Summary
Efficient derivation of endothelial cells and their progenitors from human pluripotent stem cells (hPSCs) can facilitate further studies of human vascular development, disease modeling, drug discovery and cell-based therapy. Here we provide a detailed protocol for directing hPSCs to functional endothelial cells and their progenitors in a completely defined, growth factor- and serum-free system by temporal modulation of Wnt/β-catenin signaling via small molecules. We demonstrate a 10-day, two-stage process that recapitulates endothelial cell development, in which hPSCs first differentiate to endothelial progenitors that then generate functional endothelial cells and smooth muscle cells. Methods to characterize endothelial cell identity and function are also described.
Keywords: human pluripotent stem cells, endothelial progenitors, endothelial cells, Wnt signaling, chemically-defined, growth factor-free, serum-free, small molecules
1. INTRODUCTION
Human pluripotent stem cells (hPSCs) possess great potential for the study and treatment of vascular diseases due to their capacity for unlimited self-renewal and ability to form any somatic cell type (1–4). Functional endothelial cells and their progenitors differentiated from hPSCs could be beneficial for many potential applications, including disease modeling, drug discovery and cellular therapies (5–8). To realize this potential, it is necessary to be able to control hPSC differentiation to endothelial lineages with high efficiency and reproducibility in a scalable and cost-effective manner.
Over the past decades, three major approaches: (i) co-culture of hPSCs with mouse stromal cell lines (9, 10), (ii) embryoid body (EB) formation (11–13), and (iii) 2D directed differentiation techniques (14–17), have been developed to induce endothelial differentiation from hPSCs. These protocols utilized small molecules, growth factors, and extracellular matrix proteins to specify hPSCs to EC fates. Some of these protocols have reported generating endothelial cells with a purity of 20% to 50% in different hPSC lines. However, the efficiency of these distinct differentiation protocols is highly variable between cell lines and experimental repeats within the same line. In addition, the inclusion of growth factors and xenogeneic components increases the complexity and cost of these approach, limiting their application as a model to study vascular development.
In this protocol, we provide a simple and robust differentiation platform for the generation of endothelial progenitors and functional endothelial cells from hPSCs in a completely defined, growth factor-free and serum-free system by temporal modulation of Wnt/β-catenin signaling via small molecules. This protocol is based on our earlier reports that Wnt signaling can direct hPSCs through mesoderm progenitors to cardiovascular cell types (18, 19) and is composed of three major steps: (i) induction of endothelial progenitors from hPSCs by temporal modulation of Wnt signaling, (ii) a single magnetic-activated cell sorting (MACS) step to obtain pure endothelial progenitors, and (iii) directed differentiation of endothelial progenitors into endothelial cells. We will also provide procedures for performing flow cytometry and immunostaining analysis, and cryostorage and thawing of these hPSC-derived endothelial cell progenitors. The culture system described here can generate homogenous cultures of pure endothelial cells and their progenitors within 10 days.
2. MATERIALS
Prepare all reagents and solutions in a sterile environment, or filter the unsterile solution using Stericup or Steriflip filtration systems after preparation. Prepare and store all reagents in a refrigerator (4°C) for up to 3 months unless indicated otherwise. Diligently follow all waste disposal regulations and biological safety protocols when disposing waste materials.
2.1 Cell culture and differentiation reagents
Stem cell culture media: mTeSR1 (STEMCELL Technologies, Vancouver, BC, Canada) or Essential 8™ (E8) (Life Technologies, Grand Island, NY, USA).
L-ascorbic acid stock solution (100 mg/ml): dissolve 5 g L-ascorbic acid (Sigma, St. Louis, MO, USA) into 50 ml sterile MilliQ water. Aliquot into 1 ml samples and store at −20°C for up to 1 year.
DMEM/Vc medium (500 ml): add 500 μl of 100 mg/ml L-ascorbic acid solution to 500 ml DMEM basal medium (Life Technologies, cat. no. 11965).
LaSR basal medium (500 ml): add 6.25 ml GlutaMAX (Life Technologies, Grand Island, NY, USA) and 305 μl 100 mg/ml L-ascorbic acid into 500 ml advanced DMEM/F12 medium (Life Technologies, cat. no. 12634).
Y27632 (5 mM): dissolve 10 mg Y27632 (Tocris, Minneapolis, MN, USA) in 6.24 ml sterile phosphate-buffered saline (PBS) (Life Technologies, cat. no. 10010), aliquot 100 μl samples into sterile 1.5 ml tubes and store at −20°C for up to 1 year.
CHIR99021 (36 mM): dissolve 25 mg CHIR99021 (Selleckchem, Houston, TX, USA) in 1.49 ml DMSO, aliquot and store at −20°C for up to 1 year.
Matrigel-coated plates: add 1 ml of cold (4°C) DMEM/F12 into one Matrigel aliquot (2.5 mg), and use a P1000 tip to thaw and dissolve the Matrigel (BD Biosciences, San Jose, CA, USA). Immediately transfer the solution to a 50 ml conical tube on ice that contains 23 ml cold DMEM/F12. Mix well and add 1 ml/well Matrigel in DMEM/F12 for 6-well plates, 0.5 ml/well for 12-well plates. Allow the Matrigel to sit for 30 minutes at room temperature before use.
Synthemax II-SC stock solution (1 mg/ml): dissolve 10 mg Synthemax II-SC substrate (Corning, New York, NY, USA) in 10 ml of sterile water, mix well and store at 4°C for up to 6 months.
Synthemax-coated plates: add 150 μl of 1 mg/ml Synthemax II-SC stock solution into 6 ml of sterile water and mix well by gently pipette. Add 1 ml/well Synthemax in water for 6-well plates, 0.5 ml/well for 12-well plates, and allow the Synthemax to sit at room temperature for at least 2 hours. Aspirate the remaining solution and the Synthemax-coated plates are ready to use.
Accutase (Innovative Cell Technology, San Diego, CA, USA).
2.2 Magnetic-activated cell sorting (MACS)
EasySep™ Magnet (STEMCELL Technologies, Vancouver, BC, Canada).
EasySep™ FITC Positive Selection Kit (STEMCELL Technologies, Vancouver, BC, Canada).
CD34-FITC conjugated antibodies (Miltenyi Biotec, San Diego, CA, USA).
EGM-2 BulletKit (Lonza, Basel, Switzerland).
Fetal bovine serum (FBS) (Life Technologies, Grand Island, NY, USA).
Stericup and Steriflip filtration systems (Millipore, Billerica, MA, USA).
FlowBuffer-1 (500 ml): dissolve 2.5 g BSA in 500 ml PBS and filter using a 500 ml Stericup filtration system. The solution can be stored at 4°C for up to 6 months.
40 μm Falcon cell strainer (Corning, New York, NY, USA).
Collagen IV (1 mg/ml): dissolve 5 mg collagen IV (Sigma, St. Louis, MO, USA) in 5 ml sterile water, aliquot and store at −20°C for up to 1 year.
Collagen IV-coated plates or coverslips: add 60 μl of 1 mg/ml collagen IV into 6 ml sterile water and gently pipette the solution to mix well. Immediately add 1 ml/well collagen IV in water for 6-well plates, 0.5 ml/well for 12-well plates with or without sterile coverslips. Allow the collagen IV to sit at 37°C for at least 30 minutes before use (see Note 1).
2.3 Characterization of hPSC-derived endothelial cells
DMEM10: add 50 ml FBS to 450 ml DMEM, and filter with a 500 ml Stericup filtration system.
1% and 4% formaldehyde: add 187.5 μl or 1 ml of 16% formaldehyde into 3 ml PBS. We do not recommend storing the solution.
1% Triton X-100 solution (500 ml): add 5 ml Triton X-100 into 495 ml PBS and shake the bottle to dissolve the Triton.
5% non-fat dry milk, 0.4% Triton X-100 solution: add 0.5 g non-fat dry milk powder and 4 ml of 1% Triton X-100 solution into 6 ml PBS. We don’t recommend storing this solution.
Human endothelial-SFM medium (Life Technologies, Grand Island, NY, USA).
Antibodies used in this chapter: see Table 1.
Table 1.
Antibodies used in this study.
| Antibody | Source/Isotype/clone/cat. no. | Dilution |
|---|---|---|
| CD31-APC | MiltenyiBiotec, mouse IgG1, Clone: AC128 | 1:50 |
| CD34-FITC | Miltenyi Biotec, mouse IgG2a, Clone: AC136 | 1:50 |
| CD31-FITC | Miltenyi Biotec, mouse IgG1, Clone: AC390 | 1:50 |
| VE-cadherin | Santa Cruz, mouse IgG1, Clone: F-8 sc9989 | 1:100 |
| KDR | Santa Cruz, mouse IgG1, Clone: A-3 sc6251 | 1:200 |
| vWF | Dako, rabbit IgG, Cat. no: A008202-5 | 1:500 |
| Brachyury | R&D Systems, Gotat IgG, Clone: AF2085 | 1:100 |
| Oct4 | Santa Cruz, Mouse IgG2b, sc5279 | 1:100 |
| Secondary Antibody | Alexa 488 Chicken anti-Gt IgG/A-21467 | 1:1,000 |
| Secondary Antibody | Alexa 488 Goat anti-Ms IgG1/A-21121 | 1:1,000 |
| Secondary Antibody | Alexa 488 Goat anti-Rb IgG/A-11008 | 1:1,000 |
| Secondary Antibody | Alexa 594 Goat anti-Ms IgG2b/A-21145 | 1:1,000 |
| Secondary Antibody | Alexa 594 Goat anti-Rb IgG/A-11012 | 1:1,000 |
| Secondary Antibody | Alexa 647 Goat anti-Ms IgG2b/A-21242 | 1:1,000 |
| Secondary Antibody | Alexa 647 Goat anti-Rb IgG/A-21244 | 1:1,000 |
2.4 Cryostorage and thawing of cells
Mr. Frosty™ freezing container (Thermo Scientific, Waltham, MA, USA).
EC freezing medium (50 ml): add 15 ml FBS, 5 ml DMSO and 50 μl of 5 mM Y27632 into 30 ml EGM-2 medium, and filter with a 50 ml Steriflip filtration system.
Nalgene™ Cryogenic Tubes (2 ml) (Thermo Scientific, Waltham, MA, USA).
3. METHODS
3.1 Endothelial progenitor differentiation with Gsk3 inhibitor
A summary of this protocol is shown in Fig. 1. The endothelial cell progenitors can be generated in albumin-containing LaSR basal medium with 6 μM CHIR99021 or albumin-free DMEM/Vc medium with 5 μM CHIR99021 within 5 days.
Figure 1.
Schematic of the protocol for the differentiation of endothelial progenitors from hPSCs with small-molecule modulators of canonical Wnt signaling. Bright-field images of the typical morphology of day −2, day 0, day 2 and day 5 cells from 19-9-11 are shown at ×4 magnifications along with flow cytometry analysis of indicated markers. Scale bar, 200 μm.
Culture the hPSCs on Matrigel or Synthemax-coated 6-well plates in mTeSR1 or E8 medium to 80–90% confluence using instructions provided in our previous protocol (20). Aspirate the medium and add 1 ml of room temperature Accutase to each well. Incubate the plate in a 37°C, 5% CO2 incubator for 8 minutes.
Add 0.5 ml of mTeSR1 or E8 into each well of the 6-well plate and pool all of the cells into a 15 ml conical. Mix well and count the total cell number with a hemocytometer. Centrifuge the cells at 200 × g for 5 minutes.
Aspirate the supernatant, resuspend the cells in mTeSR1 or E8 + 5 μM Y27632 at a cell density of 2 million cells/ml, and plate 0.1 to 0.5 million cells/well in each well of a 12-well plate. Add mTeSR1 or E8 + 5 μM Y27632 medium to each well to make a final volume of 1 ml in each well of the 12-well plate. This time point corresponds to day −3 (see Note 2).
Day −2 and day −1, aspirate the medium and replace with 2 ml room temperature mTeSR1 or E8 per well of the 12-well plate.
Day 0, prepare 6 μM CHIR99021 LaSR basal medium or 5 μM CHIR99021 DMEM/Vc medium. Add 4 μl of 36 mM CHIR99021 into 24 ml LaSR basal medium to make 6 μM CHIR99021 LaSR basal medium, or add 3.33 μl of 36 mM CHIR99021 into 24 ml DMEM/Vc medium to make 5 μM CHIR99021 DMEM/Vc medium. Aspirate the old medium and then add 2 ml LaSR basal medium or DMEM/Vc with CHIR99021 per well of 12-well plate (see Note 3).
Day 1, aspirate the medium from each well of the 12-well plate and replace with 2 ml room temperature 6 μM CHIR99021 LaSR basal medium or 5 μM CHIR99021 DMEM/Vc medium. Put the plate back into the 37°C, 5% CO2 incubator (see Note 4).
Day 2, day 3, and day 4, aspirate the medium and replace with 2 ml room temperature LaSR basal medium or DMEM/Vc medium per well of the 12-well plate (see Note 5).
3.2 Purification of bipotent endothelial progenitors
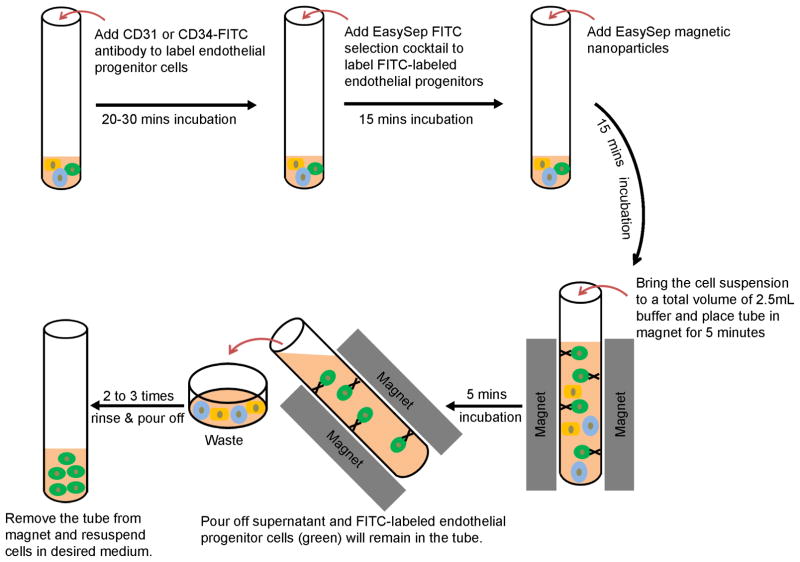
A schematic summary of this purification step is shown in Fig. 2.
Figure 2.
Schematic of the protocol for the purification of hPSC-derived endothelial progenitors.
At day 5 (or other days determined optimal for generating CD34+/CD31+ cells), aspirate the old medium, add 1 ml room temperature Accutase to each well, and incubate in a 37°C, 5% CO2 incubator for 6–10 mins.
Pipette 5–10 times with a P1000 tip to singularize the cells and then filter the cell suspensions one by one through a 40 μm Falcon cell strainer into a 50 ml conical tube containing 12 ml DMEM10 medium (see Note 6).
Count the cells with a hemocytometer, split the filtered cell suspension evenly into two 15 ml conical tubes, centrifuge the cells at 200 × g for 5 minutes, and aspirate the supernatants.
Add 10 ml FlowBuffer-1 to resuspend the two cell pellets into one single 15 ml conical tube, centrifuge the cells at 200 × g for 5 minutes, aspirate the supernatant, and resuspend the cell pellet at a concentration of 1×107 cells per 100 μl FlowBuffer-1 with a 1:50 CD31 or CD34-FITC antibody. For samples containing 1×107 cells or fewer, resuspend in 100 μl FlowBuffer-1.
Incubate the mixture in dark at room temperature for 20 to 30 min, add 2 ml FlowBuffer-1, take 100 μl samples to perform flow cytometry analysis and centrifuge the remaining cells at 200 × g for 5 minutes (see Note 7).
Aspirate the supernatant, resuspend the cell pellet at a concentration of 1×107 cells per 100 μl FlowBuffer-1, and add EasySep FITC Selection Cocktail at 10 μl per 100 μl cell mixture. Mix well and incubate at room temperature for 15 minutes.
Add the well-mixed Magnetic Nanoparticles at 5 μl per 100 μl cell mixture, mix well and incubate at room temperature for 10 minutes.
Bring the cell suspension to a total volume of 2.5 ml FlowBuffer-1, mix the cells, and then transfer into a flow tube. Next, place the flow tube (without cap) into the magnet and set aside for 5 minutes.
Pick up the magnet, and in one continuous motion invert the magnet and flow tube, pouring off the supernatant fraction. Leave the magnet and tube inverted for 2–3 seconds, then return to an upright position (see Note 8).
Remove the flow tube from the magnet, add 2.5 ml FlowBuffer-1, and mix the cell suspension by gently pipetting up and down for 2–3 times. Place the flow tube (without cap) back in the magnet and set aside for 5 minutes.
Repeat Steps 9 and 10 two to three times, and then Step 9 once more. Remove the flow tube from the magnet and resuspend cells in an appropriate amount of desired medium for further use (see Note 9).
3.3 Extended culture of hPSC-derived endothelial progenitors to endothelial cells
Take a collagen IV-coated 6-well plate from 4°C and place it at room temperature for 15 min to warm up.
Add 1 × 105 purified CD34+ cells in 2.5 ml EGM-2 medium with 5 μM Y27632 per well of a 6-well plate (see Note 10).
The next day, aspirate the medium in each well and replace with 2 ml fresh room temperature EGM-2 or Endothelial-SFM medium.
On day 3 of extended culture and every 3 day thereafter, aspirate the medium from each well and add room temperature EGM-2 or endothelial-SFM medium at a volume of 2 ml per well. On days 6–10, endothelial cells are ready for characterization.
3.4 Characterization of hPSC-derived endothelial cells and their progenitors
Cells from Steps 3.1.7 and 3.3.4 should express markers of endothelial progenitors and endothelial cells, respectively. To characterize these cells, perform immunostaining (option A) or flow cytometry analysis (option B). We recommend flow cytometry analysis for quantitative analysis of the purity of hPSC-derived endothelial cells and their progenitors. Antibody combinations of CD31/CD34 and VE-cadherin/von Willebrand Factor (vWF) are recommended for double staining.
(A) Immunostaining analysis of hPSC-derived endothelial cells and their progenitors
Day 6–10 post-addition of EGM-2 medium in Step 3.3.4, wash the differentiated cells with 1 ml PBS per well in a 6-well plate. Aspirate the PBS, add 1 ml Accutase per well, and incubate in a 37°C, 5% CO2 incubator for 10 minutes.
Pipette 5–10 times with a P1000 tip to singularize the cells and then transfer the 1 ml cell suspension into a 15 ml conical containing 2 ml DMEM10 medium.
Count the cells with a hemocytometer, centrifuge the cells at 200 × g for 5 minutes, and aspirate the supernatant.
Resuspend the cell pellet in EGM-2 medium + 5 μM Y27632 at a concentration of 100,000 cells/ml. Plate 1 ml of the resuspended cell solution in each well of a 12-well plate containing a collagen IV-coated coverslip. Incubate the plate at 37°C, 5% CO2 for 2 days without medium change to allow cell attachment (see Note 11).
After two days, aspirate the medium and add 1 ml of PBS per well to wash the cells. Aspirate the 1 ml PBS supernatant.
Add 1 ml of 4% formaldehyde per well and incubate for 15 minutes at room temperature to fix the cells. Aspirate the formaldehyde solution and then add 1 ml of PBS per well and aspirate to rinse the cells. Repeat the PBS rinse step twice.
Add 300 μl 5% non-fat dry milk, 0.4 % Triton X-100 in PBS per well and then add primary antibodies into individual wells according to Table 1. Incubate at room temperature for 1 hour or at 4°C overnight. Antibodies for cell characterization include, but are not restricted to, CD34, CD31, VE-cadherin, von Willebrand Factor (vWF) and ICAM-1.
Aspirate the antibody solution. Add 1 ml of PBS to each well and then aspirate the PBS. Repeat this wash three times.
Dilute the secondary antibodies specific to the primary IgG subtype at 1:1000 in 5% milk, 0.4% Triton X-100. Add 300 μl of secondary antibody solution to each well and then incubate for 30 min at room temperature in dark.
In dark, aspirate the secondary antibody solution. Add 1 ml of PBS to each well and then aspirate the PBS. Repeat this wash three times.
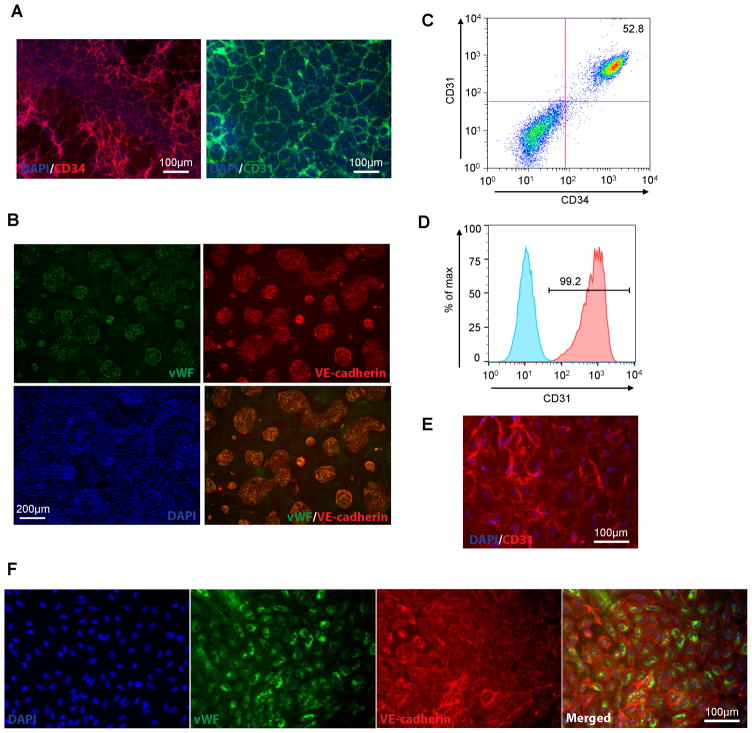
Seal the coverslips with Gold Anti-fade reagent with DAPI to glass slides. Examine the slides with an epifluorescence microscope. Typical CD34, CD31, VE-cadherin and vWF patterns of hPSC-derived endothelial progenitors (Fig. 3A, B) and endothelial cells (Fig. 3E, F) are shown in Fig. 3.
Figure 3.
Characterization of endothelial cells and their progenitors derived from hPSCs. (AC) 19-9-11 iPSCs were differentiated as illustrated in Fig. 1. At day 5, pre-sort CD34+ cells were immunostained for CD31/CD34 (A) and VE-cadherin/vWF (B) and quantitatively analyzed for CD31/CD34 (C). (D–F) the post-sort CD34+ endothelial progenitors were quantitatively analyzed for CD31 (D) and immunostained for CD31 (E) and VE-cadherin/vWF (F).
(B) Flow cytometry analysis of hPSC-derived endothelial cells and their progenitors
Wash the differentiated cells with 1 ml PBS per well in a 12-well plate (from Step 3.1.7) or 6-well plate (from Step 3.3.4), aspirate the PBS, add 1 ml Accutase per well, and incubate in a 37°C, 5% CO2 incubator for 10 min.
Pipette 5–10 times with a P1000 tip to singularize the cells and then transfer the 1 ml cell mixture into a 15 ml conical containing 2 ml DMEM10 medium.
Count the cells with a hemocytometer, centrifuge the cells at 200 × g for 5 minutes, and aspirate the supernatant.
Add 1 ml of 1% formaldehyde to resuspend the cell pellet and then incubate at room temperature for 20 minutes. Next, centrifuge the cells at 200 × g for 5 minutes, aspirate the supernatant and then resuspend the fixed cells in 1 ml of FlowBuffer-1 per tube. Calculate the cell density based on the cell count obtained from previous step (see Note 12).
Add 1 million cells into a 15-ml tube containing 2 ml of FlowBuffer-1, centrifuge the cells 200 × g for 5 minutes and then aspirate the supernatant. Repeat this wash two times to remove the formaldehyde.
Resuspend the cell pellet in 100 μl of FlowBuffer-1 with the appropriate dilution of primary antibody. Antibody combinations of CD31/CD34 and VE-cadherin/vWF are recommended for double staining. Incubate for 1 hour at room temperature or at 4 °C overnight.
Wash the cells with 2 ml of FlowBuffer-1 and resuspend the cell pellet in 100 μl of FlowBuffer-1 containing 1:1000 dilution of secondary antibody. Incubate for 30 min at room temperature in dark.
Wash the cells with 2 ml FlowBuffer-1 twice, resuspend the cell pellet in 300 μl FlowBuffer-1, and transfer into flow round-bottom tubes. Place the flow tubes on ice and perform the flow cytometric analysis with a FACSCaliber. A representative result of CD31/CD34 double staining of pre-sort endothelial progenitors is shown in Fig. 3C, and a CD31 staining result of post-sort endothelial cells is shown Fig. 3D.
3.5 Cryostorage and thawing of endothelial cells and their progenitors
-
1
Following purification of CD34+ endothelial progenitors in Step 3.2.11 or dissociation of the culture with Accutase as described in Steps 3.4 A 1–3, resuspend endothelial progenitors (from Step 3.2.11) or endothelial cells (from Step 3.4 A3) at a density of 2 × 106 cells per ml of EC freezing medium.
-
2
Aliquot 1 ml of the cell suspension into each cryovial.
-
3
Freeze in a Mr. Frosty™ freezing container at −80°C overnight.
-
4
Transfer the cryovials to liquid nitrogen for long-term storage. These cells can be stored in liquid nitrogen for at least 1 year.
Thawing cells
-
5
Partially thaw the cell vials in a 37°C water bath.
-
6
Transfer the partially thawed cells to a 15-ml conical tube containing 5 ml DMEM10 medium.
-
7
Centrifuge the cells at 200 × g for 5 minutes, and aspirate the supernatant.
-
8
Gently resuspend the cells in 1 ml of EGM-2 medium and plate into collagen-coated 6-well plate at 0.5 million cells per cm2 with 5 μM Y27632. Addition of 10% FBS or albumin may help to increase cell attachment and viability.
-
9
The next day, aspirate the medium in each well and replace with 2 ml fresh room temperature EGM-2 or endothelial-SFM medium.
-
10
Resume culturing of cells at Step 3.3.4.
Acknowledgments
This study was supported by NIH grant R01 EB007534 and NSF grant EFRI 0735903.
Footnotes
Pre-coated Corning BioCoat™ plates (laminin, collagen I, collagen IV, etc.) can also be used to culture endothelial cells and their progenitors.
Optimizing cell density is very critical for efficient endothelial cell differentiation. The initial plating density and/or the time of expansion prior to initiation of differentiation may require optimization for different cell lines or expansion conditions. We recommend plating 0.1 – 0.5 million cells per well of 12-well plate and expanding the cells for 2 or 3 days prior to initiation of differentiation.
Though we identified 6 μM or 5 μM CHIR99021 as the optimal concentrations in LaSR basal medium and DMEM/Vc medium, respectively, for the cell lines that we tested, other lines may respond to CHIR99021 treatment differently. Therefore, optimization of CHIR99021 concentration may be required. We recommend testing 4–10 μM CHIR99021.
If many cells are dead or detached from the surface, decrease the concentration of CHIR99021 or re-optimize the initial cell seeding density.
Extended culture in LaSR basal medium or DMEM/Vc medium after CHIR99021 treatment is important to achieve a high yield and purity of endothelial progenitors. Though we identified a 3-day culture after CHIR99021 treatment as the optimal condition for the cell lines that we tested, other lines may need more time to reach maximum purity. We recommend testing 3 to 5 days for the extended culture. If cells ball up during the culture, decrease initial cell seeding density.
Filtering the cell mixture is critical for the subsequent purification step since cell clusters will negatively affect the purification performance.
Flow cytometry analysis is necessary for the subsequent purification step since the purity of CD31+CD34+ cells will directly affect the performance of magnetic-activated cell sorting. It is recommended that the presort cell mixture contains at least 15% of CD34+CD31+ cells before proceeding to the next step.
Do not shake or blot off any drops that may remain hanging from the mouth of the flow tube.
Additional rounds of MACS are recommended when the CD34+ or CD31+ cell purity is not sufficient. Purified cells can be also frozen for future use.
Including a ROCK inhibitor is very important for high hPSC-derived endothelial progenitor recovery after purification. Addition of 10% FBS or albumin will also increase cell attachment and viability.
For endothelial progenitors, resuspend CD34+ cells (from Step 3.2.11) in EGM-2 medium with 5 μM Y27632 at a concentration of 100,000 cells/ml.
Do not use cold methanol or Triton X100 to permeabilize the cells since methanol and Triton X100 treatment will destroy surface proteins for subsequent flow cytometry analysis. For flow cytometry analysis of intracellular proteins such as brachyury, please follow instructions provided in our previous protocol (20).
References
- 1.Kaupisch A, Kennedy L, Stelmanis V, et al. Derivation of vascular endothelial cells from human embryonic stem cells under GMP-compliant conditions: towards clinical studies in ischaemic disease. J Cardiovasc Transl Res. 2012;5:605–17. doi: 10.1007/s12265-012-9379-2. [DOI] [PubMed] [Google Scholar]
- 2.Levenberg S, Golub JS, Amit M, et al. Endothelial cells derived from human embryonic stem cells. Proc Natl Acad Sci U S A. 2002;99:4391–6. doi: 10.1073/pnas.032074999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Van der Meer AD, Orlova VV, ten Dijke P, et al. Three-dimensional co-cultures of human endothelial cells and embryonic stem cell-derived pericytes inside a microfluidic device. Lab Chip. 2013;13:3562–8. doi: 10.1039/c3lc50435b. [DOI] [PubMed] [Google Scholar]
- 4.Wilson HK, Canfield SG, Shusta EV, Palecek SP. Concise review: Tissue-specific microvascular endothelial cells derived from human pluripotent stem cells. Stem Cells. 2014;32:3037–45. doi: 10.1002/stem.1797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ashton RS, Keung AJ, Peltier J, Schaffer DV. Progress and prospects for stem cell engineering. Annu Rev Chem Biomol Eng. 2011;2:479–502. doi: 10.1146/annurev-chembioeng-061010-114105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lian X, Selekman J, Bao X, et al. A small molecule inhibitor of SRC family kinases promotes simple epithelial differentiation of human pluripotent stem cells. PLoS One. 2013;8:e60016. doi: 10.1371/journal.pone.0060016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lian X, Bao X, Zilberter M, et al. Chemically defined, albumin-free human cardiomyocyte generation. Nat Methods. 2015;12:595–596. doi: 10.1038/nmeth.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell. 2008;132:661–80. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 9.Choi K-D, Yu J, Smuga-Otto K, et al. Hematopoietic and endothelial differentiation of human induced pluripotent stem cells. Stem Cells. 2009;27:559–67. doi: 10.1634/stemcells.2008-0922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vodyanik MA, Thomson JA, Slukvin II. Leukosialin (CD43) defines hematopoietic progenitors in human embryonic stem cell differentiation cultures. Blood. 2006;108:2095–105. doi: 10.1182/blood-2006-02-003327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.James D, Nam H, Seandel M, et al. Expansion and maintenance of human embryonic stem cell-derived endothelial cells by TGFbeta inhibition is Id1 dependent. Nat Biotechnol. 2010;28:161–6. doi: 10.1038/nbt.1605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rufaihah AJ, Huang NF, Jamé S, et al. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2011;31:e72–9. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Goldman O, Feraud O, Boyer-Di Ponio J, et al. A boost of BMP4 accelerates the commitment of human embryonic stem cells to the endothelial lineage. Stem Cells. 2009;27:1750–9. doi: 10.1002/stem.100. [DOI] [PubMed] [Google Scholar]
- 14.Sahara M, Hansson EM, Wernet O, et al. Manipulation of a VEGF-Notch signaling circuit drives formation of functional vascular endothelial progenitors from human pluripotent stem cells. Cell Res. 2014;24:820–41. doi: 10.1038/cr.2014.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.White MP, Rufaihah AJ, Liu L, et al. Limited gene expression variation in human embryonic stem cell and induced pluripotent stem cell-derived endothelial cells. Stem Cells. 2013;31:92–103. doi: 10.1002/stem.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tatsumi R, Suzuki Y, Sumi T, et al. Simple and highly efficient method for production of endothelial cells from human embryonic stem cells. Cell Transplant. 2011;20:1423–30. doi: 10.3727/096368910X547444. [DOI] [PubMed] [Google Scholar]
- 17.Wang ZZ, Au P, Chen T, et al. Endothelial cells derived from human embryonic stem cells form durable blood vessels in vivo. Nat Biotechnol. 2007;25:317–8. doi: 10.1038/nbt1287. [DOI] [PubMed] [Google Scholar]
- 18.Bao X, Lian X, Dunn KK, et al. Chemically-defined albumin-free differentiation of human pluripotent stem cells to endothelial progenitor cells. Stem Cell Res. 2015;15:122–129. doi: 10.1016/j.scr.2015.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lian X, Bao X, Al-Ahmad A, et al. Efficient Differentiation of Human Pluripotent Stem Cells to Endothelial Progenitors via Small-Molecule Activation of WNT Signaling. Stem Cell Reports. 2014;3:804–16. doi: 10.1016/j.stemcr.2014.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lian X, Zhang J, Azarin SM, et al. Directed cardiomyocyte differentiation from human pluripotent stem cells by modulating Wnt/β-catenin signaling under fully defined conditions. Nat Protoc. 2013;8:162–75. doi: 10.1038/nprot.2012.150. [DOI] [PMC free article] [PubMed] [Google Scholar]