Abstract
Background/Aims
Constructional apraxia (CA) is usually diagnosed by having patients draw figures; however, the reported assessments only evaluate the drawn figure. We designed a new assessment battery for CA (the Mie Constructional Apraxia Scale, MCAS) which includes both the shape and drawing process, and investigated its utility against other assessment methods.
Methods
We designed the MCAS, and evaluated inter- and intrarater reliability. We also investigated the sensitivity, specificity, and positive and negative predictive values in dementia patients, and compared MCAS assessment with other reported batteries in the same subjects.
Results
Moderate interrater reliability was shown for speech therapists with limited experience. Moderate to substantial intrarater reliability was shown several weeks after initial assessment. When cutoff scores and times were set at 2/3 points and 39/40 s, sensitivity and specificity were 77.1 and 70.4%, respectively, with positive and negative predictive values of 80.0 and 66.7%, respectively. Dementia patients had significantly worse scores and times for Necker cube drawing than an elderly control group on the MCAS, and on other assessments.
Conclusions
We conclude that the MCAS, which includes both the assessment of the drawn Necker cube shape and the drawing process, is useful for detecting even mild CA.
Key Words: Constructional apraxia, Necker cube, Mie Constructional Apraxia Scale, Dementia
Introduction
It is well known that the spontaneous drawing or copying of figures can be impaired as a consequence of brain damage. This deficit is termed constructional apraxia (CA), which indicates a drawing disturbance without general impairment of intelligence, visual or motor capacities [1,2]. Previous clinical studies have indicated that CA is frequently observed in patients with lesions of the posterior parietal cortex [2,3,4]. The parietal neurons encode spatial information supplied by visual input that initially encodes position as viewer-centered (retinocentric) coordinates, this is converted into spatial information-encoding position in object-centered coordinates over time, and a correlate of this transform can be detected in the parietal cortex [5].
CA is evident when patients attempt to draw copies of a simple geometric figure such as a cube [5]. Characteristic drawings from CA patients with damaged right hemispheres include a lack of accurate spatial relationships between components of objects and an incoherent, disjointed quality. In contrast, patients with damage to the left hemisphere produce qualitatively different drawings with an oversimplification of figures and a perseveration of items suggestive of planning deficits [6,7,8,9,10]. The inability to produce accurate copies could be caused by either a failure to effectively analyze the spatial structure of the model or to effectively orchestrate motor output to reproduce the structure through a sequence of actions [5,11,12]. Figure copying tests can reveal a patient's deficits in (1) visuospatial abilities, including shape processing and understanding the spatial relationships between different components of objects, (2) executive abilities involved in planning a drawing, and (3) attention to the overall extent and local aspects of a figure [6,10,13,14,15]. In short, the involvement of a widespread network of brain regions which participate in cognitive, perceptual and motor function are required for accurate copying, drawing and construction [10,14]. Although the deficits tend to be most severe following damage to the posterior parietal cortex, they can also occur following frontal damage [5,16].
It is clear that, in spite of the relative simplicity of the definition of CA, the literature suggests that CA is far from being a unitary disorder [8]. Poor figure drawing abilities resulting from a variety of constructional and executive/planning deficits have been reported in neurodegenerative disorders such as Alzheimer's disease (AD), frontotemporal dementia, and Parkinson's disease [15]; thus CA is regarded as a symptom of early-stage dementia. In AD patients, it is generally accepted that CA impairment begins during the early stages due to parietal lobe dysfunction. Some reports have proposed a method of CA assessment which involves the drawing of a simple cube and a Necker cube (table 1). The drawing of a Necker cube is considered to be more difficult than drawing a simple cube. All of the previously reported assessments qualitatively evaluated the shape of the drawn figures or some features of these figures. Strub and Black [17] reported the standard values of normal subjects and patients with AD based on age and stage of the disease. According to their report, constructional ability gradually decreases with age and disease progression. Other reports did not describe standard values.
Table 1.
Assessments of CA using a cube or Necker cube drawing
| First author [Ref.] | Journal | Year | Object | Methods |
|---|---|---|---|---|
| Strub [17] | Book | 2000 | Cube | 0: poor; 1: fair; 2: good; 3: excellent |
| Ohara [22] | J Neurol | 2001 | Necker cube, clock drawing | Qualitative assessment (good/poor) |
| Maeshima [19] | Brain Injury | 2002 | Necker cube, clock drawing | Point of connection (/8): three lines cross at the corner within 3 mm apart Plane-drawing errors: number of pairs of facing sides which are not drawn or parallel |
| Yorimitsu [20] | Higher Brain Funct Res | 2013 | Necker cube | (1) Eight vertices; (2) sides cross at a right angle at two points; (3) appropriate direction of sides; (4) from 20 to 70° of oblique side depth; (5) parallelograms of upper, lower, and side surfaces; (6) squares of frontal and rear surfaces; (7) four lines at a right angle; (8) four horizontal lines; (9) four deep oblique lines; (10) existence of right and horizontal lines (total/10) |
| Kobayashi [23] | Int Med | 2014 | Necker cube, two-dimensional figure | Qualitative assessment (good/poor) |
| Serra [21] | J Alzheimers Dis | 2014 | Cube, star, house | No time constraint. 0–4; 0: Closing-in phenomenon; 1: impossible to identify without closing-in phenomenon; 2: almost impossible to identify; 3: possible to identify but inaccurate; 4: accurate |
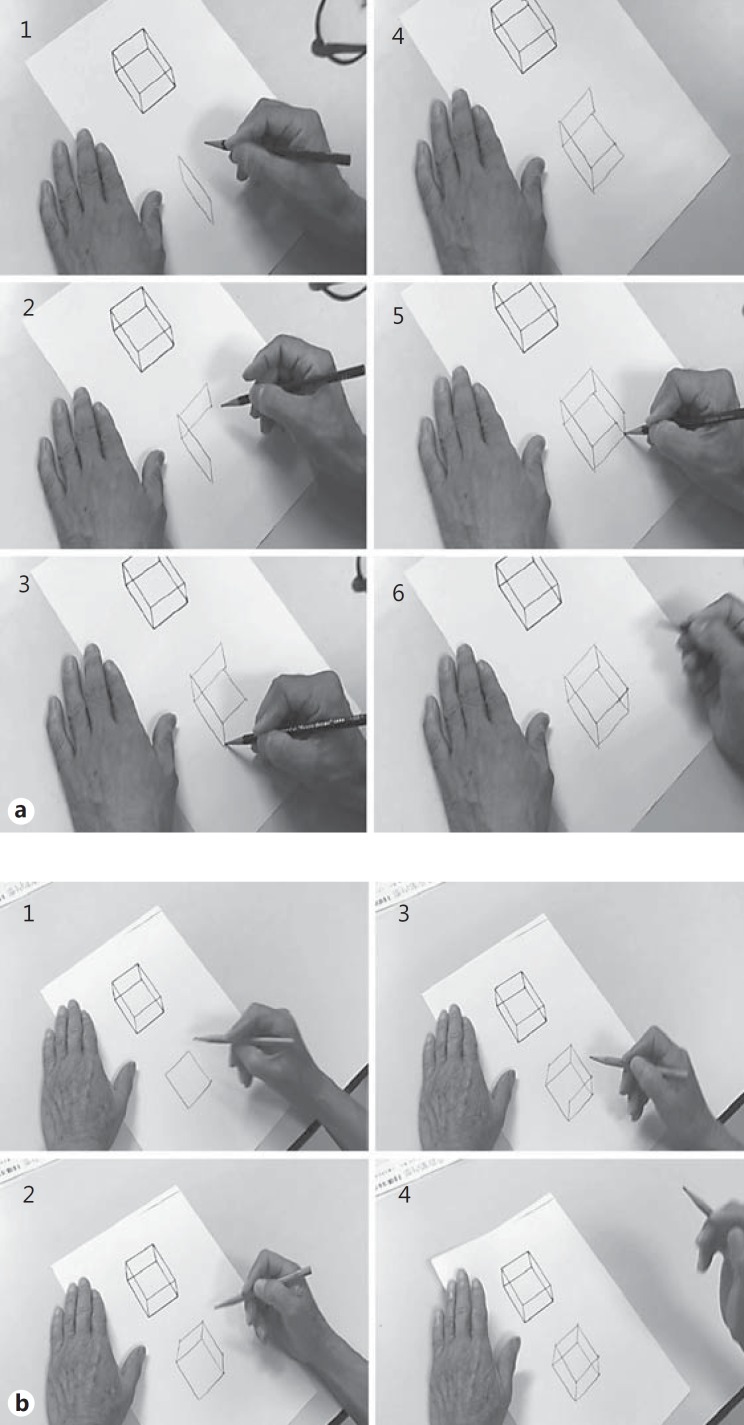
In the present study, one of the authors (M.S.) noticed that even if the drawn figures are accurate, some patients could smoothly and easily draw a Necker cube, whereas other patients drew the figure by trial-and-error and required a longer time for completion (fig. 1). It is possible that assessment of the drawn figures only may not effectively identify mild CA; thus, in order to evaluate slight impairments in constructional ability, an assessment which also includes the drawing process is necessary. The present study aimed to propose a new assessment battery for CA using a Necker cube drawing along with its drawing process. This study consisted of three experiments: (1) the creation of a new assessment battery for CA named the Mie Constructional Apraxia Scale (MCAS); (2) the evaluation of the sensitivity and specificity, and positive and negative predictive values in patients with dementia; and (3) the comparison of the MCAS results with other assessments reported in the literature using the same dementia patients. All experiments followed the Clinical Study Guidelines of the Ethics Committee of Mie University Hospital and were approved by the internal review board.
Fig. 1.
a An example of a Necker cube drawing with some difficulty. Note that the final shape is good, although the subject sometimes hesitated and had to think about how to draw the shape. The total time was 35 s. b An example of an easy and efficient Necker cube drawing. Note that the subject completed the drawing very smoothly. The total time was 32 s.
Experiment 1
The aim of this experiment was to create a new assessment battery for CA and to evaluate the inter- and intrarater reliability.
Subjects and Methods
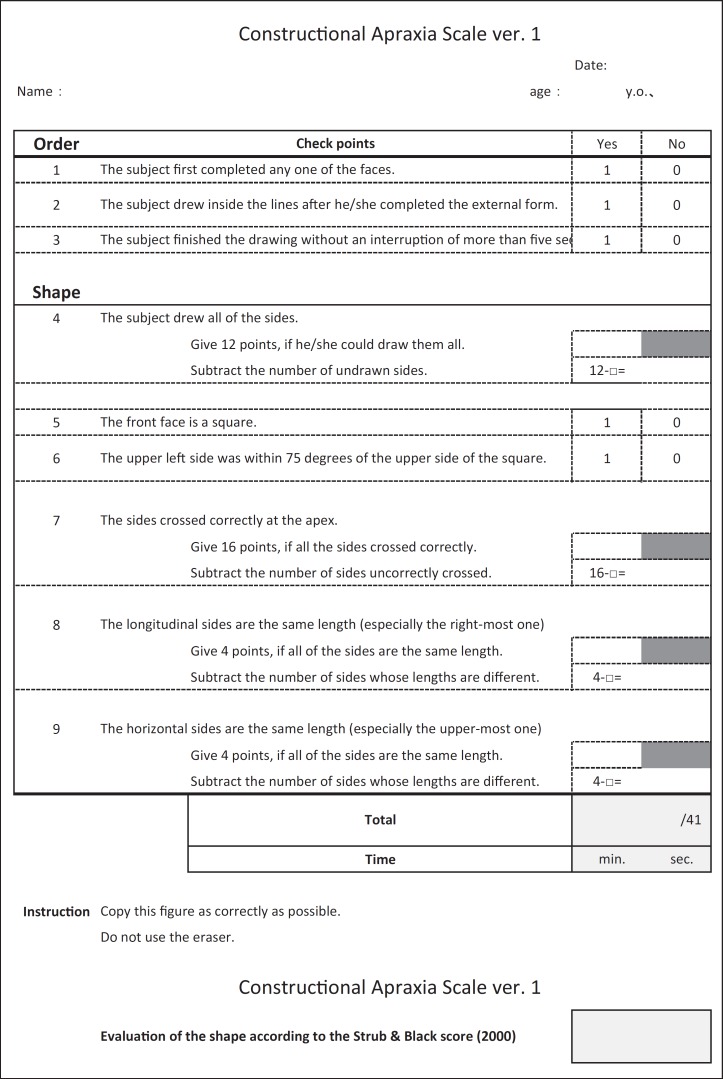
We created the original version of the new CA assessment battery (fig. 2) and evaluated it in 5-6 outpatients who sought consultation at the memory clinic of our hospital, and video-recorded their performance. The authors (M.S., C.M., K.M., Y.U.) watched the videos, then refined the contents of the battery. We then administered the new version to another group of outpatients at the memory clinic. During the evaluation process, we focused on the following points: (1) the ability to be easily used in a clinical setting, (2) the ability to evaluate abnormalities in the drawing process, (3) the total time required for the Necker cube drawing, and (4) the ability to quantitatively describe the degree of CA. For the assessment of the drawn figure, we used the methods described by Strub and Black (the S&B method) [17]. We repeated the procedures described above approximately 10 times, until the final version of the MCAS was agreed upon.
Fig. 2.
Original version of our CA assessment battery.
After the MCAS was created, we explained its use to relatively inexperienced speech therapists whose professional careers were limited to a few years. We randomly chose 13 video recordings, and had the therapists assess CA in these patients using the MCAS. Using the R2.8.1, we analyzed the interrater reliability. A few weeks after the initial assessments, 3 of the original speech therapists were chosen to reassess CA in the same videos, and we investigated the intrarater reliability using SPSS (Statistical Package for Social Science) version 21.
Results
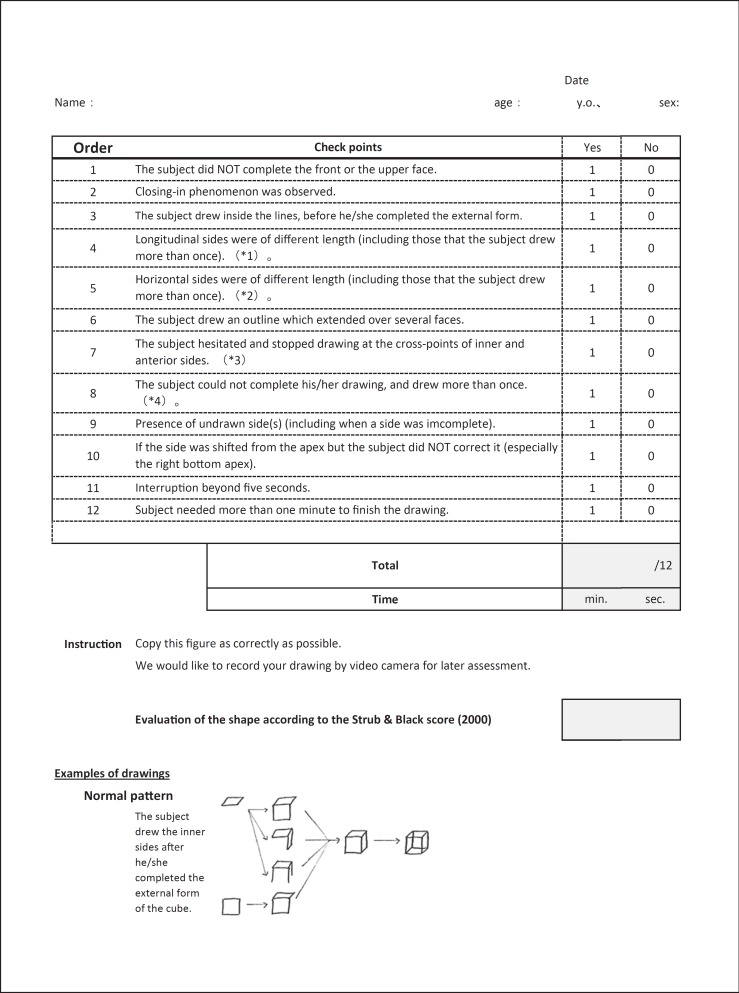
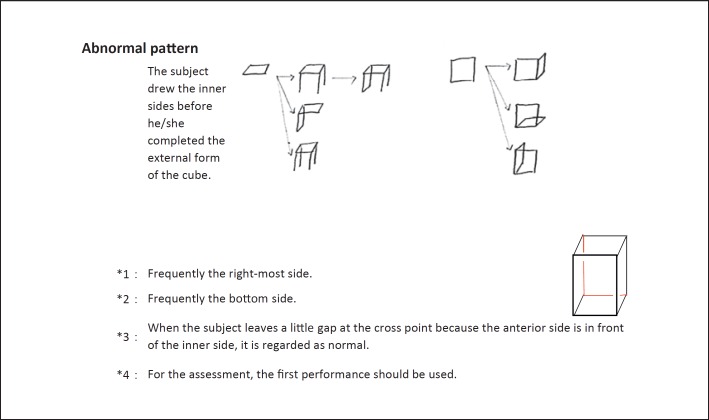
The final version of the MCAS is shown in figure 3. The main changes to the check points from the original version were as follows: (a) in the original version, a lower score indicated more severe CA, whereas in the final version of the MCAS, a higher score indicates more severe CA; (b) the score is 1 or 0, if the answer is ‘Yes’ or ‘No’, respectively, and (c) we show some examples of normal and abnormal patterns of drawing that are frequently observed.
Fig. 3.
Final version of the MCAS. Note that in contrast to the original version, CA becomes more severe as the total score increases.
The interrater reliability κ coefficient (Cohen) between relatively inexperienced speech therapists was 0.49, indicating moderate reliability. Every therapist could effectively complete the assessment after only one or two attempt(s); thus only a few minutes were required for each assessment. The intrarater reliability κ coefficients (Cohen) of three of the therapists several weeks after the first assessment were 0.634, 0.549, and 0.634, respectively, which indicated moderate to substantial reliability.
Discussion
We created the MCAS as a new assessment battery for CA. Even among inexperienced speech therapists, we obtained moderate interrater reliability and moderate to substantial intrarater reliability. These therapists easily mastered the assessment method of the MCAS within several attempts. We can reasonably conclude that the MCAS is easy to use in a clinical setting without limitations related to the experience level of the therapist.
Experiment 2
This experiment aimed to demonstrate the sensitivity, specificity, and positive and negative predictive values of the MCAS in patients with dementia.
Subjects and Methods
The subjects consisted of the following three groups. The patient group (Pt group) included 89 patients who sought consultation at the memory clinic of our hospital between October 2011 to September 2012 and were diagnosed with dementia [mean age 75.3 ± 7.1 years old; males:females = 36:53; AD: 60, mild cognitive impairment: 12, vascular dementia: 8, primary progressive aphasia: 3, idiopathic normal pressure hydrocephalus: 2, semantic dementia: 1, dementia with Lewy bodies: 1, and others: 2 (hypoxic encephalopathy and traumatic brain injury)]. The elderly control group (EC group) consisted of 54 elderly community residents who lived independently and were in normal physical and mental condition (mean age 71.2 ± 10.8 years old; males:females = 8:46). These subjects were the same ones that had participated in our previous study [18] and were video recorded during their Necker cube drawing. The young control group (YC group) consisted of 48 healthy young volunteers who did not suffer from neurological, psychiatric, or developmental disease (mean age 28.4 ± 7.4 years old; males:females = 18:30).
We administered the MCAS to subjects of the Pt, EC, and YC groups and measured their performance time. We also assessed the drawn figures using the S&B method [17]. We set various cutoff points for the MCAS and calculated the sensitivity, specificity, and positive and negative predictive values of both the MCAS and S&B method.
For the statistical analyses, the Shapiro-Wilk test was used for the normality test. If the result was parametric, a two-sample t test was used; if nonparametric, the Wilcoxon signed rank test was used.
Results
The results are summarized in tables 2, 3 and 4. The resulting scores obtained with the S&B method were the same as the standard values in the original report [17]. Comparisons between the score and time on the MCAS and the score from the S&B method showed better performance in the YC than the EC group (table 2). These results suggested that, even in normal subjects, constructional ability decreases with age. Thus, for a comparison of the Pt group, we used results from the EC group.
Table 2.
Results from the MCAS and S&B methods in the Pt, EC, and YC groups
| Pt | Control |
||
|---|---|---|---|
| EC (>65 years old) | YC (<35 years old) | ||
| MCAS | |||
| Score | 3.30 ± 2.56 | 1.83 ± 1.59 | 0.94 ± 1.06 |
| Time,s | 57.7 ± 68.9 | 35.6 ± 16.0 | 20.0 ± 8.6 |
| S&B method | 2.27 ± 0.80 | 2.54 ± 0.61 | 2.85 ± 0.36 |
Data are presented as mean ± standard deviation.
Table 3.
Comparison of results from the Pt and EC groups between the MCAS, S&B method, and neuropsychological assessments
| Pt | EC (>65 years old) | p value | |
|---|---|---|---|
| Age, years | 75.3 ± 7.1 | 71.2 ± 4.3 | <0.001 |
| Education, years | 11.0 ± 3.0 | 10.8 ± 1.8 | 0.779 |
| MCAS | |||
| Score | 3.30 ± 2.56 | 1.83 ± 1.59 | <0.001 |
| Time,s | 57.7 ± 68.9 | 35.6 ± 16.0 | <0.001 |
| S&B method | 2.27 ± 0.80 | 2.54 ± 0.61 | 0.067 |
| MMSE | 22.3 ± 4.43 | 28.2 ± 1.82 | <0.001 |
| RCPM | |||
| Score | 25.1 ± 6.21 | 27.1 ± 4.16 | 0.088 |
| Time,s | 528 ± 276 | 300 ± 80 | <0.001 |
| LM, /25 | |||
| I (immediate recall) | 4.0 ± 2.7 | 12.3 ± 3.7 | <0.001 |
| II (delayed recall) | 1.4 ± 2.3 | 11.3 ± 4.1 | <0.001 |
| TMT | |||
| A | 230 ± 138 | 129 ± 41 | <0.001 |
| B | 300 ± 136 | 175 ± 68 | <0.001 |
| WF, /min | |||
| Animal | 10.1 ± 4.0 | 15.0 ± 4.7 | <0.001 |
| Letters | 5.1 ± 2.1 | 10.3 ± 4.6 | <0.001 |
Data are presented as mean ± standard deviation. Numbers in italics show statistical significance. LM = Logical memory; MMSE = Mini-Mental State Examination; RCPM = Raven's Coloured Progressive Matrices; TMT = trail-making test; WF = word fluency.
Table 4.
Sensitivity, specificity, and positive and negative predictive values of the MCAS and S&B method
| Dementia |
Total | Sensitivity | Specificity | Predictive value |
|||
|---|---|---|---|---|---|---|---|
| yes | no | positive | negative | ||||
| MCASa | 77.1% | 70.4% | 80.0% | 66.7% | |||
| Positive | 64 | 16 | 80 | ||||
| Negative | 19 | 38 | 57 | ||||
| Total | 83 | 54 | 137 | ||||
| S&B methodb | 50.6% | 59.3% | 65.6% | 43.8% | |||
| Positive (≤2) | 42 | 22 | 64 | ||||
| Negative (3) | 41 | 32 | 73 | ||||
| Total | 83 | 54 | 137 | ||||
Cut-off: score 2/3 or time 39/40.
Cut-off: score 2/3.
In table 3, we show the age, educational history, MCAS score, S&B method score, and other neuropsychological assessments of subjects in the Pt and EC groups. The Pt group was older and more intellectually impaired. The mean score of the Mini-Mental State Examination in the Pt group was 22.3, indicating that the patients had mild dementia. It is noteworthy that the score and time on the MCAS were significantly worse in the Pt group (score p = 0.001, time p < 0.001), but the results from the S&B method could not identify significant differences between the Pt and EC groups (p = 0.067).
We next set various cutoff values for the score and time on the MCAS, and calculated the sensitivity, specificity, and positive and negative predictive values (table 4). Results showed the best sensitivity and specificity (77.1 and 70.4%, respectively) when the cutoff score and time were set at 2/3 points and 39/40 s. That is, dementia was indicated when the MCAS score was above 3 or the performance time was over 40 s. The positive and negative predictive values were 80.0 and 66.7%, respectively. The cutoff score of the S&B method was set at 2/3 [17], and according to those scores, the sensitivity and specificity were 50.6 and 59.3%, respectively, and were more than 10% worse than the MCAS scores. The positive and negative predictive values of the S&B method were 65.6 and 43.8%, respectively.
Discussion
We can strongly conclude that the constructional ability of normal subjects decreases with age. By setting the appropriate cutoff points for the score and performance time on the MCAS, all values for sensitivity, specificity, and positive and negative predictive values were better than the S&B method. It is reasonable to hypothesize that the superiority of the MCAS is attributed to the additional assessment of the drawing process versus only assessing the shape of the drawn figure. Thus, as discussed in the literature [6,8,10,13,14], cognitive functions including visuospatial, executive, and attention, might be related to constructional ability.
Experiment 3
For experiment 3, we compared the results from CA assessment in the same dementia patients and normal elderly subjects using the MCAS and other reported methods.
Subjects and Methods
We randomly chose 30 recorded videos from the Pt (AD: 21, vascular dementia: 5, primary progressive aphasia: 2, dementia with Lewy bodies: 1, frontotemporal dementia: 1) and EC groups, and assessed their performance using the MCAS, S&B method, and the batteries reported by Maeshima et al. [19], Yorimitsu et al. [20], and Serra et al. [21]. We compared the results between the Pt and EC groups for each battery. For the statistical analyses, the Shapiro-Wilk test was used to test for normality. If the result was parametric, a paired-t test was used; if nonparametric, the Mann-Whitney U test was used. Furthermore, we investigated the correlation between each assessment method with age, educational history, and the results of each neuropsychological assessment.
Results
The results of each assessment in the same subjects from the Pt and EC groups are shown in table 5. Compared to the EC group, the Pt group was significantly older and scored worse on all of the neuropsychological assessments. The difference in educational history between these two groups was insignificant. Comparisons of Necker cube drawing between the Pt and EC groups showed that the Pt group had significantly worse scores and times on the MCAS, S&B method, and the Maeshima point of connection [19], but the differences were insignificant in the assessment method by Yorimitsu et al. [20] and Serra et al. [21], and plane-drawing errors of Maeshima et al. [19]. Significant correlations were observed between each assessment method involving Necker cube drawing and other methods and the results of neuropsychological assessments (table 6). In particular, the MCAS score showed a strong correlation with logical memory-I, which was determined by assessing the immediate recall of an unfamiliar story (r = −0.708, p < 0.001).
Table 5.
Results of various CA assessments in Pt and EC using identical subjects
| Pt | EC | p value | |
|---|---|---|---|
| Number of patients (M/F) | 30 (10/20) | 30 (4/26) | |
| Age, years | 76.9 ± 6.3 | 70.1 ± 3.9 | <0.001 |
| Education, years | 11.5 ± 3.0 | 11.1 ± 2.1 | 0.179 |
| MMSE | 21.6 ± 4.5 | 28.5 ± 1.5 | <0.001 |
| RCPM | |||
| Score | 24.3 ± 6.6 | 28.0 ± 3.4 | 0.009 |
| Time,s | 562 ± 304 | 271 ± 74 | <0.001 |
| LM | |||
| I | 3.4 ± 2.3 | 12.4 ± 3.6 | <0.001 |
| II | 0.6 ± 1.3 | 10.9 ± 3.4 | <0.001 |
| TMT-A, s | 273 ± 156 | 108 ± 27 | <0.001 |
| WF-animal | 9 ± 3.0 | 16.6 ± 4.0 | <0.001 |
| S&B method | 1.8 ± 0.92 | 2.5 ± 0.78 | 0.003 |
| Maeshima | |||
| POC | 7.2 ± 0.97 | 7.8 ± 0.50 | 0.011 |
| PDE-error | 1.1 ± 1.6 | 0.4 ± 0.97 | 0.097 |
| Yorimitsu | 7.9 ± 2.4 | 8.7 ± 1.6 | 0.187 |
| Serra | 3.3 ± 0.71 | 3.6 ± 0.56 | 0.137 |
| MCAS | |||
| Score | 4.0 ± 2.7 | 1.3 ± 1.8 | <0.001 |
| Time | 69.3 ± 80.7 | 31.6 ± 12.3 | <0.001 |
Data are presented as mean ± standard deviation. Numbers in italics show statistical significance. LM = Logical memory; MMSE = Mini-Mental State Examination; RCPM = Raven's Coloured Progressive Matrices; TMT = trail-making test.
Table 6.
Spearman's rank correlation coefficients between the results of each battery and neuropsychological assessments
| Age | Educ. | MMSE | RCPM |
LM-I | LM-II | TMT-A | WF-animal | S&B | Maeshima |
Yori-mitsu | Serra | MCAS |
||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| score | time | POC | PDE | score | time | |||||||||||
| S&B | ||||||||||||||||
| r | −0.27 | 0.385 | 0.411 | 0.505 | −0.413 | 0.519 | 0.420 | −0.406 | 0.409 | 1 | 0.687 | −0.659 | 0.773 0.805 | −0.757 | −0.486 | |
| p | 0.037 | 0.002 | 0.001 | <0.001 | 0.001 | <0.001 | 0.001 | 0.001 | 0.001 | 1 | <0.001 | <0.001 | <0.001 <0.001 | <0.001 | <0.001 | |
| Maeshima | ||||||||||||||||
| POC | ||||||||||||||||
| r | −0.076 | 0.34 | 0.269 | 0.371 | −0.363 | 0.450 | 0.332 | −0.363 | 0.226 | 0.687 | 1 | −0.545 | 0.487 0.606 | −0.518 −0.39 | ||
| p | 0.562 | 0.008 | 0.038 | 0.004 | 0.004 | <0.001 | 0.010 | 0.004 | 0.082 | <0.001 | 1 | <0.001 | <0.001 <0.001 | <0.001 | 0.002 | |
| PDE | ||||||||||||||||
| r | 0.173 | −0.272 | −0.245 | −0.522 | 0.365 | −0.349 | −0.281 | 0.267 | −0.264 | −0.659 | −0.545 | 1 | −0.624 −0.703 | 0.61 | 0.425 | |
| p | 0.185 | 0.035 | 0.059 | <0.001 | 0.004 | 0.007 | 0.031 | 0.039 | 0.042 | <0.001 | <0.001 | 1 | <0.001 <0.001 | <0.001 | 0.001 | |
| Yorimitsu | ||||||||||||||||
| r | −0.144 | 0.385 | 0.267 | 0.454 | −0.383 | 0.347 | 0.264 | −0.276 | 0.253 | 0.773 | 0.487 | −0.624 | 1 0.819 | −0.573 −0.372 | ||
| p | 0.272 | 0.002 | 0.039 | <0.001 | 0.003 | 0.007 | 0.044 | 0.033 | 0.051 | <0.001 | <0.001 | <0.001 | 1 <0.001 | <0.001 | 0.003 | |
| Serra | ||||||||||||||||
| r | −0.203 | 0.42 | 0.307 | 0.484 | −0.352 | 0.343 | 0.291 | −0.264 | 0.245 | 0.805 | 0.606 | −0.703 | 0.819 1 | −0.602 | 0.369 | |
| p val. | 0.12 | 0.001 | 0.017 | <0.001 | 0.006 | 0.008 | 0.025 | 0.042 | 0.059 | <0.001 | <0.001 | <0.001 | <0.001 1 | <0.001 | 0.004 | |
| MCAS | ||||||||||||||||
| Score | ||||||||||||||||
| r | 0.34 | −0.384 | −0.602 | −0.442 | 0.515 | −0.708 | −0.685 | 0.4 | −0.598 | −0.757 | −0.518 | 0.61 | −0.573 −0.602 | 1 | 0.668 | |
| p | 0.008 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 | 0.002 | <0.001 | <0.001 | <0.001 | <0.001 | <0.001 <0.001 | 1 | <0.001 | |
| Time | ||||||||||||||||
| r | 0.289 | −0.069 | −0.528 | −0.296 | 0.583 | −0.545 | −0.541 | 0.369 | −0.51 | −0.486 | −0.39 | 0.425 | −0.372 0.369 | 0.668 | 1 | |
| p | 0.025 | 0.601 | <0.001 | 0.022 | <0.001 | <0.001 | <0.001 | 0.004 | <0.001 | <0.001 | 0.002 | 0.001 | 0.003 0.004 | <0.001 | 1 | |
LM = Logical memory; MMSE = Mini-Mental State Examination; RCPM = Raven's Coloured Progressive Matrices; TMT = trail-making test. ![]() = r: 0.7–1.0, strong correlation;
= r: 0.7–1.0, strong correlation; ![]() = r: 0.4–0.7, fair correlation;
= r: 0.4–0.7, fair correlation; ![]() = r: 0.2–0.4, minimal correlation.
= r: 0.2–0.4, minimal correlation.
Discussion
The results of each assessment method using Necker cube drawing were fairly correlated with the results of each neuropsychological assessment. These results reinforce that constructional ability might be based on various aspects of cognitive processing. Moreover, for immediate and delayed recall, the MCAS score showed strong and fair correlations, respectively. This suggests that the MCAS might be suitable for detecting subtle change in memory disturbance which is the primary early symptom of patients with dementia. Thus, the results suggest that the MCAS is superior to other reported batteries which only assess the shape of a drawn Necker cube.
General Discussion
The findings of experiments 1, 2, and 3 are summarized as follows: (a) the MCAS was optimized for the assessment of CA and includes the drawing process of a Necker cube; (b) as the MCAS score increases, the predicted impairment worsens; (c) the MCAS can be easily used in a clinical setting even by inexperienced speech therapists; (d) compared to the S&B method for which standard values have been set, the MCAS shows better sensitivity and specificity when the cutoff score and time are set at 2/3 and 39/40, respectively, and (e) the MCAS may be superior to other CA assessment batteries in the literature. In the following paragraphs, we discuss the attributes and utility of the MCAS from the perspective of cognitive processing.
All previously reported methods of CA assessment based on Necker cube drawing only evaluated the shape of the drawn figures. However, because constructional ability consists of various cognitive functions such as visuospatial, executive, and attentional processing, it might not be possible to detect constructional disability, especially in mild cases, solely by evaluating the shape of a drawn object. Furthermore, with the exception of the S&B method, standard values for normal subjects have not previously been determined. In the present study, we proposed a new assessment battery for Necker cube drawing named the MCAS, which also includes the drawing process in its assessment. In the assessment of identical subjects, plane-drawing errors of Maeshima et al. [19], and the methods of Yorimitsu et al. [20] and Serra et al. [21] could not identify significant differences between patients with dementia and normal elderly persons. This suggests that these methods are not as useful for the detection of CA. Both the MCAS and S&B method could identify significant differences between the Pt and EC groups; however, the sensitivity and specificity were better on the MCAS. Therefore, we conclude that the MCAS might be preferable for the assessment of CA.
The time required for MCAS assessment was short, and once the rater became familiar with the scoring, he/she could simultaneously score the patient as they were drawing. As such, the assessment can be completed within 1 min. Even inexperienced speech therapists can easily master the use of the MCAS with good inter- and intrarater reliability. Thus, the MCAS might be useful for the screening of dementia during health checks by both medical and nonmedical staff.
Some of the limitations and potential future investigations are described below. First, the present study targeted patients with dementia. In order to exclusively evaluate constructional ability, we need to compare results from patients with focal lesions situated within the anterior or posterior brain regions with normal intellectual function. To date, no studies have carried out such investigations. Second, it is thought that executive function, including planning, is involved in the drawing of a Necker cube. Thus, it might be possible to detect frontal lobe dysfunction by investigating drawing pattern characteristics. Lastly, the creation of a more optimized test may be possible by combining the MCAS with other neuropsychological tests for the screening of dementia. In the future, we would like to investigate in detail the differences in results on the MCAS between patients with AD, vascular dementia, dementia with Lewy bodies, and frontotemporal dementia, through the assessment of more subjects.
References
- 1.Kleist K. Gehirnpathologie. Leipzig, Bath: 1934. [Google Scholar]
- 2.Makuuchi M, Kaminaga T, Sugishita M. Both parietal lobes are involved in drawing: a functional MRI study and implications for constructional apraxia. Brain Res Cogn Brain Res. 2003;16:338–347. doi: 10.1016/s0926-6410(02)00302-6. [DOI] [PubMed] [Google Scholar]
- 3.Gainotti G. Constructional apraxia. In: Frederiks J, editor. Handbook of Clinical Neurology: Clinical Neuropsychology. Amsterdam: Elsevier; 1985. pp. 491–506. [Google Scholar]
- 4.Ogawa K, Inui T. The role of the posterior parietal cortex in drawing by copying. Neuropsychologia. 2009;47:1013–1022. doi: 10.1016/j.neuropsychologia.2008.10.022. [DOI] [PubMed] [Google Scholar]
- 5.Caminiti R, Chafee MV, Battaglia-Mayer A, Averbeck BB, Crowe DA, Georgopoulos AP. Understanding the parietal lobe syndrome from a neurophysiological and evolutionary perspective. Eur J Neurosci. 2010;31:2320–2340. doi: 10.1111/j.1460-9568.2010.07291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gainotti G, Tiacci C. Patterns of drawing disability in right and left hemispheric patients. Neuropsychologia. 1970;8:379–384. doi: 10.1016/0028-3932(70)90082-5. [DOI] [PubMed] [Google Scholar]
- 7.Guérin F, Ska B, Belleville S. Cognitive processing of drawing abilities. Brain Cogn. 1999;40:464–478. doi: 10.1006/brcg.1999.1079. [DOI] [PubMed] [Google Scholar]
- 8.Smith AD, Gilchrist ID. Drawing from childhood experience: constructional apraxia and the production of oblique lines. Cortex. 2005;41:195–204. doi: 10.1016/s0010-9452(08)70894-3. [DOI] [PubMed] [Google Scholar]
- 9.Trojano L, Conson M. Visuospatial and visuoconstructive deficits. In: Goldenberg G, Miller B, editors. Handbook of Clinical Neurology. Amsterdam: Elsevier; 2008. pp. 373–392. [DOI] [PubMed] [Google Scholar]
- 10.Russel C, Deidda C, Malhotra P, Crinion JT, Merola S, Husain M. A deficit of spatial remapping in constructional apraxia after right-hemisphere stroke. Brain. 2010;133:1239–1251. doi: 10.1093/brain/awq052. [DOI] [PubMed] [Google Scholar]
- 11.Arena R, Gainotti G. Constructional apraxia and visuoperceptive disabilities in relation to laterality of cerebral lesions. Cortex. 1978;14:463–473. doi: 10.1016/s0010-9452(78)80022-7. [DOI] [PubMed] [Google Scholar]
- 12.Mack JL, Levine RN. The basis of visual constructional disability in patients with unilateral cerebral lesions. Cortex. 1981;17:515–531. doi: 10.1016/s0010-9452(81)80059-7. [DOI] [PubMed] [Google Scholar]
- 13.Laeng B. Constructional apraxia after left or right unilateral stroke. Neuropsychologia. 2006;44:1595–1606. doi: 10.1016/j.neuropsychologia.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 14.Trojano L, Grossi D, Flash T. Cognitive neuroscience of drawing: contributions of neuropsychological, experimental and neurofunctional studies. Cortex. 2009;45:269–277. doi: 10.1016/j.cortex.2008.11.015. [DOI] [PubMed] [Google Scholar]
- 15.Chechlaz M, Novick A, Rotshtein P, Bickerton WL, Humphreys GW, Demeyere N. The neural substrates of drawing: a voxel-based morphometry analysis of constructional, hierarchical, and spatial representation deficits. J Cogn Neurosci. 2014;26:2701–2715. doi: 10.1162/jocn_a_00664. [DOI] [PubMed] [Google Scholar]
- 16.Villa G, Gainotti G, de Bonis C. Constructive disabilities in focal brain-damaged patients. Influence of hemispheric side, locus of lesion and coexistent mental deterioration. Neuropsychologia. 1986;24:497–510. doi: 10.1016/0028-3932(86)90094-1. [DOI] [PubMed] [Google Scholar]
- 17.Strub RL, Black FW. Constructional ability. In: Strub RL, Black FW, editors. The Mental Status Examination in Neurology. ed 4. Philadelphia: FA Davis Company; 2000. pp. 93–115. [Google Scholar]
- 18.Satoh M, Ogawa J, Tokita T, Nakaguchi N, Nakao K, Kida H, Tomimoto H. The effects of physical exercise with music on cognitive function of elderly people: Mihama-Kiho project. PLoS One. 2014;9:e95230. doi: 10.1371/journal.pone.0095230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maeshima S, Ueyoshi A, Matsumoto T, Boh-oka S, Yoshida M, Itakura T. Quantitative assessment of impairment in constructional ability by cube copying in patients with aphasia. Brain Injury. 2002;16:161–167. doi: 10.1080/02699050110102095. [DOI] [PubMed] [Google Scholar]
- 20.Yorimitsu M, Tsukada M, Watanabe Y, Yamada R. Development of fixed quantitative scoring method of cube copying test: from the drawings by the inpatients of brain surgery in our hospital (in Japanese) Higher Brain Funct Res. 2013;33:12–19. [Google Scholar]
- 21.Serra L, Fadda L, Perri R, Spano B, Marra C, Castelli D, Torso M, Makovac E, Cercignani M, Caltagirone C, Bozzali M. Constructional apraxia as a distinctive cognitive and structural brain feature of pre-senile Alzheimer's disease. J Alzheimer's Dis. 2014;38:391–402. doi: 10.3233/JAD-130656. [DOI] [PubMed] [Google Scholar]
- 22.Ohara S. Dressing and constructional apraxia in a patient with dentate-rubro-pallido-luysian atrophy. J Neurol. 2001;248:1106–1108. doi: 10.1007/pl00007832. [DOI] [PubMed] [Google Scholar]
- 23.Kobayashi Z, Watanabe M, Karibe Y, Nakazawa C, Numasawa Y, Tomimitsu H, Shintani S. Right hand predominant constructional apraxia due to right hemisphere infarction without corpus callosum lesions. Int Med. 2014;53:1553–1558. doi: 10.2169/internalmedicine.53.2081. [DOI] [PubMed] [Google Scholar]