Abstract
Introduction
Outbreaks of syphilis have been described among HIV-infected men who have sex with men (MSM) in Western communities, whereas reports in Asian countries are limited. We aimed to characterize the incidence and temporal trends of syphilis among HIV-infected MSM compared with HIV-infected non-MSM in Asian countries.
Methods
Patients enrolled in the TREAT Asia HIV Observational Database cohort and with a negative non-treponemal test since enrolment were analyzed. Incidence of syphilis seroconversion, defined as a positive non-treponemal test after previously testing negative, was evaluated among patients at sites performing non-treponemal tests at least annually. Factors associated with syphilis seroconversion were investigated at sites doing non-treponemal testing in all new patients and subsequently testing routinely or when patients were suspected of having syphilis.
Results
We included 1010 patients from five sites that performed non-treponemal tests in all new patients; those included had negative non-treponemal test results during enrolment and subsequent follow-ups. Among them, 657 patients were from three sites conducting regular non-treponemal testing. The incidence of syphilis seroconversion was 5.38/100 person-years (PY). Incidence was higher in MSM than non-MSM (7.64/100 PY vs. 2.44/100 PY, p<0.001). Among MSM, the incidence rate ratio (IRR) for every additional year from 2009 was 1.19 (p=0.051). MSM status (IRR 3.48, 95% confidence interval (CI) 1.88–6.47), past syphilis diagnosis (IRR 5.15, 95% CI 3.69–7.17) and younger age (IRR 0.84 for every additional 10 years, 95% CI 0.706–0.997) were significantly associated with syphilis seroconversion.
Conclusions
We observed a higher incidence of syphilis seroconversion among HIV-infected MSM and a trend to increasing annual incidence. Regular screening for syphilis and targeted interventions to limit transmission are needed in this population.
Keywords: syphilis, incidence, seroconversion, HIV, MSM
Introduction
After the development of penicillin, the number of syphilis cases fell to its lowest level in 2000 in the United States, from 20.3 cases per 100,000 people to 2.9 cases per 100,000 people. However, the overall number of new infection cases gradually increased after the early 2000s to 6.3 cases per 100,000 people in 2014 [1]. The World Health Organization reported that there were 10.6 million global new syphilis cases in 2008 [2]. Syphilis is also considered to be an significant problem in other specific areas, as more than a quarter of new cases of syphilis and other sexually transmitted infections have occurred in the Western Pacific region, including South Korea, Japan, Taiwan, China, Thailand, the Philippines and Australia [2]. Patients were predominantly male, and one surveillance reported that more than 60% of male patients were men who have sex with men (MSM) [1].
The estimated HIV prevalence in Asia is low (0.1% in East Asia and 0.3% in Southeast Asia) compared with other regions such as North America (0.5%) and Africa (4.7%) [3]. However, unlike the global decreasing trend of HIV prevalence, the estimated number of new cases has increased in East Asia since 2001 [3]. With regard to the transmission route, intravenous drug use, contaminated blood products and heterosexual contact initially played an important role in HIV transmission in Asian countries. However, the epidemic has recently changed, and the transmission of HIV among MSM has become a major threat in many Asian countries [4].
HIV and Treponema pallidum share similar routes of transmission. Syphilitic ulcers are known to disrupt the mucosal barriers, facilitating the passage of HIV [5, 6]. Syphilis is also associated with a decrease in CD4+ T lymphocyte and an increase in HIV viral load in co-infected patients [7, 8]. Some reports suggest that atypical or severe forms of syphilis are more frequent, and the course of syphilis more rapid, in HIV-infected patients [9–11].
In the 1990s, the incidence of syphilis in HIV-infected patients fell significantly, with enhanced screening, education and behavioural changes [12, 13]. However, a re-emergence of syphilis was reported in the 2000s in industrialized countries, especially among MSM [14–18]. In Western settings, a syphilis incidence of 2.9 to 6.2 per 100 PY has been reported in HIV-infected MSM [19–22]. The reasons for this resurgence of syphilis are complex, involving changes in risk behaviour and awareness of the need for testing [14, 18, 23–25]. Recently, the morbidity and mortality of HIV-infected patients has significantly decreased with prolonged life expectancy. This has enabled an increase in sexual activities, including risky sexual behaviours and serosorting of sexual partners among MSM [16, 26, 27]. Previous studies have reported that 33 to 52% of syphilis infections can be asymptomatic, creating additional challenges for diagnosis and stopping transmission [19, 28, 29]. To enhance identification and facilitate treatment in the context of concomitant HIV, US and European guidelines for management of HIV infection recommend routine screening of syphilis at least yearly among MSM [30–32].
However, routine screening of syphilis is limited in Asia. Many countries in the region have limited healthcare resources and the high false-positive rate of non-treponemal testing used for syphilis screening may make this approach unsuitable in this setting [33, 34]. Furthermore, data about rates of syphilis in Asian are inconsistent. There have been several reports of increasing syphilis co-infection among HIV-infected patients in Asian countries [4, 35, 36], while another study in Thailand found that only 1.7% of patients screened routinely had syphilis, suggesting the possible reduced importance of routine screening in this region [33]. As data remain limited, we aimed to determine trends in incidence and predictors of syphilis seroconversion, particularly among MSM compared to non-MSM, in a regional cohort of HIV-infected patients in Asia.
Methods
Study sites and population
The TREAT Asia HIV Observational Database (TAHOD) is a prospective, observational cohort study of HIV-infected patients enrolled from 21 clinical sites in 12 countries in the Asia–Pacific region, the details of which have been previously described [37]. Briefly, each site enrols 100 to 450 HIV-infected patients, both treated and untreated with antiretroviral therapy (ART). Data are collected according to a common protocol. On recruitment, all available retrospective data prior to enrolment in TAHOD are collected. Prospective data are biannually transferred to a central data management and biostatistical analysis centre. Institutional review board approvals are obtained at all participating sites, the data management and analysis centre (Kirby Institute, University of New South Wales, Sydney, Australia), and the coordinating centre (TREAT Asia/amfAR, Bangkok, Thailand). Patients provide written informed consent to participate in TAHOD, where required by local institutional review boards (IRBs).
Among the participating sites, five (one each in South Korea, Taiwan, Hong Kong, Japan and the Philippines) conduct syphilis testing of all new patients. All five countries have only one contributing site each. Three of these sites (in South Korea, Taiwan and Hong Kong) perform routine testing for syphilis at least yearly, whereas the other two sites perform testing only when patients are symptomatic or suspected of having syphilis (Table 1). We investigated the incidence of syphilis seroconversion at sites that test patients for syphilis at least annually (n=3) and described factors associated with syphilis seroconversion at sites that test all new patients (n=5). Patients with a negative Venereal Disease Research Laboratory (VDRL) test or rapid plasma regain (RPR) test after enrolment into TAHOD were eligible for inclusion in the analysis.
Table 1.
Syphilis testing practices at participating study sites
| Site country | Number of contributing patients | Testing new patients | Testing symptomatic or suspected patients | Regular screening | Increased test frequency for any patients? | Median number of reported tests per patient per year (IQR) |
|---|---|---|---|---|---|---|
| South Korea | 177 | Yes | Yes | Every six months | No | 2.07 (1.55–2.65) |
| Taiwan | 343 | Yes | Yes | Minimum every 12 months | Every six months for those subjectively assessed to be at high risk | 1.17 (0.07–2.18) |
| Hong Kong | 137 | Yes | Yes | Minimum every 12 months | Every six months for MSM | 1.84 (0.15–2.80) |
| Japan | 83 | Yes | Yes | No | No | 0.54 (0.24–1.13) |
| Philippines | 270 | Yes | Yes | No | No | 0.62 (0.40–0.84) |
IQR, interquartile range; MSM, men who have sex with men.
Study variables and definitions
The study end point was syphilis seroconversion, defined as a positive VDRL or RPR test after previously testing negative during TAHOD enrolment. VDRL and RPR tests appear to be accurate and reliable for diagnosis and monitoring treatment response in most HIV-infected patients [38]. The sensitivity of the non-treponemal test is known to be 78 to 86% in primary syphilis and 100% in secondary syphilis, with 98% specificity [38, 39]. Either VDRL or RPR tests were performed on patients according to the testing policy of each site. The positive VDRL and PRP tests included the results of both qualitative and quantitative tests. Study variables included age, sex, ethnicity, mode of HIV exposure, hepatitis B and C serology, history of prior AIDS diagnosis, CD4 cell count, HIV viral load, highly active antiretroviral therapy (HAART) regimen and past history of having positive VDRL or RPR test prior to baseline. Variables were measured at baseline. Baseline was considered as the date of first negative VDRL or RPR test during TAHOD enrolment.
Statistical analysis
Patients were only censored at the first positive syphilis test and not able to contribute more than one outcome. Follow-up was censored at the last available clinic visit date without a record of seroconversion. The total and annual incidence of syphilis seroconversion was evaluated overall and by MSM status (MSM/non-MSM). Trends in syphilis incidence were evaluated by univariate Poisson regression. Predictors of syphilis seroconversion were evaluated by Poisson regression adjusted for study site. In the analysis of predictors of syphilis seroconversion, covariates significant in the univariate model at p<0.10 were chosen for inclusion in the multivariate model. Covariates with p<0.05 in the final multivariate model were considered statistically significant. All models were adjusted for each study site, although the incidence rate ratio (IRR) for each site is not shown. Missing categories were included in the models; however, the IRRs are not reported. Stata statistical software (version 12.1; StataCorp, College Station, TX, USA) was used for all statistical analyses.
Results
From September 2003 to March 2014, data from 2135 patients receiving HIV care at one of the five sites were available for this analysis. Overall, 1047 (49.0%) patients had a negative VDRL or RPR test during enrolment, and 1010 (96.4%) had subsequent follow-up testing data. Among the three sites that routinely tested for syphilis, 1359 patients were enrolled in TAHOD, 691 (50.8%) had a negative VDRL or RPR result during enrolment and 657 (95.1%) had subsequent follow-up data. The demographics and characteristics of all patients (n=1010) and those considered for the incidence analysis (n=657) are shown in Table 2. Both groups were predominantly male. Median age, proportions having prior AIDS and using HAART at the time of syphilis seroconversion were similar among both groups. Homosexual contact was the most common mode of HIV exposure (63.0% in all patients and 65.4% in the incidence group). The median baseline CD4 cell count was 393 cells/mm3 (interquartile range (IQR) 253–551) among the overall group of patients and 410 cells/mm3 (IQR 259–561) in the incidence group. Twenty percent of the overall group and 24.7% of the incidence group had a history of syphilis infection before enrolment.
Table 2.
Baseline characteristics
| Factors | Total patients (n=1010) |
Incidence subgroup (n=657) |
|---|---|---|
| Male, n (%) | 933 (92.4) | 621 (94.5) |
| Age (years), n (%) | ||
| ≤30 | 235 (23.3) | 138 (21.0) |
| 31–40 | 335 (33.2) | 223 (33.9) |
| 41–50 | 276 (27.3) | 181 (27.5) |
| >50 | 164 (16.2) | 115 (17.5) |
| Median (IQR) | 38.2 (30.3–45.6) | 38.5 (31.1–45.7) |
| Ethnicity, n (%) | ||
| Asian | 1003 (99.3) | 653 (99.4) |
| Caucasian | 5 (0.5) | 4 (0.6) |
| Other | 2 (0.2) | 0 (0.0) |
| HIV exposure, n (%) | ||
| Heterosexual | 230 (22.8) | 136 (20.7) |
| MSM | 636 (63.0) | 430 (65.4) |
| Injecting drug use | 11 (1.1) | 10 (1.5) |
| Other | 133 (13.2) | 81 (12.3) |
| Prior AIDS diagnosis, n (%) | 354 (35.0) | 235 (35.8) |
| CD4 cell count (cells/mm3), n (%) | ||
| ≥500 | 301 (29.8) | 215 (32.7) |
| 350–499 | 248 (24.6) | 171 (26.0) |
| 200–349 | 236 (23.4) | 152 (23.1) |
| <200 | 154 (15.2) | 95 (14.5) |
| Median (IQR) | 393 (253–551) | 410 (259–561) |
| HIV viral load (copies/ml), n (%) | ||
| <400 | 546 (54.1) | 439 (66.8) |
| ≥400 | 258 (25.5) | 171 (26.0) |
| Median (IQR) | 49 (39–3410) | 49 (39–950) |
| Median years after HIV diagnosis (IQR) | 3.59 (1.5–6.6) | 3.54 (1.6–6.6) |
| Using HAART at baseline, n (%) | 887 (87.8) | 570 (86.8) |
| Positive HBsAg, n (%) | 101 (11.9) | 70 (13.4) |
| Positive hepatitis C antibody, n (%) | 43 (5.4) | 34 (6.0) |
| Past syphilis diagnosis, n (%) | 202 (20.0) | 162 (24.7) |
IQR, interquartile range; MSM, men who have sex with men; HAART, highly active antiretroviral therapy; HBsAg, hepatitis B surface antigen.
Incidence analysis
During the study period, there were a total of 127 cases of syphilis seroconversion in the incidence group. The total follow-up duration was 2359 person-years (PY) and the median follow-up time per patient was 2.92 (IQR 1.08–5.69) years. A median of 1.59 (IQR 0.80–2.54) VDRL or RPR tests per patient per year were performed. The overall incidence of syphilis seroconversion was 5.38 (95% confidence interval (CI) 4.52–6.41) per 100 PY. When assessed by MSM status, the incidence of seroconversion was 2.44 (95% CI 1.65–3.61) per 100 PY among non-MSM and 7.64 (95% CI 6.29–9.28) per 100 PY among MSM (p<0.001).
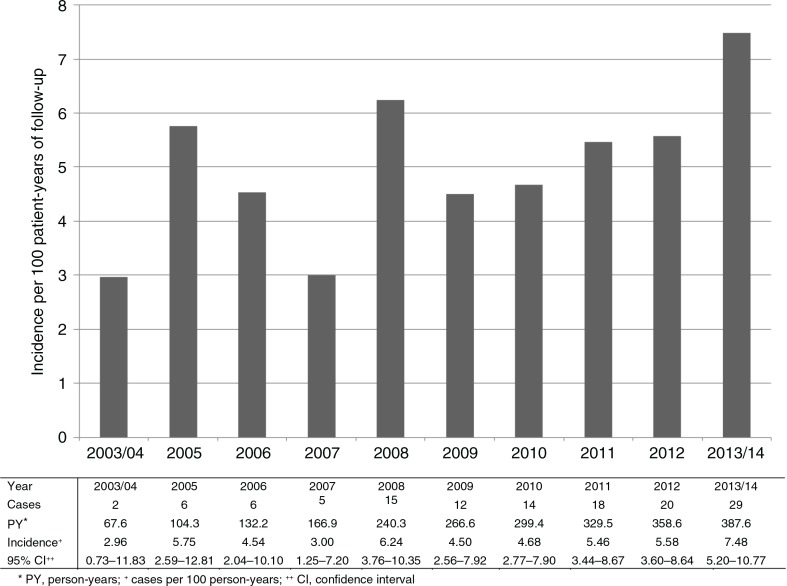
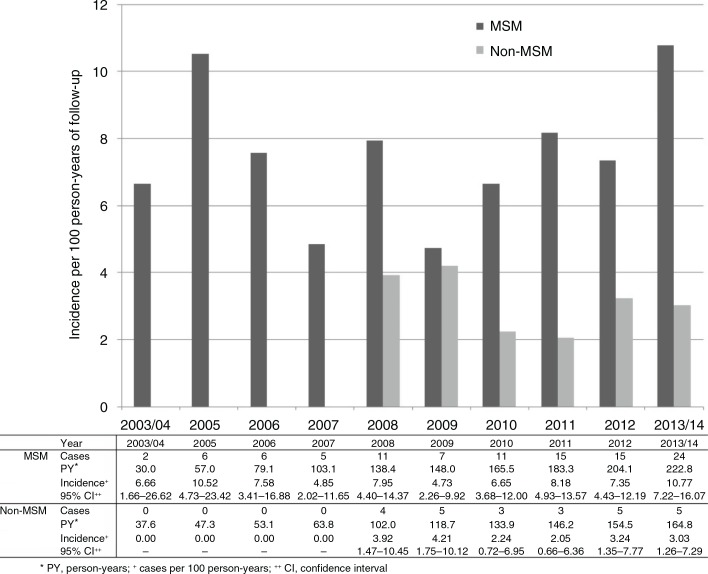
Over the study period, the rate of syphilis seroconversion was lowest in 2003 to 2004 at 2.96/100 PY and tended to show an increasing trend over time (Figure 1). Incidence was 7.48/100 PY in the period from 2013 to 2014. In particular, Figure 1 indicates a recent increase from 2009 with a visually ascending trend in annual incidence. From 2009 onwards, the univariate IRR for every additional year was 1.14 (95% CI 0.98–1.32, p=0.091). Yearly change in incidence was also evaluated by MSM status (Figure 2). In any given year, incidence was consistently higher among the MSM patients. In this group, incidence reached a peak value of 10.77/100 PY in 2013 to 2014. The univariate IRR for every additional year from 2009 onwards was 1.19 in MSM (95% CI 1.00–1.41, p=0.051) and 0.97 (95% CI 0.71–1.31, p=0.838) in non-MSM.
Figure 1.
Incidence of syphilis seroconversion among HIV-infected patients by year (n=657).
Figure 2.
Incidence of syphilis seroconversion among MSM and non-MSM by year (n=657). MSM, men who have sex with men.
Factors associated with syphilis seroconversion
Factors associated with syphilis seroconversion among all patients are shown in Table 3. In the multivariate analysis, HIV exposure via MSM contact (IRR 3.48 vs. heterosexual, 95% CI 1.88–6.47, p<0.001), past diagnosis of syphilis (IRR 5.15 vs. none, 95% CI 3.69–7.17, p<0.001) and younger age (IRR 0.84 for every additional 10 years, 95% CI 0.706–0.997, p=0.047) were significantly associated with syphilis seroconversion.
Table 3.
Factors associated with syphilis seroconversion among HIV-infected patients (n=1010)
| Baseline risk factor | Univariate IRR (95% CI) | p | Multivariate IRR (95% CI) | p |
|---|---|---|---|---|
| HIV exposure | ||||
| Heterosexual | 1 | 1 | ||
| MSM | 5.50 (2.96–10.22) | <0.001 | 3.48 (1.88–6.47) | <0.001 |
| Injecting drug use | 1.61 (0.21–12.52) | 0.649 | ||
| Other | 3.39 (1.57–7.34) | 0.002 | 2.50 (1.16–5.42) | 0.02 |
| Past syphilis diagnosis | ||||
| No | 1 | 1 | ||
| Yes | 6.06 (4.37–8.39) | <0.001 | 5.15 (3.69–7.17) | <0.001 |
| Age, every 10-year increase | 0.83 (0.70–0.98) | 0.029 | 0.84 (0.71–1.00) | 0.047 |
| Sex | ||||
| Male | 1 | |||
| Female | 0.07 (0.01–0.54) | 0.01 | ||
| Prior AIDS diagnosis | ||||
| Not known | 1 | |||
| Yes | 0.76 (0.54–1.09) | 0.134 | ||
| CD4 cell count (cells/mm3) | ||||
| ≥500 | 1 | |||
| 350–499 | 1.14 (0.75–1.73) | 0.549 | ||
| 200–349 | 0.94 (0.61–1.44) | 0.775 | ||
| <200 | 1.19 (0.71–1.98) | 0.515 | ||
| HIV viral load (copies/ml) | ||||
| <400 | 1 | |||
| ≥400 | 0.94 (0.65–1.36) | 0.742 | ||
| Time after HIV diagnosis | ||||
| Every one-year increase | 1.02 (0.98–1.06) | 0.295 | ||
| Using HAART | ||||
| Yes | 1 | |||
| No | 0.88 (0.56–1.39) | 0.593 | ||
| HBsAg status | ||||
| Negative | 1 | |||
| Positive | 1.34 (0.84–2.14) | 0.223 | ||
| Hepatitis C antibody status | ||||
| Negative | 1 | |||
| Positive | 0.95 (0.44–2.05) | 0.888 |
CI, confidence interval; IRR, incidence rate ratio; MSM, men who have sex with men; HAART, highly active antiretroviral therapy; HBsAg, hepatitis B surface antigen.
All models were adjusted for study site, though incident rate ratios for sites are not shown.
Discussion
Data about syphilis incidence from Asian countries have been limited and have shown inconsistent results [33, 35, 36, 40]. Most studies in Asia have only reported prevalence figures among HIV-infected patients or syphilis incidence in MSM regardless of HIV infection status. In a single centre study in Thailand, the prevalence of syphilis in HIV-infected patients was reported as 1.7% [33]. However, in that study, only 14.6% of the patients had reported homosexual contact risk, and the frequency of sexual contact may have further influenced the results (55.6% of the overall patients and 84% of MSM reported no recent sexual intercourse).
In our study, the overall incidence of syphilis seroconversion was higher than expected based on previous reports, which may be related to the higher proportion of MSM in our study population. Syphilis seroconversion was more frequent in the MSM group, and yearly incidence showed an increasing trend after 2009.
There are several possible reasons for the increases in syphilis seroconversions. Multiple sexual partners, use of drugs like methamphetamine or meeting partners over the Internet have been shown to increase risks of syphilis infection in HIV patients [41, 42]. Serial data suggests that risky behaviours are increasing in some HIV-infected MSM populations where syphilis rates are rising [26, 43, 44]. In one study from California, the proportion of HIV-infected MSM having 10 or more sexual partners or engaging in unprotected anal intercourse in the previous six months was reported to have increased in the early 2000s [45]. In addition, it has been shown that MSM can select their sexual partners or behaviours on the basis of their HIV infection status, in a practice known as serosorting [46–49]. This may be used to avoid the risk of transmitting HIV to an HIV-uninfected partner but could be associated with the risk of sexually transmitted infections including syphilis because risky sexual behaviour can be more common among HIV-infected MSM [46, 50–52].
In many countries, preventive interventions for HIV and syphilis among MSM have primarily targeted the HIV uninfected [46, 53]. However, the high incidence of syphilis among HIV-infected MSM in other contexts and observed in our cohort suggests that additional efforts are needed to support prevention within the MSM community.
In addition to reducing risky behaviours, another key component of a public health strategy to address syphilis in HIV-infected individuals is early detection and prompt treatment. Current US and European guidelines recommend at least annual screening for sexually transmitted infections, including syphilis, in sexually active HIV-infected patients [30, 31]. In our cohort, only 3 of 21 sites performed regular syphilis screening on all or even a subset of patients. Our findings indicate that regional health programmes may need to consider implementing routine screening in those with HIV.
There were several limitations to our study. Firstly, we considered that syphilis seroconversion defined as a newly positive VDRL or RPR test represented syphilis infection. Current guidelines recommend performing both treponemal tests and non-treponemal tests for syphilis diagnosis [31, 54]. Non-treponemal tests can have high rates of false positivity, which could overestimate the incidence of syphilis [55]. Secondly, the scope of data collection in our observational cohort did not allow for an assessment of clinical manifestations or of titres of non-treponemal tests; therefore, we could not establish whether there was a fourfold increase in the VDRL or RPR titre in order to define a syphilis infection among those with a history of syphilis and clinical diagnosis, such as primary or secondary syphilis. Similarly we were unable to correlate sexual behaviour information beyond the reported HIV exposure category or history of other sexually transmitted infections to syphilis seroconversions. In addition, only 5 of the 21 sites in our cohort conducted routine syphilis screening and were included in our analysis, and 4 of these were in high-income economies, preventing extrapolation to low- and middle-income settings.
Despite these limitations, this is the first multicentre study investigating syphilis infection among HIV-infected patients in Asia, where the epidemiology of HIV and syphilis and socio-economic status differ from Western settings. Understanding local disease epidemics and recent trends of incidence is essential for prevention and interventions.
Conclusions
The high incidence of syphilis seroconversion observed in our study, especially among HIV-infected MSM, may represent high rates of transmissions and ongoing risky sexual behaviours, and it highlights the need for targeted intervention and regular syphilis screening in this population.
The TREAT Asia HIV Observational Database
CV Mean, V Saphonn* and K Vohith, National Center for HIV/AIDS, Dermatology and STDs, Phnom Penh, Cambodia; FJ Zhang*, HX Zhao and N Han, Beijing Ditan Hospital, Capital Medical University, Beijing, China; MP Lee*‡, PCK Li, W Lam, and YT Chan, Queen Elizabeth Hospital, Hong Kong, China; N Kumarasamy*, S Saghayam and C Ezhilarasi, Chennai Antiviral Research and Treatment Clinical Research Site, YRGCARE Medical Centre, VHS, Chennai, India; S Pujari*, K Joshi, S Gaikwad and A Chitalikar, Institute of Infectious Diseases, Pune, India; TP Merati*†, DN Wirawan and F Yuliana, Faculty of Medicine, Udayana University and Sanglah Hospital, Bali, Indonesia; E Yunihastuti*, D Imran and A Widhani, Working Group on AIDS, Faculty of Medicine, University of Indonesia/Cipto Mangunkusumo Hospital, Jakarta, Indonesia; S Oka*, J Tanuma and T Nishijima, National Center for Global Health and Medicine, Tokyo, Japan; JY Choi*, S Na and JM Kim, Division of Infectious Diseases, Department of Internal Medicine, Yonsei University College of Medicine, Seoul, South Korea; BLH Sim*, YM Gani and R David, Sungai Buloh Hospital, Sungai Buloh, Malaysia; A Kamarulzaman*, SF Syed Omar, S Ponnampalavanar and I Azwa, University of Malaya Medical Centre, Kuala Lumpur, Malaysia; R Ditangco*, E Uy and R Bantique, Research Institute for Tropical Medicine, Manila, Philippines; WW Wong*, WW Ku and PC Wu, Taipei Veterans General Hospital, Taipei, Taiwan; OT Ng*, PL Lim, LS Lee and PS Ohnmar, Tan Tock Seng Hospital, Singapore; P Phanuphak*, K Ruxrungtham, A Avihingsanon, P Chusut and S Sirivichayakul, HIV-NAT/Thai Red Cross AIDS Research Centre, Bangkok, Thailand; S Kiertiburanakul*, S Sungkanuparph, L Chumla and N Sanmeema, Faculty of Medicine, Ramathibodi Hospital, Mahidol University, Bangkok, Thailand; R Chaiwarith*, T Sirisanthana, W Kotarathititum and J Praparattanapan, Research Institute for Health Sciences, Chiang Mai University, Chiang Mai, Thailand; P Kantipong* and P Kambua, Chiangrai Prachanukroh Hospital, Chiang Rai, Thailand; W Ratanasuwan* and R Sriondee, Faculty of Medicine, Siriraj Hospital, Mahidol University, Bangkok, Thailand; VK Nguyen*, VH Bui, THD Nguyen and TD Nguyen, National Hospital for Tropical Diseases, Hanoi, Vietnam; TT Pham*, DD Cuong and HL Ha, Bach Mai Hospital, Hanoi, Vietnam; AH Sohn*, N Durier* and B Petersen, TREAT Asia, The Foundation for AIDS Research, Bangkok, Thailand; DA Cooper, MG Law*, A Jiamsakul* and DC Boettiger, The Kirby Institute, University of New South Wales (UNSW) Australia, Sydney, Australia.
*TAHOD steering committee member; †steering committee chair; ‡co-chair.
Acknowledgements and funding
The TREAT Asia HIV Observational Database is an initiative of TREAT Asia, a programme of amfAR, The Foundation for AIDS Research, with support from the US National Institutes of Health's National Institute of Allergy and Infectious Diseases, Eunice Kennedy Shriver National Institute of Child Health and Human Development and National Cancer Institute, as part of the International Epidemiologic Databases to Evaluate AIDS (IeDEA; U01AI069907). TREAT Asia receives additional support from ViiV Healthcare. The Kirby Institute is funded by the Australian Government Department of Health and Ageing and is affiliated with the Faculty of Medicine, UNSW Australia (The University of New South Wales). JYC's involvement was supported by the Basic Science Research Program through the National Research Foundation of Korea (NRF), funded by the Ministry of Education, Science and Technology (NRF-2013R1A1A2005412), and BioNano Health-Guard Research Center, funded by the Ministry of Science, ICT, and Future Planning of Korea as a Global Frontier Project (Grant H-GUARD_2013M3A6B2078953). The content of this publication is solely the responsibility of the authors and does not necessarily represent the official views of any of the governments or institutions mentioned above.
Competing interests
The authors have no competing interests to declare.
Authors' contributions
All authors (JYA, DB, SK, TPM, BVH, WWW, RD, MPL, SO, ND, JYC) have read and approved the final manuscript. All authors made contributions to the conception of this study, were involved in revising the manuscript and gave final approval of the version to be published. JYA and JYC were involved in drafting the manuscript, DB and ND made contributions to the analysis and interpretation of data and SK, TPM, BVH, WWW, RD, MPL and SO made contributions to the data acquisition.
References
- 1.Centers for Disease Control and Prevention. Sexually transmitted disease surveillance 2014 [Internet] 2015. [cited 2015 Nov 1]. Available from: http://www.cdc.gov/std/stats14.
- 2.World Health Organization. Global incidence and prevalence of selected curable sexually transmitted infection 2008 [Internet] 2012. [cited 2015 Nov 1]. Available from: http://www.who.int/reproductivehealth/publications/rtis/stisestimates/en.
- 3.UNAIDS. Global report: UNAIDS report on the global AIDS Epidemic 2013 [Internet] 2013. [cited 2015 Nov 1]. Available from: http://www.unaids.org/en/resources/campaigns/globalreport2013/globalreport.
- 4.Suguimoto SP, Techasrivichien T, Musumari PM, El-saaidi C, Lukhele BW, Ono-Kihara M, et al. Changing patterns of HIV epidemic in 30 years in East Asia. Curr HIV/AIDS Rep. 2014;11(2):134–45. doi: 10.1007/s11904-014-0201-4. doi: http://dx.doi.org/10.1007/s11904-014-0201-4. [DOI] [PubMed] [Google Scholar]
- 5.Stamm WE, Handsfield HH, Rompalo AM, Ashley RL, Roberts PL, Corey L. The association between genital ulcer disease and acquisition of HIV infection in homosexual men. JAMA. 1988;260(10):1429–33. doi: http://dx.doi.org/10.1001/jama.1988.03410100119036. [PubMed] [Google Scholar]
- 6.Greenblatt RM, Lukehart SA, Plummer FA, Quinn TC, Critchlow CW, Ashley RL, et al. Genital ulceration as a risk factor for human immunodeficiency virus infection. AIDS. 1988;2(1):47–50. doi: 10.1097/00002030-198802000-00008. doi: http://dx.doi.org/10.1097/00002030-198802000-00008. [DOI] [PubMed] [Google Scholar]
- 7.Palacios R, Jimenez-Onate F, Aguilar M, Galindo MJ, Rivas P, Ocampo A, et al. Impact of syphilis infection on HIV viral load and CD4 cell counts in HIV-infected patients. J Acquir Immune Defic Syndr. 2007;44(3):356–9. doi: 10.1097/QAI.0b013e31802ea4c6. doi: http://dx.doi.org/10.1097/QAI.0b013e31802ea4c6. [DOI] [PubMed] [Google Scholar]
- 8.Buchacz K, Patel P, Taylor M, Kerndt PR, Byers RH, Holmberg SD, et al. Syphilis increases HIV viral load and decreases CD4 cell counts in HIV-infected patients with new syphilis infections. AIDS. 2004;18(15):2075–9. doi: 10.1097/00002030-200410210-00012. doi: http://dx.doi.org/10.1097/00002030-200410210-00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pialoux G, Vimont S, Moulignier A, Buteux M, Abraham B, Bonnard P. Effect of HIV infection on the course of syphilis. AIDS Rev. 2008;10(2):85–92. [PubMed] [Google Scholar]
- 10.Lynn WA, Lightman S. Syphilis and HIV: a dangerous combination. Lancet Infect Dis. 2004;4(7):456–66. doi: 10.1016/S1473-3099(04)01061-8. doi: http://dx.doi.org/10.1016/S1473-3099(04)01061-8. [DOI] [PubMed] [Google Scholar]
- 11.Hutchinson CM, Hook EW, 3rd, Shepherd M, Verley J, Rompalo AM. Altered clinical presentation of early syphilis in patients with human immunodeficiency virus infection. Ann Intern Med. 1994;121(2):94–100. doi: 10.7326/0003-4819-121-2-199407150-00003. doi: http://dx.doi.org/10.7326/0003-4819-121-2-199407150-00003. [DOI] [PubMed] [Google Scholar]
- 12.Heffelfinger JD, Swint EB, Berman SM, Weinstock HS. Trends in primary and secondary syphilis among men who have sex with men in the United States. Am J Public Health. 2007;97(6):1076–83. doi: 10.2105/AJPH.2005.070417. doi: http://dx.doi.org/10.2105/AJPH.2005.070417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fenton KA, Breban R, Vardavas R, Okano JT, Martin T, Aral S, et al. Infectious syphilis in high-income settings in the 21st century. Lancet Infect Dis. 2008;8(4):244–53. doi: 10.1016/S1473-3099(08)70065-3. doi: http://dx.doi.org/10.1016/S1473-3099(08)70065-3. [DOI] [PubMed] [Google Scholar]
- 14.Centers for Disease Control and Prevention. Trends in primary and secondary syphilis and HIV infections in men who have sex with men – San Francisco and Los Angeles, California, 1998–2002. MMWR Morb Mortal Wkly Rep. 2004;53(26):575–8. [PMC free article] [PubMed] [Google Scholar]
- 15.Couturier E, Michel A, Janier M, Dupin N, Semaille C, Syphilis Surveillance Network Syphilis surveillance in France, 2000–2003. Euro Surveill. 2004;9(12):8–10. doi: 10.2807/esm.09.12.00493-en. [DOI] [PubMed] [Google Scholar]
- 16.Dougan S, Evans BG, Elford J. Sexually transmitted infections in Western Europe among HIV-positive men who have sex with men. Sex Transm Dis. 2007;34(10):783–90. doi: 10.1097/01.olq.0000260919.34598.5b. doi: http://dx.doi.org/10.1097/01.olq.0000260919.34598.5b. [DOI] [PubMed] [Google Scholar]
- 17.Scheer S, Kellogg T, Klausner JD, Schwarcz S, Colfax G, Bernstein K, et al. HIV is hyperendemic among men who have sex with men in San Francisco: 10-year trends in HIV incidence, HIV prevalence, sexually transmitted infections and sexual risk behaviour. Sex Transm Infect. 2008;84(6):493–8. doi: 10.1136/sti.2008.031823. doi: http://dx.doi.org/10.1136/sti.2008.031823. [DOI] [PubMed] [Google Scholar]
- 18.Buchacz K, Greenberg A, Onorato I, Janssen R. Syphilis epidemics and human immunodeficiency virus (HIV) incidence among men who have sex with men in the United States: implications for HIV prevention. Sex Transm Dis. 2005;32(Suppl 10):S73–9. doi: 10.1097/01.olq.0000180466.62579.4b. doi: http://dx.doi.org/10.1097/01.olq.0000180466.62579.4b. [DOI] [PubMed] [Google Scholar]
- 19.Branger J, van der Meer JT, van Ketel RJ, Jurriaans S, Prins JM. High incidence of asymptomatic syphilis in HIV-infected MSM justifies routine screening. Sex Transm Dis. 2009;36(2):84–5. doi: 10.1097/OLQ.0b013e318186debb. doi: http://dx.doi.org/10.1097/OLQ.0b013e318186debb. [DOI] [PubMed] [Google Scholar]
- 20.Jin F, Prestage GP, Zablotska I, Rawstorne P, Imrie J, Kippax SC, et al. High incidence of syphilis in HIV-positive homosexual men: data from two community-based cohort studies. Sex Health. 2009;6(4):281–4. doi: 10.1071/SH09060. doi: http://dx.doi.org/10.1071/SH09060. [DOI] [PubMed] [Google Scholar]
- 21.Ganesan A, Fieberg A, Agan BK, Lalani T, Landrum ML, Wortmann G, et al. Results of a 25-year longitudinal analysis of the serologic incidence of syphilis in a cohort of HIV-infected patients with unrestricted access to care. Sex Transm Dis. 2012;39(6):440–8. doi: 10.1097/OLQ.0b013e318249d90f. doi: http://dx.doi.org/10.1097/OLQ.0b013e318249d90f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ivens D, Patel M. Incidence and presentation of early syphilis diagnosed in HIV-positive gay men attending a central London outpatients’ department. Int J STD AIDS. 2005;16(3):201–2. doi: 10.1258/0956462053420202. doi: http://dx.doi.org/10.1258/0956462053420202. [DOI] [PubMed] [Google Scholar]
- 23.Wheater CP, Cook PA, Clark P, Syed Q, Bellis MA. Re-emerging syphilis: a detrended correspondence analysis of the behaviour of HIV positive and negative gay men. BMC Public Health. 2003;3:34. doi: 10.1186/1471-2458-3-34. doi: http://dx.doi.org/10.1186/1471-2458-3-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zetola NM, Klausner JD. Syphilis and HIV infection: an update. Clin Infect Dis. 2007;44(9):1222–8. doi: 10.1086/513427. doi: http://dx.doi.org/10.1086/513427. [DOI] [PubMed] [Google Scholar]
- 25.Kim AA, Kent CK, Klausner JD. Increased risk of HIV and sexually transmitted disease transmission among gay or bisexual men who use Viagra, San Francisco 2000–2001. AIDS. 2002;16(10):1425–8. doi: 10.1097/00002030-200207050-00017. doi: http://dx.doi.org/10.1097/00002030-200207050-00017. [DOI] [PubMed] [Google Scholar]
- 26.Katz MH, Schwarcz SK, Kellogg TA, Klausner JD, Dilley JW, Gibson S, et al. Impact of highly active antiretroviral treatment on HIV seroincidence among men who have sex with men: San Francisco. Am J Public Health. 2002;92(3):388–94. doi: 10.2105/ajph.92.3.388. doi: http://dx.doi.org/10.2105/AJPH.92.3.388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Elford J. Changing patterns of sexual behaviour in the era of highly active antiretroviral therapy. Curr Opin Infect Dis. 2006;19(1):26–32. doi: 10.1097/01.qco.0000199018.50451.e1. doi: http://dx.doi.org/10.1097/01.qco.0000199018.50451.e1. [DOI] [PubMed] [Google Scholar]
- 28.Cohen CE, Winston A, Asboe D, Boag F, Mandalia S, Azadian B, et al. Increasing detection of asymptomatic syphilis in HIV patients. Sex Transm Infect. 2005;81(3):217–9. doi: 10.1136/sti.2004.012187. doi: http://dx.doi.org/10.1136/sti.2004.012187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Winston A, Hawkins D, Mandalia S, Boag F, Azadian B, Asboe D. Is increased surveillance for asymptomatic syphilis in an HIV outpatient department worthwhile? Sex Transm Infect. 2003;79(3):257–9. doi: 10.1136/sti.79.3.257. doi: http://dx.doi.org/10.1136/sti.79.3.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Masur H, Brooks JT, Benson CA, Holmes KK, Pau AK, Kaplan JE. Prevention and treatment of opportunistic infections in HIV-infected adults and adolescents: updated guidelines from the centers for disease control and prevention, National Institutes of Health, and HIV Medicine Association of the Infectious Diseases Society of America. Clin Infect Dis. 2014;58(9):1308–11. doi: 10.1093/cid/ciu094. doi: http://dx.doi.org/10.1093/cid/ciu094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Workowski KA, Berman S, Centers for Disease Control and Prevention Sexually transmitted diseases treatment guidelines, 2010. MMWR Recomm Rep. 2010;59(RR-12):1–110. [PubMed] [Google Scholar]
- 32.European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of HIV-infected adults [Internet] 2014. [cited 2015 Nov 1]. Available from: http://www.eacsociety.org/files/guidelines_8.0-english-revised_20160610.pdf. [DOI] [PubMed]
- 33.Kukanok S, Kiertiburanakul S. Prevalence of positive syphilis serology among HIV-infected patients: role for routine screening in Thailand. Southeast Asian J Trop Med Public Health. 2014;45(2):435–41. [PubMed] [Google Scholar]
- 34.Dorigo-Zetsma JW, Belewu D, Meless H, Sanders E, Coutinho RA, Schaap A, et al. Performance of routine syphilis serology in the Ethiopian cohort on HIV/AIDS. Sex Transm Infect. 2004;80(2):96–9. doi: 10.1136/sti.2003.005827. doi: http://dx.doi.org/10.1136/sti.2003.005827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Centers for Disease Control and Prevention. HIV and syphilis infection among men who have sex with men – Bangkok, Thailand, 2005–2011. MMWR Morb Mortal Wkly Rep. 2013;62(25):518–20. [PMC free article] [PubMed] [Google Scholar]
- 36.Zhong F, Liang B, Xu H, Cheng W, Fan L, Han Z, et al. Increasing HIV and decreasing syphilis prevalence in a context of persistently high unprotected anal intercourse, six consecutive annual surveys among men who have sex with men in Guangzhou, China, 2008 to 2013. PLoS One. 2014;9(7):e103136. doi: 10.1371/journal.pone.0103136. doi: http://dx.doi.org/10.1371/journal.pone.0103136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhou J, Kumarasamy N, Ditangco R, Kamarulzaman A, Lee CK, Li PC, et al. The TREAT Asia HIV Observational Database: baseline and retrospective data. J Acquir Immune Defic Syndr. 2005;38(2):174–9. doi: 10.1097/01.qai.0000145351.96815.d5. doi: http://dx.doi.org/10.1097/01.qai.0000145351.96815.d5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nayak S, Acharjya B. VDRL test and its interpretation. Indian J Dermatol. 2012;57(1):3–8. doi: 10.4103/0019-5154.92666. doi: http://dx.doi.org/10.4103/0019-5154.92666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sena AC, White BL, Sparling PF. Novel Treponema pallidum serologic tests: a paradigm shift in syphilis screening for the 21st century. Clin Infect Dis. 2010;51(6):700–8. doi: 10.1086/655832. doi: http://dx.doi.org/10.1086/655832. [DOI] [PubMed] [Google Scholar]
- 40.Zhang X, Wang C, Hengwei W, Li X, Li D, Ruan Y, et al. Risk factors of HIV infection and prevalence of co-infections among men who have sex with men in Beijing, China. AIDS. 2007;21(Suppl 8):S53–7. doi: 10.1097/01.aids.0000304697.39637.4c. doi: http://dx.doi.org/10.1097/01.aids.0000304697.39637.4c. [DOI] [PubMed] [Google Scholar]
- 41.Centers for Disease Control and Prevention. Outbreak of syphilis among men who have sex with men – Southern California, 2000. MMWR Morb Mortal Wkly Rep. 2001;50(7):117–20. [PubMed] [Google Scholar]
- 42.Centers for Disease Control and Prevention. Internet use and early syphilis infection among men who have sex with men – San Francisco, California, 1999–2003. MMWR Morb Mortal Wkly Rep. 2003;52(50):1229–32. [PubMed] [Google Scholar]
- 43.Chen SY, Gibson S, Katz MH, Klausner JD, Dilley JW, Schwarcz SK, et al. Continuing increases in sexual risk behavior and sexually transmitted diseases among men who have sex with men: San Francisco, Calif, 1999–2001, USA. Am J Public Health. 2002;92(9):1387–8. doi: http://dx.doi.org/10.2105/AJPH.92.9.1387-a. [PMC free article] [PubMed] [Google Scholar]
- 44.Dodds JP, Mercey DE, Parry JV, Johnson AM. Increasing risk behaviour and high levels of undiagnosed HIV infection in a community sample of homosexual men. Sex Transm Infect. 2004;80(3):236–40. doi: 10.1136/sti.2003.007286. doi: http://dx.doi.org/10.1136/sti.2003.007286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wohl AR, Johnson DF, Lu S, Frye D, Bunch G, Simon PA. Recent increase in high-risk sexual behaviors among sexually active men who have sex with men living with AIDS in Los Angeles County. J Acquir Immune Defic Syndr. 2004;35(2):209–11. doi: 10.1097/00126334-200402010-00019. doi: http://dx.doi.org/10.1097/00126334-200402010-00019. [DOI] [PubMed] [Google Scholar]
- 46.van Kesteren NM, Hospers HJ, Kok G. Sexual risk behavior among HIV-positive men who have sex with men: a literature review. Patient Educ Couns. 2007;65(1):5–20. doi: 10.1016/j.pec.2006.09.003. doi: http://dx.doi.org/10.1016/j.pec.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 47.Golden MR, Brewer DD, Kurth A, Holmes KK, Handsfield HH. Importance of sex partner HIV status in HIV risk assessment among men who have sex with men. J Acquir Immune Defic Syndr. 2004;36(2):734–42. doi: 10.1097/00126334-200406010-00011. doi: http://dx.doi.org/10.1097/00126334-200406010-00011. [DOI] [PubMed] [Google Scholar]
- 48.Centers for Disease Control and Prevention. High-risk sexual behavior by HIV-positive men who have sex with men – 16 sites, United States, 2000–2002. MMWR Morb Mortal Wkly Rep. 2004;53(38):891–4. [PubMed] [Google Scholar]
- 49.Wolitski RJ, Parsons JT, Gomez CA, Team SS, Team SS. Prevention with HIV-seropositive men who have sex with men: lessons from the Seropositive Urban Men's Study (SUMS) and the Seropositive Urban Men's Intervention Trial (SUMIT) J Acquir Immune Defic Syndr. 2004;37(Suppl 2):S101–9. doi: 10.1097/01.qai.0000140608.36393.37. doi: http://dx.doi.org/10.1097/01.qai.0000140608.36393.37. [DOI] [PubMed] [Google Scholar]
- 50.Parsons JT, Halkitis PN, Wolitski RJ, Gomez CA. Seropositive Urban Men's Study Team. Correlates of sexual risk behaviors among HIV-positive men who have sex with men. AIDS Educ Prev. 2003;15(5):383–400. doi: 10.1521/aeap.15.6.383.24043. doi: http://dx.doi.org/10.1521/aeap.15.6.383.24043. [DOI] [PubMed] [Google Scholar]
- 51.Parsons JT, Schrimshaw EW, Wolitski RJ, Halkitis PN, Purcell DW, Hoff CC, et al. Sexual harm reduction practices of HIV-seropositive gay and bisexual men: serosorting, strategic positioning, and withdrawal before ejaculation. AIDS. 2005;19(Suppl 1):S13–25. doi: 10.1097/01.aids.0000167348.15750.9a. doi: http://dx.doi.org/10.1097/01.aids.0000167348.15750.9a. [DOI] [PubMed] [Google Scholar]
- 52.Halkitis PN, Parsons JT. Intentional unsafe sex (barebacking) among HIV-positive gay men who seek sexual partners on the internet. AIDS Care. 2003;15(3):367–78. doi: 10.1080/0954012031000105423. doi: http://dx.doi.org/10.1080/0954012031000105423. [DOI] [PubMed] [Google Scholar]
- 53.King-Spooner S. HIV prevention and the positive population. Int J STD AIDS. 1999;10(3):141–50. doi: 10.1258/0956462991913763. doi: http://dx.doi.org/10.1258/0956462991913763. [DOI] [PubMed] [Google Scholar]
- 54.Kingston M, French P, Goh B, Goold P, Higgins S, Sukthankar A, et al. UK national guidelines on the management of syphilis 2008. Int J STD AIDS. 2008;19(11):729–40. doi: 10.1258/ijsa.2008.008279. doi: http://dx.doi.org/10.1258/ijsa.2008.008279. [DOI] [PubMed] [Google Scholar]
- 55.Goh BT, van Voorst Vader PC, European Branch of the International Union against Sexually Transmitted Infection, the European Office of the World Health Organization European guideline for the management of syphilis. Int J STD AIDS. 2001;12(Suppl 3):14–26. doi: 10.1258/0956462011924065. doi: http://dx.doi.org/10.1258/0956462011924065. [DOI] [PubMed] [Google Scholar]