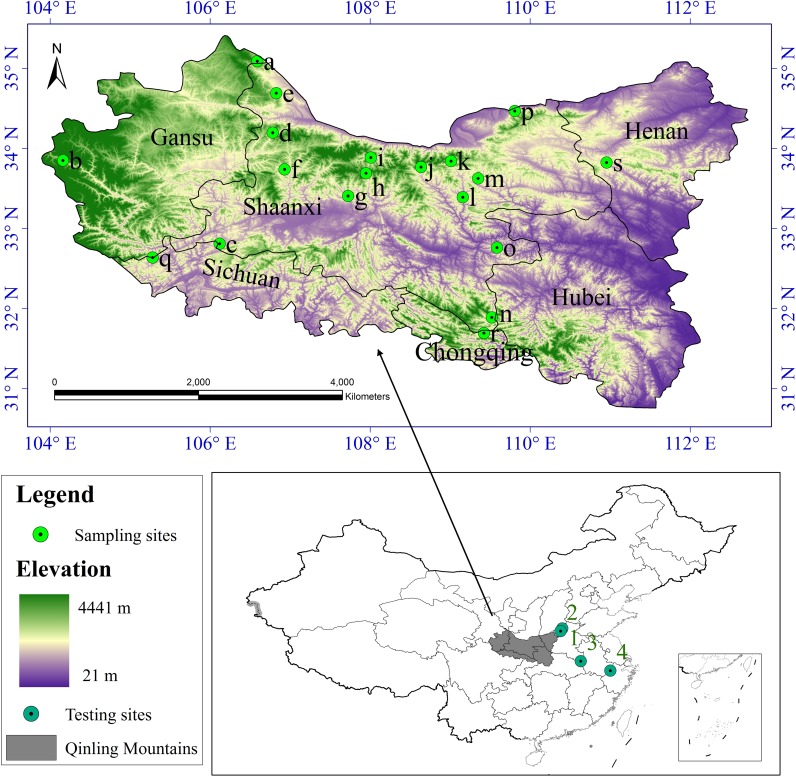
Figure 1. Study area and sampling sites distribution.
a Huating, in Gansu; b Zhouqu, in Gansu; c Ningqiang, in Shaanxi; d Fengxian, in Shaanxi; e Longxian, in Shaanxi; f Liuba, in Shaanxi; g Maoping, in Shaanxi; h Foping, in Shaanxi; i Taibai, in Shaanxi; j Ningshan, in Shaanxi; k Yingpan, in Shaanxi; l Zhen’an, in Shaanxi; m Fengzhen, in Shaanxi; n Zhenping, in Shaanxi; o Xunyang, in Shaanxi; p Huaxian, in Shaanxi; q Qingchuan, in Sichuan; r Wuxi, in Chongqing; s Lushi, in Henan. 1 Lingchuan, in Shanxi; 2 Xiuwu, in Henan; 3 Jinzhai, in Anhui; 4 Lin’an, in Zhejiang.
We collected S. sphenanthera samples throughout the range of the Qinling Mountains from 2007–2011 (Fig. 1). We chose the reasonable sampling sites by considering the different environmental conditions in traditional and original producing area of S. sphenanthera. Meanwhile, to improve the sampling accuracy, the sampling points were distributed evenly. But because of the influence of human activities, S. sphenanthera wild resources are being depleted, and some traditional areas where the plant could be found no longer host this species, at the same time in complex terrain, we are unable to obtain sample point data, so the spatial distribution of the sampling points in this study are not completely evenly distribution. At every sampling point, 10–20 plant samples had been collected, and to prevent cloning, the distance between samples was controlled more than 100 m. We used global positioning system (GPS) to record basic geographic information, including the longitude, latitude, altitude, slope and aspect. The Committee of National Pharmacopoeia (2010) has clearly defined that schisantherin A is a symbol component in the fruit of S. sphenanthera. Therefore, the content of schisantherin A in fruit of S. sphenanthera was chosen as the indicator, and it was tested by HPLC (Gu, Wei & Wang, 2008; Lu et al., 2012).
Climatic variables, future scenarios and spatial conversion
In order to work out the geographic distribution of suitable habitat of a target species, a set of characteristics about this species must be defined (Lu et al., 2012). In this study,we used 19 bioclimatic variables (Table 1) (Nix, 1986). And we obtained bioclimatic variables from the WorldClim database (www.worldclim.org/current). These variables were generated using averaged interpolated climate data during the period from 1950 to 2000 (Hijmans et al., 2005), and the resolution was 30” (about 1 km2).
Table 1. Explanatory variables used to model of the distribution for S. sphenanthera.
| Code | Description |
|---|---|
| Bio1 | Annual mean temperature |
| Bio2 | Mean diurnal temperature range |
| Bio3 | Isothermality (BIO2/BIO7) (* 100) |
| Bio4 | Temperature seasonality (standard deviation *100) |
| Bio5 | Max temperature of warmest month |
| Bio6 | Min temperature of coldest month |
| Bio7 | Temperature annual range (BIO5-BIO6) |
| Bio8 | Mean temperature of wettest quarter |
| Bio9 | Mean temperature of driest quarter |
| Bio10 | Mean temperature of warmest quarter |
| Bio11 | Mean temperature of coldest quarter |
| Bio12 | Annual precipitation |
| Bio13 | Precipitation of wettest month |
| Bio14 | Precipitation of driest month |
| Bio15 | Precipitation seasonality (coefficient of variation) |
| Bio16 | Precipitation of the wettest quarter |
| Bio17 | Precipitation of driest quarter |
| Bio18 | Precipitation of warmest quarter |
| Bio19 | Precipitation of coldest quarter |
We used the same bioclimatic variables projected into the future. Potential values for bioclimatic variables for future climate conditions in the 2020s, 2050s and 2080s, respectively, were derived from three general circulation models (GCMs): BCCR-BCM2.0 (BC), CCCMA_CGCM3 (CC), and MIROC32_medres (MI), under for the SRES-A1B, SRES-A2 and SRES-B1 emission scenarios (IPCC, 2001).The SRES was a report by the IPCC which was published in 2000. The SRES was a set of emissions scenarios. It covers a wide range of the main driving forces of future emissions, from demographic to technological and economic developments (IPCC, 2001). SRES-A1B means a balanced across energy sources in future world. The SRES-A2 driven by economic growth although at regional scale, create a diversified political and social world and regionally oriented economic growth that is more fragmented and slower than that in other storylines. The SRES-B1 is undoubtedly the “greenest” of all projections, with high levels of environmental and social consciousness and a global sustained development (IPCC, 2001).
The bioclimatic variables for the future scenarios were from the IPCC Fourth Assessment Report (available from the International Centre for Tropical Agriculture: http://ccafs-climate.org). This method assumes changes in climates only over large distances and the relationships between variables are maintained from the present towards the future (see http://ccafs-climate.org/ for more details). Future climate variables also had a resolution of 30” (about 1 km2).
Modeling procedures and testing
Based on the selection criteria of the standardization method, perfect results could be obtained by using the membership function in the fuzzy mathematics (Lu et al., 2012; Guo et al., 2013). For defining the degree of suitability of all factors in a finite set (scope) for S. sphenanthera, the values of the fuzzy membership using the statistical association between the content of schisantherin A and each factor was used to quantify the relation between habitat variables and habitat suitability. For each environmental variable, a suitability curve is defined that assigns every possible value of the variable a degree of suitability. The degree value ranges from 0 to 1. A value of 0 indicates that an attribute has complete non-membership (not suitable at all) in a fuzzy set, which means that under these environmental conditions, S. sphenanthera cannot grow or cannot completely synthesize and accumulate schisantherin A. Meanwhile, a value of 1 indicates that an attribute has a complete membership (optimal suitable) in a fuzzy set, which means that under these environmental conditions, the synthesis and accumulation of schisantherin A in S. sphenanthera is optimal, and the environment in this scenario is suitable for the growth of S. sphenanthera. Grades between 0 and 1 imply that an attribute has partial of membership in a fuzzy set. (Rüger, Schlüter & Matthies, 2005; Mouton et al., 2011).
In this study, 75 % of the distribution points were selected at random as the training data set, and the remaining points were used as the test data set. Among various existing membership functions, the Gaussian membership function (Eq. (1)) and the Trapezoidal membership function (Eqs. (2) and (3)) were used to standardize each factor (Table 2) (Lahdelma, Salminen & Kuula, 2003). The error inspection of each factor to the membership function was computed by Matlab 2011 (MathWorks, 2011).
| (1) |
where x is the independent variable, and a, σ are parameters, in this definition when x = a, the membership value is 1.
| (2) |
| (3) |
In Eqs. (2) and (3), where x is the independent variable, and a, b are parameters.
Table 2. Membership functions of factors and its fitting degree.
| Code (Unit) | Membership function | Optimal value | Threshold value | Fitting degree | Weight (%) |
|---|---|---|---|---|---|
| Bio1 ( °C) | Gaussian-type | 10.3 | 7.3, 13.2 | 85.44 | 6.37 |
| Bio2 ( °C) | Subsection Gaussian-type | 88.4 | 70, 135 | 85.70 | 5.48 |
| bio3 | Gaussian-type | 0.26 | 0.22, 0.31 | 80.52 | 3.74 |
| bio4 | Gaussian-type | 83.10 | 72.16, 4.03 | 80.36 | 4.13 |
| Bio5 ( °C) | L trapezoidal | >26.70 | 25.20 | 89.02 | 3.33 |
| Bio6 ( °C) | L trapezoidal | <−2.70 | −8.60 | 83.50 | 3.74 |
| Bio7 ( °C) | Gaussian-type | 33.00 | 30.10, 35.80 | 80.81 | 7.10 |
| Bio8 ( °C) | R trapezoid | 23.00 | 18.10 | 93.40 | 5.21 |
| Bio9 ( °C) | Gaussian-type | 5.60 | −20.00, 32.00 | 79.54 | 1.34 |
| Bio10 ( °C) | R trapezoid | 23.80 | 19.20 | 96.93 | 5.36 |
| Bio11 ( °C) | Gaussian-type | 4.04 | −21, 29 | 76.63 | 5.31 |
| Bio12 (mm) | Gaussian-type | 853.00 | 525.00, 1181.00 | 77.34 | 6.51 |
| Bio13 (mm) | Gaussian-type | 144.00 | 96.00, 192.00 | 86.52 | 5.92 |
| Bio14 (mm) | Gaussian-type | 10.14 | 0.94, 19.00 | 81.27 | 5.49 |
| Bio15 | Gaussian-type | 0.70 | 0.56, 0.82 | 84.27 | 5.82 |
| Bio16 (mm) | Gaussian-type | 415.20 | 294.00, 536.00 | 84.97 | 6.79 |
| Bio17 (mm) | Gaussian-type | 36.11 | 7.17, 65.04 | 78.75 | 5.76 |
| Bio18 (mm) | Gaussian-type | 367.20 | 251.00, 483.00 | 76.20 | 6.66 |
| Bio19 (mm) | Subsection Gaussian-type | 27.77 | 5.36 | 96.60 | 5.94 |
After establishing the factor’s fuzzy membership for S. sphenanthera, the suitability ratings were calculated. To do so, assigning the weight of different factors is very important. This is the key to the reliability of the evaluation results. The factor weight is the contribution degree of the effects of factors to the growth and quality of S. sphenanthera, which varies among different factors. To eliminate subjective influence, the current study takes advantage of the maximum entropy method (MEM) to determine the weights of factors. MEM is a parameter that describes how many different alternative approaches exist with respect to a certain attribute. Maximum information entropy weighting models embody the observed characteristics of the data, and the greatest degree of avoiding the influence of artificial factors is achieved. At the same time, according to the degree of change in the data set, the multidimensional ecological dominant factors that affect the species distribution in space are identified. Additionally, it does not require much data, which are rarely available (Phillips, Anderson & Schapire, 2006) can produce solutions with a small set of observations (Weber, 2011).
Based on the MEM and the fuzzy mathematics, membership functions were calculated to obtain the values of all factors and units, while the weights were obtained from the MEM. Then, the weighted average method [Eq. (4)] was used to comprehensively assess the values of S. sphenanthera ecological suitability in the study area (Lu et al., 2012; Guo et al., 2013).
| (4) |
where yi (integrated ecological suitability index) refers to the comprehensive value of assessment of S. sphenanthera ecological suitability in each evaluation unit, n is the number of factors, wj is the weight of a factor, and xij is the value of the ith grid cell for the jth variable. The scope of yi was defined as 0–1. The higher the yi value means the greater the contribution of ecological factors to the quality level of S. sphenanthera. We applied spatial analysis using ArcGIS 9.3 (Redlands, CA, USA) to calculate the raster and to set the cell size at the maximum of the inputs. Therefore, the maps of the habitat have a suitability assessment resolution of 30” (about 1 km2).
The root mean square error (RMSE) and determination coefficient (R-squared) were used to validate the results of the habitat suitability assessment for this plant. RMSE and R-squared were calculated as (Eq. (5)) (Willmott, 1984) and (Eq. (6)) (Magee, 1990):
| (5) |
| (6) |
where N is the number of samples, Pi is the ith predicted value and Qi represents the ith observed value and is the mean of the observed value. The smaller the RMSE is, the better the interpolation method (Li & Heap, 2011). R-squared summarize the discrepancy between observed values and their predicted values, the values of R-squared vary from 0 to 1. Meanwhile, to test the applicability of our model in other habitats besides the Qinling Mountains, 60 samples from four locations (Fig. 1, 1–4) were collected, and the data were assessed using the above mentioned habitat suitability assessment model.
Potential change in the distributions of species
We used the above mentioned model to study the potential changes in the distributions of S. sphenanthera. The future scenarios were regionalized for the study area from three general circulation models (GCM) for the SRES-A1B, SRES-A2 and SRES-B1 (IPCC, 2001), and for the three time (2020s, 2050s and 2080s) periods. All operations were automated with a script made in python programming language and incorporated as a toolbox in ArcGIS 9.3 (Redlands, CA, USA).
Results
The determination of the fuzzy membership functions
The membership function was established to explore the relationships between factors and membership degrees by cure-fitting and drawing scatter diagrams. Because the interaction between the growth and quality of S. sphenanthera and ecological factors was quite different, different types of fuzzy membership functions were chosen to express their relationships. Therefore, in this research, gaussian-type functions were used for quantitative factors because most factors have upper and lower boundaries in which the membership degree is zero. The L trapezoidal function was used for quantitative factors that only had lower boundaries, and the R trapezoid type function was used for quantitative factors that only had upper boundaries. The membership degrees, extending from 0 to 1, can be calculated according to these membership functions. When the membership degree is 1, it is considered to be the best scenario of ecological factors suitable for the growth of S. sphenanthera. However, when the membership degree is 0, the ecological factor has a serious deficiency. The fitting degree of all membership functions varies from 76.20 % to 96.93 % (Table 2), with an average of 84.09 %. This verifies our model’s accuracy.
Distribution of suitable habitats in the current climate environment
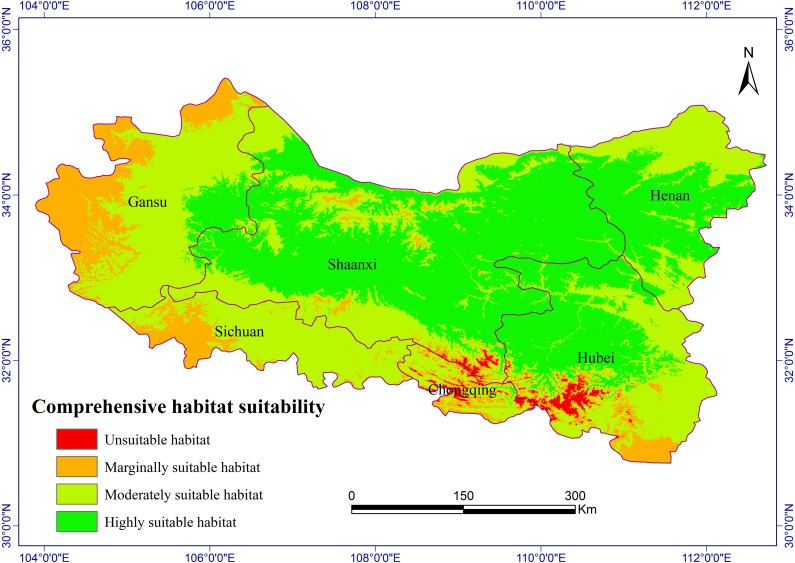
The Chinese Pharmacopoeia clearly states that the minimum content of schisantherin A in S. sphenanthera fruit is 0.2 % (Committee of National Pharmacopoeia, 2010). In this study, the content of schisantherin A in the 307 samples was 0.015–1.948 %, we designated the content proportions of schisantherin A less than 0.2 % as unsuitable. According to the content of schisantherin A and the ecological suitability index calculated by our model, the habitat suitability of S. sphenanthera in the Qinling Mountains can be classified into four grades: unsuitable, marginally suitable, moderately suitable, and highly suitable (yi < 0.3, unsuitable habitat; 0.3 ≤ yi < 0.5, marginally suitable habitat; 0.5 ≤yi < 0.7, moderately suitable habitat; and yi ≥ 0.7, highly suitable habitat). We have applied spatial analysis using ArcGIS 9.3 (Redlands, CA, USA) to draw the comprehensive distribution map of habitat suitability of S. sphenanthera in the Qinling area (Fig. 2), and we calculated the area of all of the habitat suitability grades. The results show that the proportions of unsuitable, marginally suitable, moderately suitable, and highly suitable habitats for S. sphenanthera in the Qinling Mountains are 1.12 %, 11.76 %, 43.69 % and 43.43 %, respectively. The highly suitable habitats are mainly located in southern Shaanxi, eastern Gansu, western Henan, and northern Hubei province, and their surrounding areas, with an elevation of 800 to 2000 m.
Figure 2. Habitat suitability comprehensive distribution of S. sphenanthera in Qinling area.
The RMSE of the habitat suitability assessment for S. sphenanthera is 0.056 and the R-squared is 0.76, indicating that the simulation values are highly consistent with the observed values. Therefore, the model was built in the study is accurate and available. Moreover, the RMSE is 0.075 and the R-squared is 0.68 for the data from the 60 sample sites outside of the Qinling Mountains district, which demonstrates that the model is applicable to other areas besides the Qinling Mountains.
Projecting the effects of climate change on the distribution and quality of S. sphenanthera in the Qinling Mountains
The habitat suitability assessment model based on GIS and the fuzzy mathematics could be used to accurately determine the habitat suitability of S. sphenanthera and to quantify the area of suitable habitat. Using it as a base, the future distributions of S. sphenanthera in the 2020s, 2050s and 2080s under the climate change scenarios of SRES-A1B (Fig. 3), SRES-A2 (Fig. 4) and SRES-B1 (Fig. 5) from IPCC were projected. The SRES scenarios were constructed to explore future developments in the global environment with special reference to the production of greenhouse gases and aerosol precursor emissions, which is an important means of evaluating the future climate change.
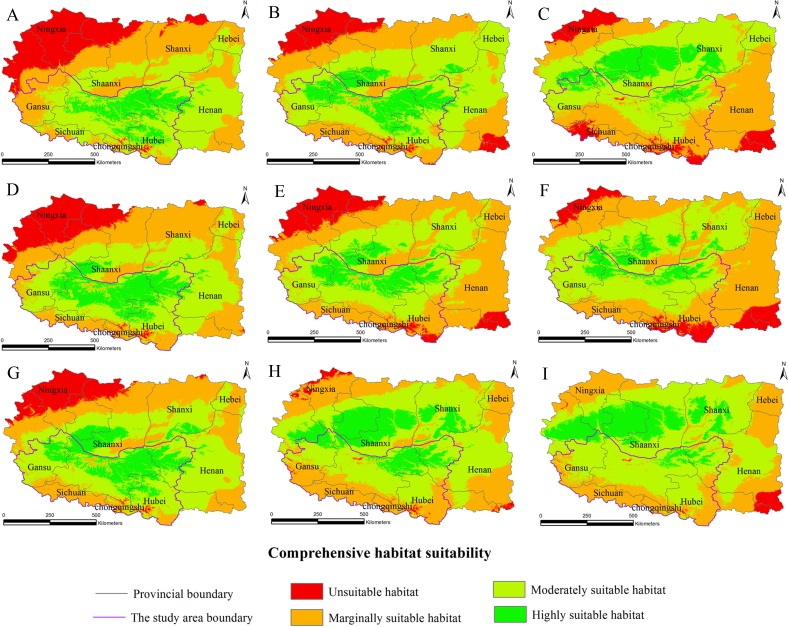
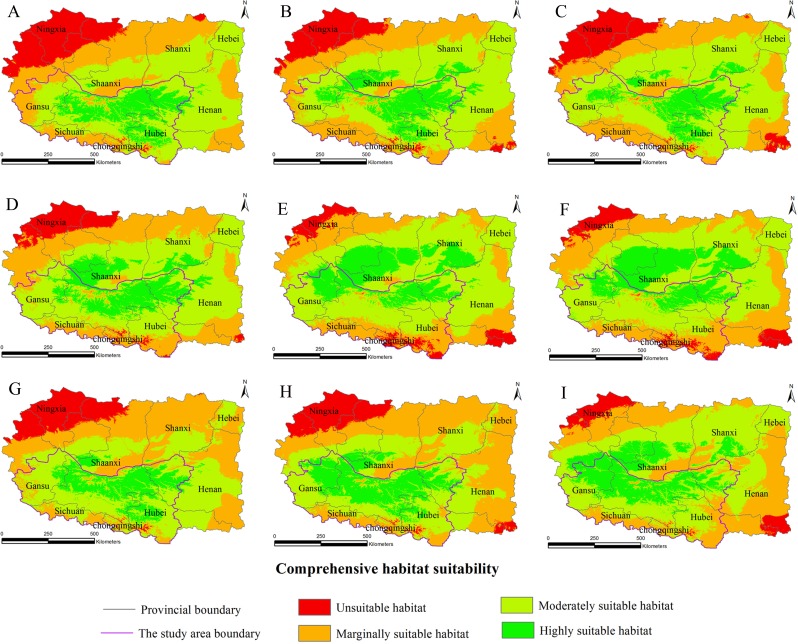
Figure 3. Distribution map of habitat acclimatization for S. sphenanthera based on three GCM in SRES-A1B.
(A) 2020s in GCM of BC; (B) 2050s in GCM of BC; (C) 2080s in GCM of BC; (D) 2020s in GCM of CC; (E) 2050s in GCM of CC; (F) 2080s in GCM of CC; (G) 2020s in GCM of MI; (H) 2050s in GCM of MI; (I) 2080s in GCM of MI. Future scenarios were regionalized within the study area from the following general circulation models (GCM): BCCR-BCM2.0 (BC), CCCMA_CGCM3 (CC), and MIROC32_medres (MI).
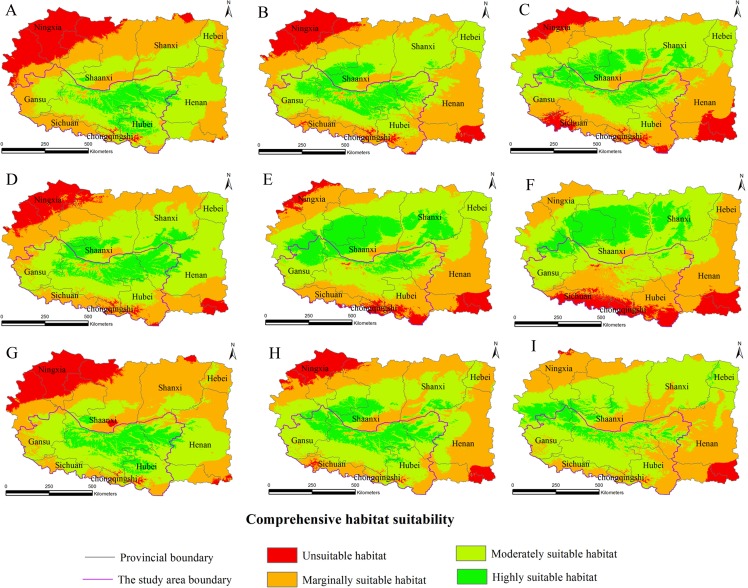
Figure 4. Distribution map of habitat acclimatization for S. sphenanthera based on three GCM in SRES-A2.
(A) 2020s in GCM of BC; (B) 2050s in GCM of BC; (C) 2080s in GCM of BC; (D) 2020s in GCM of CC; (E) 2050s in GCM of CC; (F) 2080s in GCM of CC; (G) 2020s in GCM of MI; (H) 2050s in GCM of MI; (I) 2080s in GCM of MI. Future scenarios were regionalized within the study area from the following general circulation models (GCM): BCCR-BCM2.0 (BC), CCCMA_CGCM3 (CC), and MIROC32_medres (MI).
Figure 5. Distribution map of habitat acclimatization for S. sphenanthera based on three GCM in SRES-B1.
(A) 2020s in GCM of BC; (B) 2050s in GCM of BC; (C) 2080s in GCM of BC; (D) 2020s in GCM of CC; (E) 2050s in GCM of CC; (F) 2080s in GCM of CC; (G) 2020s in GCM of MI; (H) 2050s in GCM of MI; (I) 2080s in GCM of MI. Future scenarios were regionalized within the study area from the following general circulation models (GCM): BCCR-BCM2.0 (BC), CCCMA_CGCM3 (CC), and MIROC32_medres (MI).
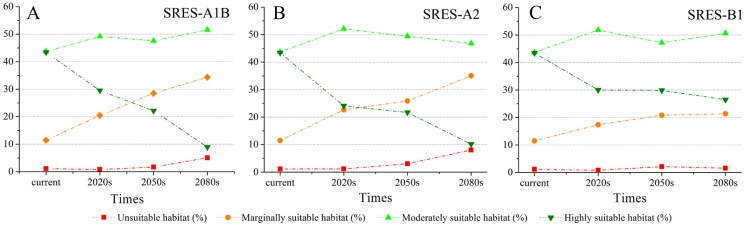
Using the projected climate data for the studied area, we reassessed the current S. sphenanthera distribution to reflect a changing climate. These predictions provide a comprehensive assessment of S. sphenanthera ecological suitability. Using the same standard of classification, the future habitat suitability of S. sphenanthera in the Qinling Mountains is classified into four grades. The data were derived from the average of the three types of GCMs (Fig. 6). The model forecast indicates that in the study area, the unsuitable habitats of S. sphenanthera under climate change scenarios SRES-A1B and SRES-A2 were gradually increased. As time goes on, unsuitable habitat areas will expand in the northeast of Chongqing and the northwest of Hubei (Figs. 3 and 4). The unsuitable habitat of SRES-B1 does not represent a large change, even a slight reduction with time. The marginally suitable habitat of S. sphenanthera under the three climate change scenarios would rapidly increase. The difference between the climate changes scenarios is a higher increasing rate in the SRES-A1B and SRES-A2 scenarios. The moderately suitable habitat of S. sphenanthera under all climate change scenarios remained relatively stable. The highly suitable habitat of S. sphenanthera under the climate change scenarios of SRES-A1B, SRES-A2 and SRES-B1 gradually decreased. Some of the differences between the climate change scenarios are worth mentioning. Generally, in the study area, a larger decline rate of highly suitable habitat area in both GCMs refers to the SRES-A1B and SRES-A2.
Figure 6. The variation of the percentage of areas of habitat suitability comprehensive distribution of S. sphenanthera under different climate change scenarios.
(A) SRES-A1B; (B) SRES-A2; (C) SRES-B1.
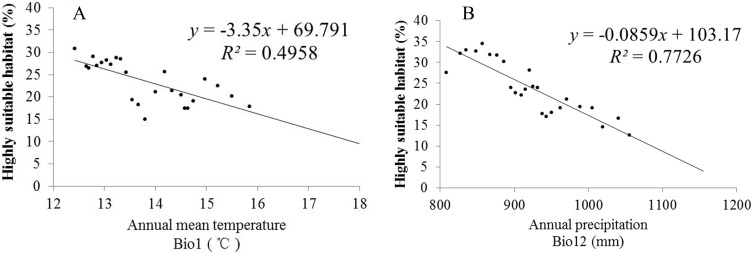
Climate change will significantly affect plant distribution. With the support of the ArcGIS 9.3 (Redlands, CA, USA) platform, we carried out statistics that can represent the future climate data in the model operations. To explore the relationships between climatic change and the distribution of S. sphenanthera, SPSS 19.0 software (IBM, Armonk, North Castle, NY, USA) was used to determine the correlations between Bio1 (annual mean temperature), Bio12 (annual precipitation) and the area proportion of the four habitat types of S. sphenanthera in the studied area. Table 3 shows that Bio1, Bio12 and the area of highly suitable habitat have a significant negative correlation. As Bio1 and Bio12 increased, the highly suitable habitat area gradually decreased. To further explore this quantitative relation and future change trend, we performed linear regression analysis (Fig. 7). The results indicate that when Bio1 of the studied area exceeds 20 °C and Bio12 exceeds 1200 mm, the study area will no longer have highly suitable habitat areas for S. sphenanthera. The result showed that climate change will affect the distribution and quality of S. sphenanthera, which will further increase the rate of this species’ extinction.
Table 3. The correlation between Bio1, Bio 12 and S. sphenanthera’s different grades of habitat suitability in the studied area.
| Code | Unsuitable habitat | Marginally suitable habitat | Moderately suitable habitat | Highly suitable habitat |
|---|---|---|---|---|
| Bio1 | .151 | .380* | .292 | −.514** |
| Bio12 | .326 | .558** | .051 | −.598** |
Notes.
Correlation is significant at the 0.05 level (2-tailed),
Correlation is significant at the 0.01 level (2-tailed).
Figure 7. Linear regression analysis of Bio1, Bio12 and its corresponding percentage of highly suitable habitats of S. sphenanthera.
(A) Bio1 (Annual mean temperature); (B) Bio12 (Annual precipitation).
Discussion
Advantages of the methods and implications for ecological modeling
The reliability of the evaluation results depends on the integrity of the database and the rationality of the evaluation methods (Lu et al., 2012; Guo et al., 2013). GIS is an important tool for the analysis of medicinal plants’ ecological suitability using species presence records associated with environmental variables to determine the essential environmental requirements of a particular species. The suitability evaluation model was integrated with the GIS software ArcGIS, which could quickly provide evaluation results after inputting the parameters of the model. Fuzzy theory was widely used in ecological environment modeling (Rocchini, 2014; Mouton et al., 2011), and how to determine membership function is a crux of this theory. Usually, the parameters of fuzzy membership functions were calculated based on the expert knowledge (Zhu et al., 2010; Wang, Hong & Tseng, 2000). But in this study, to make sure the consistency between the sampling data and the bioclimatic data, we extract bioclimatic variables values by sampling sites, and then get the bioclimatic data sequence for model training. Finally, using this data, the fuzzy membership function was defined, which include the information on relationship between the value of 19 climatic variables and schisantherin A content of S. sphenanthera samples. This method can standardize variables with no prior knowledge as well as retain information about the original data.
There are many methods for determining the weight of factors, such as the Delphi (Lehtonen & Tykkyläinen, 2014), AHP (Houshyara et al., 2014) and principal component analysis (Alfaro et al., 2014). The methods such as Delphi and AHP need priori knowledge or additional expertise, but S. sphenanthera is a wildlife species, and is lacking sufficient expert knowledge and conventional established definitions. For principal component analysis, to get reasonable results, the model requires a lot of data, so it is not suitable for this research. MEM is an objective approach using information provided by the observation data to determine the weight of climatic variables. It can objectively evaluate the habitat suitability and eliminate subjective influence. MEM emphasizes the influence of strongly restrictive factors on targets. Moreover it determines the dominant influencing factor of schisantherin A.
Habitat requirements and spatial distribution of species are essential to biological conservation and management. In this study, our aim is to establish a universal model for simulation quality of wild herbs in different environmental conditions, which means For wild medical plant without explicit habitat requirements, this study can only be use the information provided by the statistical data to build up a model, and according to the research target, this model should not only forecast spatial variation of medicinal plants quality, but also can determine the mathematical relationship between value of each impact factor and the content of effective composition in wild herbs. Therefore, the general statistical model cannot meet the requirements of this study. Moreover, the samples data in this study were collected by field work in Qinling Mountains, every data consumed extremely labor, material, and financial resources, especially in mountains or regions of complex terrain. Thus, only limited data was provided for model building, it can’t meet the requirements of other machine learning model such as artificial neural network model, support vector machine, and random forest. At the same time, most of the machine learning models are a black-box model, which cannot show relationship between the value of each impact factor and the content of effective composition. This means machine learning models are also not suitable for this study. However, Fuzzy mathematics provides a way to solve this problem. Firstly, fuzzy membership function can standardize the factors with no prior knowledge and retain the information of original data. Secondly, through this method we can obtain suitable range of each factor for medical plant, and this information is significant for wildlife protection. Finally, this method does not require large amounts of data, and gives a satisfactory result.
The reasonability of the bioclimatic variables
Bioclimatic variables are derived from the monthly temperature and rainfall values in order to generate more biologically meaningful variables. These variables were created by the thin-plate smoothing spline algorithm implemented for interpolation, using latitude, longitude, and elevation as independent variables, and the uncertainty arising from the input data and the interpolation was quantified by mapping weather station density, elevation bias in the weather stations, and elevation variation within grid cells and through data partitioning and cross validation. All of this guarantees the accuracy of the bioclimatic data (Hijmans et al., 2005). Furthermore, those data had been proven to be useful for SDMs; at the same time, it’s also one of the most widely used data (Rödder & Lötters, 2009).
Previous researches indicated that temperature and the moisture factor (especially in spring and autumn) are the main climate factors which control the growth of plants (Trisurat, Shrestha & Kjelgren, 2011). Only under the circumstances of appropriate temperatures and sufficient water can plants have normal growth and accumulate particular organics. S. sphenanthera is a woody vine, supporting itself on rocks, shrubs, and trees in broad-leaved evergreen forests or coniferous mixed forests (Committee of Flora of China, 1996). On the base of phenological observation, we have a preliminary understanding of the mechanism of the S. spenanthera. In S. sphenanthera growth process, the period of sap flow begin in early March, and the sprout leaves period is from late March to mid-April, in this period, low temperature or low rainfall will kill the buds or delay sprout leaves period. In late April S. spenanthera begin to blossom, and the blossom and young fruit period continue to end of June, at the same time it’s also a peak-growth period. The developing fruit period is begin in early July, and the fruit ripe period begin in early August, then its followed by abscission period which last from October to November, and from July to September is the warmest quarter in study area and usually, the wettest month also happen in this three months, it is the key period for generation and accumulation of schisantherin A. Therefore, in the whole growth period, suitable temperature and precipitation is significant for S. sphenanthera. The bioclimatic variables represent annual trends, seasonality, and extreme or limiting temperature and precipitation factors in plant life history, so they are sufficient for this research.
Lu et al. (2012) found that temperature and precipitation in the growing period have important effects on the distribution of S. sphenanthera. Hu et al. (2012) found that, according to MaxEnt, Bio13 (precipitation of the wettest month), Bio18 (precipitation of the warmest quarter), and Bio1 (annual mean temperature) have important effects on the distribution of Schisandra chinensis. S. chinensis, another species in the genus. In this study, according to the contributions of individual factors to the content of schisantherin A (Table 2), five dominant climatic factors controlled S. sphenanthera distribution. These five dominant features are Bio7 (temperature annual range), Bio16 (precipitation of the wettest quarter), Bio18 (precipitation of the warmest quarter), Bio12 (annual precipitation) and Bio1 (annual mean temperature). The weight of each of these features is higher than 6 %. Our results are therefore somewhat similar with those of Lu et al. (2012) and Hu et al. (2012).
The effects of climate change on the highly suitable habitat of S. sphenanthera
Over time, a new suitable habitat for S. sphenanthera would be generated while its normally suitable habitat would be lost. To show this change, we expanded the areas around the studied area to generate the future suitable habitat of S. sphenanthera. The highly suitable habitat of S. sphenanthera under the climate change scenarios of SRES-A1B and SRES-A2 was shifted to the north and gradually reduced (Figs. 3 and 4). By the 2020s, the highly suitable habitat area in eastern Gansu would be significantly reduced. The same situation would occur in Shaanxi, Hubei and Henan provinces with habitat fragmentation in the study area (Figs. 3A, 3D, 3G, 4A, 4D and 4G). By the 2050s, there would be a highly suitable habitat in the east of Gansu, which is located in the north of the studied area. There was little or almost no highly suitable habitat in Hubei. The highly suitable habitats of S. sphenanthera would continue to reduce in Shaanxi and Henan, with further habitat fragmentation occurring in the study area. In the meantime, outside of the studied area, new highly suitable habitats are likely to appear in northern Gansu, central Shaanxi and central Shanxi (Figs. 3B, 3E, 3H, 4B, 4E and 4H). By the 2080s, in the study area, the highly suitable habitat of S. sphenanthera would fall sharply and only a little area in parts of southern Shaanxi and west Henan would remain. However, outside of the study area in northern Gansu, central Shaanxi and central Shanxi, new highly suitable habitat areas would be further expanded, leading to a new distribution area of S. sphenanthera (Figs. 3C, 3F, 3I, 4C, 4F and 4I).
The other factors which affect the distribution of S. sphenanthera
In addition to climate change, human interference is also an important factor in influencing the distribution of S. sphenanthera. Theoretically, S. sphenanthera can grow in all of the highly suitable habitats within the scope of its distribution area. Due to the effects of human activities such as over-grazing and wanton picking, wild S. sphenanthera resources are facing depletion. Traditional habitats no longer host this species. The phenomenon of habitat fragmentation is irreversible. The proliferation ability of S. sphenanthera is also a key factor affecting its distribution. Because S. sphenanthera is a woody vine, its growth has more stringent environmental requirements. Research has shown that land cover types in the highly suitable habitats of S. sphenanthera include broad-leaved evergreen forests that provide support to climbing plants (Lu et al., 2012). Future climate warming will impact the highly suitable habitats of S. sphenanthera. Additionally, the distribution of the highly suitable habitats of S. sphenanthera would shift to higher latitudes in response to climate change. However, in the studied area, like northern Shaanxi and some parts of the eastern Gansu area, the present vegetation type is not suitable for S. sphenanthera growth. At present, because the understanding level and the technical conditions are limiting, this study cannot include all factors influencing the distribution and quality of S. sphenanthera. If all factors were considered, we can deduce that in the study area, the suitable habitat of S. sphenanthera under the climate change scenarios would reduce by a larger range, and new suitable habitat range will be smaller.
Conclusions
Medicinal plants represent a significant contribution to human health, and with the continuous development of global warming, some of wild medicinal plants would change their distribution patterns as well as quality. This study provided a new case for that theory. Firstly, samples of wild herb plant S. sphenanthera were collected form field work in Qinling Mountains, and the content of schisantherin A in the fruit were examined by HPLC, then with this data combining fuzzy theory and a maximum entropy model, we developed a method to project the spatial assessment of medicinal plants quality, and used this model we predict future quality and distribution of S. spenanthera in Qinling Mountains. The result showed that under future climate scenarios, the habitats of high quality S. sphenanthera will continue to decrease and draw near to extinction. As the prediction of species distribution is influenced by multiple factors, this study predicted the effects of climate change on the distribution and quality of medicinal plants. Further research is required, and the present study only provides a reference for related research and native habitat protection.
Supplemental Information
Funding Statement
This work is supported by the National Natural Science Foundation of China (No.31070293), the National Eleventh-Five Year Science and Technology Support Program from Ministry of Science and the Technology of the People’s Republic of China (No.2006BAI06A13-06). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Contributor Information
Haiyan Wei, Email: weihy@snnu.edu.cn.
Wei Gu, Email: weigu@snnu.edu.cn.
Additional Information and Declarations
Competing Interests
The authors declare there are no competing interests
Author Contributions
Yanlong Guo conceived and designed the experiments, performed the experiments, analyzed the data, contributed reagents/materials/analysis tools, wrote the paper, prepared figures and/or tables, reviewed drafts of the paper.
Haiyan Wei conceived and designed the experiments, performed the experiments, analyzed the data, reviewed drafts of the paper.
Chunyan Lu reviewed drafts of the paper, have made great contributions to revision.
Bei Gao analyzed the data, contributed reagents/materials/analysis tools, prepared figures and/or tables, reviewed drafts of the paper.
Wei Gu conceived and designed the experiments, performed the experiments, contributed reagents/materials/analysis tools, reviewed drafts of the paper.
Data Availability
The following information was supplied regarding data availability:
The raw data has been supplied as a Supplemental File.
References
- Adhikari, Barik & Upadhaya (2012).Adhikari D, Barik SK, Upadhaya K. Habitat distribution modelling for reintroduction of Ilex khasiana Purk., a critically endangered tree species of northeastern India. Ecological Engineering. 2012;40:37–43. doi: 10.1016/j.ecoleng.2011.12.004. [DOI] [Google Scholar]
- Alfaro et al. (2014).Alfaro CA, Aydin B, Valencia CE, Bullitt E, Ladha A. Dimension reduction in principal component analysis for trees. Computational Statistics and Data Analysis. 2014;74:157–179. doi: 10.1016/j.csda.2013.12.007. [DOI] [Google Scholar]
- Araújo et al. (2011).Araújo MB, Alagador D, Cabeza M, Nogués-Bravo D, Thuiller W. Climate change threatens European conservation areas. Ecology Letters. 2011;14:484–492. doi: 10.1111/j.1461-0248.2011.01610.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araújo & Luoto (2007).Araújo MB, Luoto M. The importance of biotic interactions for modelling species distributions under climate change. Global Ecology and Biogeography. 2007;16:743–753. doi: 10.1111/j.1466-8238.2007.00359.x. [DOI] [Google Scholar]
- Araújo & Rahbek (2006).Araújo MB, Rahbek C. How does climate change affect biodiversity? Science. 2006;313:1396–1397. doi: 10.1126/science.1131758. [DOI] [PubMed] [Google Scholar]
- Austin & Van Niel (2011).Austin MP, Van Niel KP. Improving species distribution models for climate change studies: variable selection and scale. Journal of Biogeography. 2011;38:1–8. doi: 10.1111/j.1365-2699.2010.02416.x. [DOI] [Google Scholar]
- Bartholomeus et al. (2011).Bartholomeus RP, Witte JPM, Van Bodegom PM, Van Dam JC, Aerts R. Climate change threatens endangered plant species by stronger and interacting water-related stresses. Journal of Geophysical Research Atmospheres. 2011;116(G4):116–120. doi: 10.1029/2011JG001693. [DOI] [Google Scholar]
- Bean et al. (2014).Bean WT, Prugh LR, Stafford R, Butterfield HS, Westphal M, Brashares JS. Species distribution models of an endangered rodent offer conflicting measures of habitat quality at multiple scales. Journal of Applied Ecology. 2014;51:1116–1125. doi: 10.1111/1365-2664.12281. [DOI] [Google Scholar]
- Bertrand et al. (2011).Bertrand R, Lenoir J, Piedallu C, Riofrío-Dillon G, Ruffray PD, Vidal C, Pierrat JC, Gégout JC. Changes in plant community composition lag behind warming in lowland forests. Nature. 2011;479:517–520. doi: 10.1038/nature10548. [DOI] [PubMed] [Google Scholar]
- Bystriakova, Peregrym & Dragicevic (2015).Bystriakova N, Peregrym M, Dragicevic S. Effect of environment on distributions of rock ferns in the mediterranean climate: the case of the genus asplenium in montenegro. Flora - Morphology Distribution Functional Ecology of Plants. 2015;215:84–91. doi: 10.1016/j.flora.2015.07.003. [DOI] [Google Scholar]
- Committee of Flora of China (1996). Committee of Flora of China . Flora of China. vol.30. Science Press; Beijing: 1996. pp. pp. 231–269. (in Chinese) [Google Scholar]
- Committee of National Pharmacopoeia (2010). Committee of National Pharmacopoeia . Pharmacopoeia of the People’s Republic of China. vol. 1. China Medical Science Press; Beijing: 2010. pp. pp. 227–228. (in Chinese) [Google Scholar]
- Dang et al. (2010).Dang H, Zhang Y, Zhang K, Jiang M, Zhang Q. Age structure and regeneration of subalpine fir (Abies fargesii) forests across an altitudinal range in the Qinling Mountains, China. Forest Ecology & Management. 2010;259:547–554. doi: 10.1016/j.foreco.2009.11.011. [DOI] [Google Scholar]
- Euskirchen, Carman & McGuire (2014).Euskirchen ES, Carman TB, McGuire AD. Changes in the structure and function of northern Alaskan ecosystems when considering variable leaf-out times across groupings of species in a dynamic vegetation model. Global Change Biology. 2014;20:963–978. doi: 10.1111/gcb.12392. [DOI] [PubMed] [Google Scholar]
- Fan et al. (2014).Fan J, Li J, Xia R, Hu L, Wu X, Li G. Assessing the impact of climate change on the habitat distribution of the giant panda in the Qinling Mountains of China. Ecological Modelling. 2014;274:12–20. doi: 10.1016/j.ecolmodel.2013.11.023. [DOI] [Google Scholar]
- Gu, Wei & Wang (2008).Gu W, Wei NY, Wang ZZ. LC analysis of lignans from Schisandra sphenanthera Rehd. et Wils. Chromatographia. 2008;67:979–983. doi: 10.1365/s10337-008-0621-7. [DOI] [Google Scholar]
- Guo et al. (2013).Guo YL, Gu W, Lu CY, Wei HY. Deoxyschizandrin and γ-schizandrin content in wild Schisandra sphenanthera to determine potential distribution in the Qinling Mountains. Chinese Bulletin of Botany. 2013;48:411–422 (in Chinese). doi: 10.3724/SP.J.1259.2013.00411. [DOI] [Google Scholar]
- Guo et al. (2014).Guo YL, Wei HY, Lu CY, Zhang HL, Gu W. Predictions of potential geographical distribution of Sinopodophyllum hexandrum under climate change. Chinese Journal of Plant Ecology. 2014;38:249–261 (in Chinese). doi: 10.3724/SP.J.1258.2014.00022. [DOI] [Google Scholar]
- Hijmans et al. (2005).Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. International Journal of Climatology. 2005;25:1965–1978. doi: 10.1002/joc.1276. [DOI] [Google Scholar]
- Houshyara et al. (2014).Houshyara E, Javad SheikhDavoodi M, Almassi M, Almassia M, Bahramia H, Azadib H, Omidic M, Sayyadd G, Witlox F. Silage corn production in conventional and conservation tillage systems. Part I: sustainability analysis using combination of GIS/AHP and multi-fuzzy modeling. Ecological Indicators. 2014;39:102–114. doi: 10.1016/j.ecolind.2013.12.002. [DOI] [Google Scholar]
- Hu et al. (2012).Hu LL, Zhang HY, Qin L, Yan BQ. Current distribution of Schisandra chinensis in China and its predicted responses to climate change. Chinese Journal of Applied Ecology. 2012;23:2445–2450. (in Chinese) [PubMed] [Google Scholar]
- IPCC (2001). IPCC . Emission scenarios: a special report of working group III of the intergovernmental panel on climate change. Cambridge University Press; Cambridge: 2001. pp. pp. 595–600. [Google Scholar]
- Kang & Zhu (2007).Kang MY, Zhu Y. Discussion and analysis on the geo-ecological boundary in Qinling Range. Acta Ecologica Sinica. 2007;27:2774–2784. (in Chinese) [Google Scholar]
- Lahdelma, Salminen & Kuula (2003).Lahdelma R, Salminen P, Kuula M. Testing the efficiency of two pairwise comparison methods in discrete multiple criteria problems. European Journal of Operational Research. 2003;145:496–508. doi: 10.1016/S0377-2217(02)00214-X. [DOI] [Google Scholar]
- Lehtonen & Tykkyläinen (2014).Lehtonen O, Tykkyläinen M. Delphi path simulator for unveiling development opportunities in the forest industries by contrasting forest management practices-the case of North Karelia. Technological Forecasting & Social Change. 2014;84:171–185. doi: 10.1016/j.techfore.2013.08.005. [DOI] [Google Scholar]
- Lenoir et al. (2008).Lenoir J, Gégout JC, Marquet PA, De Ruffray P, Brisse H. A significant upward shift in plant species optimum elevation during the 20th century. Science. 2008;320:1768–1771. doi: 10.1126/science.1156831. [DOI] [PubMed] [Google Scholar]
- Li & Heap (2011).Li J, Heap AD. A review of comparative studies of spatial interpolation methods in environmental sciences: performance and impact factors. Ecological Informatics. 2011;6:228–241. doi: 10.1016/j.ecoinf.2010.12.003. [DOI] [Google Scholar]
- Li et al. (2013).Li X, Tian H, Wang Y, Li R, Song Z, Zhang F, Xu M, Li D. Vulnerability of 208 endemic or endangered species in China to the effects of climate change. Regional Environmental Change. 2013;13:843–852. doi: 10.1007/s10113-012-0344-z. [DOI] [Google Scholar]
- Liu & Chen (1986).Liu JD, Chen YW. New geography of China. Shanghai People’s Press; Shanghai: 1986. pp. pp. 26–27. (in Chinese) [Google Scholar]
- Lu et al. (2012).Lu CY, Gu W, Dai AH, Wei HY. Assessing habitat suitability based on geographic infomation system (GIS) and fuzzy: a case study of Schisandra sphenanthera Rehd. et Wils. in Qinling Mountains, China. Ecological Modelling. 2012;242:105–115. doi: 10.1016/j.ecolmodel.2012.06.002. [DOI] [Google Scholar]
- Madaleno (2010).Madaleno IM. Traditional uses of organic medicinal plant species. In XXVIII international horticultural congress on science and horticulture for people (IHC2010): a new look at medicinal and aromatic plants seminar. ISHS Acta Horticulturae. 2010;925:321–326. [Google Scholar]
- Magee (1990).Magee L. R2 measures based on Wald and likelihood ratio joint significance tests. American Statistician. 1990;44(3):250–253. doi: 10.2307/2685352. [DOI] [Google Scholar]
- MathWorks (2011). MathWorks . MATLAB 2011a. MathWorks, Inc; USA: 2011. [Google Scholar]
- Mouton et al. (2011).Mouton AM, Alcaraz-Hernández JD, De Baets B, Goethals PLM, Martínez-Capel F. Data-driven fuzzy habitat suitability models for brown trout in Spanish Mediterranean rivers. Environmental Modelling & Software. 2011;26:615–622. doi: 10.1016/j.envsoft.2010.12.001. [DOI] [Google Scholar]
- Nix (1986).Nix HA. A biogeographic analysis of Australian elapid snakes. In: Longmore R, editor. Atlas of Elapid Snakes of Australia. Australian Government Publishing Service; Canberra: 1986. pp. 4–15. [Google Scholar]
- Parmesan & Yohe (2003).Parmesan C, Yohe G. A globally coherent fingerprint of climate change impacts across natural systems. Nature. 2003;421:37–42. doi: 10.1038/nature01286. [DOI] [PubMed] [Google Scholar]
- Phillips, Anderson & Schapire (2006).Phillips SJ, Anderson RP, Schapire RE. Maximum entropy modeling of species geographic distributions. Ecological Modelling. 2006;190:231–259. doi: 10.1016/j.ecolmodel.2005.03.026. [DOI] [Google Scholar]
- Rocchini (2014).Rocchini D. Fuzzy species distribution models: a way to represent plant communities spatially. Journal of Vegetation Science. 2014;78(25):317–318. doi: 10.1111/jvs.12152. [DOI] [Google Scholar]
- Root et al. (2003).Root TL, Price JT, Hall KR, Schneider SH, Rosenzweig C, Pounds JA. Fingerprints of global warming on wild animals and plants. Nature. 2003;421:57–60. doi: 10.1038/nature01333. [DOI] [PubMed] [Google Scholar]
- Rödder & Lötters (2009).Rödder D, Lötters S. Niche shift versus niche conservatism? Climatic characteristics of the native and invasive ranges of the Mediterranean house gecko (Hemidactylus turcicus) Global Ecology and Biogeography. 2009;18(4):674–687. doi: 10.1111/j.1466-8238.2009.00477.x. [DOI] [Google Scholar]
- Rüger, Schlüter & Matthies (2005).Rüger N, Schlüter M, Matthies M. A fuzzy habitat suitability index for Populus euphratica in the Northern Amudarya delta (Uzbekistan) Ecological Modelling. 2005;184:313–328. doi: 10.1016/j.ecolmodel.2004.10.010. [DOI] [Google Scholar]
- Smith (1947).Smith AC. The families Illiciaceae and Schisandraceae. Sargentia. 1947;7:1–244. [Google Scholar]
- Trisurat, Shrestha & Kjelgren (2011).Trisurat Y, Shrestha RP, Kjelgren R. Plant species vulnerability to climate change in Peninsular Thailand. Applied Geography. 2011;31:1106–1114. doi: 10.1016/j.apgeog.2011.02.007. [DOI] [Google Scholar]
- Wang, Hong & Tseng (2000).Wang CH, Hong TP, Tseng SS. Integrating membership functions and fuzzy rule sets from multiple knowledge sources. Fuzzy Sets & Systems. 2000;112(1):141–154. doi: 10.1016/S0165-0114(97)00385-0. [DOI] [Google Scholar]
- Weber (2011).Weber TC. Maximum entropy modeling of mature hardwood forest distribution in four US states. Forest Ecology & Management. 2011;261:779–788. doi: 10.1016/j.foreco.2010.12.009. [DOI] [Google Scholar]
- Williamson et al. (2013).Williamson EM, Lorenc A, Booker A, Robinso N. The rise of traditional Chinese medicine and its materia medica: a comparison of the frequency and safety of materials and species used in Europe and China. Journal of Ethnopharmacology. 2013;149:453–462. doi: 10.1016/j.jep.2013.06.050. [DOI] [PubMed] [Google Scholar]
- Willmott (1984).Willmott CJ. On the evaluation of model performance in physical geography. In: Gaile GL, Willmott CJ, editors. Spatial Statistics and Models. D. Reidel Publishing Company; Boston: 1984. pp. 443–460. [Google Scholar]
- Wu et al. (2010).Wu S, Yin Y, Zhao D, Huang M, Shao X, Dai E. Impact of future climate change on terrestrial ecosystems in China. International Journal of Climatology. 2010;30:866–873. doi: 10.1002/joc.1938. [DOI] [Google Scholar]
- Zhang et al. (2014).Zhang MG, Zhou ZK, Chen WY, Cannon CH, Raes N, Slik JW. Major declines of woody plant species ranges under climate change in Yunnan, China. Diversity & Distributions. 2014;20:405–415. doi: 10.1111/ddi.12165. [DOI] [Google Scholar]
- Zhu et al. (2010).Zhu AX, Yang L, Li BL, Qin CZ, Pei T, Liu BY. Construction of membership functions for predictive soil mapping under fuzzy logic. Geoderma. 2010;155:164–174. doi: 10.1016/j.geoderma.2009.05.024. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The following information was supplied regarding data availability:
The raw data has been supplied as a Supplemental File.