Abstract
The Alberta Stroke Program Early CT Score (ASPECTS) is a useful scoring system for assessing early ischemic signs on noncontrast computed tomography (CT). Cerebral blood volume (CBV) on CT perfusion defines the core lesion assumed to be irreversibly damaged. We aim to explore the advantages of CBV_ASPECTS over CT_ASPECTS in the prediction of final infarct volume according to time.
Methods
Consecutive patients with anterior circulation stroke who underwent endovascular reperfusion according to initial CT_ASPECTS ≥7 were studied. CBV_ASPECTS was assessed blindly later on. Recanalization was defined as thrombolysis in cerebral ischemia score 2b-3. Final infarct volumes were measured on follow-up imaging. We compared ASPECTS on CBV and CT images, and defined ASPECTS agreement as: CT_ASPECTS - CBV_ASPECTS ≤1.
Results
Sixty-five patients, with a mean age of 67 ± 14 years and a median National Institutes of Health Stroke Scale score of 16 (range 10–20), were studied. The recanalization rate was 78.5%. The median CT_ASPECTS was 9 (range 8–10), and the CBV_ASPECTS was 8 (range 8–10). The mean time from symptoms to CT was 219 ± 143 min. Fifty patients (76.9%) showed ASPECTS agreement. The ASPECTS difference was inversely correlated to the time from symptoms to CT (r = −0.36, p < 0.01). A ROC curve defined 120 min as the best cutoff point after which the ASPECTS difference becomes more frequently ≤1. After 120 min, 89.5% of the patients showed ASPECTS agreement (as compared with 37.5% for <120 min, p < 0.01). CBV_ASPECTS but not CT_ASPECTS correlated with final infarct (r = −0.33, p < 0.01). However, if CT was done >2 h after symptom onset, CT_ASPECTS also correlated to final infarct (r = −0.39, p = 0.01).
Conclusions
In acute stroke, CBV_ASPECTS correlates with the final infarct volume. However, when CT is performed after 120 min from symptom onset, CBV_ASPECTS does not add relevant information to CT_ASPECTS.
Key Words: Computed tomography, Computed tomography perfusion, Stroke, Thrombectomy
Introduction
The optimal imaging paradigm for selecting acute ischemic stroke patients (AIS) for reperfusion therapies is not defined yet. The Alberta Stroke Program Early CT Score (ASPECTS) [1] is a useful scoring system to assess the extent of early ischemic signs in the middle cerebral artery (MCA) territory on noncontrast computed tomography (CT). The ASPECTS has been demonstrated to be associated with outcome in patients receiving intravenous as well as endovascular reperfusion therapies [2, 3, 4]. However, there is no complete agreement on considering ASPECTS a good prognostic marker [5, 6]. Its main limitations are the modest interobserver agreement (especially when dichotomized at 7 [7]) and the lack of information on the extent of ischemic penumbra [8]. Furthermore, the meaning of CT early ischemic changes is not unequivocal: only parenchymal hypoattenuation (focal hypodensity and/or loss of gray-white matter differentiation) represents irreversibly injured brain tissue while isolated cortical swelling may suggest penumbral or oligemic tissue [9].
In the last years, multimodal CT [including CT, CT angiography (CTA) and CT perfusion (CTP)] has been extensively used for the selection of AIS patients for endovascular reperfusion treatments in clinical trials. EXTEND-IA [10] demonstrated the effectiveness of mechanical thrombectomy in patients with large-vessel occlusion and salvageable tissue on CTP. In MR RESCUE [11], the subgroup of patients with a ‘penumbral pattern’ on CTP achieved a good functional outcome regardless of treatment assignment.
Cerebral blood volume (CBV) maps on CTP have been classically used to define the core lesion assumed to be irreversibly damaged [12, 13]. ASPECTS can be applied to CTP maps to improve the prediction of the final infarct extent and stroke outcome [14]. However, recent evidences suggest that CBV maps could overestimate the final infarct volume [15]. Therefore, the optimal CBV ASPECTS threshold to discriminate between AIS patients with good and poor clinical outcome remains to be established [14, 16, 17, 18]. Moreover, whether CBV provides more additional information compared to CT in the initial assessment of AIS patients is still a matter of controversy. We aim to explore the advantages of CBV_ASPECTS over CT_ASPECTS in the prediction of final infarct volume, as a surrogate marker of clinical outcome.
Methods
From January 2012 to July 2015, we studied consecutive patients with MCA (M1 and proximal M2) or internal carotid artery (ICA) occlusion below 8 h from symptom onset who received a multimodal CT at baseline and underwent endovascular reperfusion treatment. All patients were selected for endovascular treatment according to our local protocol, including a baseline favorable functional status [modified Rankin scale (mRS) score <3] and an initial CT_ASPECTS ≥7. Mechanical thrombectomies were performed by experienced interventionalists using commercially available stent retrievers and aspiration catheters. At the end of the procedure, recanalization was determined by the thrombolysis in cerebral ischemia (TICI) score. Complete recanalization was defined as TICI score 2b or 3. Long-term outcome was assessed at 3 months by means of the mRS considering as good outcome an mRS score <3. The study was approved by our local Ethics Committee. Informed consent was obtained from each patient or from his/her close relatives on admission.
Imaging Protocol
Multimodal CT study was performed on Definition AS Siemens (Siemens, Erlangen, Germany). All patients underwent noncontrast CT to rule out hemorrhage and patients with large early parenchymal ischemic changes (ASPECTS <7). CTA was performed to determine the presence of large-vessel occlusion, and select patients for endovascular reperfusion treatments. Parameters for CTP were: collimator of 32 × 1.5 mm, 80 kVp, and 200 mAs with total coverage of 86 mm. The plane of imaging was parallel to the floor of the anterior cranial fossa starting just above the orbits. Thirty cycles were obtained with a total scan time of 46 s.
Image Analysis
ASPECTS was computed on nonenhanced CT by a radiologist in the emergency setting, and revised subsequently by two experienced neuroradiologists (P.C. and J.C). When there was a discrepancy, decision about the ASPECTS was taken by consensus.
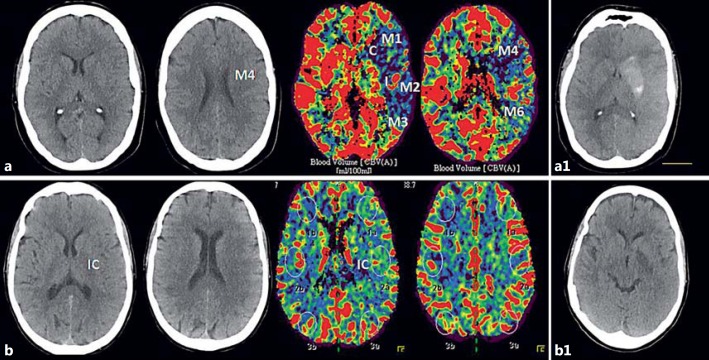
All the images were transferred to a separate workstation for analysis using a DICOM viewer (Osirix 64-bit; Pixmeo, Geneva, Switzerland). CBV_ASPECTS was assessed later on on baseline CTP maps by a vascular neurologist (M.P.) blinded to CT_ASPECTS evaluation and clinical data. ASPECTS in noncontrast CT and CBV were compared. We defined as ASPECTS agreement a difference between CT_ASPECTS and CBV_ASPECTS ≤1. If the difference was higher than 1, it was considered as ASPECTS disagreement (fig. 1).
Fig. 1.
CT_ASPECTS and CBV_ASPECTS comparison in 2 patients with ASPECTS disagreement (a) and ASPECTS agreement (b). a Patient with 110 min of global aphasia and right-side hemiparesis. Left M1-MCA occlusion treated with intravenous recombinant tissue plasminogen activator and endovascular reperfusion treatment, with complete recanalization after 215 min from symptom onset. b Patient with 200 min of mild right hemiparesis and motor aphasia. Distal left M1-MCA occlusion, with complete recanalization at 245 min from symptom onset after primary endovascular thrombectomy. a1, b1 24-hour CT scan showing final infarct lesion in both patients.
Final infarct volumes were measured on follow-up noncontrast CT scan at 24–48 h using the ABC/2 method [19].
Statistical Analysis
Descriptive and frequency statistical analysis were obtained using SPSS 17.0 software. Categorical variables are presented as absolute values and percentages, while the continuous variables are presented as median ± SD if normally distributed, or median (interquartile ranges, IQR) if not normally distributed. Correlation between continuous variables was assessed by Spearman's correlation coefficient. A ROC curve analysis was used to calculate the best cutoff time point after which the ASPECTS difference becomes ≤1. Statistical significance for intergroup differences was assessed by Pearson's χ2 or Fisher's exact test for categorical variables and by the Student t test for continuous variables. Not normally distributed variables were evaluated by the Mann-Whitney U test. A p value <0.05 was considered significant for all tests.
Results
Baseline clinical and radiological data are presented in table 1. Sixty-five patients (25 men), with a mean age of 67 ± 14 years (range 36–86) were studied. Median National Institutes of Health Stroke Scale (NIHSS) at onset was 16 (IQR 10–20).
Table 1.
Radiological and clinical data
| Female/male | 25/40 |
| Age (mean ± SD), years TOAST | 67±14 |
| TOAST | |
| Atherothrombotic | 14/65 (21.5) |
| Cardioembolic | 36/65 (55.4) |
| Undetermined | 10/65 (15.6) |
| Other determined (dissections) | 5/65 (7.5) |
| Median NIHSS score at entry | 16 (10–20) |
| Time to CT (mean ± SD), min | 219±43 |
| Unknown time of onset (wake-up stroke) | 8/65 (12) |
| Median CT_ASPECTS on admission | 9 (8–10) |
| Median CBV_ASPECTS on admission | 8 (8–10) |
| Occlusion location on CTA at admission, n/total | |
| MCA-M1 | 30/65 (4.2) |
| MCA-M2 | 13/65 (20) |
| Proximal ICA | 6/65 (9.2) |
| IV rtPA, n/total | 35/65 (53.8) |
| Time to groin (mean ± SD), min | 304±178 |
| Time to recanalization or end procedure, mean ± SD, min Final TICI score, n/total | 347±148 |
| 0–1 | 5/65 (7.5) |
| 2a | 9 (13.4) |
| 2b | 26 (38) |
| 3 | 25 (37.3) |
| Median NIHSS score at 24 h | 9 (3–18) |
| Final infarct volume on CT at 24 h (mean ± SD), ml | 31.6±48.9 |
| Symptomatic intracranial hemorrhage, n/total | 3/65 (1.95) |
| 3-month mRS score <3, n/total | 30/65 (46.1) |
Figures in parentheses are percentages or IQR, unless indicated otherwise. TOAST = Trial of ORG 10172 in acute stroke treatment; IV rtPA = intravenous recombinant tissue plasminogen activator.
Initial angiography identified 6 proximal ICA occlusions (9.2%), 16 terminal ICA occlusions (24.6%), 30 M1 occlusions (46.2%) and 13 proximal M2 occlusions (20%). All the patients underwent endovascular reperfusion treatment; 53.8% (35 patients) after intravenous thrombolysis. Recanalization (defined as TICI score 2b–3) occurred in 51 patients (78.5%). The median NIHSS score at 24 h was 9 (IQR 3–18). The mean infarct volume in the 24- to 48-hour control CT was 31.6 ± 48.9 ml. Thirty patients (46.1%) achieved a good functional outcome (mRS score <3) at 3 months (table 1).
Mean time from symptom onset to CT was 219 ± 143 min. On baseline CT, median CT_ASPECTS was 9 (IQR 8–10), and in CTP, median CBV_ASPECTS was 8 (IQR 8–10). In 50 patients (76.9%), the CT_ASPECTS and CBV_ASPECTS difference was ≤1 (ASPECTS agreement).
We evaluated whether any clinical or radiological variable could be related to the presence of ASPECTS agreement. In the univariate analysis, ASPECTS disagreement was associated with lower CBV_ASPECTS and larger infarct volumes. Time to CT also differed significantly between patients with and without ASPECTS agreement (table 2).
Table 2.
Clinical and radiological comparison between patients with and without ASPECTS_agreement
| AD | AA | p value | |
|---|---|---|---|
| Age (mean ± SD), years | 72±15 | 65±13 | 0.11 |
| Female/male | 9/6 | 31/19 | 0.89 |
| Hypertension | 10/15 | 31/50 | 0.74 |
| Atrial fibrillation | 6/15 | 13/50 | 0.30 |
| Diabetes mellitus | 3/15 | 10/50 | 1.00 |
| CHD | 2/15 | 6/50 | 1.00 |
| Dyslipidemia | 7/15 | 18/50 | 0.46 |
| Statin therapy | 6/15 | 17/50 | 0.71 |
| Previous stroke | 1/15 | 11/50 | 0.27 |
| Glycemia (mean ± SD), mg/dl | 122±34 | 130±53 | 0.61 |
| Systolic blood pressure at onset (mean ± SD), mm Hg | 150±29 | 144±34 | 0.58 |
| Diastolic blood pressure at onset (mean ± SD), mm Hg | 81±14 | 78±17 | 0.57 |
| Time to CT (mean ± SD), min | 150±141 | 244±137 | 0.02* |
| Time to CT <120 min | 10/15 | 7/42 | <0.01* |
| Site of vessel occlusion | 0.25 | ||
| MCA-M1 | 7/15 | 23/50 | |
| MCA-M2 | 2/15 | 11/50 | |
| Proximal ICA | 0/15 | 6/50 | |
| Terminal ICA | 6/15 | 10/50 | |
| Recanalization (TICI score 2b–3) | 12/15 | 39/50 | 1.00 |
| Final infarct volume on 24 h CT (mean ± SD), ml | 55±74 | 24±35 | <0.01* |
| 3-month mRS score <3 | 6/15 | 24/50 | 0.77 |
AD = ASPECTS disagreement; AA = ASPECTS agreement; CHD = coronary artery disease; TOAST = trial of Org 10172 in Acute Stroke Treatment.
p < 0.05 statistical significance for intergroup differences. It was assessed by Pearson's χ2 or Fisher's exact test for categorical variables and by Student t test for continuous variables.
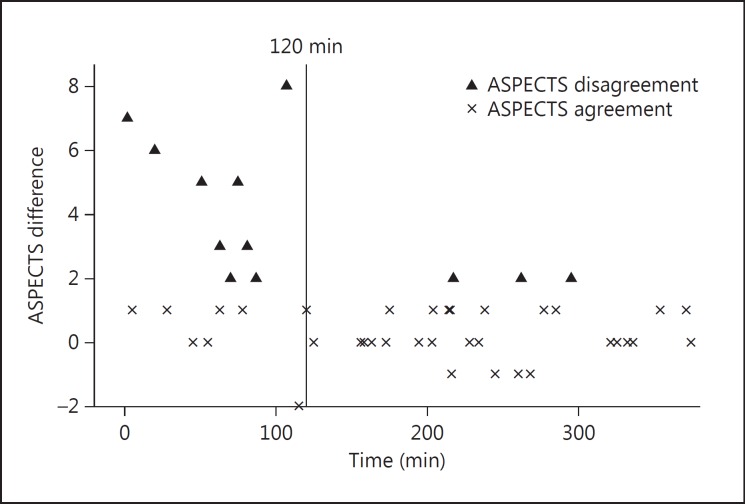
The ASPECTS difference was inversely correlated to the time from symptom onset to CT (r = −0.36, p < 0.01). A ROC curve defined 120 min (sensibility: 0.83, specificity: 0.67) as the best cutoff time point after which the ASPECTS difference becomes ≤1. After 120 min (fig. 2), almost 90% of patients showed ASPECTS agreement (89.5 vs. 37.5% for <120 min; p < 0.01). CBV_ASPECTS (r = −0.33, p < 0.01) but not CT_ASPECTS (r < −0.20, p = 0.08) correlated with the final infarct volume. However, if CT was done >2 h after symptom onset, then CT_ASPECTS also correlated to the final infarct volume (r = −0.39, p = 0.01).
Fig. 2.
Scatter plot showing ASPECTS difference according to time to CT.
Eight patients (12%) presented with stroke of unknown time of onset because of wake-up stroke; all of them have ASPECTS agreement and were excluded for the time to CT analysis.
Median CT_ASPECTS was 10 (IQR 9–10) in patients who achieved a good functional outcome and 9 (IQR 8–10) in patients with poor functional outcome (p = 0.12), while median CBV_ASPECTS was respectively 9 (IQR 8–10) and 8 (IQR 7–9) in these subgroups (p > 0.05). There was a trend towards higher CBV_ASPECTS in patients who achieved functional independency, but it did not reach statistical significance (p = 0.09).
However, considering only patients who achieved recanalization, both CT and CBV_ASPECTS correlate with 3-month mRS score (p = 0.01 and p = 0.04, respectively).
Discussion
In this study, we explored the advantages of CBV_ASPECTS over CT_ASPECTS in the prediction of final infarct volume, as a surrogate marker of clinical outcomes [20, 21]. Previous studies have shown a higher prognostic value of CTV_ASPECTS compared to CT_ASPECTS [14, 16, 17, 22] and this is confirmed by our data. In fact, CBV_ASPECTS but not CT_ASPECTS correlated to the final infarct volume. However, previous reports did not take into account the time from symptom onset to CT. Our study suggests that the predictive accuracy of CBV_ASPECTS is time-dependent. After 120 min, CBV_ASPECTS did not provide additional information about ischemic core compared to CT_ASPECTS.
Early ischemic changes on baseline CT are related to the development of cytotoxic and subsequently ionic edema. The ability of the observers to detect these changes is influenced by the size of the infarction, the severity of ischemia and the time to CT [1, 23, 24]. It is known that CT reliability is lower in the ultra-early stroke presenters (within 90 min from symptom onset) [25]. As time goes by, the CT_ASPECTS is probably more accurate to detect these ischemic changes, and therefore, the ASPECTS difference decreases over time. On the other hand, CTP directly derives information on cerebral perfusion by analyzing the first passage through the cerebral vessels of an intravenous contrast bolus. The software generates the pixel-based color-coded parametric maps on the basis of the integration of the time density curves and deconvolution calculations [26]. Theoretically, the infarct core in CTP is the area with reduced cerebral blood flow and CBV (cerebral blood flow/CBV match) [27]. Using the ASPECTS methodology, we demonstrated that CTP was more reliable than plain CT at predicting final infarct volume in the first 2 h from symptom onset. In our series, all patients with unknown time of symptom onset (because of wake-up stroke) presented with ASPECTS agreement. We hypothesized that these patients were probably not early presenters.
However, our study showed that, when CT was done after 2 h from symptom onset, CT_ASPECTS also correlated to the final infarct volume. In this time frame, CBV_ASPECTS was similar to CT_ASPECTS in almost 90% of the patients. Therefore, no additional information about ischemic core was provided by CBV in the majority of cases. Furthermore, ASPECTS disagreement was more frequent in patients with lower CBV_ASPECTS, and consequently, larger final infarct volumes.
The ischemic core is associated with the risk of hemorrhagic transformation and outcome after reperfusion therapies [18]. Theoretically, CTP adds information about the tissue at risk in the penumbral area. However, there are still uncertainties about which are the best CTP parameters to define core and penumbra [28, 29]. Furthermore, recent evidences have shown the limited reliability of CTP in acute infarct volume measurements compared with multiparametric MRI [30].
The ESCAPE trial [31] selected patients for intra-arterial reperfusion therapies by using collateral assessment on CTA along with CT_ASPECTS. In concordance with our study, we believe that, after 2 h from symptom onset, CT_ASPECTS associated with CTA could be an adequate technique for selecting acute stroke patients for reperfusion therapies. This could represent a time, radiation and contrast-dose sparing imaging protocol for AIS patients who are not early presenters.
Our study presents several limitations; first of all its retrospective nature and the small sample size. Moreover, we performed an ASPECTS analysis of CBV maps visually, without using quantitative thresholds, and not a volumetric automated analysis similar to EXTEND-IA [10] or SWIFT PRIME [32] studies. Our findings should be confirmed in future larger studies, also using a volumetric analysis of the CBV maps, even better with standardized automated processing. Also, infarct volume was determined by the ABC/2 instead of volumetric measurements.
In conclusion, in acute stroke patients undergoing endovascular reperfusion therapies, baseline CBV_ASPECTS correlates with final infarct volume. However, when CT is performed beyond 120 min from symptom onset, CBV_ASPECTS does not add relevant information to CT_ASPECTS about the ischemic irreversible lesion.
Statement of Ethics
The study was approved by the local Ethics Committee. Informed consent was obtained from each patient or from his/her close relatives on admission. Details have been removed from the case descriptions to ensure anonymity.
Disclosure Statement
All the authors disclose no conflicts of interest related with this research. This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
References
- 1.Barber PA, Demchuk AM, Zhang J, Buchan AM. Validity and reliability of a computed tomography score in predicting outcome of hyperacute stroke before thrombolytic therapy. Lancet. 2000;355:1670–1674. doi: 10.1016/s0140-6736(00)02237-6. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Menon BK, Coutts SB, Hill MD, Demchuk AM, Penumbra Pivotal Stroke Trial Investigators, Calgary Stroke Program, and the Seaman MR Research Center Effect of baseline CT scan appearance and time to recanalization on clinical outcomes in endovascular thrombectomy of acute ischemic strokes. Stroke. 2011;42:93–97. doi: 10.1161/STROKEAHA.110.594481. [DOI] [PubMed] [Google Scholar]
- 3.Yoo AJ, Chaudhry ZA, Berkhemer OA, González RG, Goyal M, Demchuk AM, Menon BK, Mualem E, Ueda D, Buell H, Sit SP, Bose A, Penumbra Pivotal and Penumbra Imaging Collaborative Study (PICS) Investigators Impact of pretreatment noncontrast CT Alberta Stroke Program Early CT Score on clinical outcome after intra-arterial stroke therapy. Stroke. 2014;45:746–751. doi: 10.1161/STROKEAHA.113.004260. [DOI] [PubMed] [Google Scholar]
- 4.Hill MD, Demchuk AM, Goyal M, Jovin TG, Foster LD, Tomsick TA, von Kummer R, Yeatts SD, Palesch YY, Broderick JP, IMS3 Investigators Alberta Stroke Program early computed tomography score to select patients for endovascular treatment: Interventional Management of Stroke (IMS)-III Trial. Stroke. 2014;45:444–449. doi: 10.1161/STROKEAHA.113.003580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dzialowski I, Hill MD, Coutts SB, Demchuk AM, Kent DM, Wunderlich O, von Kummer R. Extent of early ischemic changes on computed tomography (CT) before thrombolysis: prognostic value of the Alberta Stroke Program Early CT Score in ECASS II. Stroke. 2006;37:973–978. doi: 10.1161/01.STR.0000206215.62441.56. [DOI] [PubMed] [Google Scholar]
- 6.McTaggart RA, Jovin TG, Lansberg MG, Mlynash M, Jayaraman MV, Choudhri OA, Inoue M, Marks MP, Albers GW, DEFUSE 2 Investigators Alberta stroke program early computed tomographic scoring performance in a series of patients undergoing computed tomography and MRI: reader agreement, modality agreement, and outcome prediction. Stroke. 2015;46:407–412. doi: 10.1161/STROKEAHA.114.006564. [DOI] [PubMed] [Google Scholar]
- 7.Gupta AC, Schaefer PW, Chaudhry ZA, Leslie-Mazwi TM, Chandra RV, González RG, Hirsch JA, Yoo AJ. Interobserver reliability of baseline noncontrast CT Alberta Stroke Program Early CT Score for intra-arterial stroke treatment selection. AJNR Am J Neuroradiol. 2012;33:1046–1049. doi: 10.3174/ajnr.A2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bivard A, Parsons M. ASPECTaSaurus (a dinosaur)? Int J Stroke. 2012;7:564. doi: 10.1111/j.1747-4949.2012.00854.x. [DOI] [PubMed] [Google Scholar]
- 9.Puetz V, Dzialowski I, Hill MD, Demchuk AM. The Alberta Stroke Program Early CT Score in clinical practice: what have we learned? Int J Stroke. 2009;4:354–364. doi: 10.1111/j.1747-4949.2009.00337.x. [DOI] [PubMed] [Google Scholar]
- 10.Campbell BC, Mitchell PJ, Kleinig TJ, Dewey HM, Churilov L, et al. EXTEND-IA Investigators Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med. 2015;12(372):1009–1018. doi: 10.1056/NEJMoa1414792. [DOI] [PubMed] [Google Scholar]
- 11.Kidwell CS, Jahan R, Gornbein J, Alger JR, Nenov V, Ajani Z, Feng L, Meyer BC, Olson S, Schwamm LH, Yoo AJ, Marshall RS, Meyers PM, Yavagal DR, Wintermark M, Guzy J, Starkman S, Saver JL, MR RESCUE Investigators A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med. 2013;7(368):914–923. doi: 10.1056/NEJMoa1212793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wintermark M, Flanders AE, Velthuis B, Meuli R, van Leeuwen M, Goldsher D, Pineda C, Serena J, van der Schaaf I, Waaijer A, Anderson J, Nesbit G, Gabriely I, Medina V, Quiles A, Pohlman S, Quist M, Schnyder P, Bogousslavsky J, Dillon WP, Pedraza S. Perfusion-CT assessment of infarct core and penumbra: receiver operating characteristic curve analysis in 130 patients suspected of acute hemispheric stroke. Stroke. 2006;37:979–985. doi: 10.1161/01.STR.0000209238.61459.39. [DOI] [PubMed] [Google Scholar]
- 13.Murphy BD, Fox AJ, Lee DH, Sahlas DJ, Black SE, Hogan MJ, Coutts SB, Demchuk AM, Goyal M, Aviv RI, Symons S, Gulka IB, Beletsky V, Pelz D, Hachinski V, Chan R, Lee TY. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke. 2006;37:1771–1777. doi: 10.1161/01.STR.0000227243.96808.53. [DOI] [PubMed] [Google Scholar]
- 14.Parsons MW, Pepper EM, Chan V, Siddique S, Rajaratnam S, Bateman GA, Levi CR. Perfusion computed tomography: prediction of final infarct extent and stroke outcome. Ann Neurol. 2005;58:672–679. doi: 10.1002/ana.20638. [DOI] [PubMed] [Google Scholar]
- 15.Copen WA, Morais LT, Wu O, Schwamm LH, Schaefer PW, González RG, Yoo AJ. In acute stroke, can CT perfusion-derived cerebral blood volume maps substitute for diffusion-weighted imaging in identifying the ischemic core? PLoS One. 2015;10:e0133566. doi: 10.1371/journal.pone.0133566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Aviv RI, Mandelcorn J, Chakraborty S Gladstone D, Malham S, Tomlinson G, Fox AJ, Symons S. Alberta Stroke Program Early CT Scoring of CT perfusion in early stroke visualization and assessment. AJNR Am J Neuroradiol. 2007;28:1975–1980. doi: 10.3174/ajnr.A0689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lin K, Rapalino O, Lee B, Do KG, Sussmann AR, Law M, Pramanik BK. Correlation of volumetric mismatch and mismatch of Alberta Stroke Program Early CT Scores on CT perfusion maps. Neuroradiology. 2009;51:17–23. doi: 10.1007/s00234-008-0454-y. [DOI] [PubMed] [Google Scholar]
- 18.Lum C, Ahmed ME, Patro S, Thornhill R, Hogan M, Iancu D, Lesiuk H, Dos Santos M, Dowlatshahi D, Ottawa Stroke Research Group (OSRG) Computed tomographic angiography and cerebral blood volume can predict final infarct volume and outcome after recanalization. Stroke. 2014;45:2683–2688. doi: 10.1161/STROKEAHA.114.006163. [DOI] [PubMed] [Google Scholar]
- 19.Sims JR, Gharai LR, Schaefer PW, Vangel M, Rosenthal ES, Lev MH, Schwamm LH. ABC/2 for rapid clinical estimate of infarct, perfusion, and mismatch volumes. Neurology. 2009;72:2104–2110. doi: 10.1212/WNL.0b013e3181aa5329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Padroni M, Bernardoni A, Tamborino C, et al. Cerebral blood volume ASPECTS is the best predictor of clinical outcome in acute ischemic stroke: a retrospective, combined semi-quantitative and quantitative assessment. PLos One. 2016;11:e0147910. doi: 10.1371/journal.pone.0147910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vagal AS, Sucharew H, Prabhakaran S, et al. Final infarct volume discriminates outcome in mild strokes. Neuroradiol J. 2015;28:404–408. doi: 10.1177/1971400915609347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kloska SP, Dittrich R, Fischer T, Nabavi DG, Fischbach R, Seidensticker P, Osada N, Ringelstein EB, Heindel W. Perfusion CT in acute stroke: prediction of vessel recanalization and clinical outcome in intravenous thrombolytic therapy. Eur Radiol. 2007;17:2491–2498. doi: 10.1007/s00330-007-0696-9. [DOI] [PubMed] [Google Scholar]
- 23.Hirano T, Yonehara T, Inatomi Y, Hashimoto Y, Uchino M. Presence of early ischemic changes on computed tomography depends on severity and the duration of hypoperfusion: a single photon emission-computed tomographic study. Stroke. 2005;36:2601–2608. doi: 10.1161/01.STR.0000189990.31225.82. [DOI] [PubMed] [Google Scholar]
- 24.Demchuk AM, Coutts SB. Alberta Stroke Program Early CT Score in acute stroke triage. Neuroimaging Clin N Am. 2005;15:409–419. doi: 10.1016/j.nic.2005.06.008. [DOI] [PubMed] [Google Scholar]
- 25.Bal S, Bhatia R, Menon BK, Shobha N, Puetz V, Dzialowski I, Modi J, Goyal M, Hill MD, Smith EE, Demchuk AM. Time dependence of reliability of noncontrast computed tomography in comparison to computed tomography angiography source image in acute ischemic stroke. Int J Stroke. 2015;10:55–60. doi: 10.1111/j.1747-4949.2012.00859.x. [DOI] [PubMed] [Google Scholar]
- 26.Cianfoni A, Colosimo C, Basile M, Wintermark M, Bonomo L. Brain per-fusion CT: principles, technique and clinical applications. Radiol Med. 2007;112:1225–1243. doi: 10.1007/s11547-007-0219-4. [DOI] [PubMed] [Google Scholar]
- 27.Murphy BD, Fox AJ, Lee DH, Sahlas DJ, Black SE, Hogan MJ, Coutts SB, Dem-chuk AM, Goyal M, Aviv RI, Symons S, Gulka IB, Beletsky V, Pelz D, Hachinski V, Chan R, Lee TY. Identification of penumbra and infarct in acute ischemic stroke using computed tomography perfusion-derived blood flow and blood volume measurements. Stroke. 2006;37:1771–1777. doi: 10.1161/01.STR.0000227243.96808.53. [DOI] [PubMed] [Google Scholar]
- 28.Campbell BC, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, Parsons MW. Cerebral blood flow is the optimal CT perfusion parameter for assessing infarct core. Stroke. 2011;42:3435–3440. doi: 10.1161/STROKEAHA.111.618355. [DOI] [PubMed] [Google Scholar]
- 29.Campbell BC, Christensen S, Levi CR, Desmond PM, Donnan GA, Davis SM, Parsons MW. Comparison of computed tomography perfusion and magnetic resonance imaging perfusion-diffusion mismatch in ischemic stroke. Stroke. 2012;43:2648–2653. doi: 10.1161/STROKEAHA.112.660548. [DOI] [PubMed] [Google Scholar]
- 30.Schaefer PW, Souza L, Kamalian S, Hirsch JA, Yoo AJ, Kamalian S, Gonzalez RG, Lev MH. Limited reliability of computed tomographic perfusion acute infarct volume measurements compared with diffusion-weighted imaging in anterior circulation stroke. Stroke. 2015;46:419–424. doi: 10.1161/STROKEAHA.114.007117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goyal M, Demchuk AM, Menon BK, Eesa M, Rempel JL, Thornton J, et al. ES-CAPE Trial Investigators Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med. 2015;372:1019–1030. doi: 10.1056/NEJMoa1414905. [DOI] [PubMed] [Google Scholar]
- 32.Saver JL, Goyal M, Bonafe A, Diener HC, et al. SWIFT PRIME Investigators Stent-retriever thrombectomy after intravenous t-PA vs t-PA alone in stroke. N Engl J Med. 2015;372:2285–2295. doi: 10.1056/NEJMoa1415061. [DOI] [PubMed] [Google Scholar]