Abstract
Techniques such as the real-time reverse transcription-polymerase chain reaction (qRT-PCR) can detect RNA in samples with a low viral load. However, these amplicons typically are either too short or at insufficient concentrations for use in subsequent sequencing reactions for genotyping and detection confirmation. The assay developed in this study detects rotavirus G genotypes and P genotypes with viral loads as low as 6.2 and 8.2 copies per reaction, respectively. The assay was validated using a panel of 91 stool samples, 32 reference rotavirus strains, and 6 non-target enteric virus samples.
Keywords: Rotavirus, Nested, RT-PCR, Sensitivity, Specificity
1. Introduction
Group A rotaviruses (RVA) are estimated to cause 453,000 deaths among infants and young children each year (Tate et al., 2012). The genome of RVA, members of the Reoviridae family, consists of 11 double-stranded RNA (dsRNA) segments that encode six viral structural proteins (VP1-VP4, VP6 and VP7) and five or six nonstructural proteins (NSP1-NSP5/6) (Estes and Kapikian, 2007). Traditionally, viral classification was based on the serological characteristics and sequence diversity of the outer capsid proteins, VP7 (glycosylated, G-type) and VP4 (protease-sensitive, P-type) (Iturriza-Gomara et al., 2004). Although, at least 32G genotypes and 46P genotypes have been identified to date (http://rega.kuleuven.be/cev/viralmetagenomics/virus-classification) (Matthijnssens et al., 2011), 5 strains (G1P[8], G2P[4], G3P[8], G4P[8] and G9P[8]) are associated with 80–90% of the global RVA disease burden (Patel et al., 2011).
Numerous techniques are available for detection of RVA in stool samples, including virus isolation in cell culture, electron microscopy (EM), enzyme immunoassays (EIA), coupled reverse transcription and PCR amplification (RT-PCR), and real-time reverse transcriptase-polymerase chain reactions (qRT-PCR) (Mijatovic-Rustempasic et al., 2013). Molecular techniques are more rapid and sensitive than cell culture-based techniques or EM. For example, reverse transcription-polymerase chain reaction (RT-PCR) assays targeting the RVA VP4, VP6 and VP7 gene segments have shown increases of 15–27% in the rate of RVA detection in comparison with EIA assays (Gouvea et al., 1991; Pang et al., 1999; Wilde et al., 1992; Xu et al., 1990). However, these approaches are not as sensitive as qRT-PCR. Recently, we described a one-step quantitative, highly sensitive and specific qRT-PCR assay targeting the NSP3 gene which can detect one genome copy per reaction (Mijatovic-Rustempasic et al., 2013). Using this assay, we were able to detect 17% and 10% more RVA positives as compared to a commercially available EIA assay and hemi-nested RT-PCR, respectively (Das et al., 1994; Gentsch et al., 1992; Gouvea et al., 1990). However, a majority of RVA samples detected only with qRT-PCR had a Ct value greater than 30, indicating that the viral load in the sample was less than 100 copies per qRT-PCR assay. In addition, the qRT-PCR amplicon is 87 bp in length, too small to use for sequencing and not useful as a genotyping tool since it is amplified from the NSP3 (segment 7) gene. In this study, we developed nested primers that target the VP7 and VP4 genes of RVA in order to genotype by sequencing stool samples with low concentrations of RVA (100 copies per qRT-PCR assay or qRT-PCR Ct ≥ 30). The advantage of the nested assay is that it permits G and P typing of samples with low copy numbers that are detected with qRT-PCR at concentration too low for direct sequencing.
2. Materials and methods
The nucleotide sequences of VP7 (G genotypes G1-G27) and VP4 (P genotypes P[1]-P[35]) gene segments available in Gen-Bank were aligned using GeneDoc version 2.7.000 (Nicholas and Nicholas, 1997) and Multalign (Corpet, 1988). Multiple consensus and internal primer sets were designed manually and degenerate bases were introduced into the primer sequences to account for sequence variation observed in the sequence alignments of the genes mentioned above. The primer sequences were checked for specificity using BLAST (http://blast.ncbi.nlm.nih.gov/Blast.cgi). All primers were checked for self-annealing sites, hairpin loop formation, 3′ complementarity and melting temperatures (Tm) using the IDT oligonucleotide calculator (http://www.idtdna.com/analyzer/Applications/OligoAnalyzer/). All the primers were synthesized by the Biotechnology Core Facility at the Centers for Disease Control and Prevention (CDC), Atlanta, GA.
Stool samples (n = 91) from surveillance studies and outbreak investigations in the USA and international surveillance studies (Cardemil et al., 2012; Cortes et al., 2012; Gentsch et al., 2009; Hull et al., 2011) were tested in this study. All samples were de-identified and could not be traced back to patient or hospital case identifiers. All specimens were collected between 2008 and 2014. Out of 91 samples, 64 (non-typeable samples with approximately 100 copies per qRT-PCR assay or qRT-PCR Ct ≥ 30) and 27 samples (previously genotyped samples with more than 100 copies per qRT-PCR assay or qRT-PCR Ct ≤ 30) were used to assess sensitivity and specificity of the assay, respectively. Samples shown in Table 1 were previously analysed by EIA (Premier™ Rotaclone® Rotavirus Detection Kit, Meridian Diagnostics, Inc., Cincinnati OH), hemi-nested RT-PCR (Hull et al., 2011), and qRT-PCR targeting the NSP3 gene (Mijatovic-Rustempasic et al., 2013). Of the 64 samples that were positive by qRT-PCR, all were negative by EIA and 60 were negative by RT-PCR. A majority of the samples (50 out of 64 samples) had fewer than 10 copies of the NSP3 gene per qRT-PCR reaction. Four samples tested by RT-PCR and qRT-PCR were positive with the VP7 assay, but were negative with the VP4 assay (samples 3, 8, 55 and 56, Table 1). Ten percent suspensions of each sample were prepared in PBS (10% PBS suspension) and RNA was extracted using the automated KingFisher extraction system (Thermo Electron Corporation, Vantaa, Finland) in accordance with the manufacturers’ instructions using program AM1836 with a volume input of 50 μL 10% PBS suspension and volume output of 75 μL RNA in elution buffer. An additional 27 samples (Table 2), previously genotyped, had more than 100 copies of the NSP3 gene per qRT-PCR reaction and were used to check specificity of the assay. Samples were chosen based on their genotype to include the major circulating genotypes, G1-4, G9, G12, P[4], P[6] and P[8].
Table 1.
Summary of EIA, hemi-nested and qRT-PCR results for samples with a low viral load which were genotyped by hemi-nested assay amplification and sequencing.
| Sample # | EIA | Genotype by hemi nested PCR | NSP3 Ct value | NSP3 copy number | VP7 genotype by nested PCR and sequencing | VP4 genotype by nested PCR and sequencing |
|---|---|---|---|---|---|---|
| 1 | 0.063 | GNT P[NT] | 41.6 | ≤1 | G1 | P[8] |
| 2 | 0.049 | GNT P[NT] | 39.3 | ≤1 | Negative | Negative |
| 3 | 0.048 | G1 P[NT] | 39.3 | ≤1 | G1 | P[8] |
| 4 | 0.055 | GNTP[NT] | 38.6 | ≤1 | G1 | P[8] |
| 5 | 0.049 | GNT P[NT] | 38.6 | ≤1 | G1 | P[8] |
| 6 | 0.052 | GNT P[NT] | 38.2 | ≤1 | G3 | P[8] |
| 7 | 0.060 | GNT P[NT] | 36.7 | 1.1 | G3 | Negative |
| 8 | 0.061 | G1 P[NT] | 39.8 | ≤1 | G1 | P[4] |
| 9 | 0.095 | GNT P[NT] | 38.2 | ≤1 | G1 | P[8] |
| 10 | 0.088 | GNT P[NT] | 36.5 | 1.2 | G1 | P[8] |
| 11 | 0.104 | GNT P[NT] | 40.8 | ≤1 | G3 | P[8] |
| 12 | 0.104 | GNT P[NT] | 33.0 | 13.0 | G1 | P[8] |
| 13 | 0.088 | GNT P[NT] | 38.7 | ≤1 | G1 | Negative |
| 14 | 0.086 | GNT P[NT] | 36.5 | 1.2 | G1 | P[8] |
| 15 | 0.099 | GNT P[NT] | 31.5 | 36.2 | G1 | P[8] |
| 16 | 0.093 | GNT P[NT] | 27.2 | 685.2 | G3 | P[8] |
| 17 | 0.056 | GNT P[NT] | 40.0 | ≤1 | Negative | P[8] |
| 18 | 0.056 | GNT P[NT] | 38.7 | ≤1 | G1 | P[8] |
| 19 | 0.052 | GNT P[NT] | 40.4 | ≤1 | Negative | P[8] |
| 20 | 0.060 | GNT P[NT] | 37.0 | ≤1 | G9 | P[8] |
| 21 | 0.059 | GNT P[NT] | 36.3 | 1.4 | G3 | P[8] |
| 22 | 0.058 | GNT P[NT] | 34.5 | 4.7 | G3 | P[6] |
| 23 | 0.057 | GNT P[NT] | 40.4 | ≤1 | G3 | P[8] |
| 24 | 0.056 | GNT P[NT] | 29.4 | 161.1 | G1 | P[8] |
| 25 | 0.053 | GNT P[NT] | 37.3 | ≤1 | G1 | P[8] |
| 26 | 0.052 | GNT P[NT] | 38.4 | ≤1 | G1 | P[8] |
| 27 | 0.055 | GNT P[NT] | 38.8 | ≤1 | G1 | P[8] |
| 28 | 0.059 | GNT P[NT] | 40.0 | ≤1 | G1 | P[8] |
| 29 | 0.060 | GNT P[NT] | 36.2 | 1.5 | G1 | P[8] |
| 30 | 0.057 | GNT P[NT] | 35.9 | 1.9 | Negative | P[6] |
| 31 | 0.054 | GNT P[NT] | 32.3 | 21.1 | G1 | P[8] |
| 32 | 0.061 | GNT P[NT] | 31.6 | 33.8 | G1 | P[8] |
| 33 | 0.053 | GNT P[NT] | 30.4 | 80.3 | G3 | P[8] |
| 34 | 0.052 | GNT P[NT] | 35.3 | 2.8 | G1 | P[8] |
| 35 | 0.054 | GNT P[NT] | 35.6 | 2.3 | Negative | Negative |
| 36 | 0.054 | GNT P[NT] | 39.8 | ≤ 1 | G3 | P[8] |
| 37 | 0.053 | GNT P[NT] | 34.2 | 5.7 | G1 | P[8] |
| 38 | 0.065 | GNT P[NT] | 38.2 | ≤1 | G1 | P[8] |
| 39 | 0.097 | GNT P[NT] | 35.5 | 2.4 | G1 | P[8] |
| 40 | 0.077 | GNT P[NT] | 39.5 | ≤1 | G1 | P[8] |
| 41 | 0.055 | GNT P[NT] | 38.9 | ≤1 | G1 | P[8] |
| 42 | 0.046 | GNT P[NT] | 39.4 | ≤1 | Negative | P[8] |
| 43 | 0.057 | GNT P[NT] | 39.2 | ≤1 | G1 | P[8] |
| 44 | 0.093 | GNT P[NT] | 30.4 | 80.4 | G3 | P[8] |
| 45 | 0.073 | GNT P[NT] | 35.2 | 2.9 | G1 | P[8] |
| 46 | 0.090 | GNT P[NT] | 36.5 | 1.2 | G1 | P[8] |
| 47 | 0.088 | GNT P[NT] | 40.1 | ≤1 | Negative | P[8] |
| 48 | 0.077 | GNT P[NT] | 33.5 | 9.3 | Negative | P[4] |
| 49 | 0.074 | GNT P[NT] | 33.7 | 8.3 | G1 | P[8] |
| 50 | 0.092 | GNT P[NT] | 31.6 | 35.3 | G3 | P[6] |
| 51 | 0.149 | GNT P[NT] | 37.8 | ≤1 | G1 | P[8] |
| 52 | 0.086 | GNT P[NT] | 33.3 | 11.0 | G1 | P[8] |
| 53 | 0.074 | GNT P[NT] | 34.0 | 6.7 | Negative | Negative |
| 54 | 0.069 | GNT P[NT] | 32.0 | 26.4 | G3 | P[8] |
| 55 | 0.068 | G2 P[NT] | 27.0 | 804.7 | G2 | P[8] |
| 56 | 0.054 | G2 P[NT] | 44.0 | ≤1 | G1 | P[8] |
| 57 | 0.059 | GNT P[NT] | 39.0 | ≤1 | G3 | P[8] |
| 58 | 0.057 | GNT P[NT] | 30.0 | 103.5 | G8 | P[8] |
| 59 | 0.068 | GNT P[NT] | 32.0 | 26.4 | G1 | P[8] |
| 60 | 0.072 | GNT P[NT] | 35.0 | 3.4 | G2 | P[4] |
| 61 | 0.048 | GNT P[NT] | 41.0 | ≤1 | G1 | P[8] |
| 62 | 0.080 | GNT P[NT] | 43.0 | ≤1 | G3 | P[8] |
| 63 | 0.080 | GNT P[NT] | 35.0 | 3.4 | G1 | P[8] |
| 64 | 0.080 | GNT P[NT] | 41.0 | ≤1 | G1 | P[8] |
NT = non-typeable
Table 2.
Hemi-nested and qRT-PCR results for well-characterized samples which were genotyped by hemi-nested assay amplification and sequencing.
| Sample # | Genotype by hemi nested PCR or sequencing | NSP3 Ct value | NSP3 copy number | VP7 genotype by nested PCR and sequencing | VP4 genotype by nested PCR and sequencing |
|---|---|---|---|---|---|
| 65 | G1P[8] | 29.0 | 2.1E + 02 | G1 | P[8] |
| 66 | G12P[6] | 28.9 | 2.2E + 02 | G12 | P[6] |
| 67 | G12P[6] | 13.0 | 1.2E + 07 | G12 | P[6] |
| 68 | G12P[6] | 21.0 | 4.0E + 04 | G12 | P[6] |
| 69 | G9P[8] | 17.2 | 6.5E + 05 | G9 | P[8] |
| 70 | G9P[6] | 22.0 | 2.5E + 04 | G9 | P[6] |
| 71 | G9P[8] | 22.3 | 2.0E + 04 | G9 | P[8] |
| 72 | G2P[4] | 14.3 | 4.7E + 06 | G2 | P[4] |
| 73 | G2P[4] | 27.0 | 8.0E + 02 | G2 | P[4] |
| 74 | G2P[4] | 19.1 | 1.8E + 05 | G2 | P[4] |
| 75 | G9P[8] | 18.2 | 3.3E + 05 | G9 | P[8] |
| 76 | G1P[8] | 17.8 | 4.3E + 05 | G1 | P[8] |
| 77 | G9P[8] | 13.8 | 6.7E + 06 | G9 | P[8] |
| 78 | G1P[8] | 19.1 | 1.8E + 05 | G1 | P[8] |
| 79 | G3P[6] | 16.8 | 8.6E + 05 | G3 | P[6] |
| 80 | G12P[8] | 17.2 | 6.5E + 05 | G12 | P[8] |
| 81 | G4P[6] | 21.2 | 4.2E + 04 | G4 | P[6] |
| 82 | G4P[6] | 20.2 | 8.4E + 04 | G4 | P[6] |
| 83 | G4P[6] | 22.5 | 1.7E + 04 | G4 | P[6] |
| 84 | G1P[8] | 27.1 | 7.5E + 02 | G1 | P[8] |
| 85 | G3P[6] | 14.5 | 4.1E + 06 | G3 | P[6] |
| 86 | G3P[6] | 15.2 | 2.6E + 06 | G3 | P[6] |
| 87 | G2P[4] | 17.1 | 7.0E + 05 | G2 | P[4] |
| 88 | G12P[8] | 14.1 | 5.4E + 06 | G12 | P[8] |
| 89 | G12P[8] | 15.5 | 2.1E + 06 | G12 | P[8] |
| 90 | G3P[6] | 14.2 | 5.1E + 06 | G3 | P[6] |
| 91 | G3P[6] | 14.0 | 5.8E + 06 | G3 | P[6] |
Thirty two reference RVA strains, previously propagated in MA104 cells, were extracted by the same protocol used for the stool samples except that undiluted stocks were used. The strains used were: Wa (G1P[8]), DS-1 (G2P[4]), ST3 (G4P[6]), P (G3P[8]), US1205 (G9P[6]), 1076 (G2P[6]), 116E (G9P[11]), 69M (G8P[10]), AU-1 (G3P[9]), B223 (G10P[11]), CC425 (G3P[9]), F123 (G14P[12]), HOCHI (G4P[8]), K8 (G1P[9]), L26 (G12P[4]), NCDV (G6P[1]), OSU (G5P[7]), Ro1845 (G3P[3]), S2 (G2P[4]), Se584 (G4P[9]), WI61 (G9P[8]), WC3 (G6P[5]), 157C (G3P[11]), F45 (G9P[8]), KU (G1P[8]), VA70 (G4P[8]), EDIM (G16P[16]), L338 (G13P[18]), SA-11 (G3P[1]), B37(G8P[X]), LAMB (G10P[15]), and UK (G6P[5]). RNA extracts were stored at −80 °C until testing.
The first round of the nested RT-PCR assay was performed using the Qiagen One-Step RT-PCR Kit (Qiagen, Inc. Valencia, CA). Each 30 μL reaction mixture contained 15 μL of RNase-Free Water, 6 μL of 5× QIAGEN OneStep RT-PCR Buffer, 300 μM dNTP Mix, 2U QIA-GEN OneStep RT-PCR Enzyme Mix, 1 μM each forward and reverse primer and 4 μL of RNA extract. For the reaction targeting VP7, the forward primer, N-VP7F1, 5′-TAG CTC CTT TTR ATG TAT GGT A-3′, and the reverse primer, N-VP7R1, 5′-GTN GGC CAT CCT TTN GT-3′, were used to amplify a 333 bp amplicon (spanning nucleotides 37–370). For the VP4 reaction, the forward primer, N-VP4F1, 5′-GGC TAT AAA ATG GYT TCN YT-3′, and the reverse primer, N-VP4R1, 5′-ARY ADC CAR TAA TCR NYD RGT G-3′, were used to amplify a 257 bp amplicon (spanning nucleotides 1–257). After denaturing the RVA dsRNA and oligonucleotides for 5 min at 97 °C, the reaction mixture was added and reverse-transcription and amplification were carried out on a GeneAMP PCR System 9700 thermocycler (Applied Biosystems, Foster City, CA, USA). Thermocycling conditions consisted of a 30 min hold at 50 °C for reverse transcription, 15 min at 95 °C, and 35 cycles of 30 s at 95 °C, 30 s at 50 °C and 45 s at 72 °C, with a final hold of 7 min at 72 °C.
The second round of the nested RT-PCR assay was performed using the AmpliTaq® DNA Polymerase with Buffer II (Applied Biosystems, Foster City, CA, USA). Each 50 μL reaction mixture contained 26.5 μL Nuclease-Free Water (Ambion®, Austin TX), 5 μL of 10X PCR Buffer II, 2 mM MgCl2 Solution, 8 μL 10 mM GeneAmp® dNTP Blend (Applied Biosystems, Foster City, CA, USA), 2.5 U Ampli-Taq DNA Polymerase, 800 nM each forward and reverse primer and 2 μL of the 1st round RT-PCR reaction. For the reaction targeting the VP7 forward primer, N-VP7F2, 5′-ATG TAT GGT ATT GAA TAT ACC AC-3′, and the reverse primer, N-VP7R2, 5′-GTR TCC ATD GAT CCA GTN ATT GG-3′, were used to amplify a 193 bp amplicon (spanning nucleotides 49–242). For the VP4 reaction forward primer, N-VP4F2, 5′-ATG GYT TCN YTM ATT TAT AGA CA-3′, and reverse primer, N-VP4R2, 5′-GNT GGY TGA TAW GGA CCR TCK A-3′, were used to amplify a 214 bp amplicon (spanning nucleotides 10–224). Thermocycling conditions consisted of 30 cycles of 45 s at 94 °C, 30 s at 42 °C and 60 s at 72 °C, with a final hold of 7 min at 72 °C.
Second round amplicons were analyzed by gel electrophoresis using 3% agarose gels made of 1% low-melting-temperature NuSieve® GTG® Agarose (Lonza Inc., Rockland, ME) and 2% SeaKem® ME Agarose (Lonza Inc., Rockland, ME) or 3% SeaKem® ME Agarose gels. The VP7 amplicons were subsequently excised and purified with a QIAquick Gel Extraction kit (Qiagen, Inc., Valencia, CA) following the manufacturer’s instructions. A majority of the VP4 amplicons produced single light bands on the gel. Therefore, the PCR products were purified using ExoSAP-IT® for PCR Product Clean-Up (Affymetrix, Santa Clara, CA) following the manufacturer’s protocol. DNA cycle sequencing was accomplished utilizing a Big Dye Terminator Cycle Sequencing kit v3.1 (Applied Biosystems, Inc., Foster City, CA) with individual PCR primers used for generating the second round product. Cycle sequenced products were purified using an in-house magnetic bead technique (Mijatovic-Rustempasic et al., 2012) and were analyzed on an ABI Prism 3130XL automated sequencer (Applied Biosystems, Foster City, CA, USA). Overlapping sequence fragments were assembled and edited in Sequencher 4.8 (Gene Codes Corporation, Inc., Ann Arbor, MI). The genotype for each amplicon was determined using BLAST to search the GenBank sequence database for highly similar matches to reference RVA strains.
Two positive control RNA templates were generated from the RVA VP4 and VP7 genes of laboratory strain Wa (G1P[8]) RNA, using a protocol described previously (Mijatovic-Rustempasic et al., 2013). Additionally, G2, G3 and G9 transcripts were generated using the same protocol. The 10-fold serial dilutions of dsRNA transcripts were prepared in Nuclease-Free Water (Ambion®, Austin TX) containing 100 μg/mL of carrier yeast RNA (Bio-Rad laboratories, Hercules CA). Positive controls were analysed on a LabChip GX Separation System using a DNA 1 K Reagent kit (Caliper Life Sciences-Perkin Elmer, Waltham, MA).
A second set of primers for amplification of VP7 was designed to address the observation that the quantity of the second round VP7 product was not dependent on the concentration of the positive control input when the Wa positive dsRNA RNA transcript is used: first round, N-VP7F537, 5′-GAR INC AAY TTR TGG ATI KCN ATG GG-3′ and N-VP7F903, 5′-TCN CAN AYN GTR TAR AAN ACT TGC CAC CA-3′; and second round, N-VP7F481, 5′-AYG AGT GGY TNT GYA AYC CIA TGG A-3′ and N-VP7R794, 5′-CCN CCI ATY TGI ATI AYN GCN ACY TT-3′. The same protocol described for RT-PCR and nested PCR of the VP7 gene was then used to amplify the VP7 positive control.
3. Results
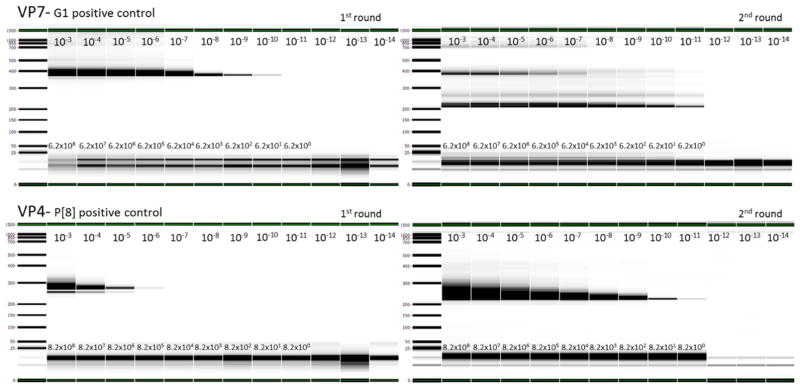
The limit of detection (LOD) is the lowest quantity of an analyte that can be distinguished from the absence of that analyte (IUPAC, 1997). In order to establish the LOD of the new assay, 10-fold serial dilutions of the G1 and P[8] dsRNA transcripts were prepared and used as standard controls in assay runs. The concentrations of undiluted (devoid of carrier yeast RNA) G1 and P[8] transcripts were determined using the Nanodrop ND-1000 to be 352.6 ng/μL and 1028.0 ng/μL, respectively. Eleven 10-fold serial dilutions (10−3–10−14), corresponding to copy numbers of 6.2 × 108 to 0 for the G1 transcript and 8.2 × 108 to 0 for the P[8] transcript, were tested using the assay conditions as described above. The LODs for VP7 and VP4 transcripts were calculated to be 6.2 and 8.2 copies per reaction, respectively, as shown in Fig. 1. For VP7, we observed both the first round RT-PCR and second round nested PCR products when we used a transcript to amplify the VP7 gene. This necessitated separation of products by gel electrophoresis followed by purification of templates from gel slices for sequencing both the first and second round PCR products (Fig. 1). We observed a single band for amplification of VP4 after two rounds of amplification. This product was a second round PCR amplicon which we were able to sequence by purifying the PCR product directly without gel separation. The size of the second round PCR amplicon for VP4 is linearly dependent on the concentration of the P[8] transcript, however a similar pattern was not observed for the VP7 amplicon when G1, G2, G3 or G9 transcript are used. To address this observation, additional primers were designed to amplify VP7 in a different area of the gene. A similar pattern of 2nd round amplification was observed for this set of primers as well, where the size of the second round amplicon was not linearly dependent on the sample input.
Fig. 1.
Nested amplification of 10-fold serial dilutions of the G1 and P[8] dsRNA positive control transcript with VP7 and VP4 1st and 2nd round primers. Lanes: molecular weight marker (lane 1), 10−3–10−14 transcript dilutions (lanes 2–13) as indicated on the upper part of the lane. Numbers on the lower part of each lane (scientific notation) indicate copies per qRT-PCR reaction. Amplicon sizes for first and second round VP7 amplification are 333 bp and 193 bp and for VP4 amplification, 257 bp and 214 bp, respectively. 1st and 2nd round reactions were visualized using a LabChip® GX instrument (PerkinElmer, Inc., Waltham, MA).
A total of 91 stool samples were extracted and analysed using the VP4 and VP7 nested PCRs (Tables 1 and 2). Out of 64 samples with a low viral load, we were able to detect and sequence VP4 and VP7 for 59 and 55 stool samples, respectively (Table 1). RVA strains represented in the stool samples tested included monotypic genotypes G1P[8], G2P[8], G3P[8], G9P[8], G3P[6], G1P[4], G2P[4] and G8P[4]. Three samples did not yield amplicons for both the VP4 and VP7 nested PCRs after the second PCR round. NSP3 copy numbers for these three stool samples were ≤1, 2.3, and 6.7 copies per qRT-PCR reaction. No evidence of PCR inhibition was found in these 3 samples by attempted amplification of diluted RNA. Increases of 85% and 92% in detection rates were recorded for nested VP7 and VP4 PCRs when compared to previously described hemi-nested VP7 and VP4 RT-PCR assays, respectively (Table 1). One discrepancy was found when comparing nested assay results to the previously determined genotype for sample 56 (Table 1).
An additional 27 samples, which were genotyped previously, were used to confirm the specificity of the assay. Genotyping results obtained with the nested assay described in this report matched the genotyping results obtained previously by genotyping or sequencing in previous studies (Table 2).
The nested RT-PCR assay was able to detect all 32 reference RVA strains (data not shown). When nucleic acid extracts for non-RVA gastroenteritis viruses (Norovirus types GI and GII, Sapovirus types GI.2 and GIV, Astrovirus types 3, 7 and 8, Adenovirus types 40 and 41) were tested with our assay, no amplicon was generated for any of the non-target viruses tested (data not shown).
4. Discussion
We developed and validated a highly sensitive nested RT-PCR assay for detecting RVA in stool samples with low viral load that targets all G and P genotypes. RVA can be shed at very high concentrations, up to approximately 1011 particles per g of stool (Flewett, 1983); therefore, samples are typically easy to genotype or sequence. However samples with low viral load, such as those collected during mild, asymptomatic, or late stage infections, are more difficult to genotype. Recently, we described a qRT-PCR assay targeting the NSP3 gene that can detect one genome copy per reaction (Mijatovic-Rustempasic et al., 2013), allowing us to identify 10% more RVA positives as compared to hemi-nested RT-PCR assays that target the most common genotypes (Das et al., 1994; Gentsch et al., 1992; Gouvea et al., 1990). However, a majority of RV samples detected only with qRT-PCR had viral loads of less than 100 copies per qRT-PCR assay and could not be sequenced directly or genotyped. This assay will permit typing of these low viral load samples.
The nested PCR reaction requires two set of primers that are used in two successive PCR reactions. The first amplicon is produced by an RT-PCR reaction using one set of primers while the second amplicon is produced by a PCR reaction using a second set of primers that bind inside the first amplicon. Nested RT-PCR has the advantage of improved sensitivity and specificity over conventional RT-PCR. Specificity improves because two sets of primers are used for nested RT-PCR reactions. Sensitivity increases because two rounds of PCR are performed. Nested PCR gained popularity in the early 1990s after Nichol and colleagues used nested PCR reactions to identify a novel hantavirus during an outbreak of a highly pathogenic acute respiratory illness (Nichol et al., 1993). Nested PCR reactions have been employed to detect a number of viruses (Aghasadeghi et al., 2013; Lam et al., 2007; Song et al., 2009), but not yet for RVA until this study.
Previously published hemi-nested RT-PCR assays targeted common RVA genotypes (Das et al., 1994; DiStefano et al., 2005; Gentsch et al., 1992; Gouvea et al., 1990; Iturriza-Gomara et al., 1999, 2000, 2004; Simmonds et al., 2008). The assay of Gouvea et al., a hemi-nested assay, targets VP7 genotypes G1-4, G8 and G9 by using genotype-specific primers along with a 3′ end consensus primer (Gouvea et al., 1990). Similarly, Gentsch et al. focused on the detection of common P-types such as P[4], P[6], P[8], P[9] and P[10] (Gentsch et al., 1992). The assay’s limit of detection was determined by amplifying 10-fold serial dilutions of the four cultured stains and was found to be 104–105 virus particles. Later, a revised version of the assay targeting P[8] was published (Iturriza-Gomara et al., 2000). Das et al. developed a hemi-nested assay targeting the VP7 gene for amplification of G1-4 and G9 genotypes (Das et al., 1994). Other hemi-nested primers targeting outer capsid genes follow a similar pattern of amplification with genotype specific primers while employing random primers from the RT step (Iturriza-Gomara et al., 1999). This assay can detect 10 virus particles/mL of 10% faecal suspension based on comparison of the 10-fold serial dilutions of cDNA generated with random primers and amplicons generated with consensus-specfic primers. As RVA evolves, primers used in these assays are constantly updated (Iturriza-Gomara et al., 2000; Iturriza-Gomara et al., 2004; Simmonds et al., 2008). There are other RT-PCR assays which employ sequencing in order to determine genotypes (DiStefano et al., 2005; Simmonds et al., 2008), but none are in a nested PCR format.
The VP7 gene has 6 antigenic regions that are highly conserved among strains of the same genotype, but highly diverse between genotypes (Gouvea et al., 1990). The nested VP7 primers developed in this study were designed to contain as many variable regions as possible to clearly distinguish between the genotypes. Sequences ranging obtained from the second round VP7 amplicon (excluding primer sequences) contain complete antigenic regions B and C and partial antigenic region D. The assay described in this report was designed and validated to detect and genotype broad range of RVA genotypes by subsequent sequencing.
The nested VP7 PCR developed in this study for both 1st round (RT-PCR) and 2nd round (nested PCR) is sensitive, (a LOD of 62.4 and 6.2 copies per reaction, respectively) and both products could be sequenced if gel purified. For the second round VP4 assay, only the 2nd round product is observed almost exclusively. We hypothesize that this is a result of the greater efficiency of the first round for the VP7 RT-PCR (62.4 copies per reaction) compared to the first round for the VP4 RT-PCR (8.2 × 105 copies per reaction; Fig. 1). Both nested PCRs, however, have comparable limits of detection (less than 10 copies per reaction).
A limitation of this study is that a small number of genotypes for stool samples with low viral load were tested due to limited availability of low viral load samples. However, previously genotyped strains with higher viral load, as well as reference strains tested in this study have provided a large diversity of genotypes tested. Including stool samples and reference strains, we have successfully amplified 27 genotypes, which include G1, G2, G3, G4, G5, G6, G8, G9, G10, G12, G13, G14, G16, P[1], P[3], P[4], P[5], P[6], P[7], P[8], P[9], P[10], P[11], P[12], P[15], P[16] and P[18].
In conclusion, a highly sensitive and highly specific nested RT-PCR assay targeting a large range of VP4 and VP7 genotypes, which can detect low viral load samples, has been developed and validated for the detection and genotyping of RVA. This is the first nested RT-PCR that targets all RVA genotypes and the first RT-PCR method targeting RVA that uses a double-stranded transcript to determine the true LOD of the assay.
Acknowledgments
We wish to thank Jennifer J. Hull for her expertise in propagating cell culture strains, as well as for her time teaching cell culture propagation. We thank M. Leanne Ward for editorial assistance. We also would like to thank Rashi Gautam, Sunando Roy, Kunchala Rungsrisuriyachai and Jan Vinje. Funding for this study was provided by the Centers for Disease Control and Prevention.
Footnotes
Disclaimer: the findings and conclusions in this report are those of the author(s) and do not necessarily represent the official position of the Centers for Disease Control and Prevention. Names of specific vendors, manufacturers, or products are included for public health and informational purposes; inclusion does not imply endorsement of the vendors, manufacturers, or products by the Centers for Disease Control and Prevention or the US Department of Health and Human Services.
References
- Aghasadeghi MR, Mohraz M, Bahramali G, Aghakhani A, Banifazl M, Foroughi M, Ahmadi F, Eslamifar A, Sadat SM, Ramezani A. Frequency and genotype of hepatitis d virus infection in patients infected with HIV and those undergoing hemodialysis. Hepat Mon. 2013 May 5;13:e7481. doi: 10.5812/hepatmon.7481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardemil CV, Cortese MM, Medina-Marino A, Jasuja S, Desai R, Leung J, Rodriguez-Hart C, Villarruel G, Howland J, Quaye O, Tam KI, Bowen MD, Parashar UD, Gerber SI Rotavirus Investigation T. Two rotavirus outbreaks caused by genotype G2P[4] at large retirement communities: cohort studies. Ann Intern Med. 2012;157:621–631. doi: 10.7326/0003-4819-157-9-201211060-00006. [DOI] [PubMed] [Google Scholar]
- Corpet F. Multiple sequence alignment with hierarchical clustering. Nucleic Acids Res. 1988;16:10881–10890. doi: 10.1093/nar/16.22.10881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortes J, Arvelo W, Lopez B, Reyes L, Kerin T, Gautam R, Patel M, Parashar U, Lindblade KA. Rotavirus disease burden among children <5 years of age–Santa Rosa, Guatemala, 2007–2009. Trop Med Int Health. 2012;17:254–259. doi: 10.1111/j.1365-3156.2011.02911.x. [DOI] [PubMed] [Google Scholar]
- Das BK, Gentsch JR, Cicirello HG, Woods PA, Gupta A, Ramachandran M, Kumar R, Bhan MK, Glass RI. Characterization of rotavirus strains from newborns in New Delhi, India. J Clin Microbiol. 1994;32:1820–1822. doi: 10.1128/jcm.32.7.1820-1822.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiStefano DJ, Kraiouchkine N, Mallette L, Maliga M, Kulnis G, Keller PM, Clark HF, Shaw AR. Novel rotavirus VP7 typing assay using a one-step reverse transcriptase PCR protocol and product sequencing and utility of the assay for epidemiological studies and strain characterization, including serotype subgroup analysis. J Clin Microbiol. 2005;43:5876–5880. doi: 10.1128/JCM.43.12.5876-5880.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estes MK, Kapikian AZ. Rotaviruses. In: Knipe DM, Howley PM, Griffin DE, Lamb RA, Martin MA, Roizman B, Straus SE, editors. Fields Virology. Lippincott Williams & Wilkins; 2007. pp. 1917–1974. [Google Scholar]
- Flewett TH. Rotavirus in the home and hospital nursery. Br Med J. 1983;287:568–569. doi: 10.1136/bmj.287.6392.568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch JR, Glass RI, Woods P, Gouvea V, Gorziglia M, Flores J, Das BK, Bhan MK. Identification of group A rotavirus gene 4 types by polymerase chain reaction. J Clin Microbiol. 1992;30:1365–1373. doi: 10.1128/jcm.30.6.1365-1373.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gentsch JR, Hull JJ, Teel EN, Kerin TK, Freeman MM, Esona MD, Griffin DD, Bielfelt-Krall BP, Banyai K, Jiang B, Cortese MM, Glass RI, Parashar UD. G and P types of circulating rotavirus strains in the United States during 1996–2005: nine years of prevaccine data. J Infect Dis. 2009;200(Suppl 1):S99–S105. doi: 10.1086/605038. [DOI] [PubMed] [Google Scholar]
- Gouvea V, Glass RI, Woods P, Taniguchi K, Clark HF, Forrester B, Fang ZY. Polymerase chain reaction amplification and typing of rotavirus nucleic acid from stool specimens. J Clin Microbiol. 1990;28:276–282. doi: 10.1128/jcm.28.2.276-282.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouvea V, Allen JR, Glass RI, Fang ZY, Bremont M, Cohen J, McCrae MA, Saif LJ, Sinarachatanant P, Caul EO. Detection of group B and C rotaviruses by polymerase chain reaction. J Clin Microbiol. 1991;29:519–523. doi: 10.1128/jcm.29.3.519-523.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hull JJ, Teel EN, Kerin TK, Freeman MM, Esona MD, Gentsch JR, Cortese MM, Parashar UD, Glass RI, Bowen MD. United States rotavirus strain surveillance from 2005 to 2008: genotype prevalence before and after vaccine introduction. Pediatr Infect Dis J. 2011;30:S42–7. doi: 10.1097/INF.0b013e3181fefd78. [DOI] [PubMed] [Google Scholar]
- IUPAC. Compendium of Chemical Terminology. 2. Blackwell Scientific Publications; 1997. the Gold Book. [Google Scholar]
- Iturriza-Gomara M, Green J, Brown DW, Desselberger U, Gray JJ. Comparison of specific and random priming in the reverse transcriptase polymerase chain reaction for genotyping group A rotaviruses. J Virol Methods. 1999;78:93–103. doi: 10.1016/s0166-0934(98)00168-2. [DOI] [PubMed] [Google Scholar]
- Iturriza-Gomara M, Green J, Brown DW, Desselberger U, Gray JJ. Diversity within the VP4 gene of rotavirus P[8] strains: implications for reverse transcription-PCR genotyping. J Clin Microbiol. 2000;38:898–901. doi: 10.1128/jcm.38.2.898-901.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iturriza-Gomara M, Kang G, Gray J. Rotavirus genotyping: keeping up with an evolving population of human rotaviruses. J Clin Virol. 2004;31:259–265. doi: 10.1016/j.jcv.2004.04.009. [DOI] [PubMed] [Google Scholar]
- Lam WY, Yeung AC, Tang JW, Ip M, Chan EW, Hui M, Chan PK. Rapid multiplex nested PCR for detection of respiratory viruses. J Clin Microbiol. 2007;45:3631–3640. doi: 10.1128/JCM.00280-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthijnssens J, Ciarlet M, McDonald SM, Attoui H, Banyai K, Brister JR, Buesa J, Esona MD, Estes MK, Gentsch JR, Iturriza-Gomara M, Johne R, Kirkwood CD, Martella V, Mertens PP, Nakagomi O, Parreno V, Rahman M, Ruggeri FM, Saif LJ, Santos N, Steyer A, Taniguchi K, Patton JT, Desselberger U, Van Ranst M. Uniformity of rotavirus strain nomenclature proposed by the Rotavirus Classification Working Group (RCWG) Arch Virol. 2011;156:1397–1413. doi: 10.1007/s00705-011-1006-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mijatovic-Rustempasic S, Frace MA, Bowen MD. Cost-effective paramagnetic bead technique for purification of cycle sequencing products. Sequencing 2012. 2012 Article ID 767959. [Google Scholar]
- Mijatovic-Rustempasic S, Tam KI, Kerin TK, Lewis JM, Gautam R, Quaye O, Gentsch JR, Bowen MD. Sensitive and specific quantitative detection of rotavirus A by one-step real-time reverse transcription-PCR assay without antecedent double-stranded-RNA denaturation. J Clin Microbiol. 2013;51:3047–3054. doi: 10.1128/JCM.01192-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nichol ST, Spiropoulou CF, Morzunov S, Rollin PE, Ksiazek TG, Feldmann H, Sanchez A, Childs J, Zaki S, Peters CJ. Genetic identification of a hantavirus associated with an outbreak of acute respiratory illness. Science. 1993;262:914–917. doi: 10.1126/science.8235615. [DOI] [PubMed] [Google Scholar]
- Nicholas KB, Nicholas HBJ. Genedoc: a tool for editing and annotating multiple sequence alignments. 1997 http://www.psc.edu/biomed/genedoc.
- Pang XL, Joensuu J, Hoshino Y, Kapikian AZ, Vesikari T. Rotaviruses detected by reverse transcription polymerase chain reaction in acute gastroenteritis during a trial of rhesus-human reassortant rotavirus tetravalent vaccine: implications for vaccine efficacy analysis. J Clin Virol. 1999;13:9–16. doi: 10.1016/s1386-6532(98)00013-4. [DOI] [PubMed] [Google Scholar]
- Patel MM, Steele D, Gentsch JR, Wecker J, Glass RI, Parashar UD. Real-world impact of rotavirus vaccination. Pediatr Infect Dis J. 2011;30:S1–5. doi: 10.1097/INF.0b013e3181fefa1f. [DOI] [PubMed] [Google Scholar]
- Simmonds MK, Armah G, Asmah R, Banerjee I, Damanka S, Esona M, Gentsch JR, Gray JJ, Kirkwood C, Page N, Iturriza-Gomara M. New oligonucleotide primers for P-typing of rotavirus strains: strategies for typing previously untypeable strains. J Clin Virol. 2008;42:368–373. doi: 10.1016/j.jcv.2008.02.011. [DOI] [PubMed] [Google Scholar]
- Song MK, Chang J, Hong Y, Hong S, Kim SW. Direct multiplex reverse transcription-nested PCR detection of influenza viruses without RNA purification. J Microbiol Biotechnol. 2009;19:1470–1474. doi: 10.4014/jmb.0905.5012. [DOI] [PubMed] [Google Scholar]
- Tate JE, Burton AH, Boschi-Pinto C, Steele AD, Duque J, Parashar UD The WHO-coordinated Global Rotavirus Surveillance Network. 2008 estimate of worldwide rotavirus-associated mortality in children younger than 5 years before the introduction of universal rotavirus vaccination programmes: a systematic review and meta-analysis. Lancet Infect Dis. 2012;12:136–141. doi: 10.1016/S1473-3099(11)70253-5. [DOI] [PubMed] [Google Scholar]
- Wilde J, Van R, Pickering L, Eiden J, Yolken R. Detection of rotaviruses in the day care environment by reverse transcriptase polymerase chain reaction. J Infect Dis. 1992;166:507–511. doi: 10.1093/infdis/166.3.507. [DOI] [PubMed] [Google Scholar]
- Xu L, Harbour D, McCrae MA. The application of polymerase chain reaction to the detection of rotaviruses in faeces. J Virol Methods. 1990;27:29–37. doi: 10.1016/0166-0934(90)90143-4. [DOI] [PubMed] [Google Scholar]