Abstract
Background
Rural populations experience several barriers to accessing clinical facilities for malaria diagnosis. Increasing penetration of ICT and mobile-phones and subsequent m-Health applications can contribute overcoming such obstacles.
Methods
GIS is used to evaluate the feasibility of m-Health technologies as part of anti-malaria strategies. This study investigates where in Uganda: (1) malaria affects the largest number of people; (2) the application of m-Health protocol based on the mobile network has the highest potential impact.
Results
About 75% of the population affected by Plasmodium falciparum malaria have scarce access to healthcare facilities. The introduction of m-Health technologies should be based on the 2G protocol, as 3G mobile network coverage is still limited. The western border and the central-Southeast are the regions where m-Health could reach the largest percentage of the remote population. Six districts (Arua, Apac, Lira, Kamuli, Iganga, and Mubende) could have the largest benefit because they account for about 28% of the remote population affected by falciparum malaria with access to the 2G mobile network.
Conclusions
The application of m-Health technologies could improve access to medical services for distant populations. Affordable remote malaria diagnosis could help to decongest health facilities, reducing costs and contagion. The combination of m-Health and GIS could provide real-time and geo-localized data transmission, improving anti-malarial strategies in Uganda. Scalability to other countries and diseases looks promising.
Keywords: Remote diagnosis, Malaria mapping, Mobile phones, Rapid diagnostic tests (RDTs), Process innovation, Healthcare, Information communication technology (ICT), Geospatial health technology, Geographic information systems (GIS)
Background
Rapid diagnosis of malaria is difficult, especially in backward rural areas. The gold standard to diagnose malaria is the bright-field microscopy of Giemsa-stained thick and thin blood smears, which allows parasitological confirmation in blood samples and identification of the malaria species. However, light microscopy requires specialized staff and laboratory equipment, not available in forsaken environments. The current alternative to microscopy in remote areas is the rapid diagnostic test (RDT), which detects parasite antigens. RDTs are easier to perform and can be used by operators with less training [1]. However, RDTs have several limitations (such as the impossibility of showing parasite density or detecting persisting antigens once the parasites are cleared) that can lead to misdiagnosis and, therefore, mistreatment [2].
For many reasons rural populations have a preference toward home-based malaria testing [3]. The cost of malaria testing at healthcare facilities is a financial barrier for poor rural households. Travel to the main urban areas where healthcare facilities are mostly located is often difficult and long due to geographical barriers and the lack of good transportation systems. Moreover, shortage of equipment and specialized staff cause congestion at healthcare facilities in the case of disease outbreaks, increasing waiting times for testing and treatment. Many people cannot afford to access healthcare facilities and thus prefer to see if the fever resolves on its own or use RDTs rather than travel to a healthcare spot. Furthermore, the perception of scarce trustworthiness of healthcare facilities equipment and personnel discourage people from accessing appropriate medical structures.
Diffusion of information and communication technologies (ICT) offers unprecedented opportunities to overcome the hindrances to accessing clinical facilities and thus to increase the rate of appropriate treatment of malaria and other infectious diseases. The integration between traditional medical approach and ICT (i.e. e-Health), especially with Smartphone technologies (m-Health), [4] represents a process innovation that could provide cheap, real-time, and geo-referenced data transmission for on-time response to disease outbreaks.
Scientific literature shows the lack of alignment between medical research and ICT developments that can provide cheaply available, but still largely unexploited technological tools. Not much has been done to integrate the several research fields and technologies [5] due also to the inherent interdisciplinary of m-Health [6].
m-Health technology
Nowadays m-Health technology has gained attention because of the latest developments in medical technology [7]. The application of m-Health on chronic disease outcomes in low- and middle-income countries shows positive impacts [8]. Projects based on m-Health are increasing in Africa and their effectiveness has been proven at several levels, including specialist advice in remote areas [9], drugs supply and stock management, and contribution to national health management [10]. One of the main challenges for m-Health applied to malaria is the reliability of the parasites screening technologies.
Several kinds of m-Health technology and, specifically, of mobile microscopy applications are already described in the scientific literature regarding the detection of soil-transmitted parasite worms [11] or hematologic and infectious diseases [12]. The Cellscope mobile microscope (a mobile phone connected through a frame to a lens) meets the Digital Imaging and Communications in Medicine (DICOM) standards [13]. A reversed-lens mobile phone microscope can generate imaging with little distortion and sufficiently high resolution to be employed for the detection of parasites in blood and stool samples [14]. This solution has excellent potential for further developments especially considering the minimal cost of the apparatus (approximately 30 USD, excluding the mobile phone). Potential application to diagnose malaria was demonstrated through the detection of haemozoin crystals in the blood smear [15]. Therefore, m-Health, and in particular mobile microscopy, can provide a low-cost method of detecting malaria in remote areas, but also could become a precious source of real-time and geo-located data on malaria. Consequently, the integration of m-Health and GIS with existing health systems will boost the chances of identifying the spatial and temporal pattern of malaria and responding accordingly [16]. This will also highlight the environmental [17], climatic [18], and socio-economic [19] risk factors for malaria. As a matter of fact, GIS has become a central component of the vector-borne disease risk-assessment process in public health and epidemiology, and it is recognized as a functional element in the roadmap for malaria elimination [20]. However, the adoption of m-Health technologies in rural areas is subject to the ICT infrastructure bottleneck. In the case of an inadequate ICT backbone, m-Health could widen the existing gap between urban and rural populations.
This paper aims to evaluate theoretically if the ICT infrastructure in a developing setting could be able to support m-Health technology in reaching the portion of the population with limited access to proper health diagnosis. Furthermore, the paper examines the feasibility of m-Health technology for malaria detection in rural settings.
Methods
This study investigates Ugandan districts where malaria affects the largest number of people and where the application of m-Health technologies, based on the mobile network, has the highest potential impact. The theoretical model to assess the feasibility and the impacts of this technique for malaria detection in developing countries and remote areas requires several types of geographic data. GIS is then used to evaluate the feasibility of m-Health technologies as part of anti-malaria strategies.
The design and the setting of the study are based on:
The mobile network coverage to determine in which areas it is possible to use m-Health technologies, as they are based on mobile devices that should be connected to the ICT network to work properly.
The combination of geographical models of the human population density with the clinical incidence of malaria, which allows an estimate of the spatial distribution of malaria cases. Overlapping data on mobile network coverage with data on the spatial distribution of malaria cases determines the potential impact of the m-Health technology.
An estimation of how many malaria cases covered by the mobile network reside too distant (in terms of travel time) from a healthcare facility. The introduction of m-Health technologies could be initially implemented only in such areas where the benefit is the greatest, thus, where a large number of people affected by malaria do not have immediate access to healthcare facilities.
This model can be applied in every country where data is available and can be extended to other infective diseases such as TB or water-borne parasites. It can be easily implemented using standard GIS software to overlay and analyse different geographical datasets and to produce significant maps and outputs. The present paper focuses on the execution of the feasibility model in Uganda for its characteristics about malaria morbidity and ITC penetration.
GIS application in Uganda: a case study
According to World Bank statistics [21], Uganda has a population of 37.78 million people (2014), out of which 19.5% are below the poverty line (in 2012, down from 33.8% in 1999). Life expectancy at birth is 59 years. Average monthly temperature (1901–2009) ranges from 23.9 °C in February to 21.6 °C in July, whereas average monthly rainfall peaks in April (149.5 mm) and floors in December (34.4 mm). According to WHO, 48% of the population in 2013 is aged under 15 and only 4% is over 60 [22]. About 85% of the population lives in rural areas.
According to IndexMundi [23], which refers to CIA World Factbook (August 2014), the major infective diseases in Uganda are:
Food or waterborne diseases: bacterial diarrhoea, hepatitis A and E, and typhoid fever,
Vector-borne diseases: malaria, dengue fever, and trypanosomiasis-Gambiense (African sleeping sickness),
Water contact disease: schistosomiasis,
Animal contact disease: rabies.
However, Malaria is recognized as the leading cause of morbidity in Uganda with 90–95% of the population at risk and it contributes to approximately 13% of the mortality of under five-year-old children [24]. According to the President’s Malaria Initiative (PMI) [25] malaria prevalence in children (up to 59 months) is 42%, but in rural areas in the northern region, this can climb up to 63%. Malaria is highly endemic in most of the country, and Uganda has some of the highest transmission rates in the world. Falciparum is the major source of infection, responsible for 99% of malaria cases. Pyrethroid and carbamate insecticide resistance has been documented in the country. Therefore, malaria places a huge burden on the Ugandan health system accounting for 30–50% of outpatient visits and 15–20% of hospital admissions and 9–14% of patient deaths [25]. The overall malaria-specific mortality is estimated to be between 70,000 and 100,000 child deaths annually in Uganda [26].
The Ugandan national health system is organized at the national level and in district level health centres [25]. At the top of the national health service are the two national referral hospitals, which offer general hospital and comprehensive specialist services, in addition to training and research facilities for medical students. Regional Referral hospitals (14 in total) offer general hospital plus some specialist services. At district level health facilities are organized in general hospitals and four categories of health centres: HC-I, II, III, and IV, which respectively serve at the community, parish, sub-county and county level. Only general hospitals, HC-III and HC-IV have laboratory facilities. HC-II only provide outpatient care and community outreach, while HC-I does not even have physical infrastructure but consist of groups of volunteers (Village Health Teams, VHT) that engage mainly in primary healthcare, prevention campaigns and promoting health services at community level.
In Uganda there are 25.3 million mobile phones, thus a SIM penetration of 64% (2014) and mobile network coverage of 75% of the population and 65% of the land (2013) [27]. Connections, SIM card penetration, and mobile network coverage are growing fast.
To assess m-Health feasibility in Uganda, the authors mapped the distribution of cellular base stations (2G and 3G), the clinical incidence of falciparum, the modelled population density and the travel time to main cities.
The Uganda Communication Commission produced a map of the distribution of 2G and 3G cellular base stations in Uganda [27]. Semi-automatic capture screen software elaborated this map creating a mosaic of images which was assembled and geo-referenced using Esri ArcGIS (version 10.0). The position of both types of the cellular base stations was digitalized. The mobile network coverage was estimated considering that the signal from a base station can cover a radius of approximately 10 km. This assumption was adopted because of the undetermined topographic position and, most important, the undocumented type, brand, and version of the cellular base stations deployed in Uganda. Therefore, using GIS software, a 10 km buffer was mapped around each cellular base station to represent the network coverage.
The modelled clinical incidence of falciparum malaria geo-dataset [28] was multiplied by the population distribution geo-dataset [29] in Uganda using GIS. The output represents the number of persons that are affected by falciparum malaria according to the model per unit of surface.
Two final assumptions are made: first, the equipped healthcare structures are located only in major cities and second, that people would not like to spend more than 1 h to travel to healthcare facilities. The extra time that people spend waiting for testing in the healthcare facility is not considered. Using the global map of accessibility [30] calculations are made about how many patients affected by falciparum malaria live more than 1 h away from a major city and thus are unwilling to travel to the healthcare facilities.
In GIS environment, the zone statistic tool allowed calculating the number of modelled falciparum malaria patients per district, the number of falciparum malaria patients that are within 10 km from the 2G and 3G mobile network, and the number of falciparum malaria patients covered by mobile network residing more than 1 h of travel away from a major city (i.e. healthcare facility equipped with laboratory facilities).
Results
Figure 1 shows the distribution of 2G and 3G cellular base stations overlaid with the modelled clinical incidence of falciparum malaria in Uganda. The simple overlay between modelled clinical malaria incidence in Uganda and the cellular base stations shows that the area with a large amount of affected people (in dark brown) are well covered by the 2G cellular base stations (Fig. 1). The eastern region of Uganda has very few cellular base stations, but this area, together with the south-western region, has the lowest malaria incidence values. At a first look, the belt from south–east to north–west (along the Nile River) represents the areas where the highest malaria incidence values are recorded. This belt is well covered by the 2G cellular base stations (green dot).
Fig. 1.
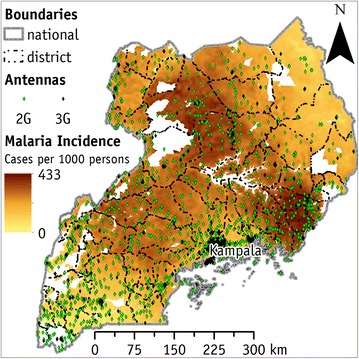
The incidence of falciparum malaria and the distribution of cellular base station in Uganda. Uganda is largely affected by falciparum malaria. Areas with the lowest incidence are the eastern and the south west region. Two-G cellular base station network is diffused in most of the country at the same density, except in the eastern part, where their density is low
Figure 2 shows the absolute number of clinical cases of falciparum malaria per year and hectare. The white areas are zones without population, therefore without cases of falciparum malaria. The main falciparum malaria hotspots are the city of Kampala and the south-eastern portion of the country (centred on Mbale district). Secondary hotspots are (1) the Victoria Lake coastal zone, (2) the central part of the country (between Lira, Apac, and Gulu district) and (3) the western border region (from Arua to Kabarole district).
Fig. 2.
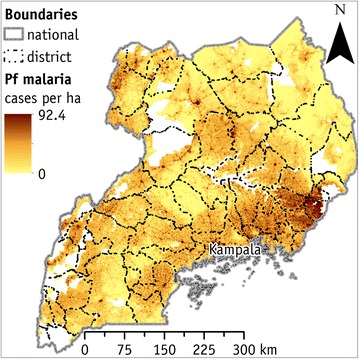
The modelled number of falciparum malaria cases per year and per hectare in Uganda. Integration between the spatial distributions of falciparum malaria incidence (falciparum malaria cases × 1000 people) and of population density model (people × hectare). East region has the lowest number of cases
The land surface conjunctly covered by 2G and 3G (Fig. 3) mobile networks corresponds to about 143,000 km2. It is about 68% of the surface of Uganda (excluding bodies of water). This percentage is not significantly different from the 65% claimed by the Uganda Communication Commission of land covered by the mobile network in 2013. Therefore this model is the nearest to the mobile network coverage in Uganda and the assumption that each cellular base station can cover an area of 10 km of radius is realistic.
Fig. 3.
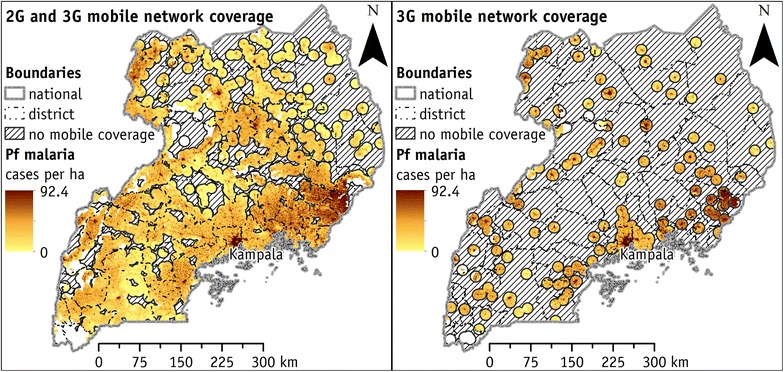
Cases of falciparum malaria covered by mobile network coverage in Uganda. Figure on the left represents falciparum malaria cases covered by the 2G cellular base station network. Figure on the right shows falciparum malaria cases covered by the 3G cellular base station network. Area without mobile coverage is represented with diagonal hatching
Table 1 summarizes the results of the paper. Specifically, it figures out the potential impact of m-Health adoption on a national scale (first line below table heading) and by district (listed in the first column). In Uganda, there are more than 7.1 million falciparum malaria cases per year (second column) according to the modelled clinical incidence (2015), of which approximately three-quarters (third column) reside more than an hour away from main cities. In the fourth column, the table shows the number of modelled falciparum malaria cases located in areas where there is some mobile network coverage (2G and 3G). They amount to circa 6.6 million cases or 93% (fifth column) of the total number of falciparum malaria cases. Except for a few districts, circled with a red line, the mobile network coverage could reach more than 80% of the falciparum malaria cases. Only three districts have a small area covered by mobile network but they represent only 185,000 cases of falciparum malaria (2% of the total).
Table 1.
Number of modelled clinical cases of falciparum malaria and cases covered by the mobile network (2G and 3G) by the district
| District | Falciparum malaria cases | Falciparum malaria cases 2G and 3G network coverage | Falciparum malaria cases 3G network coverage | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Total | >1 h | Cases | % Cases | >1 h | % >1 h | Cases | % Cases | >1 h | |
| Uganda | 7,111,817 | 5,248,041 | 6,620,175 | 93.1 | 4,780,161 | 91.1 | 3,017,262 | 42.4 | 1,719,229 |
| Adjumani | 56,340 | 56,340 | 42,252 | 75.0 | 42,252 | 75.0 | 21,624 | 38.4 | 21,624 |
| Apac | 287,892 | 275,183 | 245,277 | 85.2 | 233,245 | 84.8 | 31,988 | 11.1 | 31,843 |
| Arua | 254,091 | 254,091 | 231,365 | 91.1 | 231,365 | 91.1 | 125,647 | 49.4 | 125,647 |
| Bugiri | 114,148 | 108,314 | 110,916 | 97.2 | 99,089 | 97.0 | 35,367 | 31.0 | 31,515 |
| Bundibugyo | 60,021 | 51,182 | 57,696 | 96.1 | 49,059 | 95.9 | 31,955 | 53.2 | 28,379 |
| Bushenyi | 142,203 | 127,916 | 141,654 | 99.6 | 127,382 | 99.6 | 73,422 | 51.6 | 62,616 |
| Busia | 54,652 | 54,580 | 54,643 | 100.0 | 54,568 | 100.0 | 21,754 | 39.8 | 21,730 |
| Gulu | 192,240 | 88,992 | 162,643 | 84.6 | 77,286 | 74.5 | 91,655 | 47.7 | 18,023 |
| Hoima | 126,662 | 126,662 | 123,371 | 97.4 | 123,371 | 97.4 | 56,020 | 44.2 | 56,020 |
| Iganga | 298,180 | 200,010 | 293,397 | 98.4 | 200,447 | 97.7 | 98,568 | 33.1 | 49,163 |
| Jinja | 91,841 | 20,358 | 91,841 | 100.0 | 20,358 | 100.0 | 77,437 | 84.3 | 12,096 |
| Kabale | 2649 | 2649 | 2647 | 99.9 | 2647 | 99.9 | 339 | 12.8 | 339 |
| Kabarole | 101,984 | 47,464 | 101,794 | 99.8 | 47,281 | 99.6 | 63,495 | 62.3 | 24,291 |
| Kaberamaido | 50,600 | 34,356 | 46,006 | 90.9 | 30,561 | 89.0 | 5198 | 10.3 | 3939 |
| Kalangala | 8295 | 7527 | 7156 | 86.3 | 6387 | 84.8 | 1584 | 19.1 | 1584 |
| Kampala | 196,253 | 0 | 196,253 | 100.0 | 0 | 0.0 | 196,253 | 100.0 | 0 |
| Kamuli | 278,226 | 235,210 | 270,785 | 97.3 | 227,844 | 96.9 | 43,950 | 15.8 | 28,516 |
| Kamwenge | 81,022 | 74,970 | 77,960 | 96.2 | 68,907 | 95.9 | 26,678 | 32.9 | 21,677 |
| Kanungu | 26,395 | 26,395 | 26,366 | 99.9 | 26,366 | 99.9 | 12,374 | 46.9 | 12,374 |
| Kapchorwa | 42,995 | 36,145 | 42,243 | 98.3 | 35,397 | 97.9 | 17,937 | 41.7 | 13,456 |
| Kasese | 98,293 | 95,307 | 97,716 | 99.4 | 94,727 | 99.4 | 45,447 | 46.2 | 44,871 |
| Katakwi | 93,144 | 73,369 | 73,727 | 79.2 | 55,776 | 76.0 | 20,417 | 21.9 | 10,960 |
| Kayunga | 86,261 | 66,305 | 79,698 | 92.4 | 59,759 | 90.1 | 138 | 0.2 | 110 |
| Kibale | 165,812 | 165,812 | 159,074 | 95.9 | 159,074 | 95.9 | 61,195 | 36.9 | 61,195 |
| Kiboga | 86,042 | 86,042 | 80,506 | 93.6 | 80,506 | 93.6 | 0 | 0.0 | 0 |
| Kisoro | 2880 | 2880 | 2467 | 85.7 | 2467 | 85.7 | 561 | 19.5 | 561 |
| Kitgum | 87,522 | 87,522 | 68,621 | 78.4 | 68,621 | 78.4 | 28,489 | 32.6 | 28,489 |
| Kotido | 107,843 | 107,843 | 57,844 | 53.6 | 57,844 | 53.6 | 20,081 | 18.6 | 20,081 |
| Kumi | 163,477 | 86,452 | 149,553 | 91.5 | 77,849 | 90.0 | 86,100 | 52.7 | 41,243 |
| Kyenjojo | 135,578 | 117,711 | 122,328 | 90.2 | 99,499 | 88.8 | 24,968 | 18.4 | 19,687 |
| Lira | 249,699 | 245,238 | 229,175 | 91.8 | 224,723 | 91.6 | 76,388 | 30.6 | 74,594 |
| Luwero | 148,063 | 102,884 | 141,360 | 95.5 | 96,205 | 93.5 | 75,173 | 50.8 | 40,975 |
| Masaka | 221,199 | 96,998 | 219,846 | 99.4 | 96,286 | 99.3 | 150,699 | 68.1 | 55,790 |
| Masindi | 174,348 | 174,348 | 163,868 | 94.0 | 163,868 | 94.0 | 104,225 | 59.8 | 104,225 |
| Mayuge | 66,331 | 41,338 | 61,058 | 92.1 | 36,035 | 87.2 | 8337 | 12.6 | 1272 |
| Mbale | 312,315 | 133,592 | 312,315 | 100.0 | 133,503 | 99.9 | 229,919 | 73.6 | 84,596 |
| Mbarara | 188,492 | 153,222 | 184,781 | 98.0 | 150,986 | 97.9 | 59,885 | 31.8 | 39,972 |
| Moroto | 36,405 | 36,405 | 15,808 | 43.4 | 15,808 | 43.4 | 5712 | 15.7 | 5712 |
| Moyo | 54,214 | 54,214 | 49,703 | 91.7 | 49,703 | 91.7 | 14,984 | 27.6 | 14,984 |
| Mpigi | 127,126 | 97,693 | 118,941 | 93.6 | 89,674 | 91.8 | 50,616 | 39.8 | 31,608 |
| Mubende | 242,633 | 230,370 | 231,000 | 95.2 | 218,753 | 95.0 | 56,582 | 23.3 | 52,350 |
| Mukono | 174,959 | 79,240 | 167,137 | 95.5 | 68,708 | 90.5 | 112,335 | 64.2 | 31,737 |
| Nakapiripirit | 40,738 | 40,738 | 24,746 | 60.7 | 24,746 | 60.7 | 8077 | 19.8 | 8077 |
| Nakasongola | 46,156 | 46,156 | 40,230 | 87.2 | 40,230 | 87.2 | 10,098 | 21.9 | 10,098 |
| Nebbi | 139,188 | 139,188 | 133,317 | 95.8 | 133,317 | 95.8 | 46,020 | 33.1 | 46,020 |
| Ntungamo | 46,067 | 44,328 | 44,937 | 97.5 | 43,194 | 97.4 | 11,862 | 25.7 | 10,513 |
| Pader | 108,060 | 106,295 | 79,466 | 73.5 | 75,982 | 73.4 | 23,619 | 21.9 | 23,619 |
| Pallisa | 262,041 | 145,323 | 251,115 | 95.8 | 135,556 | 93.3 | 105,524 | 40.3 | 57,139 |
| Rakai | 104,828 | 88,756 | 98,943 | 94.4 | 83,272 | 93.8 | 47,494 | 45.3 | 33,317 |
| Rukungiri | 40,408 | 40,408 | 39,908 | 98.8 | 39,908 | 98.8 | 19,343 | 47.9 | 19,343 |
| Sembabule | 57,389 | 45,351 | 53,212 | 92.7 | 41,438 | 91.4 | 21,028 | 36.6 | 15,375 |
| Sironko | 98,985 | 71,724 | 96,646 | 97.6 | 68,413 | 96.8 | 10,987 | 11.1 | 5612 |
| Soroti | 139,009 | 54,074 | 124,553 | 89.6 | 44,154 | 85.4 | 52,332 | 37.6 | 10,229 |
| Tororo | 272,450 | 153,936 | 266,788 | 97.9 | 150,779 | 96.7 | 186,946 | 68.6 | 92,483 |
| Wakiso | 185,698 | 27,160 | 185,698 | 100.0 | 27,160 | 100.0 | 166,175 | 89.5 | 15,301 |
| Yumbe | 81,479 | 81,479 | 67,822 | 83.2 | 67,822 | 83.2 | 42,260 | 51.9 | 42,260 |
In Uganda 4.78 (sixth column) out of 5.25 (third column) million people with falciparum malaria who live in remote areas (1 h away from a major city) have the availability of some mobile network coverage (2G and 3G). This corresponds to about 91% of the remote population (seventh column) at a national scale. Specifically, at the district level, only six districts, namely Kotido, Moroto, Nakapiripirit Adjumani, Gulu, and Pader, have 75% or less of the falciparum malaria affected population covered by the mobile network. In all remaining districts, the 2G and 3G mobile networks cover the vast majority of the modelled falciparum malaria cases even among remote populations. Figure 4 suggests the districts in which the implementation of m-Health technologies could have the greatest effect to supply malaria diagnosis to remote populations. The mapped indicator quantifies the number of cases (i.e. people infected by falciparum malaria, living in remote areas and covered by some mobile network) that could take potential benefit from the introduction of m-Health technologies.
Fig. 4.
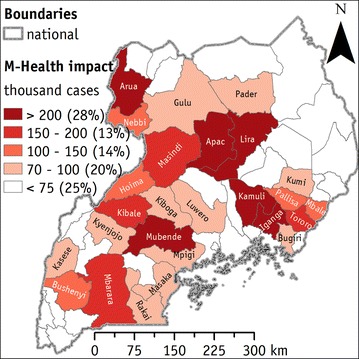
Percentage of m-Health potential cases, i.e. falciparum malaria among people living in remote areas covered by some mobile network. Districts where the implementation of m-Health (i.e. Cellscope mobile) may reach the largest impacts (thousands of potential beneficiaries). Percentages in brackets indicate the portions of the potential beneficiaries cumulated in each class
Six districts (Arua, Apac, Lira, Kamuli, Iganga, and Mubende) have more than 200,000 cases each one that could be approached using m-Health technologies. They represent about 28% of the total m-Health potential cases in Uganda. An additional 13% of m-Health potential cases is localized in four districts (Masindi, Kibale, Tororo, and Mbarara) where there are between 150,000 and 200,000 of m-Health potential cases per district. Further, between 14 and 20% of m-Health potential cases are in other five to eleven districts. Lastly, 30 districts, mapped in white, have less than 75,000 of m-Health potential cases per district, and they amount to 25% of the total m-Health potential cases in Uganda.
The 3G mobile network coverage currently available in Uganda is limited. About three million (eighth column) falciparum malaria cases are in the areas covered by the 3G network, which is 42% (ninth column) of all modelled falciparum malaria cases. Under present conditions, few districts (marked in red in the ninth column: namely Jinja, Kampala, Mbale, and Wakiso) have the vast majority of modelled falciparum malaria cases covered by the 3G mobile network. The 3G network is still in its launch phase and can support m-Health technology only in few Ugandan districts. The last (tenth) column reports the number of falciparum malaria cases among the remote population with available 3G mobile network coverage.
Discussion
This paper highlights that ICT infrastructures and technologies have the potential to aid public health managers, in particular becoming a pivotal tool for the anti-malarial strategies in a developing country. Uganda was selected as a case study to demonstrate the feasibility of the application of ICT to malaria control and prevention. Specifically, m-Health technologies, based on 2G mobile network, are currently applicable almost everywhere in Uganda. Unfortunately, the current 3G network is not sufficient to support a mass application of m-Health technologies.
In remote areas where only the 2G mobile network is available, however, mobile microscopy could be conveniently used in offline mode if the Smartphone is equipped with automated cell-count software, which would enable field operators to diagnose malaria without a remote consultation through m-Health. In this case, the diagnosis would be instantly made on site at the remote point-of-care while the patient’s record would be transmitted to a larger health centre when faster (3G) connectivity is restored.
From traditional to the integrated m-Health/GIS malaria healthcare system
The implementation of m-Health will drastically transform the public health management system. Figure 5 represents a traditional malaria management system (blue arrows represent physical movement, red arrows decision fluxes, green arrows information and data flow, and yellow arrows drugs supply).
Fig. 5.
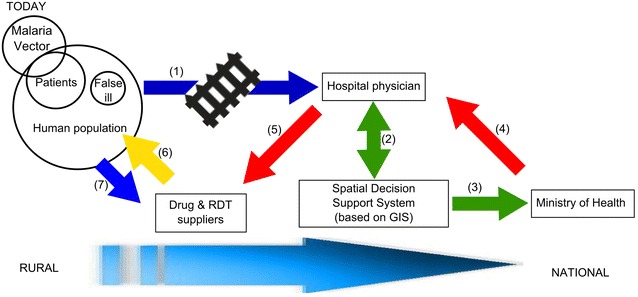
Current malaria management process. Actual malaria management system in Uganda. Blue arrows represent physical movement, red arrows decision fluxes, green arrows information and data flow, and yellow arrow drugs supply. Each step (numbers in brackets) is described in the text
Patients with malaria symptoms reach a healthcare facility where trained medical staff can diagnose malaria (step 1 in Fig. 5). However, there are some barriers to adequate diagnosis and treatment: (a) remoteness of villages and lack of reliable transportation infrastructure, (b) poor equipment, (c) little specialist training, and (d) economic constraints (endemic poverty of rural population). Alternatively, the remote population can use the rapid diagnostic test (RDT) provided by drug suppliers (step 7 in Fig. 5). RDTs, however, have several limitations (e.g. less accurate diagnosis). Data collected at the healthcare facility is analysed by spatial and decision support systems (SDSS) (step 2 in Fig. 5), computerized management systems aimed to smoothen complicated geographic issues [31]. These systems are usually based on a GIS platform and aim to back a better-informed decision of public health authorities (such as Ministry of Health) on malaria elimination (step 3 in Fig. 5).
An example of SDSS applied to malaria is represented by the system that has been developed in the South West Pacific archipelago to automatically locate and map the distribution of confirmed malaria cases, rapidly classify significant transmission centres, and guide targeted responses [32, 33]. As the SDSS relies on effective case detection, the performance of this system is dependent upon the quality of case reporting. However, healthcare facilities collect data not in real-time and with poor spatial accuracy [34]. Indeed, official records are likely to be linked to a health unit, a district, a municipality, or another level of spatial aggregation. Although data can be stored at the individual patient level, the spatial dimension is restricted to an aggregation that can hide crucial local diversity and thus hinder control efforts. Consequently, public health authorities cannot design efficient and flexible responses to malaria disease and cannot adequately direct the healthcare personnel (step 4 in Fig. 5). Moreover, doctors in healthcare facilities do not have a clear picture of transmission in the rural area because they are not onsite, and malaria testing offsite could be unreliable due to errors and inaccuracy. Therefore, they give directives to drug suppliers (step 5 in Fig. 5) to provide medicine to the population in an approximate way, resulting in inappropriate drugs delivery to the rural population and a waste of medical resources.
Figure 6 shows the same process with the innovative introduction of m-Health technologies integrated with GIS. The main innovation is the establishment of mobile points of care equipped with m-Health technologies (i.e. mobile microscopy). They help overcome some of the barriers obstructing the access of rural populations to proper malaria diagnosis and treatment (step 8 in Fig. 6). As a matter of fact, remote diagnosis helps to decongest health facilities where sick patients fuel malaria diffusion, and it significantly reduces costs and the challenges of timely transportation. In particular, in Uganda was proven that introduction of community healthcare services can reduce significantly the number of patient visits presenting as malaria and change the profile of cases seen at health facilities [35].
Fig. 6.
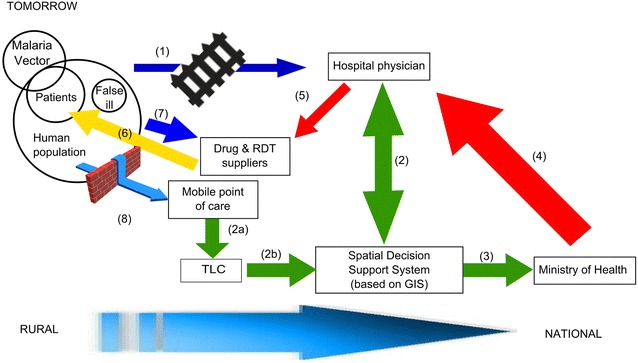
An hypothesis for a future malaria management process after the implementation of m-Health approach. Potential benefit of the introduction of m-Health approach and GIS to manage malaria in Uganda. Blue arrows represent physical movement; red arrows decision fluxes, green arrows information and data flow, and yellow arrow drugs supply. Size of the arrows gives a semi-quantitative measure of the increase of flow of people, material, data, information and decision. Each step (numbers in brackets) is described in the text
Moreover, the performance of any SDSS depends on a correct approach to key health system components, specifically at healthcare facilities and community levels [36]. The introduction of m-Health technology will automatically link case recording with the geographic position of the detection, almost in real time, limiting the discretion of the operators that record cases. This will help to overcome conventional SDSS challenges.
First, the m-Health/GIS integration will strengthen community engagement and malaria monitoring at local level encouraging initial treatment. As other scholars suggest vigilance at the community level is a best practice in the anti-malaria strategy in remote areas [37, 38].
Second, the diagnosis and case reporting will be more accurate, timely, and useful along the chain from local communities to healthcare structures, district authorities, and the Ministry of Health. To this extent, the preservation and safeguarding of the information flow will require the introduction of TLC stakeholders in the malaria management scheme (step 2a in Fig. 6).
Third, timely and accurate reporting (including geographical information) granted by m-Health technologies is a key factor for the effectiveness of any surveillance-response system. Indeed, the ability of health system components to geo-reference data and properly define cases influences the capability of the SDSS to routinely map the allocation of cases and precisely categorize malaria transmission hotspots.
The combination between m-Health technologies and GIS in an integrated SDSS could enable time and space shortcuts. The intensity of edge-connecting nodes (e.g. ill patients to diagnostic centres) depends on TLC signal quality (3G versus 2G standards). The integrated SDSS provides an efficient protocol to visualize and communicate the distribution pattern of malaria transmission a high degree of accuracy. Moreover, integrated GIS queries could enable malaria programme managers to place specific positive cases at a detailed (i.e. household) level; recognize, pick and map areas of main concern (e.g. malaria hotspot) prioritising the intervention; steer the choice of suitably focused, exclusive reaction; and dig out comprehensive additional statistics and facts.
The necessity of gathering more high quality data over a broad geographical area has already been indicated by malaria specialists in Uganda [25]. A network of sentinel sites (UMSN—Uganda malaria surveillance network) has been set up to collect data, ranging from malaria morbidity to mortality of carriers after exposure to insecticides. However, the UMSN is too small to allow a meaningful comparative analysis. The new technology proposed in this paper would allow creating a rich database from which patterns and trends could be identified through Big Data analysis. In order to gain a deeper understanding of the link between malaria and environmental, social and economical conditions it is, therefore, advisable to have a multidisciplinary approach to the subject, gathering data from fields such as climatology, entomology, education, genetics, geography, economics, and more. Big Data and GIS would help discover and visualize the emerging patterns and suggest a preferred course of action.
Assembling this comprehensive dataset facilitates rapid and efficient decision-making by public health authorities in marginal and remote areas and permits rapid and well-organized budget determination, resource allotment and workforce recruitment to aid the execution of actions within acknowledged disease hotspot (step 4 in Fig. 6). Initially, an RDT approach will be integrated into the new system (step 7 in Fig. 6) with the target to be substituted by mobile microscopy. Finally, the distribution of drugs will be enhanced and will be delivered to the appropriate patients avoiding the waste of resources on persons who are not sick (step 6 in Fig. 6).
Upgrading to 3G or 4G standards can substantially improve the efficacy of m-Health technologies. Network optimization exemplified in Fig. 6, softens location problems. Physical barriers will be partly overcome by technology, and organization will change sensibly. As a consequence:
New stakeholders will emerge (TLC, software developers, GIS specialists, IT engineers);
New equipment will be adopted;
Information flows will be faster and more accurate across a wider network;
Policy makers will have different data set on which to work (in real-time and more precisely geo-localized);
Patients will have easier access to health services through mobile point-of-care;
Drugs will be supplied, stored and utilized more efficiently;
Prevention campaigns will be more targeted; legal experts will be involved in managing privacy issues arising from data transmission and cloud storage and computing.
Technological and socio-economic aspects to be considered for implementing an integrated m-Health/GIS healthcare system
Considering the current trend to develop open source software for spatial analysis and geographic visualization, implementing such a system with those capabilities does not require significant financial investment on hardware or software, but only the human-resources investment to set up and maintain the system. A strategy of developing self-paced training packages that can be used to training individuals could possibly be used to customize some GIS software to minimize end-user skill requirements for its use [37].
The present study highlights also that the current 3G network is not sufficiently extended to support a mass application of m-Health technologies. Therefore, in remote areas where only the 2G mobile network is available, mobile microscopy could be conveniently used in offline mode if the Smartphone is equipped with automated cell-count software, which would enable field operators to diagnose malaria without a remote consultation through m-Health. In this case, the diagnosis would be instantly made on site at the remote point-of-care while the patient’s record would be transmitted to a larger health centre when faster (3G) connectivity is restored.
Future implementation of m-Health in the diagnosis and treatment of malaria should address some technological challenges:
The majority of mobile phones in rural areas are “feature phones” (i.e. low-end phones with limited capabilities, often only 2G, with low computing capacity and limited memory). However, the cheap smart phones and apps will be deployed only for field operatives, with little investment. Should mobile microscopy be used by a larger number of people (for example enabling VHTs at village level to carry out tests—but need to consider training issues), then it would be necessary to upgrade from feature phone to Smartphone;
Data transmission (e.g. sending a mobile microscope image from a village to a major health centre or hospital) can be difficult and slow without a 3G connection. Data compression is required, but this means a loss of data quality. Therefore, the development and testing of a suitable compression method represents a technological target;
Data should be transferred in a readable format that could be integrated into the most widely used electronic health records to create, or integrate, a digital patient’s record.
An important concern is the quality of the data that governments have to manage and input in any disease control and surveillance system. Data transmission and management should be reliable, accurate, and safe, and at the same time the financial costs of m-Health/GIS system implementation should have the minimum impact on the poorest part of the population. If not properly addressed these issues will have three results:
Very remote locations lacking healthcare and ITC infrastructure will be more marginalized and underreported than now, cumulating the infrastructural (i.e. roads) and the digital divides;
If poor quality services are provided, people are likely to rely on local healers or ‘‘street doctors’’. Therefore, it is very important to take into consideration the social perception that population has of the m-Health technologies; and
The poorest may not have financial resources to pay for m-Health services and thus only seek care when severely ill [38].
Therefore, future research should focus not only technological aspects (mobile microscopy and GIS) of the innovation process but also on the social and economic impact of new technology on the stakeholders described in Fig. 6 (e.g. network analysis of stakeholders’ relationship).
Further refinement and validation of integration between SDSS and m-Health technologies should include a cost/benefit assessment of the surveillance systems. While the application of m-Health strategies involves initial costs, subsequent savings should rapidly bring to break-even and then to a positive payback.
Further studies to improve the effectiveness of the m-Health feasibility model should address healthcare facilities location—a central node for both the remote diagnosis and treatment. Identification of rural communities that have complicated access to the healthcare system is also crucial.
This study is limited to malaria in Uganda. However, exploring additional applications for various other vector-borne and infectious diseases such as dengue, filariasis, chikungunya, kala-azar, and Japanese encephalitis are very promising [39].
Conclusions
This paper demonstrates the feasibility of an m-Health approach for malaria detection in a developing country (i.e. Uganda). The m-Health approach could have the largest impact, in terms of the remote population potentially involved in the system, in the west and the central-southeast regions of Uganda where m-Health could outreach the vastest portion of the remote population. In particular, six districts (Arua, Apac, Lira, Kamuli, Iganga, and Mubende) account for approximately 28% of the remote population affected by falciparum malaria with access to the 2G mobile network. This technology can improve access to low-cost, valuable and safe diagnostic protocol, making healthcare affordable even in forsaken environments. Further, malaria diagnosis in remote areas could help to decongest health facilities, reducing costs and contagion. Moreover, the combination of m-Health and GIS could provide real-time and geo-localized data transmission, enhancing anti-malaria strategies.
While empirical evidence of this study is limited to falciparum malaria in Uganda, wider applications, regarding other countries and pathologies, seem possible. Scalability can so be vertical and horizontal, geographically in other countries and concerning other diseases, such as TB or parasite-borne illnesses, separately or jointly with malaria.
Further research, to be conducted with an interdisciplinary approach, may comprehensively analyse in an innovative way classical topics such as:
Integrated (and customized) medical history, through ICT recording of diagnosis and treatment;
Digitalization of key information (malaria morbidity, healthcare/ICT spots, etc.) for big data processing;
Just in time logistics, remembering that flexible m-Health networks may show much-wanted resilience when illnesses become pandemic;
Healthcare facilities location that represents a central node for both the remote diagnosis and treatment, to improve the effectiveness of the m-Health feasibility model.
Authors’ contributions
All authors were jointly responsible for the study design and writing. AL collected and analysed ICT data; MM elaborated and interpreted GIS maps and statistics. RMV focused on the paper framework, methodology, and jointly with MM, on the discussion. All authors read and approved the final manuscript.
Acknowledgements
The authors wish to thank Uganda Communication Commission for providing network coverage data and Dr. Emmanuel Ochola (Lacor Hospital, Gulu, Uganda) for his helpful comments. The usual disclaimer applies.
Competing interests
The authors declare that they have no competing interests.
Availability of data and material
The dataset are freely downloadable from repository mentioned in the references list, except for the distribution of mobile network antennas, whose map was supplied by the Uganda Communication Commission.
Consent for publication
There are no case presentations that require disclosure of participants’ identifying data in this study.
Contributor Information
Alberto Larocca, Email: alb.larocca@gmail.com.
Roberto Moro Visconti, Email: roberto.morovisconti@morovisconti.it.
Michele Marconi, Email: mik.marconi@gmail.com.
References
- 1.Mbabazi P, Hopkins H, Osilo E, Kalungu M, Byakika-Kibwika P, Kamya MR. Accuracy of two malaria rapid diagnostic tests (RDTs) for initial diagnosis and treatment monitoring in a high transmission setting in Uganda. Am J Trop Med Hyg. 2015;92:530–536. doi: 10.4269/ajtmh.14-0180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.WHO. Malaria rapid diagnostic test performance results of WHO product testing of malaria RDTs: round 3 (2010–2011). Geneva: World Health Organization, 2011. http://www.who.int/tdr/publications/documents/rdt3.pdf?ua=1. Accessed 7 Sept 2016.
- 3.Ranasinghe S, Ansumana R, Lamin JM, Bockarie AS, Bangura U, Buanie JAG, et al. Attitudes toward home-based malaria testing in rural and urban Sierra Leone. Malar J. 2015;14:80. doi: 10.1186/s12936-015-0582-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Adibi S. Mobile health. A technology road map. Berlin: Springer; 2015. [Google Scholar]
- 5.Fiordelli M, Diviani N, Schulz PJ. Mapping m-Health research: a decade of evolution. J Med Internet Res. 2013;15:e95. doi: 10.2196/jmir.2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dàvalos ME, French MT, Burdick A, Simmons SC. Economic evaluation of telemedicine: review of the literature and research guidelines for benefit-cost analysis. Telemed J E Health. 2009;15:933–948. doi: 10.1089/tmj.2009.0067. [DOI] [PubMed] [Google Scholar]
- 7.Dhanalakshmi TG, Bharathi N. Secure m-Health patient monitoring and emergency alert system framework. Res J Pharm Biol Chem Sci. 2015;6:476–484. [Google Scholar]
- 8.Beratarrechea A, Lee AG, Willner JM, Jahangir E, Ciapponi A, Rubinstein A. The impact of mobile health interventions on chronic disease outcomes in developing countries: a systematic review. Telemed J E Health. 2014;20:75–82. doi: 10.1089/tmj.2012.0328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chihanga S, Tatarsky A, Mosweunyane T, Motlaleng M, Bewlay L, Digovich K, et al. Toward malaria elimination in Botswana: a pilot study to improve malaria diagnosis and surveillance using mobile technology. Malar J. 2012;11:96. doi: 10.1186/1475-2875-11-S1-P96. [DOI] [Google Scholar]
- 10.Kamanga A, Moono P, Stresman G, Mharakurwa S, Shiff C. Rural health centres, communities and malaria case detection in Zambia using mobile telephones: a means to detect potential reservoirs of infection in unstable transmission conditions. Malar J. 2010;9:96. doi: 10.1186/1475-2875-9-96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bogoch II, Andrewa JR, Speich B, Utzinger J, Ame SH, Ali SM, Keiser J. Mobile phone microscopy for the diagnosis of soil-transmitted helminth infections: a proof-of-concept study. Am J Trop Med Hyg. 2013;88:626–629. doi: 10.4269/ajtmh.12-0742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breslauer DN, Maamari RN, Switz NA, Lam WA, Fletcher DA. Mobile phone based clinical microscopy for global health applications. PLoS ONE. 2009;4:e6320. doi: 10.1371/journal.pone.0006320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Skandarajah A, Reber CD, Switz NA, Fletcher DA. Quantitative imaging with a mobile phone microscope. PLoS ONE. 2014;9:e96906. doi: 10.1371/journal.pone.0096906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Switz NA, D’Ambrosio MW, Fletcher DA. Low-cost mobile phone microscopy with a reversed mobile phone camera lens. PLoS ONE. 2014;9:e95330. doi: 10.1371/journal.pone.0095330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pirnstill CW, Coté GL. Malaria diagnosis using a mobile phone polarized microscope. Sci Rep. 2015;5:13368. doi: 10.1038/srep13368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Xia J, Cai S, Zhang H, Lin W, Fan Y, Qiu J, et al. Spatial, temporal, and spatiotemporal analysis of malaria in Hubei Province, China from 2004–2011. Malar J. 2015;14:145. doi: 10.1186/s12936-015-0650-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Oliveira EC, dos Santos ES, Zeilhofer P, Souza-Santos R, Atanaka-Santos M. Geographic information systems and logistic regression for high-resolution malaria risk mapping in a rural settlement of the southern Brazilian Amazon. Malar J. 2013;12:420. doi: 10.1186/1475-2875-12-420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gwitira I, Murwira A, Zengeya FM, Masocha M. Modelled habitat suitability of a malaria causing vector (Anopheles arabiensis) relates well with human malaria incidences in Zimbabwe. Appl Geogr. 2015;60:130–138. doi: 10.1016/j.apgeog.2015.03.010. [DOI] [Google Scholar]
- 19.Qayum A, Arya R, Kumar P, Lynn AM. Socio-economic, epidemiological and geographic features based on GIS-integrated mapping to identify malarial hotspots. Malar J. 2015;14:192. doi: 10.1186/s12936-015-0685-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chanda E, Ameneshewa B, Angula HA, Iitula I, Uusiku P, Trune D, et al. Strengthening tactical planning and operational frameworks for vector control: the roadmap for malaria elimination in Namibia. Malar J. 2015;14:302. doi: 10.1186/s12936-015-0785-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.The World Bank. Uganda. http://data.worldbank.org/country/uganda. Accessed 7 Sept 2016.
- 22.World Health Organization. Uganda. http://www.who.int/countries/uga/en/. Accessed 7 Sept 2016.
- 23.Index mundi. Uganda major infectious diseases. http://www.indexmundi.com/uganda/major_infectious_diseases.html. Accessed 7 Sept 2016.
- 24.Roberts D, Matthews G. Risk factors of malaria in children under the age of five years old in Uganda. Malar J. 2016;15:246. doi: 10.1186/s12936-016-1290-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.President’s Malaria Initiative. Fighting malaria and saving lives, 2015. https://www.pmi.gov/docs/default-source/default-document-library/country-profiles/uganda_profile.pdf?sfvrsn=16. Accessed 7 Sept 2016.
- 26.Lynch KI, Beach R, Asamoa K, Adeya G, Nambooze J, Janowsky E. President’s malaria initiative. Rapid assessment report—Uganda. Washington: USAID; 2005. [Google Scholar]
- 27.Uganda Communications Commission. http://www.ucc.co.ug/data/qmenu/3/Facts-and-Figures.html. Accessed 7 Sept 2016.
- 28.Malaria Atlas Project. http://www.map.ox.ac.uk/. Accessed 7 Sept 2016.
- 29.World Pop. http://www.worldpop.org.uk/. Accessed 7 Sept 2016.
- 30.Nelson A. Estimated travel time to the nearest city of 50,000 or more people in year 2000. Global Environment Monitoring Unit—Joint Research Centre of the European Commission, Ispra Italy. 2008. http://www.forobs.jrc.ec.europa.eu/products/gam/ Accessed 7 Sept 2016.
- 31.Densham DJ. Spatial decision support systems. In: Maguire DJ, Goodchild MF, Harlom RDW, editors. Geographical information systems volume 1: principle. London: Longman Scientific & Technical; 1991. pp. 403–412. [Google Scholar]
- 32.Kelly GC, Tanner M, Vallely A, Clements A. Malaria elimination: moving forward with spatial decision support systems. Trends Parasitol. 2012;28:297–304. doi: 10.1016/j.pt.2012.04.002. [DOI] [PubMed] [Google Scholar]
- 33.Kelly GC, Hale E, Donald W, Bataril W, Bugoro H, Nausien J, et al. A high-resolution geospatial surveillance-response system for malaria elimination in Solomon Islands and Vanuatu. Malar J. 2013;12:108. doi: 10.1186/1475-2875-12-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Atkinson JA, Johnson ML, Wijesinghe R, Bobogare A, Losi L, O’Sullivan M, et al. Operational research to inform a sub-national surveillance intervention for malaria elimination in Solomon Islands. Malar J. 2012;11:101. doi: 10.1186/1475-2875-11-101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lal S, Ndyomugenyi R, Alexander ND, Lagarde M, Paintain L, Magnussen P, et al. Health facility utilisation changes during the introduction of community case management of malaria in South Western Uganda: an interrupted time series approach. PLoS ONE. 2015;10:e0137448. doi: 10.1371/journal.pone.0137448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Sullivan M, Kenilorea G, Yamaguchi Y, Bobogare A, Losi L, Atkinson JA, et al. Malaria elimination in Isabel Province. Solomon Islands: establishing a surveillance-response system to prevent introduction and reintroduction of malaria. Malar J. 2011;10:235. doi: 10.1186/1475-2875-10-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Asuo-Mante E, Awoonor-Williams JK, Yelifari L, Boyer C, Schmitt ML, Phillip JF. The application of geographic information systems (GIS) to improving health systems in the Upper East Region of Ghana. J Glob Health Care Syst. 2016;6:1–14. [Google Scholar]
- 38.De Savigny D, Binka F. Monitoring future impact on malaria burden in sub-Saharan Africa. Am J Trop Med Hyg. 2004;71:224–231. [PubMed] [Google Scholar]
- 39.Cuevas LE, Jeanne I, Molesworth A, Bell M, Savory EC, Connor SJ, et al. Risk mapping and early warning systems for the control of meningitis in Africa. Vaccine. 2007;25:12–17. doi: 10.1016/j.vaccine.2007.04.034. [DOI] [PubMed] [Google Scholar]


