Abstract
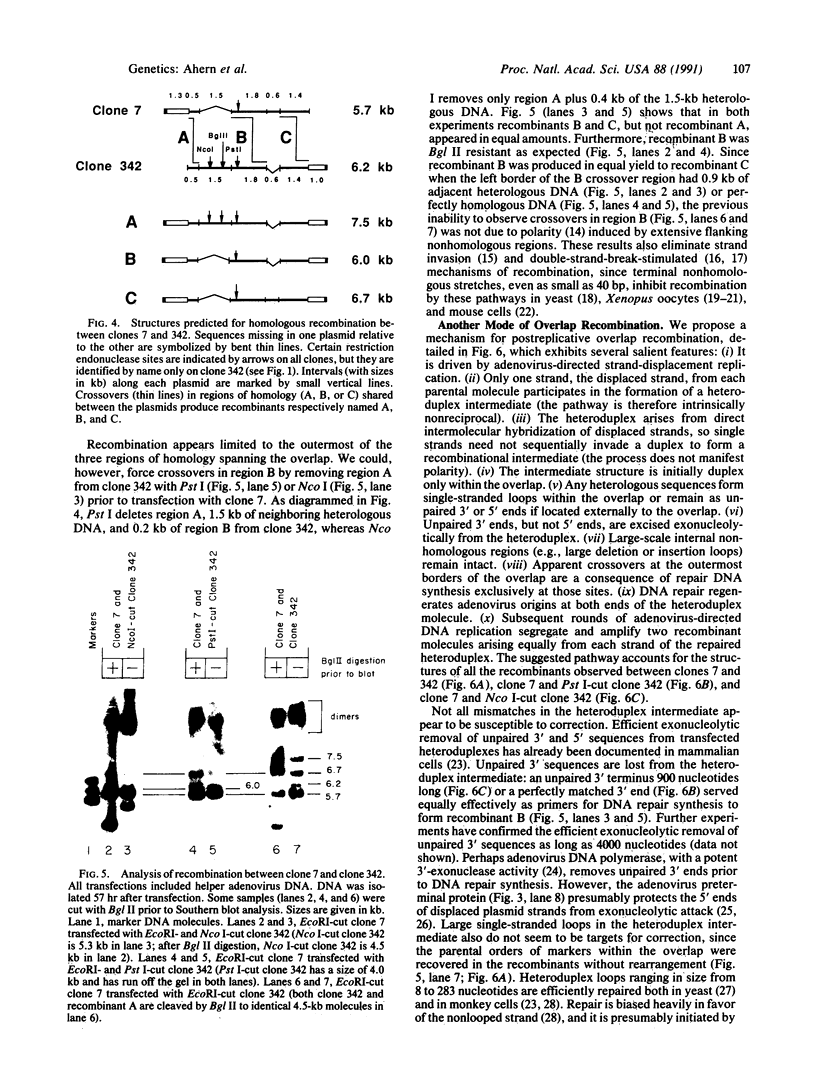
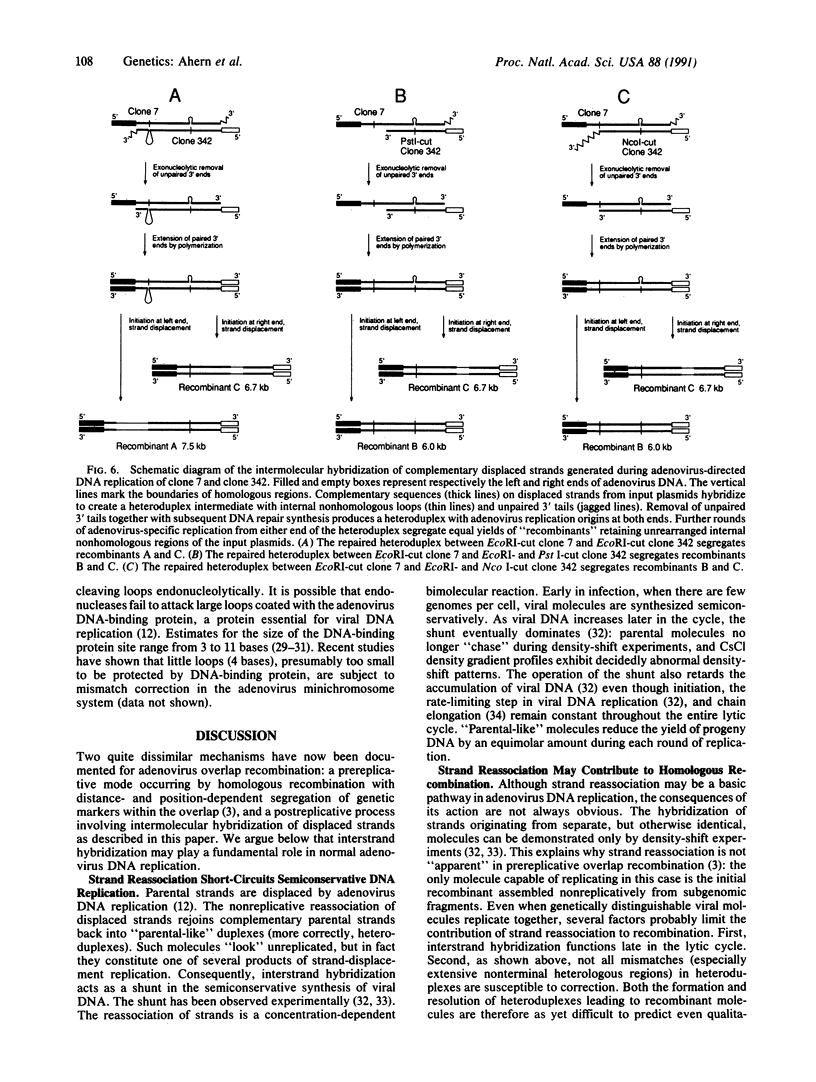
We describe a postreplicative mechanism for adenovirus overlap recombination. An adenovirus minichromosome system was used to study overlap recombination driven by adenovirus DNA replication. Crossing-over appeared to occur equally at, but not within, the borders of the overlap between partner molecules. We propose that recombination in the minichromosome system proceeds through an intermediate formed by direct hybridization of complementary sequences on displaced strands generated by adenovirus-specific DNA replication. Some, but not all, heterologous regions in the intermediate are susceptible to mismatch correction. This pathway is intrinsically nonreciprocal and differs significantly from other adenovirus recombinational mechanisms that have been described previously.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abastado J. P., Darche S., Godeau F., Cami B., Kourilsky P. Intramolecular recombination between partially homologous sequences in Escherichia coli and Xenopus laevis oocytes. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6496–6500. doi: 10.1073/pnas.84.18.6496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayares D., Ganea D., Chekuri L., Campbell C. R., Kucherlapati R. Repair of single-stranded DNA nicks, gaps, and loops in mammalian cells. Mol Cell Biol. 1987 May;7(5):1656–1662. doi: 10.1128/mcb.7.5.1656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berkner K. L., Sharp P. A. Generation of adenovirus by transfection of plasmids. Nucleic Acids Res. 1983 Sep 10;11(17):6003–6020. doi: 10.1093/nar/11.17.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop D. K., Kolodner R. D. Repair of heteroduplex plasmid DNA after transformation into Saccharomyces cerevisiae. Mol Cell Biol. 1986 Oct;6(10):3401–3409. doi: 10.1128/mcb.6.10.3401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodnar J. W., Pearson G. D. Kinetics of adenovirus DNA replication II. Initiation of adenovirus DNA replication. Virology. 1980 Sep;105(2):357–370. doi: 10.1016/0042-6822(80)90037-9. [DOI] [PubMed] [Google Scholar]
- Bodnar J. W., Pearson G. D. Kinetics of adenovirus DNA replication. I. Rate of adenovirus DNA replication. Virology. 1980 Jan 15;100(1):208–211. doi: 10.1016/0042-6822(80)90570-x. [DOI] [PubMed] [Google Scholar]
- Brenner D. A., Smigocki A. C., Camerini-Otero R. D. Double-strand gap repair results in homologous recombination in mouse L cells. Proc Natl Acad Sci U S A. 1986 Mar;83(6):1762–1766. doi: 10.1073/pnas.83.6.1762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll D., Wright S. H., Wolff R. K., Grzesiuk E., Maryon E. B. Efficient homologous recombination of linear DNA substrates after injection into Xenopus laevis oocytes. Mol Cell Biol. 1986 Jun;6(6):2053–2061. doi: 10.1128/mcb.6.6.2053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carusi E. A. Evidence for blocked 5'-termini in human adenovirus DNA. Virology. 1977 Jan;76(1):380–394. doi: 10.1016/0042-6822(77)90310-5. [DOI] [PubMed] [Google Scholar]
- Challberg M. D., Kelly T. J. Animal virus DNA replication. Annu Rev Biochem. 1989;58:671–717. doi: 10.1146/annurev.bi.58.070189.003323. [DOI] [PubMed] [Google Scholar]
- Chinnadurai G., Chinnadurai S., Brusca J. Physical mapping of a large-plaque mutation of adenovirus type 2. J Virol. 1979 Nov;32(2):623–628. doi: 10.1128/jvi.32.2.623-628.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Halluin J. C., Milleville M. Initiation rate of adenovirus DNA synthesis in infected cell. Biochim Biophys Acta. 1984 May 15;782(1):67–75. doi: 10.1016/0167-4781(84)90107-6. [DOI] [PubMed] [Google Scholar]
- Dunsworth-Browne M., Schell R. E., Berk A. J. Adenovirus terminal protein protects single stranded DNA from digestion by a cellular exonuclease. Nucleic Acids Res. 1980 Feb 11;8(3):543–554. doi: 10.1093/nar/8.3.543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Field J., Gronostajski R. M., Hurwitz J. Properties of the adenovirus DNA polymerase. J Biol Chem. 1984 Aug 10;259(15):9487–9495. [PubMed] [Google Scholar]
- Graham F. L., Smiley J., Russell W. C., Nairn R. Characteristics of a human cell line transformed by DNA from human adenovirus type 5. J Gen Virol. 1977 Jul;36(1):59–74. doi: 10.1099/0022-1317-36-1-59. [DOI] [PubMed] [Google Scholar]
- Graham F. L., van der Eb A. J. A new technique for the assay of infectivity of human adenovirus 5 DNA. Virology. 1973 Apr;52(2):456–467. doi: 10.1016/0042-6822(73)90341-3. [DOI] [PubMed] [Google Scholar]
- Grzesiuk E., Carroll D. Recombination of DNAs in Xenopus oocytes based on short homologous overlaps. Nucleic Acids Res. 1987 Feb 11;15(3):971–985. doi: 10.1093/nar/15.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hay R. T., Stow N. D., McDougall I. M. Replication of adenovirus mini-chromosomes. J Mol Biol. 1984 Jun 5;175(4):493–510. doi: 10.1016/0022-2836(84)90181-5. [DOI] [PubMed] [Google Scholar]
- Horton R. M., Hunt H. D., Ho S. N., Pullen J. K., Pease L. R. Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene. 1989 Apr 15;77(1):61–68. doi: 10.1016/0378-1119(89)90359-4. [DOI] [PubMed] [Google Scholar]
- McGrory W. J., Bautista D. S., Graham F. L. A simple technique for the rescue of early region I mutations into infectious human adenovirus type 5. Virology. 1988 Apr;163(2):614–617. doi: 10.1016/0042-6822(88)90302-9. [DOI] [PubMed] [Google Scholar]
- Meselson M. S., Radding C. M. A general model for genetic recombination. Proc Natl Acad Sci U S A. 1975 Jan;72(1):358–361. doi: 10.1073/pnas.72.1.358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munz P. L., Young C. S. Polarity in adenovirus recombination. Virology. 1984 Jun;135(2):503–514. doi: 10.1016/0042-6822(84)90204-6. [DOI] [PubMed] [Google Scholar]
- Orr-Weaver T. L., Szostak J. W., Rothstein R. J. Yeast transformation: a model system for the study of recombination. Proc Natl Acad Sci U S A. 1981 Oct;78(10):6354–6358. doi: 10.1073/pnas.78.10.6354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pettersson U., Sambrook J. Amount of viral DNA in the genome of cells transformed by adenovirus type 2. J Mol Biol. 1973 Jan;73(1):125–130. doi: 10.1016/0022-2836(73)90164-2. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Schechter N. M., Davies W., Anderson C. W. Adenovirus coded deoxyribonucleic acid binding protein. Isolation, physical properties, and effects of proteolytic digestion. Biochemistry. 1980 Jun 10;19(12):2802–2810. doi: 10.1021/bi00553a041. [DOI] [PubMed] [Google Scholar]
- Sharp P. A., Moore C., Haverty J. L. The infectivity of adenovirus 5 DNA-protein complex. Virology. 1976 Dec;75(2):442–456. doi: 10.1016/0042-6822(76)90042-8. [DOI] [PubMed] [Google Scholar]
- Stow N. D. The infectivity of adenovirus genomes lacking DNA sequences from their left-hand termini. Nucleic Acids Res. 1982 Sep 11;10(17):5105–5119. doi: 10.1093/nar/10.17.5105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Struhl K. Effect of deletion and insertion on double-strand-break repair in Saccharomyces cerevisiae. Mol Cell Biol. 1987 Mar;7(3):1300–1303. doi: 10.1128/mcb.7.3.1300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szostak J. W., Orr-Weaver T. L., Rothstein R. J., Stahl F. W. The double-strand-break repair model for recombination. Cell. 1983 May;33(1):25–35. doi: 10.1016/0092-8674(83)90331-8. [DOI] [PubMed] [Google Scholar]
- Volkert F. C., Young C. S. The genetic analysis of recombination using adenovirus overlapping terminal DNA fragments. Virology. 1983 Feb;125(1):175–193. doi: 10.1016/0042-6822(83)90072-7. [DOI] [PubMed] [Google Scholar]
- Wang K., Pearson G. D. Adenovirus sequences required for replication in vivo. Nucleic Acids Res. 1985 Jul 25;13(14):5173–5187. doi: 10.1093/nar/13.14.5173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss U., Wilson J. H. Repair of single-stranded loops in heteroduplex DNA transfected into mammalian cells. Proc Natl Acad Sci U S A. 1987 Mar;84(6):1619–1623. doi: 10.1073/pnas.84.6.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams J., Grodzicker T., Sharp P., Sambrook J. Adenovirus recombination: physical mapping of crossover events. Cell. 1975 Feb;4(2):113–119. doi: 10.1016/0092-8674(75)90117-8. [DOI] [PubMed] [Google Scholar]
- Young C. S. Heat-stable variant of human adenovirus type 5: characterization and use in three-factor crosses. J Virol. 1975 May;15(5):1168–1175. doi: 10.1128/jvi.15.5.1168-1175.1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Amerongen H., van Grondelle R., van der Vliet P. C. Interaction between adenovirus DNA-binding protein and single-stranded polynucleotides studied by circular dichroism and ultraviolet absorption. Biochemistry. 1987 Jul 28;26(15):4646–4652. doi: 10.1021/bi00389a009. [DOI] [PubMed] [Google Scholar]
- van der Vliet P. C., Keegstra W., Jansz H. S. Complex formation between the adenovirus type 5 DNA-binding protein and single-stranded DNA. Eur J Biochem. 1978 May 16;86(2):389–398. doi: 10.1111/j.1432-1033.1978.tb12321.x. [DOI] [PubMed] [Google Scholar]