Abstract
Neurodegenerative diseases are incurable and debilitating conditions that result in the progressive degeneration of nerve cells, which affect the cognitive activity. Currently, as a result of multiple studies linking Alzheimer's disease (AD) to oxidative damage, the uses of natural antioxidant to prevent, delay, or enhance the pathological changes underlying the progression of AD has received considerable attention. Therefore, this study was aimed at examining the effect of ethanolic extracts of Phyllanthus emblica (EEPE) ripe (EEPEr) and EEPE unripe (EEPEu) fruits on cognitive functions, brain antioxidant enzymes, and acetylcholinesterase (AChE) activity in rat. The effects of EEPEr and EEPEu fruits (i.e., 100 and 200 mg/kg b.w.) were examined in Swiss albino male rats for 12 days and its effect on cognitive functions, brain antioxidant enzymes, and AChE activity determined. Learning and memory enhancing activity of EEPE fruit was examined by using passive avoidance test and rewarded alternation test. Antioxidant potentiality was evaluated by measuring the activity of antioxidant enzymes such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), glutathione reductase, reduced glutathione (GSH), glutathione-S-transferase, and the contents of thiobarbituric acid reactive substances (TBARS) in entire brain tissue homogenates. AChE activity was determined using colorimetric method. Administration of the highest dose (i.e., 200 mg/kg b.w.) of EEPEr fruit significantly (p < 0.01) and both lowest and highest doses (i.e., 100 and 200 mg/kg b.w.) of EEPEu fruit markedly (p < 0.05, p < 0.001) increased step-through latency in rats on 6th, 11th, and 12th day with respect to the control group. For aforementioned doses, the percentage of memory retention (MR) was considerably (p < 0.05, p < 0.01) increased in rats on 10th, 11th, and 12th days with respect to the control group. The extract, particularly highest dose (i.e., 200 mg/kg b.w.) of EEPEr fruit markedly (p < 0.05) and lowest and highest doses (i.e., 100 and 200 mg/kg b.w.) of EEPEu fruit significantly (p < 0.01) increased the correct responses in rats on 6th, and 12th day related to the control group. In case of this test, the percentage of MR was significantly (p < 0.05, p < 0.01) increased in rats treated with aforementioned doses on 12th day with respect to the control group. The highest dose (i.e., 200 mg/kg b.w.) of EEPEr fruit suggestively (p < 0.05) and both lowest and highest doses (i.e., 100 and 200 mg/kg b.w.) of EEPEu fruit suggestively (p < 0.05, p < 0.01, p < 0.001) increased the levels of SOD, CAT, GSH, GSH-Px and expressively (p < 0.01) decreased the TBARS level compared to the control group. Treatment with the highest dose (i.e., 200 mg/kg b.w.) of EEPEr fruit significantly (p < 0.05) and both lowest and highest doses (i.e., 100 and 200 mg/kg b.w.) of EEPEu fruit markedly (p < 0.01, p < 0.001) decreased the level of AChE activity compared to that of the control group. The present study shows that EEPE fruit possesses an excellent source for natural cognitive enhancer which could be developed in the treatment of AD and other neurodegenerative diseases.
Key Words: Phyllanthus emblica, Cognitive performance, Brain antioxidant markers, Acetylcholinesterase, Alzheimer's disease
Introduction
Cognitive functions are brain-based superior topographies of human beings that cover all mental functions, mental processes, and states of intelligent entities for processing information, applying wisdom, and changing choice [1]. Memory is a set of programmed neural connections in the brain, to encode, store, and recover information. It is linked, but is different from learning, which is the collection of new information from the surrounding environment [2]. Cognitive impairments have long been known as severe and consistent in some neurological disease conditions such as Alzheimer's disease (AD) [3]. The pathophysiology of this degenerative brain disease is closely connected to the injury and death of neurons that usually starts slowly and gets worse over time [4]. Worldwide, 35 million people are affected by AD, including 5.5 million Americans, and it is projected that in 2050 more than 115 million people will have dementia [5,6].
This disease is characterized by the formation of senile plaques, amyloid-β (Aβ) deposits, and neurofibrillary tangles (NFTs) in the hippocampus and cortex [7]. Senile plaques are spherical collections of dilated, tortuous, dystrophic neuritis around a central amyloid core, microglia and reactive astrocytes at the periphery. Amyloid core is composed of aggregated neurotoxic Aβ (1-42) peptide and a 40- or 42-amino acid peptide [8]. Enzymatic cleavage and improper amyloid precursor protein processing is responsible for the formation of Aβ. Deposition of Aβ constitutes the fundamental AD abnormality. Aβ peptides are intermediate soluble oligomers that readily aggregate and can be directly synaptotoxic. Aggregates also stimulate an inflammatory response that is blamed for progressive damage through mediator release [9]. NFTs are bundles of paired helical filaments in neuronal cytoplasm, largely containing hyperphosphorylated tau protein as well as ubiquitin and other microtubule-associated molecules [10]. Tau is a microtubule-associated protein that enhances microtubule assembly. Once hyperphosphorylated, it can no longer sustain microtubule assembly, causing disintegration and eventual cell death. In case of senile plaque and NFTs, 4-hydroxy-2-nonenal (HNE) and acrolein are significantly elevated [11]. Patients affected with AD have higher levels of free HNE in amygdala, hippocampus, and parahippocampal gyrus. An elevation of free HNE levels in ventricular cerebrospinal fluid and serum serves as a biomarker for this disease [12]. HNE may associate with protein and this is observed in 3 stages of AD. In fact association of HNE with protein serves as an indication of Michael addition of HNE to protein [13]. HNE is responsible for activating the p38 and c-Jun signaling cascades which are responsible for stimulating cellular apoptosis. Research indicates that the accumulation of Aβ in the brain due to oxidative stress is responsible for the formation of senile plaques [14].
Oxidative stress is an important factor in the pathogenesis of AD [15]. Several studies suggest that Aβ-induced neurotoxicity, tau pathology, mitochondria dysfunction, and metal dyshomeostasis seen among Alzheimer patients are due to oxidative stress [16]. Oxidative stress, which facilitates the neurotoxicity initiated by various factors such as abnormal accumulation of Aβ and tau proteins, may increase Aβ production and aggregation in addition to assisting tau phosphorylation and polymerization and further stimulating multiple neurotoxic events such as reactive oxygen species (ROS) production, thereby forming a dangerous cycle that enhances the initiation and progression of AD. Apart from a primary or secondary event, oxidative stress plays a significant role in the development of AD [16,17]. Acetylcholinesterase (AChE) is the primary cholinesterase in the body that plays an important role in the regulation of central cholinergic systems (CCS). In the brain, damage to the CCS due to lack of AChE has been shown to be possibly linked to the memory and cognition deficits associated with AD. In recent times, the drugs available to treat AD are AChE inhibitors and N-methyl-D-aspartate receptor antagonist. Currently, 4 reversible AChE inhibitors are approved for the treatment of mild to moderate AD. They are donepezil, galantamine, rivastigmine, and tacrine [18].
Medicinal plants have been identified and used to treat diseases from ancient times. For the treatment of various minor and major diseases, people of the developing and low income countries depend on medicinal plants [19]. Including Bangladesh, in a number of poor and developing countries, about 80% people rely on traditional medicines. The use of traditional medicines is not only limited to developing countries, but also to a number of industrialized countries, where many people frequently use several forms of traditional medicines, with England (47%), Canada (70%), and Germany (75%) being examples [20]. WHO stated that more than 80% of the world's population depends on medicinal plants for their primary healthcare needs [21]. Medicinal plants are the greatest source of natural nootropics. In the treatment of AD, natural nootropics (i.e., smart drugs, memory enhancers, neuro enhancers, cognitive enhancers, and intelligence enhancers) play an important role due to the side effects associated with synthetic learning and memory enhancers such as piracetam and cholinesterase inhibitors [22]. As a result, the search for new natural remedies is ongoing. Ginkgo biloba[23], Bacopa monnieri [24,] and Huperzia serrata[25] are well-known natural cognitive enhancers for the treatment of AD.
The plant Phyllanthus emblica (PE) L. is known in Bengali as Amloki and belongs to the Euphorbiaceae family [26]. This plant is indigenous to tropical regions of Southeast Asia. PE is also widely distributed throughout the forests of Chittagong, Chittagong Hill Tracts, Cox's Bazar, Tangail, Dinajpur, Sylhet, and villages of Bangladesh [27]. The fruit of this plant is almost spherical, quite smooth, 18-25 mm wide and 15-20 mm long, light greenish yellow in color, with 6 vertical streaks, and hard in appearance [28]. Traditionally, the fruit is beneficial as an anti-inflammatory, antipyretic, astringent, diuretic, laxative, stomachic, liver, and hair tonic [29,30]. This fruit is a great source of numerous phytoconstituents such as alkaloids, flavonoids, terpenoids, tannins, and pectin [31]. The important pharmacological actions of this fruit are antioxidant, analgesic, anti-inflammatory, neuroprotective, antitussive, anti-atherogenic, adaptogenic, cardioprotective, immunomodulatory, gastroprotective, antiviral, antiemetic, anthelmintic, nephroprotective, and anticancer activities [32,33,34,35].
Preliminary studies have shown that PE fruit has antioxidant and neuro-enhancing properties [36,37]. No study has been conducted so far to demonstrate such an effect of PE ripe and unripe fruit that is of benefit in the treatment of AD. Therefore, the purpose of this study was to investigate the effect of ethanolic extract of PE (EEPE) ripe (EEPEr) and EEPE unripe (EEPEu) fruits on learning and memory improvement in rats by behavioral tests such as passive avoidance (PA) test, rewarded alternation (RA) test, and the activity of brain antioxidant enzymes by biochemical tests such as superoxide dismutase (SOD), catalase (CAT), glutathione peroxidase (GSH-Px), glutathione reductase (GSR), reduced glutathione (GSH), glutathione-S-transferase (GST), estimation of contents of thiobarbituric acid (TBA) reactive substances (TBARS), and AChE activity in rat brain tissue homogenates.
Materials and Methods
Chemicals and Drug
Acetyl thiocholine iodide (ATCI), 5,5-dithiobis-2-nitrobenzoate ion (DTNB), tris amino methane hydrochloride (Tris-HCl), bovine serum albumin (BSA), phenazine methosulphate, sulfosalicylic acid, sodium pyrophosphate, sodium azide, GSR, GSH, oxidized glutathione, reduced GSH, ethylenediaminetetraacetic acid (EDTA), nicotinamide adenine dinucleotide phosphate (NADPH), 1-chloro-2,4-dinitrobenzene (CDNB), TBA, and trichloroacetic acid (TCA) all were purchased from Sigma-Aldrich, USA. All other chemicals were of analytical grade, unless otherwise specified and purchased from indigenous sources. Donepezil hydrochloride powder was obtained from Incepta Pharmaceuticals Ltd., Dhaka, Bangladesh, as gift.
Collection and Identification of Plant Materials
The fresh leaves of PE were collected from Sitakunda Upazila under the Chittagong district, Bangladesh, in October 2015, and identified by an expert taxonomist from the Bangladesh National Herbarium, Mirpur, Dhaka, Bangladesh. A voucher specimen was preserved in the herbarium for future reference. Accession number: DACB-42531 for PE.
Drying and Grinding of Plant Materials
The fresh ripe and unripe fruits of the plant were collected (weighing each 5 kg), washed properly to remove dirt and shade dried for 30 min. Then seeds were separated from the fruits and shade dried for 7 days with irregular sun drying. These dried fruits were then dried in an oven for 24 h at considerably lower temperatures for better grinding. The dried fruits were milled separately into coarse powder by using a grinding machine and kept in an airtight container for extraction.
Extraction of Plant Materials
Each powdered plant material (fruits), having a weight of 500 g, was taken in an amber colored glass bottle and soaked in 2.5 liter of 98% ethanol. The bottle with its contents was sealed and kept at room temperature and allowed to stand for 7 days with irregular shaking. Then the extracts were filtered through cotton and then through Whatman (No. 1) filter paper. The liquid filtrates were concentrated and evaporated to dry at 50°C temperature by using a rotary evaporator under reduced pressure to get the crude extract (12.35 g for EEPEr and 10.62 g for EEPEu fruit). Finally, dried crude ethanolic extracts were stored at 4°C for further tests.
Animals
All experiments were carried out using 46 healthy adult male, Swiss albino rats weighing about 190-180 g purchased from ICDDR, B, Dhaka, Bangladesh. The rats were housed in 6 per animal cage and placed under standard environmental conditions (25 ± 2°C temperature, 60 ± 5% relative humidity) with a half day light and dark cycle. Standard laboratory food and water were administered properly. The care and use of the animals were monitored along with the guide for laboratory animals of the National Institutes of Health (NIH) [38]. The protocol of the experiment was approved by the Animal Ethics Committee of the Department of Pharmacy, Southeast University, Dhaka, Bangladesh.
Administration of Drugs and Test Compounds
A solution of donepezil hydrochloride was prepared by using normal saline having pH 7.4 and administered orally to experimental rats at 1 mg/kg body weight (b.w.). Weighed quantity of EEPE was suspended in normal saline (pH 7.4) and administered orally to rats at 100 and 200 mg/kg b.w. The duration of the study and doses of donepezil hydrochloride and EEPE were adjusted based on literature searches [39,40]. Every day, standard drug and the suspension of extract were prepared freshly and administered before 30 min of the experiment.
Experimental Design
Rats were divided randomly into 6 groups as follows:
Group 1: standard food and water were administered for 12 days to rats (Con),
Group 2: donepezil hydrochloride at a dose of 1 mg/kg b.w. was administered orally for 12 days to rats (Don),
Group 3: ripe fruit extract at a dose of 100 mg/kg b.w. was administered orally for 12 days to rats (EEPEr 100),
Group 4: unripe fruit extract at a dose of 100 mg/kg b.w. was administered orally for 12 days to rats (EEPEu 100),
Group 5: ripe fruit extract at a dose of 200 mg/kg b.w. was administered orally for 12 days to rats (EEPEr 200),
Group 6: unripe fruit extract at a dose of 200 mg/kg b.w. was administered orally for 12 days to rats (EEPEu 200).
Acute Toxicity Study
The acute oral toxicity test of the EEPE was performed according to the Organization for Economic Cooperation and Development guidelines in healthy male Swiss albino rats [41]. For this test, rats were arbitrarily divided into 6 groups with 6 animals in each group, and normal saline was used to prepare the suspension of the extract. The rats were kept on fasting for 3-4 h with supplementation of water prior to oral dosing. Then the extract was administered orally at different dose levels (i.e., 25, 50, 100, 500, 1,000, and 2,000 mg/kg b.w.) with the help of intragastric tube. After dose administration, food was withdrawn for 1-2 h. The rats were observed uninterruptedly for next 24 h for behavioral change, any adverse change and subsequently 14 days for any lethality and death.
Behavioral Study
One week training was conducted to prepare rats for behavioral study. During the training period, they received only food and water. The completely trained rats were selected for the study. Behavioral studies were conducted between 10.00 a.m. and 3.00 p.m. in a sound resistant room.
PA Test
The sensitive memory of rats based on contextual fear conditioning learning and instrumental learning was assessed by using the PA test [42], which consists of light and dark compartments, each measuring 270 (depth) × 370 (width) × 360 (height) mm. The light compartment and the dark compartment were made by transparent and black vinyl chloride plates, respectively. The 2 compartments of this apparatus were connected by a sliding door having 90 mm diameter in the middle part. The floor of this apparatus consisted of a metal grid spaced 0.9 cm apart and connected to a shock generator, able to generate shock in the range of 0.5 mA. Lighting in the light compartment was provided by fluorescent lamp [43]. Each test consists of 2 distinct trials such as acquisition trial and retention trial. For the acquisition trial, each rat was placed in the light compartment in front of the wall opposite to the sliding door. After adaptation for 15 s, the sliding door was opened and when the rat entered into the dark compartment, an electrical foot shock of 0.5 mA was administered for 3 s [44]. The latency times, once the rat had entered the dark compartment was recorded as initial transfer latency (ITL) with the help of stopwatch. Subsequently, the rat was returned to its home cage. A retention trial was performed after 24 h of the acquisition trial, in which no shock was given when the rat entered the dark compartment and latency times to re-enter the dark chamber were measured as step-through latency (STL) up to 300 s [45]. After the determination of ITL on 5th and 9th days, STL was measured on 6th and 10th day (i.e., 24 h later in the acquisition trial), yet again STL was determined on 11th and 12th day (i.e., 48, 72 h later in the acquisition trial), respectively to determine the long-term memory. The percent of memory retention (MR) was calculated by using the formula given below:
An increase in percentage of MR directed enhanced retention of memory [46]. The apparatus was cleaned after each test with 70% ethanol to avoid instinctive odorant clues [47].
RA Test
The spatial working memory of rats was assessed by using the RA test [48], which consists of 3 identical arms, each measuring 500 (length) × 100 (width) × 100 (height of the side walls) mm. The arms were connected by a central square in the middle of the maze to form a T-shape. These 3 arms were designated as start arm, force arm, and novel arm. Each day, each rat was subjected to 6 trials, and each test consisted of 2 distinct trials, forced run trial, and choice run trial. For the forced run trial, the novel arm was blocked and each rat was placed in the start arm facing the central square and forced to the force arm to consume the pellet placed previously. Subsequently, the rat was returned to its home cage. A choice run trial was performed after 60 s of the forced run trial, in which the novel arm was opened (i.e., both the arms were free for the rat to choose). In this choice run trial, the force arm was kept empty and pellets were placed in the novel arm. During the choice run trial, if the rat entered into the novel arm, then the response was considered as correct response (CR). If it entered into the force arm, then it was considered a wrong response (WR) [49,50]. The number of CR and WR were measured on 6th and 12th day. The percentage of MR (i.e., learned task) was calculated by using the formula given below:
where TCRs = total number of CRs, TTs = total number of trials. An increase in percentage of MR was considered as an index of improved cognition (i.e., learning and memory) [49,50]. The apparatus was cleaned after each test with 70% ethanol to avoid instinctive odorant clues [47].
Biochemical Study
The rats were sacrificed under light anesthesia on 13th day by cervical decapitation, and the whole brain was collected and placed on an ice-chilled petri dish for cleaning. The cerebellum was rapidly removed from the whole brain tissue and the remaining brain was rinsed with ice-cold 0.9% NaCl and finally each hemisphere was separated. One of the 2 hemispheres was used to prepare 10% brain homogenate by using ice-cold 30 mM Na2HPO4, pH 7.6 in a homogenizer. Then the homogenate was centrifuged at 20,000 rpm for 2 h at 4°C to remove cellular debris, and the supernatant was used for the determination of SOD, CAT, GSH-Px, GSH, GSH, GST, and TBARS activities. Remaining hemispheres were homogenized (10% w/v) with a glass homogenizer in ice-cold 30 mM Na2HPO4, pH 7.6 and centrifuged at 20,000 rpm for 2 h at 4°C to obtain the salt soluble (SS) part. The pellets were re-extracted with an equal volume of ice-cold phosphate buffer containing 1% Triton X-100 and centrifuged at 20,000 rpm for 2 h at 4°C to recover the detergent soluble (DS) part [45]. Supernatant obtained from both extraction procedures were stored at −20°C and used for AChE activity. The protein concentration was determined by using BSA [51].
SOD Assay
The method of Kakkar et al. [52] was used for the determination of the SOD activity. The total volume of the reaction mixture for this test was 1.6 ml; it consisted of 0.1 ml of 186 μM phenazine methosulphate, 1.2 ml of 0.052 mM sodium pyrophosphate buffer having pH 7.0, and 0.3 ml of supernatant after centrifugation (1,500 g, 10 min followed by 10,000 g, 15 min) of 10% brain tissue homogenate. To start the enzyme reaction, 0.2 ml of 780 μM NADPH was added to the reaction mixture. After 1 min incubation, enzyme reaction was stopped by adding 1 ml of glacial acetic acid. The changes in absorbance of the reaction mixture were determined at 560 nm by using spectrophotometer and expressed as U/mg protein.
CAT Assay
The method of Chance and Maehly with some modification was used for the determination of the CAT activity [53]. The total volume of the reaction mixture for this test was 3.0 ml; it consisted of 2.5 ml of 50 mM phosphate buffer having pH 5.0, 0.4 ml of 5.9 mM H2O2, and 0.1 ml of 10% brain tissue homogenate. After 1 min incubation, the changes in absorbance of the reaction mixture were determined at 240 nm by using spectrophotometer. Here one unit of CAT activity was defined as an absorbance change of 0.01 and expressed as U/min.
GSR Assay
The method of Carlberg and Mannervik was used for the determination of the GSR activity [54]. The total volume of the reaction mixture was 2.0 ml; it consisted of 1.65 ml of 0.1 M phosphate buffer having pH 7.6, 0.1 ml of 0.5 mM EDTA, 0.1 ml of 0.1 mM NADPH, 0.05 ml of 1 mM oxidized GSH, and 0.1 ml of 10% brain tissue homogenate. The changes in absorbance of the reaction mixture, that is, disappearance of NADPH at 25°C were determined at 340 nm by using spectrophotometer and expressed as nM NADPH oxidized/min/mg protein using a molar extinction coefficient of 6.22 × 103 M-1 cm-1.
GSH Assay
The method of Jollow et al. [56] was used for the determination of the GSH activity. For this test, 1.0 ml of 10% brain tissue homogenate was precipitated with 1.0 ml of 4% sulfosalicylic acid. The samples were kept at 4°C for 1 h and then centrifuged (1,200 g for 20 min) at 4°C. The total volume of the reaction mixture was 3.0 ml; it consisted of 0.1 ml filtered aliquot, 2.7 ml of 0.1 M phosphate buffer having pH 7.4, and 0.2 ml of 100 mM DTNB. The yellow color of the mixture was developed and the absorbance of the reaction mixture was determined at 412 nm by using spectrophotometer and expressed as μM GSH/g protein.
GST Assay
The method of Habig et al. [57] was used for the determination of the GST activity. The total volume of the reaction mixture was 2.0 ml; it consisted of 1.475 ml of 0.1 M phosphate buffer having pH 6.5, 0.025 ml of 1 mM CDNB, 0.2 ml of 1 mM reduced GSH, and 0.3 ml of 10% brain tissue homogenate. The changes in absorbance of the reaction mixture were determined at 340 nm by using spectrophotometer and expressed as nM CDNB conjugate formed/min/mg protein using a molar extinction coefficient of 9.6 × 103 M-1 cm-1.
GSH-Px Assay
The method of Mohandas et al. [54] was used for the determination of the GSH-Px activity. The total volume of the reaction mixture for this test was 2.0 ml; it consisted of 1.49 ml of 0.1 M phosphate buffer having pH 7.4, 0.1 ml of 1 mM sodium azide, 0.05 ml of 1 IU/ml GSR, 0.05 ml of 1 mM GSH, 0.1 ml of 1 mM EDTA, 0.1 ml of 0.2 mM NADPH, 0.01 ml of 0.25 mM H2O2, and 0.1 ml of 10% brain tissue homogenate. The changes in absorbance of the reaction mixture, that is, disappearance of NADPH at 25°C were determined at 340 nm by using spectrophotometer and expressed as nM NADPH oxidized/min/mg protein using a molar extinction coefficient of 6.22 × 103 M-1 cm-1.
Lipid Peroxidation (TBARS) Assay
The method of Iqbal et al. [58] was used for the determination of the TBARS activity. The total volume of the reaction mixture was 1.0 ml; it consisted of 0.58 ml of 0.1 M phosphate buffer having pH 7.4, 0.2 ml of 100 mM ascorbic acid, 0.02 ml of 100 mM ferric chloride, and 0.2 ml of 10% brain tissue homogenate. The reaction mixture was incubated at 37°C in a shaking water bath for 1 h. Then 1.0 ml of 10% TCA was added to stop the reaction. Following the addition of 1.0 ml 0.67% TBA, all the test tubes were boiled in a water bath for 20 min. Then the test tubes were shifted to crushed ice bath before centrifuging (2,500 g for 10 min). The amount of TBARS formed in each of the samples was determined by measuring the optical density of the supernatant at 535 nm by using spectrophotometer against a reagent blank and expressed as nM TBARS/min/mg protein at 37°C using a molar extinction coefficient of 1.56 × 105 M-1 cm-1.
AChE Assay
The method of Ellman et al. [59] was used for the determination of the AChE activity. For this test, 25 μl of 15 mM ATCI, 75 μl of 3 mM DTNB, and 75 μl of 50 mM Tris-HCl having pH 8.0, containing 0.1% BSA were added in the 96 well plates and incubated for 5 min at 25°C. Then absorbance was determined at 405 nm by using spectrophotometer. Any increase in the absorbance owing to the regular hydrolysis of the substrate was adjusted by subtracting the rate of the reaction before adding the enzyme. Then 25 μl of brain tissue homogenates (SS and DS portion) were added and the absorbance was determined again after incubation for 5 min at 25°C. The AChE activity was expressed as M/min/g protein.
Statistical Analysis
Results were presented as mean ± SEM and analyzed with one-way analysis of variance. Tukey's post-hoc test was performed for behavioral studies and in case of biochemical studies the least significant difference was determined using post-hoc testing for inter group comparisons at a probability level of 0.05 and 0.01%. SPSS 14.0 (Chicago, Ill., USA) and Microsoft Excel 2010 (Roselle, Ill., USA) were used for the statistical and graphical evaluations. The results were considered as statistically significant at p < 0.05 compared to the control group.
Results
Determination of Acute Toxicity
Oral administration of EEPE was shown to be safe up to the dose level of 2,000 mg/kg b.w. in rats. In any rat, the extracts did not influence any toxicological effect. During the experimental stage, any change in behavioral, motor, and neuronal functions and mortality were not claimed in addition to the monitoring of skin, fur, and eyes of the rats that remained constant so that the extracts were considered safe.
Effect of EEPE on Learning and Memory of Rats Using PA Test
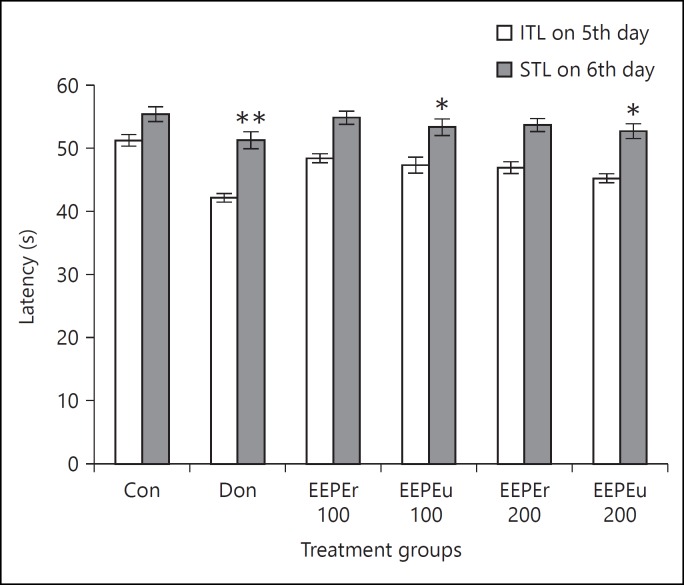
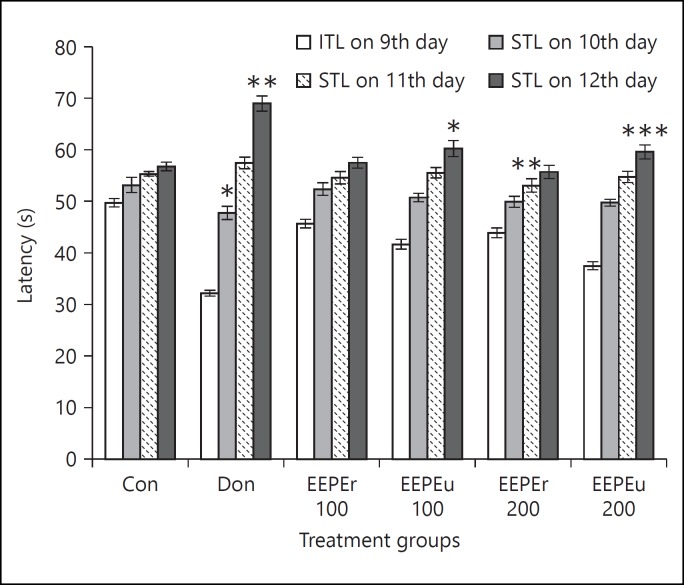
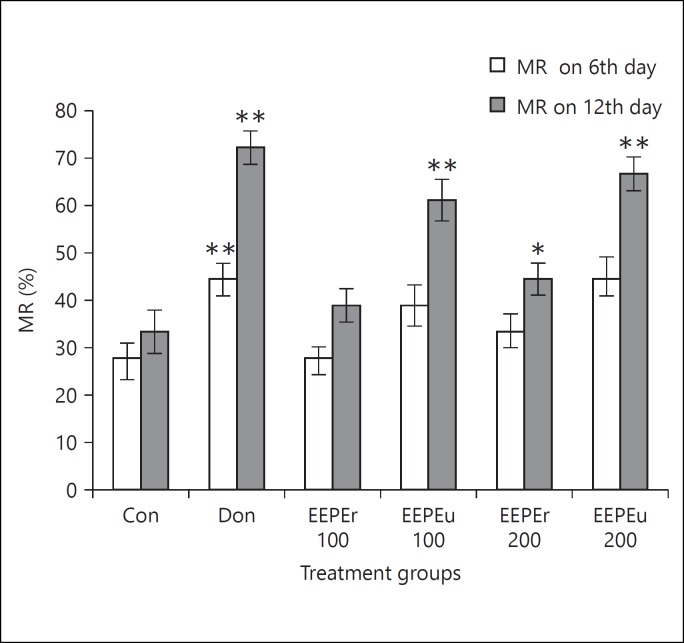
Effect of EEPE on ITL and STL of rats is shown in figures 1 and 2. The rats treated with EEPEr and EEPEu fruits (i.e., 100 and 200 mg/kg, b.w.) and donepezil hydrochloride showed dose-dependent alteration of ITL and STL. The highest dose (i.e., 200 mg/kg b.w.) of EEPEr fruit considerably (p < 0.01) increased STL in rats on 11th day as compared to the control group. For EEPEu fruit, both lowest and highest doses (i.e., 100 and 200 mg/kg b.w.) significantly (p < 0.05, p < 0.001) increased the STL of rats on 6th and 12th day with respect to the control group. For all extract treated groups, the value of STL was greater on 11th and 12th day, respectively, than the 10th day STL. The rats treated with donepezil hydrochloride showed significant (p < 0.05, p < 0.01) increase in STL on successive days compared to the control group. Figure 3 presented the percentage of MR of rats in which an increase in MR after 24, 48, and 72 h, respectively, indicated improved retention of learning task. The percentage of MR was significantly (p < 0.05, p < 0.01) increased in rats treated with highest dose (i.e., 200 mg/kg b.w.) of EEPEr fruit and both lowest and highest doses (i.e., 100 and 200 mg/kg b.w.) of EEPEu fruit on 10th, 11th, and 12th day as compared to the control group.
Fig. 1.
Effect of EEPE on ITL and STL of rats on 5th and 6th day using PA test. Values were expressed as mean ± SEM (n = 6/group). * p < 0.05, ** p < 0.01 significant difference from the control group.
Fig. 2.
Effect of EEPE on ITL and STL of rats on 9th and 10th, 11th and 12th day using PA test. Values were expressed as mean ± SEM (n = 6/group). * p < 0.05, ** p < 0.01, *** p < 0.001 significant difference from the control group.
Fig. 3.
Effect of EEPE on the percentage of MR of rats using PA test. Values were expressed as mean ± SEM (n = 6/group). * p < 0.05, ** p < 0.01, *** p < 0.001 significant difference from the control group.
Effect of EEPE on Learning and Memory of Rats Using RA Test
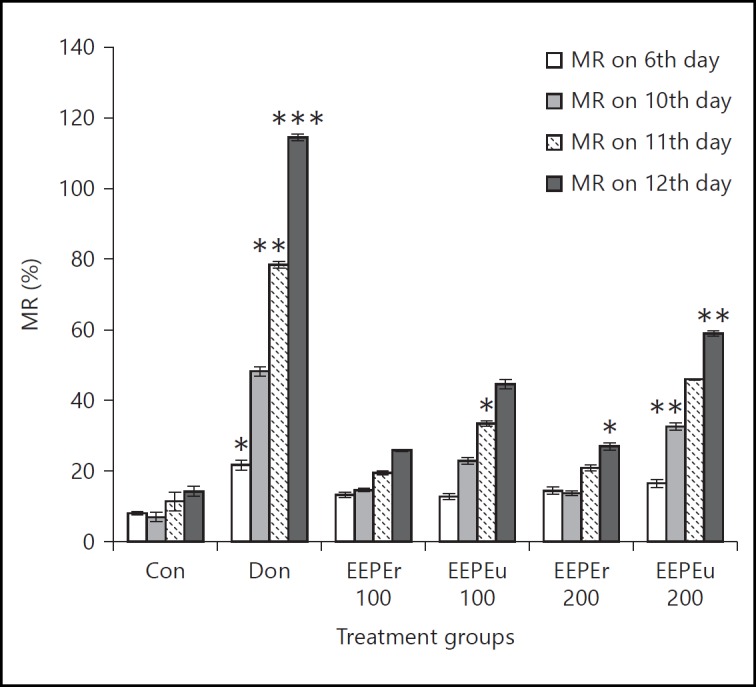
Figure 4 shows the results of the RA test in which the EEPE fruit showed a dose-dependent increase in the number of CR of rats as compared to the control group. Donepezil hydrochloride treated group showed marked (p < 0.05, p < 0.01) increases in the number of CR on 6th and 12th day with respect to the control group. The rats treated with the highest dose (i.e., 200 mg/kg, b.w.) of EEPEr fruit significantly (p < 0.05) increased the CR on 12th day as compared to the control group. In fact, the lowest and highest doses (i.e., 100 and 200 mg/kg b.w.) of EEPEu fruit showed noticeable (p < 0.01) activity on 12th day. Percentage of MR of rats is given in figure 5, in which an increase in MR indicated improved learning and memory. The rats treated with highest dose (i.e., 200 mg/kg b.w.) of EEPEr fruit and both lowest and highest doses (i.e., 100 and 200 mg/kg b.w.) of EEPEu fruit showed considerably (p < 0.05, p < 0.01) increased percentage of MR on 12th day as compared to the control group.
Fig. 4.
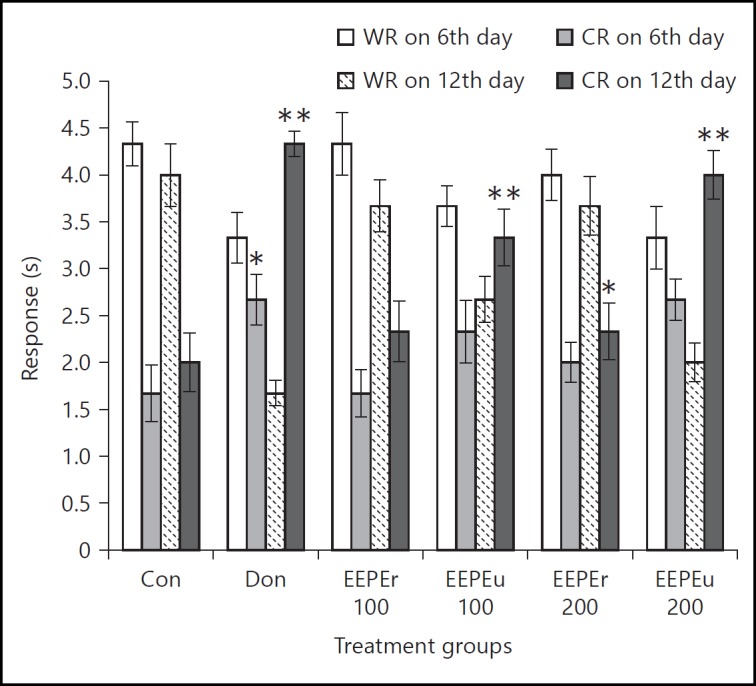
Effect of EEPE on WR and CR of rats using RA test. Values were expressed as mean ± SEM (n = 6/group). * p < 0.05, ** p < 0.01 significant difference from the control group.
Fig. 5.
Effect of EEPE on percentage of MR of rats using RA test. Values were expressed as mean ± SEM (n = 6/group). * p < 0.05, ** p < 0.01 significant difference from the control group.
Effect of EEPE on Brain Oxidative Status
Alteration of antioxidant enzyme activities in rat's brain tissue homogenates is displayed in table 1. Administration of EEPEr and EEPEu fruits and donepezil hydrochloride significantly altered the activities of SOD, CAT, GSH-Px, GSR, GSH, GST, and TBARS in a dose-dependent way. EEPEr fruit at 200 mg/kg b.w. considerably (p < 0.05) increased the level of CAT and GST, while markedly (p < 0.05) reduced the concentration of TBARS to that of the control group. For EEPEu fruit (i.e., 100 and 200 mg/kg b.w.), significantly (p < 0.05, p < 0.01, p < 0.001) increased concentration of SOD, CAT, GSH, GSH-Px and markedly (p < 0.01) reduced concentration of TBARS was detected as compared to the control group. In case of donepezil-treated rats, significant (p < 0.05, p < 0.01) increase in the level of CAT, GSR, GSH, GSH-Px and noticeable (p < 0.01) decrease in the level of TBARS were reported.
Table 1.
Effect of EEPE on biochemical parameters of rat brain antioxidant defense system
| Treatment | SOD, U/mg protein | CAT, U/min | GSR, nM/min/mg protein | GSH, µM/g protein | GST, nM/min/mg protein | GSH-Px, nM/min/mg protein | TBARS, nM/min/mg protein |
|---|---|---|---|---|---|---|---|
| Con | 10.17±1.20 | 8.07±0.83 | 126.42±4.48 | 50.69±7.42 | 102.49±4.40 | 27.32±1.41 | 190.71±3.49 |
| Don | 20.25±0.64 | 19.67±0.90* | 257.98±5.86** | 133.48±5.60** | 172.23±3.01 | 74.84±3.61** | 141.2±4.13** |
| EEPEr 100 | 11.40±1.23 | 10.45±0.42 | 130.64±3.89 | 63.77±3.53 | 113.27±7.54 | 30.55±4.62 | 179.05±4.62 |
| EEPEu 100 | 12.93±1.01* | 14.78±0.87* | 167.14±1.06 | 74.00±8.22** | 131.82±2.75 | 35.23±1.29 | 164.47±3.56** |
| EEPEr 200 | 13.05±0.70 | 12.26±1.68* | 145.47±5.74 | 79.54±2.83 | 117.05±5.56* | 32.88±4.63 | 172.52±4.32* |
| EEPEu 200 | 15.72±1.12** | 16.43±1.48** | 203.48±4.97 | 107.34±3.81** | 140.14±4.80 | 49.04±1.07** | 159.6±4.58** |
The rats brain biochemical parameters were expressed as mean ± SEM values (n = 6/group).
p < 0.05
p < 0.01
p < 0.001 significant difference from the control group.
Effect of EEPE on Brain AChE Activity
The activity of AChE in the SS and DS portions of rat brain tissue homogenate is given in table 2. Treatment with EEPEr and EEPEu fruits and donepezil hydrochloride extensively decreases the concentration of AChE level in a dose-dependent manner. Donepezil treatment substantially (p < 0.01) decreased the AChE level in both SS and DS portions of rat's brain tissue homogenate compared to the control group. EEPEr fruit at concentration of 200 mg/kg b.w. noticeably (p < 0.05) decreased the AChE activity in SS brain tissue homogenate of rats with respect to the control group. Administration of lowest and highest doses (i.e., 100 and 200 mg/kg b.w.) of EEPEu fruit suggestively (p < 0.01, p < 0.001) decreases the AChE activity in both SS and DS portions of brain tissue homogenate of rats as compared to the control group.
Table 2.
Effect of EEPE on AChE activity in rat brain
| Treatment | SS AchE, M/min/g protein | DS AchE, M/min/g protein |
|---|---|---|
| Con | 0.183±0.002 | 0.905±0.021 |
| Don | 0.102±0.002** | 0.323±0.010** |
| EEPEr 100 | 0.165±0.003 | 0.746±0.021 |
| EEPEu 100 | 0.126±0.004** | 0.479±0.013 |
| EEPEr 200 | 0.141±0.003* | 0.531±0.015 |
| EEPEu 200 | 0.110±0.003** | 0.395±0.011*** |
The AChE activity for each group was expressed as mean ± SEM values (n = 6/group).
p < 0.05
p < 0.01
p < 0.001 significant difference from the control group.
Discussion
Medicinal plants are rich sources of phytochemicals, which have a significant role in the treatment of various diseases [60]. The phytoconstituents of medicinal plants play an important role in the treatment of cognitive impairment associated with aging and neurodegenerative disorder [61]. Natural nootropic plants such as Ginkgo biloba, Bacopa monnieri, and Huperzia serrata has been extensively examined for their anti-Alzheimer effect [62]. In this study, EEPEr and EEPEu fruits was administered for 12 days showed momentous memory enhancing effect in rats.
This is the first experiment showing memory enhancing activity of EEPEr and EEPEu fruits in rats. In PA test, neuronal activity (i.e., emotional memory) of rats was examined in which ITL and STL were the measured parameters. STL is the measure of memory in rats after an aversive stimulus. In this test, an increase in STL on 6th (after 24 h of ITL), 10th, 11th, and 12th day (after 24, 48 and 72 h of ITL) after the ITL on 5th and 9th day, respectively, indicated improvement of learning and memory of rats with respect to the control group. Percentage of MR was also determined that indicate strong linkage with long-term memory. Valcheva-Kuzmanova et al. [63] assessed the effect of Aronia melanocarpa fruit juice on memory in rats by the PA task and also reported similar findings. The RA test was used to examine WR and CR. Increased number of CRs was used as a parameter to measure the MR compared to the control group. In this study, dose-dependent improvement of percentage of MR of EEPEr and EEPEu on 6th and 12th day was used as a measure of learning and memory. To examine the neuroenhancing potentiality of Acacia auriculiformis leaves, Sharma et al. [64] used the RA test and monitored better learning and memory enhancing potentiality in rats. Our results proposed that out of the 2 operative doses of EEPE (i.e., 100 and 200 mg/kg, b.w.), in case of PA test, higher dose of EEPEr (i.e., 200 mg/kg, b.w.), and both lowest and highest doses of EEPEu (i.e., 100 mg and 200 mg/kg, b.w.) and for RA test aforementioned doses of EEPEr and EEPEu fruits improved learning and memory in rats as compared to the control group.
SOD is a group of metalloenzymes, which acts as both antioxidant and anti-inflammatory agent, helps to protect oxygen-metabolizing cells, and repairs cell damage that can be usually done by superoxide free-radicals [65]. Studies revealed that SOD catalyzes to form molecular oxygen (O2) and hydrogen peroxide (H2O2) by the breakdown of the superoxide (O2-·) radical. This generated H2O2 can be neutralized by the CAT and glutathione peroxidase systems by converting it to H2O and O2, although H2O2 is less damaging [66]. CAT is a well-known antioxidant present in all cells responsible for the protection of cells from highly reactive hydroxyl radicals (OH·) [67]. During normal metabolic processes, H2O2 is produced as a harmful by-product. To prevent damage to cells by H2O2, it must be quickly converted into other, less dangerous substances. This function is performed by CAT. It neutralizes the H2O2 by converting it to H2O and O2[68]. GSH is an important antioxidant responsible for preventing oxidative damage caused by ROS and providing the first line defense to the body [69]. Total GSH pool is more than 90% in healthy cells and tissues. It exerts its function by donating a reducing equivalent to free radicals, peroxides, lipid peroxides, heavy metals, and various electrophilic compounds [70]. GSR and GST are key antioxidant enzymes because they are involved in the reduction of oxidative damage caused by ROS and help in maintaining the reducing environment of the cell [71]. Oxidized glutathione (GSSG) represents less than 10% of total glutathione. GSR is responsible for the reduction of GSSG to the GSH [72]. GST is considered as primary cellular defense antioxidant that detoxifies ROS. It also catalyzes the nucleophilic attack by GSH on electrophilic substances and consequently prevents oxidative damage [73]. GSH-Px is another main antioxidant enzyme which is responsible for the reduction of H2O2 to H2O by reacting with GSH and converting it to GSSG, which is finally reduced to GSH by GSR [74]. In the progression of neurodegenerative disorder, free radical-mediated lipid peroxidation plays a vital role. TBARS are highly evident in several neurodegenerative diseases, especially AD [75]. In the reduction of oxidative stress and increasing cognition, brain antioxidant enzymes such as SOD, CAT, GSH-Px, GSR, GSH, and GST play a vital role [76]. The study of the effect of aerial parts of Persicaria flaccida on brain antioxidant markers and cognitive performance of rats by Uddin et al. [77] also reported analogous outcomes. Acetylcholine is a neurotransmitter released by nerve cells to send signals to other cells. The cholinergic system (i.e., acetylcholine) is imperative for making memory or encoding, the creation of long-term memory from the new memory or storage and the recall of the memory or recovery [78]. In this study, AChE activity was decreased in both SS and DS portions, with significant effects in rats treated with unripe fruit extract as compared to the control group. Uddin et al. [79] showed that the administration of Phyllanthus acidus fruit increases the brain acetylcholine levels and enhances the cognitive function in rats, while attenuating memory deficits in animal model of amnesia. Our results projected that administration of EEPEr fruit, especially higher dose (i.e., 200 mg/kg b.w.) and both lowest and highest doses of EEPEu fruit (i.e., 100 mg and 200 mg/kg, b.w.) for 12 days increased the levels of SOD, CAT, GSH-Px, GSR, GSH, GST and decreased the levels of TBARS and AChE activity in rat brain tissue homogenates.
The aforementioned behavioral and biochemical effects suggest that EEPE has the ability to improve cognitive function by the regulation of the antioxidant system.
Conclusion
This study clearly demonstrates that EEPE fruits showed marked beneficial effects for improving the learning, memory, and antioxidant potential. Among ripe and unripe fruits, significant cognitive enhancing effects were observed by unripe fruit which is comparable with the standard. Thus, this plant extract can be useful in the treatment of various cognitive disorders, dementia, and neurodegenerative disorders, especially AD. In spite of these findings, further studies are in progress for isolating and identifying promising nootropic compound(s).
Ethical Approval
The study protocol was approved by the Ethics Committee of the Department of Pharmacy, Southeast University, Dhaka, Bangladesh. The care and use of animals were followed in accordance with the principles of NIH.
Authors' Contributions
This work was carried out in collaboration among all authors. M.S.U. and M.A. designed the study, wrote the protocol, managed the analyses of the study, and prepared the draft of the manuscript. M.S.U., A.A.M. and M.S.H. performed the laboratory experiments. F.A. collected and prepared the plant extract and performed literature review. M.A.I. participated in data analysis and interpretation. All authors read and approved the final manuscript.
Disclosure Statement
The authors proclaim that they have no competing interests.
Acknowledgements
The authors wish to thank the Department of Pharmacy, Southeast University, Dhaka, Bangladesh for providing research facilities.
References
- 1.Uddin MS, Mamun AA, Iqbal MA, Islam A, Hossain MF, Khanum S, Rashid M. Analyzing nootropic effect of Phyllanthus reticulatus Poir. on cognitive functions, brain antioxidant enzymes and acetylcholinesterase activity against aluminium-induced Alzheimer's model in rats: applicable for controlling the risk factors of Alzheimer's disease. Adv Alzheimer Dis. 2016;5:87–102. [Google Scholar]
- 2.Solomon Arunodhayan Sam D, Henri C, Sushmitha, et al. Neuron the memory unit of the brain. J Compu Enginee. 2015;17:48–61. [Google Scholar]
- 3.Selnes OA, Vinters HV. Vascular cognitive impairment. Nat Clin Pract Neuro. 2006;2:538–547. doi: 10.1038/ncpneuro0294. [DOI] [PubMed] [Google Scholar]
- 4.Chauhan V, Chaudhary A. Alzheimer's disease: a literature review. Eur J Pharma Pand Med Res. 2015;2:201–207. [Google Scholar]
- 5.Querfurth HW, LaFerla FM. Alzheimer's disease. N Engl J Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 6.Wimo A, Winblad B, Aguero-Torres H, et al. The magnitude of dementia occurrence in the world. Alzheimer Dis Assoc Disord. 2003;17:63–67. doi: 10.1097/00002093-200304000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Mamun AA, Uddin MS, Wahid F, Iqbal MA, Rahman MM. Neurodefensive effect of Olea europaea L. in alloxan-induced cognitive dysfunction and brain tissue oxidative stress in mice: incredible natural nootropic. J Neuro Neurosci. 2016;7:1. [Google Scholar]
- 8.Serrano-Pozo A, Frosch MP, Masliah E, et al. Neuropathological alterations in Alzheimer disease. Cold Spring Harb Perspect Med. 2011;1:a006189. doi: 10.1101/cshperspect.a006189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wray S, Noble W. Linking amyloid and tau pathology in Alzheimer's disease: the role of membrane cholesterol in Abeta-mediated tau toxicity. J Neurosci. 2009;29:9665–9667. doi: 10.1523/JNEUROSCI.2234-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- 11.Kuhla B, Haase C, Flach K, et al. Effect of pseudophosphorylation and cross-linking by lipid peroxidation and advanced glycation end product precursors on tau aggregation and filament formation. J Biol Chem. 2007;282:6984–6991. doi: 10.1074/jbc.M609521200. [DOI] [PubMed] [Google Scholar]
- 12.Selley M, Close DR, Stern SE. The effect of increased concentrations of homocysteine on the concentration of (E)-4-hydroxy-2-nonenal in the plasma and cerebrospinal fluid of patients with Alzheimer's disease. Neurobiol Aging. 2002;23:383–388. doi: 10.1016/s0197-4580(01)00327-x. [DOI] [PubMed] [Google Scholar]
- 13.Bradley MA, Markesbery WR, Lovell MA. Increased levels of 4-hydroxynonenal and acrolein in the brain in preclinical Alzheimer disease. Free Radic Biol Med. 2010;48:1570–1576. doi: 10.1016/j.freeradbiomed.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sultana R, Butterfield DA. Role of oxidative stress in the progression of Alzheimer's disease. J Alzheimers Dis. 2010;19:341–353. doi: 10.3233/JAD-2010-1222. [DOI] [PubMed] [Google Scholar]
- 15.Uddin MS, Mamun AA, Khanum S, Begum Y, Alam MS. Analysis of in vitro antioxidant activity of Caryota urens L. leaves: a traditional natural remedy. J Coast Life Med. 2016;4:483–489. [Google Scholar]
- 16.Zhao Y, Zhao B. Oxidative stress and the pathogenesis of Alzheimer's disease. Oxid Med Cell Longev. 2013;2013:316523. doi: 10.1155/2013/316523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agostinho P, Cunha RA, Oliveira C. Neuroinflammation, oxidative stress and the pathogenesis of Alzheimer's disease. Curr Pharm Des. 2010;16:2766–2778. doi: 10.2174/138161210793176572. [DOI] [PubMed] [Google Scholar]
- 18.Asaduzzaman M, Uddin MJ, Kader MA, et al. In vitro acetylcholinesterase inhibitory activity and the antioxidant properties of Aegle marmelos leaf extract: implications for the treatment of Alzheimer's disease. Psychogeriatrics. 2014;14:1–10. doi: 10.1111/psyg.12031. [DOI] [PubMed] [Google Scholar]
- 19.Mwitari PG, Ayeka PA, Ondicho J, et al. Antimicrobial activity and probable mechanisms of action of medicinal plants of Kenya: Withania somnifera, Warbugia ugandensis, Prunus Africana and Plectrunthus barbatus. PLoS One. 2013;8:e65619. doi: 10.1371/journal.pone.0065619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kayne SB. Traditional Medicine. ed 1. London: Pharmaceutical Press; 2010. [Google Scholar]
- 21.Duraipandiyan V, Ayyanar M, Ignacimuthu S. Antimicrobial activity of some ethnomedicinal plants used by Paliyar tribe from Tamil Nadu, India. BMC Complement Altern Med. 2006;6:35. doi: 10.1186/1472-6882-6-35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bhattacharjee A, Shashidhara SC, Saha S. Nootropic activity of Crataeva nurvala Buch-Ham against scopolamine induced cognitive impairment. EXCLI J. 2015;14:335–345. doi: 10.17179/excli2014-541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oken BS, Storzbach DM, Kaye JA. The efficacy of Ginkgo biloba on cognitive function in Alzheimer disease. Arch Neurol. 1998;55:1409–14015. doi: 10.1001/archneur.55.11.1409. [DOI] [PubMed] [Google Scholar]
- 24.Goswami S, Saoji A, Kumar N, et al. Effect of Bacopa monnierion cognitive functions in Alzheimer's disease patients. Int J Colla Res Int Medi Pub Heal. 2011;3:285–289. [Google Scholar]
- 25.Skolnick AA. Old Chinese herbal medicine used for fever yields possible new Alzheimer disease therapy. JAMA. 1997;277:776. [PubMed] [Google Scholar]
- 26.Zhu X, Wang J, Ou Y, et al. Polyphenol extract of Phyllanthus emblica (PEEP) induces inhibition of cell proliferation and triggers apoptosis in cervical cancer cells. Eur J Med Res. 2013;18:46. doi: 10.1186/2047-783X-18-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hossen Moazzem SM, Sarkar R, Mahmud S, et al. Medicinal potential of Phyllanthus emblica (Linn.) fruits extracts: biological and pharmacological activities. Bri J Pharma Re. 2014;4:1486–1499. [Google Scholar]
- 28.Krishnaveni M, Mirunalini S. Amla - the role of ayurvedic therapeutic herb in cancer. Asi J Pharma Cli Res. 2011;4:13. [Google Scholar]
- 29.Zhang YJ, Tanaka T, Iwamoto Y, et al. Phyllaemblic acid, a novel highly oxygenated norbisabolane from the roots of Phyllanthus emblica. Tetrahedron Lett. 2000;41:1781–1784. [Google Scholar]
- 30.Perianayagam JB, Sharma SK, Joseph A, et al. Evaluation of anti-pyretic and analgesic activity of Emblica officinalis Gaertn. J Ethnopharmacol. 2004;95:83–85. doi: 10.1016/j.jep.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 31.Krishnaveni M, Mirunalini S. Therapeutic potential of Phyllanthus emblica (amla): the ayurvedic wonder. J Basic Clin Physiol Pharmacol. 2010;21:93–105. doi: 10.1515/jbcpp.2010.21.1.93. [DOI] [PubMed] [Google Scholar]
- 32.Dasaroju S, Gottumukkala KM. Current trends in the research of Emblica officinalis (Amla): a pharmacological perspective. Int J Pharm Sci Rev Res. 2014;24:150. [Google Scholar]
- 33.Ihantola-Vormisto A, Summanen J, Kankaanranta H, et al. Anti-inflammatory activity of extracts from leaves of Phyllanthus emblica. Planta Med. 1997;63:518–524. doi: 10.1055/s-2006-957754. [DOI] [PubMed] [Google Scholar]
- 34.Dhale DA, Mogle UP. Phytochemical screening and antibacterial activity of Phyllanthus emblica (L.) Sci Res Reporte. 2011;1:138–142. [Google Scholar]
- 35.Raghu HS, Ravindra P. Antimicrobial activity and phytochemical study of Phyllanthus emblica Linn. Int J Pharma Stud Res. 2010;1:30–33. [Google Scholar]
- 36.Iamsaard S, Arun S, Burawat J, et al. Phenolic contents and antioxidant capacities of Thai-Makham Pom (Phyllanthus emblica L.) aqueous extracts. J Zhejiang Univ Sci B. 2014;15:405–408. doi: 10.1631/jzus.B1300284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ashwlayan VD, Singh R. Reversal effect of Phyllanthus emblica (euphorbiaceae) rasayana on memory deficits in mice. Int J App Pharmace. 2011;3:10–15. [Google Scholar]
- 38.National Research Council . Guide for the Care and Use of Laboratory Animals. ed 8. Washington: National Academies Press; 2011. [Google Scholar]
- 39.Weon JB, Lee J, Eom MR, et al. The effects of Loranthus parasiticus on scopolamine-induced memory impairment in mice. Evid Based Complement Alternat Med. 2014;2014:860180. doi: 10.1155/2014/860180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tasanarong A, Kongkham S, Itharat A. Antioxidant effect of Phyllanthus emblica extract prevents contrast-induced acute kidney injury. BMC Complement Altern Med. 2014;14:138. doi: 10.1186/1472-6882-14-138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Organisation for Economic Cooperation and Development . OECD Guidelines for the Testing of Chemicals: Acute Oral Toxicity - Acute Toxic Class Method. Paris: OECD; 2002. [Google Scholar]
- 42.Akar F, Mutlu O, Komsuoglu Celikyurt I, et al. Zaprinast and Rolipram enhances spatial and emotional memory in the elevated plus maze and passive avoidance tests and diminishes exploratory activity in naive mice. Med Sci Monit Basic Res. 2014;20:105–111. doi: 10.12659/MSMBR.891149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.van der Staay FJ, Schuurman T, van Reenen CG, et al. Emotional reactivity and cognitive performance in aversively motivated tasks: a comparison between four rat strains. Behav Brain Funct. 2009;5:50. doi: 10.1186/1744-9081-5-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wang J, Wang X, Lv B, et al. Effects of Fructus akebiae on learning and memory impairment in a scopolamine-induced animal model of dementia. Exp Ther Med. 2014;8:671–675. doi: 10.3892/etm.2014.1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. J Neurosci Methods. 1984;11:47–60. doi: 10.1016/0165-0270(84)90007-4. [DOI] [PubMed] [Google Scholar]
- 46.Saadipour K, Sarkaki A, Alaei H, et al. Forced exercise improves passive avoidance memory in morphine-exposed rats. Pak J Biol Sci. 2009;12:1206–1211. doi: 10.3923/pjbs.2009.1206.1211. [DOI] [PubMed] [Google Scholar]
- 47.Benchenane K, Castel H, Boulouard M, et al. Anti-NR1 N-terminal-domain vaccination unmasks the crucial action of tPA on NMDA-receptor-mediated toxicity and spatial memory. J Cell Sci. 2007;120((pt 4)):578–585. doi: 10.1242/jcs.03354. [DOI] [PubMed] [Google Scholar]
- 48.Sossin WS, Lacaille JC, Castellucci VF, et al. Progress in Brain Research; Essence of Memory. ed 1. Netherland: Elsevir; 2008. [DOI] [PubMed] [Google Scholar]
- 49.Rao MK, Rao MS, Rao GS. Treatment with Centalla asiatica (Linn) fresh leaf extract enhances learning ability and memory retention power in rats. Neurosciences (Riyadh) 2007;12:236–241. [PubMed] [Google Scholar]
- 50.Deacon RM, Rawlins JN. T-maze alternation in the rodent. Nat Protoc. 2006;1:7–12. doi: 10.1038/nprot.2006.2. [DOI] [PubMed] [Google Scholar]
- 51.Kameyama T, Nabeshima T, Kozawa T. Step-down-type passive avoidance- and escape-learning method. Suitability for experimental amnesia models. J Pharmacol Methods. 1986;16:39–52. doi: 10.1016/0160-5402(86)90027-6. [DOI] [PubMed] [Google Scholar]
- 52.Kakkar P, Das B, Viswanathan PN. A modified spectrophotometric assay of superoxide dismutase. Indian J Biochem Biophys. 1984;21:130–132. [PubMed] [Google Scholar]
- 53.Chance B, Maehly AC. Assay of catalase and peroxidases. Meth Enzymo. 1955;11:764–775. [Google Scholar]
- 54.Mohandas J, Marshall JJ, Duggin GG, et al. Differential distribution of glutathione and glutathione-related enzymes in rabbit kidney. Possible implications in analgesic nephropathy. Biochem Pharmacol. 1984;33:1801–1807. doi: 10.1016/0006-2952(84)90353-8. [DOI] [PubMed] [Google Scholar]
- 55.Bhaskar M, Chintamaneni M. Investigating the role of Eclipta alba on brain antioxidant markers, cognitive performance and acetylcholinesterase activity of rats. Int J Pharm Phytopharmacol Res. 2014;3:390–394. [Google Scholar]
- 56.Jollow DJ, Mitchell JR, Zampaglione N, et al. Bromobenzene-induced liver necrosis. Protective role of glutathione and evidence for 3,4-bromobenzene oxide as the hepatotoxic metabolite. Pharmacology. 1974;11:151–169. doi: 10.1159/000136485. [DOI] [PubMed] [Google Scholar]
- 57.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–7139. [PubMed] [Google Scholar]
- 58.Iqbal M, Sharma MD, Rezazadeh HR, et al. Glutathione metabolizing enzymes and oxidative stress in ferric nitrilotriacetate mediated hepatic injury. Redox Report. 1996;2:385–391. doi: 10.1080/13510002.1996.11747079. [DOI] [PubMed] [Google Scholar]
- 59.Ellman GL, Courtney KD, Andres V, Jr, et al. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 60.Ncube NS, Afolayan AJ, Okoh AI. Assessment techniques of antimicrobial properties of natural compounds of plant origin: current methods and future trends. Afr J Biotechnol. 2008;7:1797–1806. [Google Scholar]
- 61.Kumar GP, Khanum F. Neuroprotective potential of phytochemicals. Pharmacogn Rev. 2012;6:81–90. doi: 10.4103/0973-7847.99898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gong X, Sucher NJ. Stroke therapy in traditional Chinese medicine (TCM): prospects for drug discovery and development. Trends Pharmacol Sci. 1999;20:191–196. doi: 10.1016/s0165-6147(98)01276-0. [DOI] [PubMed] [Google Scholar]
- 63.Valcheva-Kuzmanova SV, Eftimov MT, Tashev RE, et al. Memory effects of Aronia melanocarpa fruit juice in a passive avoidance test in rats. Folia Med (Plovdiv) 2014;56:199–203. doi: 10.2478/folmed-2014-0029. [DOI] [PubMed] [Google Scholar]
- 64.Sharma A, Shetty M, Parida A, et al. Effect of ethanolic extract of Acacia auriculiformis leaves on learning and memory in rats. Pharmacognosy Res. 2014;6:246–250. doi: 10.4103/0974-8490.132605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Salin ML, McCord JM. Free radicals and inflammation. Protection of phagocytosine leukocytes by superoxide dismutase. J Clin Investi. 1975;56:1319–1323. doi: 10.1172/JCI108208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Ansari MA, Scheff SW. Oxidative stress in the progression of Alzheimer disease in the frontal cortex. J Neuropathol Exp Neurol. 2010;69:155–167. doi: 10.1097/NEN.0b013e3181cb5af4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Celik VK, Ersan E, Ersan S, et al. Plasma catalase, glutathione-S-transferase and total antioxidant activity levels of children with attention deficit and hyperactivity disorder. Adv Biosci Biotech. 2013;4:183–187. [Google Scholar]
- 68.Fernandez C, San Miguel E, Fernandez-briera A. Superoxide dismutase and catalase: tissue activities and relation with age in the long-lived species Margaritifera margaritifera. Biol Res. 2009;42:57–68. [PubMed] [Google Scholar]
- 69.Yadav P, Sarkar S, Bhatnagar D. Action of Capparis decidua against alloxan-induced oxidative stress and diabetes in rat tissues. Pharmacol Res. 1997;36:221–228. doi: 10.1006/phrs.1997.0222. [DOI] [PubMed] [Google Scholar]
- 70.Meister A. Glutathione-ascorbic acid antioxidant system in animals. J Biol Chem. 1994;269:9397–9400. [PubMed] [Google Scholar]
- 71.Fang YZ, Yang S, Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/s0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 72.Deponte M. Glutathione catalysis and the reaction mechanisms of glutathione-dependent enzymes. Biochim Biophys Acta. 2013;1830:3217–3266. doi: 10.1016/j.bbagen.2012.09.018. [DOI] [PubMed] [Google Scholar]
- 73.Hayes JD, Flanagan JU, Jowsey IR. Glutathione transferases. Annu Rev Pharmacol Toxicol. 2005;45:51–88. doi: 10.1146/annurev.pharmtox.45.120403.095857. [DOI] [PubMed] [Google Scholar]
- 74.Maritim AC, Sanders RA, Watkins JB., 3rd Effects of alpha-lipoic acid on biomarkers of oxidative stress in streptozotocin-induced diabetic rats. J Nutr Biochem. 2003;14:288–294. doi: 10.1016/s0955-2863(03)00036-6. [DOI] [PubMed] [Google Scholar]
- 75.Galasko D, Montine TJ. Biomarkers of oxidative damage and inflammation in Alzheimer's disease. Biomark Med. 2010;4:27–36. doi: 10.2217/bmm.09.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.El-Missiry MA. Antioxidant enzyme. ed 1. Croatia: InTech; 2012. [Google Scholar]
- 77.Uddin MS, Nasrullah M, Hossain MS, Rahman MM, Sarwar MS, Amran MS, et al. Evaluation of nootropic activity of Persicaria flaccida on cognitive performance, brain antioxidant markers and acetylcholinesterase activity in rats: implication for the management of Alzheimer's disease. Ame J Psy Neurosci. 2016;4:26–37. [Google Scholar]
- 78.McIntyre CK, Pal SN, Marriott LK, et al. Competition between memory systems: acetylcholine release in the hippocampus correlates negatively with good performance on an amygdala-dependent task. J Neurosci. 2002;22:1171–1176. doi: 10.1523/JNEUROSCI.22-03-01171.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Uddin MS, Mamun AA, Hossain MS, Ashaduzzaman M, Noor Asif MA, Hossain MS, Uddin MJ, Sarker J, Asaduzzaman M. Neuroprotective effect of Phyllanthus acidus L. on learning and memory impairment in scopolamine-induced animal model of dementia and oxidative stress: natural wonder for regulating the development and progression of Alzheimer's disease. Adv Alzheimer Dis. 2016;5:53–72. [Google Scholar]