Abstract
Objective:
The aim of this multicenter, case-control study was to investigate the prevalence and severity of impulsive-compulsive behaviors (ICBs) in a cohort of patients with parkin-associated Parkinson disease (PD) compared to a group of patients without the mutation.
Methods:
We compared 22 patients with biallelic parkin mutations (parkin-PD) and 26 patients negative for parkin, PINK1, DJ-1, and GBA mutations (PD-NM), matched for age at onset, disease duration, levodopa, and dopamine agonist equivalent daily dose. A semistructured interview was used to diagnose each of the following ICBs: compulsive sexual behavior, compulsive buying, binge eating, punding, hobbyism, and compulsive medication use. The Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease–Rating Scale (QUIP-RS) was adopted to rate ICB severity.
Results:
Frequency of patients with at least one ICB was comparable between parkin-PD and PD-NM. Nevertheless, when analyzing the distribution of specific ICBs, a higher frequency of compulsive shopping, binge eating, and punding/hobbyism was found in the parkin-PD group. Compared to PD-NM, parkin-PD patients with ICB had younger onset age and higher frequency of smokers; in 5 patients, ICB had predated PD onset. Total and partial (compulsive buying, compulsive sexual behavior, binge eating, hobbyism/punding) QUIP-RS scores were higher in patients with parkin-PD compared to patients with PD-NM. Logistic regression analysis showed that the presence of parkin mutations was associated with smoking status and higher QUIP-RS total score.
Conclusions:
Our data expand the parkin-associated phenotypic spectrum demonstrating higher frequency and severity of specific ICBs, and suggesting an association between the parkin genotype, smoking status, and ICB severity.
The term early-onset Parkinson disease (EOPD) refers to Parkinson disease (PD) with age at onset between the third and the fifth decade, slow progression, good response to levodopa treatment, and low burden of cognitive symptoms.1,2 However, patients with EOPD are more likely to develop psychiatric and behavioral disturbances, specifically depression, anxiety, and impulsive-compulsive behaviors (ICBs).3 These include impulse control disorders such as pathologic gambling, compulsive sexual behavior, compulsive buying and binge eating, and compulsive behaviors such as punding and compulsive use of dopamine replacement therapy (also known as dopamine dysregulation syndrome).4
EOPD might be inherited in autosomal recessive fashion, and it is most frequently caused by biallelic mutations in the parkin gene.5 Previous studies aiming to define the neuropsychiatric profile of parkin-associated PD (parkin-PD) have failed to demonstrate a higher frequency of either depression6 or obsessive-compulsive symptoms (OCS)7 in symptomatic carriers of parkin mutations as compared to noncarriers. Nevertheless, no study has systematically explored the occurrence and spectrum of ICB in parkin-PD. Therefore, it remains unclear whether ICB might be related to specific genetic mutations or simply to an early age at onset, as previously reported.4
The aim of this multicenter, case-control study was to investigate the prevalence and severity of ICB in a cohort of patients with parkin-PD compared to a group of patients with nonmutated PD (PD-NM) matched for demographic and clinical features.
METHODS
Selection of patients.
From the genetic database at the CSS Mendel Institute in Rome, including an overall number of 2,124 patients with PD, of whom 791 had EOPD (age at onset <50 years), we identified 36 patients who were carriers of pathogenic biallelic mutations of the parkin gene (parkin-PD). Twenty-two patients with parkin-PD agreed to take part in this study (table e-1 at Neurology.org). Referral centers were 4 tertiary movement disorders outpatient clinics based in Rome, Naples, Salerno, and Messina. From the same database, we selected a cohort of 26 controls with EOPD (PD-NM) in whom we excluded point mutations and exon rearrangements in the parkin gene. Moreover, in all cases and controls, we excluded the occurrence of point mutations, small insertions/deletions, and splice-site mutations in the PINK1, DJ-1, and GBA genes by direct Sanger sequencing; we also excluded the presence of single or multiexon deletions or multiplications of PINK1, DJ-1, and SNCA by multiplex ligation-dependent probe amplification analysis. Furthermore, we used TaqMan genotyping to exclude the Contursi mutation p.A53T in the SNCA gene and the common mutations p.G2019S and p.R1441C in the LRRK2 gene.
Parkin-PD and PD-NM patients were matched for the following demographic and clinical features: sex, age at study entry, age at disease onset, disease duration, PD phenotype, most affected side, and levodopa equivalent daily dose (LEDD).8 LEDD was also considered in terms of dopamine agonist (D-Ag) LEDD. Smoking habits were also retrieved (smoker/nonsmoker at study entry). In all patients, the motor section of the Unified Parkinson's Disease Rating Scale, Part III, was assessed in the “on” state to evaluate disease severity. The presence/absence of the following psychiatric and cognitive features was recorded: psychosis (illusions, hallucinations, delusions) according to National Institute of Neurological Disorders and Stroke/National Institute of Mental Health criteria,9 and depression and dementia according to DSM-IV criteria. The presence of rapid eye movement sleep behavior disorder was diagnosed based on a single question as in Postuma et al.10: “Have you ever been told, or suspected yourself, that you seem to ‘act out your dreams’ while asleep (for example, punching, flailing your arms in the air, making running movements, etc.)?”
ICB assessment.
A semistructured interview using accepted diagnostic criteria11 for pathologic gambling, compulsive buying, compulsive sexual behavior, binge eating, punding, and compulsive use of dopamine replacement therapy (anytime in the past 6 months) was used to reach the diagnosis of each of these ICBs. In addition, the Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease–Rating Scale (QUIP-RS), a rating scale designed to measure severity of ICB in PD, was administered to all patients.12,13 The QUIP-RS is a patient-filled questionnaire composed by 4 questions for each ICB, which have to be answered on a 5-point Likert scale. Total impulse control disorder score ranges from 0 to 64; total QUIP-RS score ranges from 0 to 112. In all patients diagnosed with ICB, we also evaluated duration of ICB, their occurrence before disease onset, and presence of a positive family history of ICB or alcohol and drug abuse.
Standard protocol approvals, registrations, and patient consents.
The study was approved by the ethic committee of the recruiting centers and conformed to the Declaration of Helsinki. All patients gave their written informed consent before participation.
Statistical analysis.
Comparisons between groups were made using the Fisher exact test or the χ2 test for categorical variables. Nonparametric Mann-Whitney U test was used to compare continuous variables. Correlations between QUIP-RS and age at onset, disease duration, LEDD, D-Ag LEDD, and ICB duration were explored by Spearman rank correlation. Logistic regression was used to verify the association between ICB (presence/absence) and the following variables: age at onset, LEDD, D-Ag LEDD, and smoking status. Moreover, logistic regression was also used to test possible associations between mutation carrier status (presence/absence of biallelic mutations in the parkin gene) and the following variables: QUIP-RS, ICB duration, smoking status, and ICB before PD onset. Significance level was set at p < 0.05. Unless otherwise stated, data are given as mean ± SD.
RESULTS
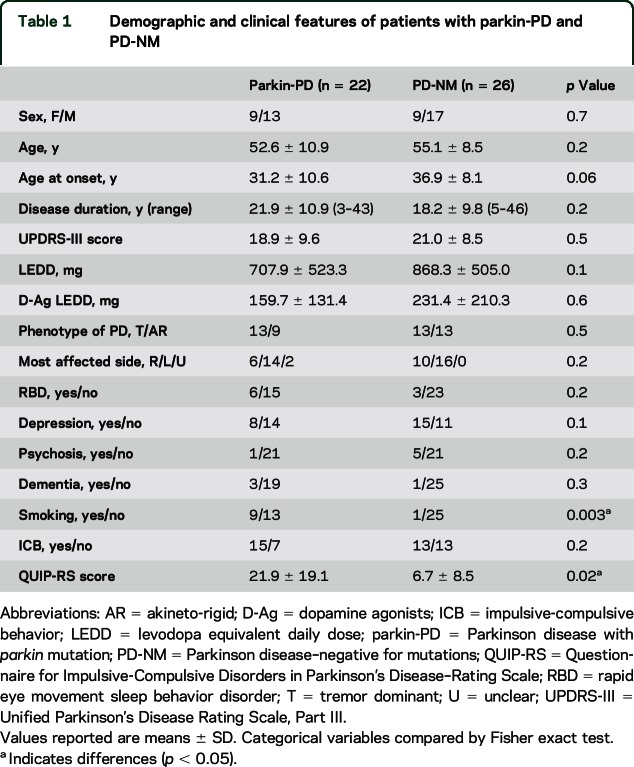
Parkin-PD (n = 22) and PD-NM (n = 26) were comparable for all the demographic and clinical variables taken into account but smoking habit, which was more frequent in the parkin-PD group (p = 0.003; table 1).
Table 1.
Demographic and clinical features of patients with parkin-PD and PD-NM
The frequency of patients with at least one ICB was comparable between parkin-PD and PD-NM (table 1). Nevertheless, when analyzing distribution of specific ICBs in each group, Fisher exact test disclosed differences between the 2 groups, with higher frequency of compulsive shopping, binge eating, and punding/hobbyism in the parkin-PD group (figure 1). Moreover, QUIP-RS scores were higher in the parkin-PD compared to the PD-NM group (table 1).
Figure 1. ICBs in parkin-PD and nonmutated PD.
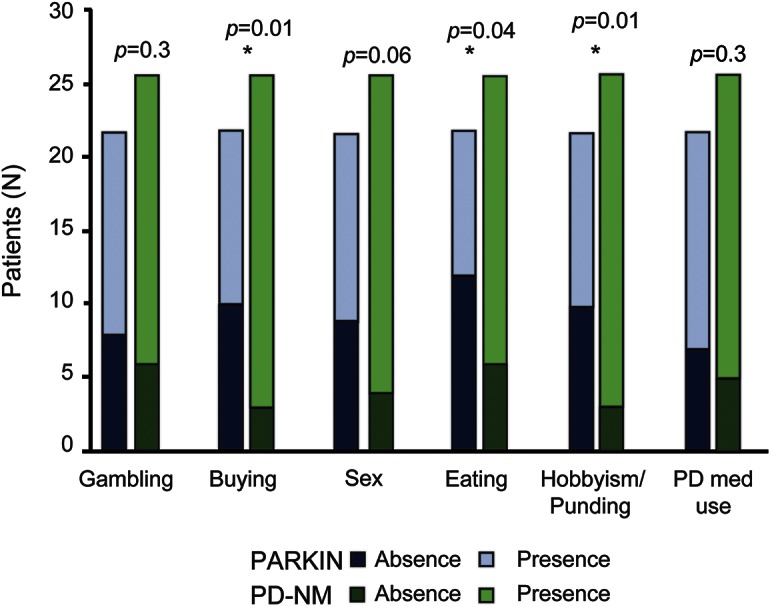
Frequency of specific impulsive-compulsive behaviors (ICBs) in parkin homozygous and in nonmutated Parkinson disease (PD-NM). Parkin-PD disclosed a higher frequency of compulsive shopping, binge eating, punding/hobbysim, and abuse of PD medications.
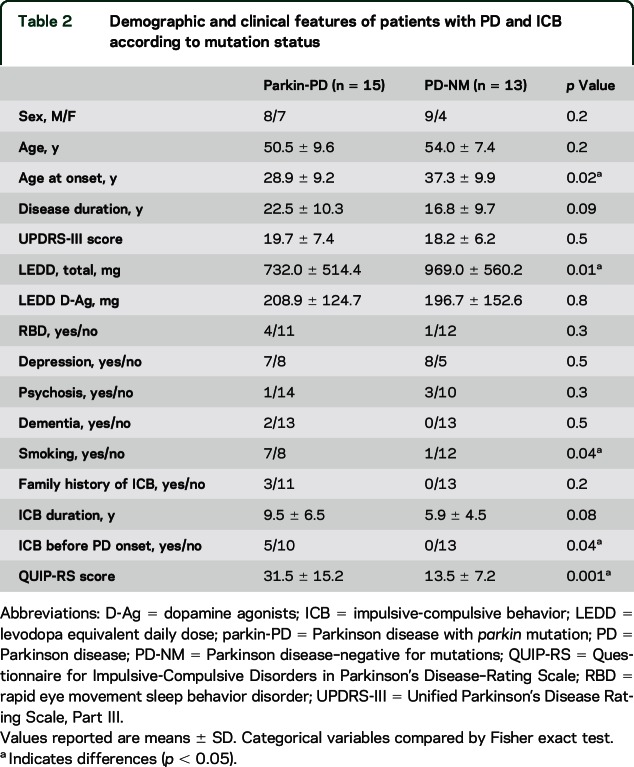
When comparing only patients with ICB, the 2 groups of patients remained similar for most demographical and clinical features, except for a younger age at PD onset, a higher frequency of smokers than in the PD-NM group, and a lower LEDD in parkin-PD (table 2). Again, the severity of ICB, as measured by total QUIP-RS, was greater in the parkin-PD group compared to PD-NM (table 2), with higher values in the subscores related to compulsive buying, compulsive sexual behavior, binge eating, and hobbyism/punding (figure 2). Accordingly, 2-way analysis of covariance adjusted for age at onset confirmed that patients with parkin-PD scored higher at QUIP-RS (F = 9.5, p = 0.005).
Table 2.
Demographic and clinical features of patients with PD and ICB according to mutation status
Figure 2. Severity of ICB in parkin-PD and nonmutated PD.
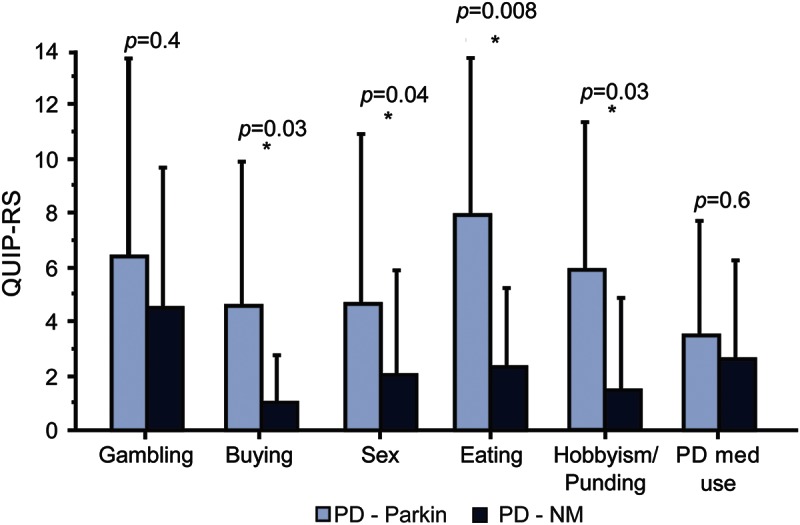
Patients with parkin-PD disclose higher Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease–Rating Scale (QUIP-RS) scores for the following impulsive-compulsive behaviors (ICBs) compared to nonmutated Parkinson disease (PD-NM): compulsive shopping, hypersexuality, punding/hobbyism, abuse of PD medication.
In addition, we compared in the parkin-PD group patients with (n = 15) and without (n = 7) ICB; we were unable to disclose any difference for age (p = 0.1), age at onset (p = 0.07), disease duration (p = 0.7), total LEDD (p = 0.2), or D-Ag LEDD (p = 0.1). Similar findings were demonstrated in the PD-NM group categorizing patients by presence (n = 13) or absence (n = 13) of ICB (age: p = 0.6, age at onset: p = 0.6, disease duration: p = 0.2, total LEDD: p = 0.6, or D-Ag LEDD: p = 0.9).
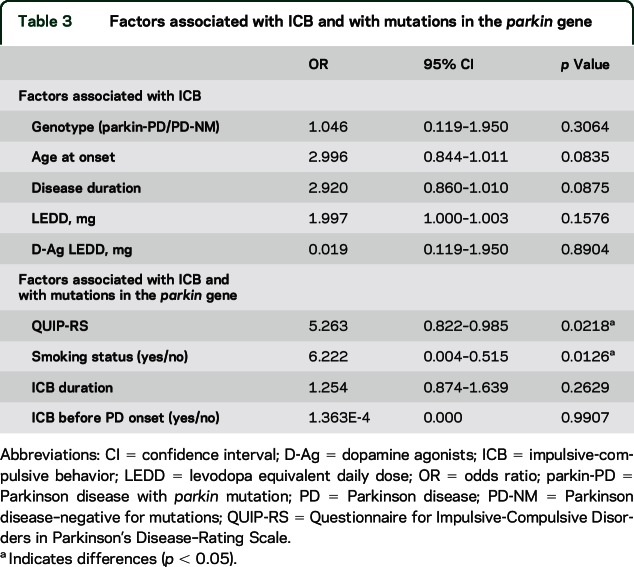
Univariate correlational analysis in patients with ICB disclosed a positive relation between QUIP-RS and ICB duration (p = 0.03, r = 0.4) but not with age at onset (p = 0.09, r = −0.3). No correlations were found between QUIP-RS and disease duration, total LEDD, or D-Ag LEDD. Logistic regression analysis (table 3) did not demonstrate any association of ICB with presence of parkin mutations, age at onset, or D-Ag LEDD, whereas association with age at onset and disease duration showed a statistical trend. Nevertheless, the parkin genotype was associated with smoking status and higher QUIP-RS total score (table 3). Finally, multiple linear regression analysis did not show an association of QUIP-RS with total LEDD (p = 0.27), D-Ag LEDD (p = 0.50), age at onset (p = 0.15), or disease duration (p = 0.48).
Table 3.
Factors associated with ICB and with mutations in the parkin gene
DISCUSSION
Several conditions have been established as risk factors for ICB in PD, including younger age, male sex, exposure to D-Ag and levodopa, smoking habit, marital status, and family history of gambling problems.4,14 However, it is still unknown whether genetic mutations represent predisposing factors for ICB. Recently, ICB was reported in 3 patients carrying mutations in PINK1, another gene responsible for autosomal recessive EOPD. They all presented hypersexuality, compulsive shopping, and binge eating; in addition, punding was also present in one patient.1 A similar behavioral profile was reported in 2 brothers with PINK1 mutation respectively presenting with pathologic gambling many years before PD onset and hypersexuality, punding, and hypomania15 and in one patient with PINK1 mutation presenting with compulsive use of dopaminergic medications.16 Besides these case reports, no study has systematically evaluated the frequency and severity of these behavioral complications in autosomal recessive EOPD.
In this case-control study, we sought to determine whether parkin-related EOPD might be associated with a higher frequency of ICB by assessing 2 cohorts of patients, one with parkin-mutated PD and one with nonmutated PD comparable for several variables involved in ICB development (age, age at onset, disease duration, LEDD, D-Ag LEDD). Although the occurrence of ICB did not correlate with the parkin genotype, parkin mutations influenced both the onset and severity of specific behavioral disturbances in the impulsive-compulsive spectrum. In particular, ICB was overall more severe in parkin carriers who also disclosed a higher frequency and severity of compulsive shopping, binge eating, and punding. Regarding hypersexuality, patients with parkin-PD reported a similar frequency but a more severe clinical expression. Moreover, ICB tended to occur earlier in patients with the parkin mutation, sometimes predating PD onset.
Univariate correlational analysis revealed a correlation of QUIP-RS with ICB duration. In line with this finding, it is possible to speculate that the observed increased severity in patients with the parkin mutation may be at least in part influenced by the age at PD onset, which tended to be younger in the parkin-PD group. However, the effect of genotype on QUIP-RS remained when adjusting for age at onset; in addition, age at onset was not correlated with QUIP-RS, neither in the univariate analysis nor in multiple linear regression analysis. These differences are also unlikely to depend on disparities in dopaminergic medication dosage, since patients with and without parkin mutation were matched both for total LEDD and D-Ag LEDD; nevertheless, the patients with parkin-PD and ICB had a lower total LEDD, supporting the hypothesis that they might be more sensitive to developing ICB with a lower dose of dopaminergic medications.
A novel datum from our study is the association between the parkin genotype and smoking status. This might have also accounted for the association between smoking and ICB, considering that smoking habit was more frequent in patients with parkin-PD and ICB compared to the nonmutated ICB group. Only one study had reported an inverse association between parkin mutation and smoking, demonstrating a higher frequency of parkin mutations among never-smoker Sikh Indians, in whom smoking is forbidden by religious texts.17 The relationship between smoking and PD has been explored in several studies in sporadic PD, which demonstrated an inverse association between PD and smoking,18 likely to be caused by low-sensation-seeking personality traits caused by dopaminergic denervation.19 Conversely, smoking has been positively associated with ICB in PD,4,20 in a dose-dependent manner and also independently by D-Ag use.21 Recent studies showed that smoking habits can result in motor and nonmotor correlates in PD, and, thus, nicotine and other cigarette components have been suggested to have a broad action on the heterogeneity of the clinical phenotype.22 Thus, it is tempting to speculate that smoking habit could somehow influence the severity of ICB in patients with parkin mutation. Supporting this hypothesis, IV administration of nicotine in the rat is associated with increased glucose utilization and increased dopaminergic transmission in the shell of the nucleus accumbens,23 a limbic area involved in reward and addiction. Alternatively, patients with parkin-PD might be more prone to smoking as a manifestation of their ICB, as smoking and pathologic gambling also frequently co-occur in nonparkinsonian patients.24
OCS were also reported to be more severe in patients with PD and ICB.7 Of note, a recent study showed lower levels of OCS in parkin-mutated compared to nonmutated PD, but highlighted a trend for higher OCS levels in asymptomatic parkin mutation carriers compared to noncarriers, suggesting that OCS may represent an early nonmotor dopamine-dependent feature.7 In this light, although we were not able to assess OCS in our patients, our observation of ICB predating PD onset in some parkin mutation carriers could also point at ICB as a potential early sign of dopaminergic dysfunction. Further studies are needed to thoroughly explore the association between OCS and ICB in patients with parkin-PD.
Besides these potential correlations, the mechanism underlying the increased ICB severity in parkin-associated PD still remains to be elucidated. An intriguing explanation may relate to the selective neurodegeneration of frontostriatal-limbic structures observed in parkin carriers, as patients with PD and ICB were found to exhibit alterations of cortical thickness in the frontostriatal-limbic circuitry.13 Indeed, a functional neuroimaging study using an automated segmentation MRI method demonstrated decrease of gray matter volume in the caudate nuclei of parkin-PD compared to PD-NM24; a similar finding was reported also by functional imaging studies by PET and SPECT, which demonstrated a severe dopaminergic denervation both in the caudate nuclei and the putamen in symptomatic parkin-PD.25,26 The caudate nucleus is part of the ventral striatum and is connected with the orbitofrontal cortex, an area involved in reward and stimulus–reinforcement association learning27; specifically, the caudate nucleus receives converging projections from orbitofrontal cortex, dorsolateral prefrontal cortex, and parietal regions, supporting the role of this structure in the integration of reward, attention, and executive processes that occurs during spatial reinforcement learning.28 In this light, it can be foreseen that functional neuroimaging studies would greatly help in the understanding of the neural basis of ICB symptoms in patients with parkin-PD.
We acknowledge that the number of study participants is small, because of the rarity of these genetic conditions. Indeed, our study sample is comparable to previous cohort studies exploring cognition and depressive symptoms in parkin-PD.6,7,29 Further studies in distinct cohorts of patients with and without the parkin mutation will be required to replicate our findings.
Our data expand the spectrum of parkin-associated PD phenotype demonstrating a higher frequency and severity of specific ICB related to compulsive behaviors; furthermore, our findings suggest an association between the parkin genotype and smoking status, which needs to be further explored in relation to the development of impulsive-compulsive behaviors.
Supplementary Material
GLOSSARY
- D-Ag
dopamine agonist
- DSM-IV
Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition)
- EOPD
early-onset Parkinson disease
- ICB
impulsive-compulsive behavior
- LEDD
levodopa equivalent daily dose
- OCS
obsessive-compulsive symptoms
- parkin-PD
Parkinson disease with parkin mutation
- PD
Parkinson disease
- PD-NM
Parkinson disease–negative for mutations
- QUIP-RS
Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease–Rating Scale
Footnotes
Supplemental data at Neurology.org
Editorial, page 1426
AUTHOR CONTRIBUTIONS
Francesca Morgante and Enza Maria Valente: study concept and design, acquisition of data, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. Alfonso Fasano: study concept and design, analysis and interpretation, critical revision of the manuscript for important intellectual content, study supervision. Monia Ginevrino, Simona Petrucci, Lucia Ricciardi, Francesco Bove, Chiara Criscuolo, Marcello Moccia, Anna De Rosa, Chiara Sorbera: acquisition of data, critical revision of the manuscript for important intellectual content. Anna Rita Bentivoglio, Paolo Barone, Giuseppe De Michele, Maria Teresa Pellecchia: analysis and interpretation, critical revision of the manuscript for important intellectual content.
STUDY FUNDING
This work was partly supported by the EU grant FP7 MEFOPA.
DISCLOSURE
F. Morgante has received honoraria as a consultant and on advisory boards from Medtronic and Chiesi. She has received honoraria for speaking from UCB Pharma, Medtronic, Lundbeck, Chiesi, AbbVie. She serves on the editorial board of Movement Disorders Clinical Practice and Frontiers in Movement Disorders. A. Fasano has received grant support from the University of Toronto, the McLaughlin Centre, and the Michael J. Fox Foundation; he received speaking honoraria from UCB Pharma, Medtronic, Boston Scientific, AbbVie, Novartis, Chiesi Pharmaceutical, and TEVA; he is on an advisory board for AbbVie and provided consultancies for UCB Pharma, Medtronic, Boston Scientific, and AbbVie. M. Ginevrino, S. Petrucci, L. Ricciardi, F. Bove, C. Criscuolo, M. Moccia, A. De Rosa, C. Sorbera, and A. Bentivoglio report no disclosures relevant to the manuscript. P. Barone has received honoraria as a consultant and on advisory boards from Lundbeck, UCB, Zambon, PIAM. He has received honoraria for speaking from UCB, Zambon, Lundbeck, Chiesi, AbbVie. G. De Michele and M. Pellecchia report no disclosures relevant to the manuscript. E. Maria Valente has received grant support from the Italian Ministry of University and Research, the Italian Ministry of Health, Telethon Foundation Italy, the Pierfranco and Luisa Mariani Foundation ONLUS, and the European Research Council. She received honoraria for speaking from Medtronic. She serves on the editorial board of BMC Neurology, Pediatric Research and Current Molecular Medicine. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Ricciardi L, Petrucci S, Guidubaldi A, et al. Phenotypic variability of PINK1 expression: 12 years' clinical follow-up of two Italian families. Mov Disord 2014;29:1561–1566. [DOI] [PubMed] [Google Scholar]
- 2.Alcalay RN, Caccappolo E, Mejia-Santana H, et al. Frequency of known mutations in early-onset Parkinson disease: implication for genetic counseling: the Consortium on Risk for Early Onset Parkinson Disease Study. Arch Neurol 2010;67:1116–1122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Weintraub D, Burn DJ. Parkinson's disease: the quintessential neuropsychiatric disorder. Mov Disord 2011;26:1022–1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Weintraub D, Koester J, Potenza MN, et al. Impulse control disorders in Parkinson disease: a cross-sectional study of 3090 patients. Arch Neurol 2010;67:589–595. [DOI] [PubMed] [Google Scholar]
- 5.Kitada T, Asakawa S, Hattori N, et al. Mutations in the parkin gene cause autosomal recessive juvenile parkinsonism. Nature 1998;392:605–608. [DOI] [PubMed] [Google Scholar]
- 6.Srivastava A, Tang MX, Mejia-Santana H, et al. The relation between depression and parkin genotype: the CORE-PD study. Parkinsonism Relat Disord 2011;17:740–744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sharp ME, Caccappolo E, Mejia-Santana H, et al. The relationship between obsessive-compulsive symptoms and PARKIN genotype: the CORE-PD study. Mov Disord 2015;30:278–283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord 2010;25:2649–2653. [DOI] [PubMed] [Google Scholar]
- 9.Ravina B, Marder K, Fernandez HH, et al. Diagnostic criteria for psychosis in Parkinson's disease: report of an NINDS, NIMH work group. Mov Disord 2007;22:1061–1068. [DOI] [PubMed] [Google Scholar]
- 10.Postuma RB, Arnulf I, Hogl B, et al. A single-question screen for rapid eye movement sleep behavior disorder: a multicenter validation study. Mov Disord 2012;27:913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Weintraub D, Hoops S, Shea JA, et al. Validation of the questionnaire for impulsive-compulsive disorders in Parkinson's disease. Mov Disord 2009;24:1461–1467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Weintraub D, Mamikonyan E, Papay K, Shea JA, Xie SX, Siderowf A. Questionnaire for Impulsive-Compulsive Disorders in Parkinson's Disease–Rating Scale. Mov Disord 2012;27:242–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Biundo R, Weis L, Facchini S, et al. Patterns of cortical thickness associated with impulse control disorders in Parkinson's disease. Mov Disord 2015;30:688–695. [DOI] [PubMed] [Google Scholar]
- 14.Weintraub D. Impulse control disorders in Parkinson's disease: prevalence and possible risk factors. Parkinsonism Relat Disord 2009;15(suppl 3):S110–S113. [DOI] [PubMed] [Google Scholar]
- 15.Ephraty L, Porat O, Israeli D, et al. Neuropsychiatric and cognitive features in autosomal-recessive early parkinsonism due to PINK1 mutations. Mov Disord 2007;22:566–569. [DOI] [PubMed] [Google Scholar]
- 16.Criscuolo C, Volpe G, De Rosa A, et al. PINK1 homozygous W437X mutation in a patient with apparent dominant transmission of parkinsonism. Mov Disord 2006;21:1265–1267. [DOI] [PubMed] [Google Scholar]
- 17.Prabhakar S, Vinish M, Das CP, Anand A. Occurrence of PARK2 mutations in a never-smoker population with Parkinson's disease in North India. Neuroepidemiology 2010;35:152–159. [DOI] [PubMed] [Google Scholar]
- 18.Nicoletti A, Pugliese P, Nicoletti G, et al. Voluptuary habits and clinical subtypes of Parkinson's disease: the FRAGAMP case-control study. Mov Disord 2010;25:2387–2394. [DOI] [PubMed] [Google Scholar]
- 19.Evans AH, Lawrence AD, Potts J, et al. Relationship between impulsive sensation seeking traits, smoking, alcohol and caffeine intake, and Parkinson's disease. J Neurol Neurosurg Psychiatry 2006;77:317–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bastiaens J, Dorfman BJ, Christos PJ, Nirenberg MJ. Prospective cohort study of impulse control disorders in Parkinson's disease. Mov Disord 2013;28:327–333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Valenca GT, Glass PG, Negreiros NN, et al. Past smoking and current dopamine agonist use show an independent and dose-dependent association with impulse control disorders in Parkinson's disease. Parkinsonism Relat Disord 2013;19:698–700. [DOI] [PubMed] [Google Scholar]
- 22.Moccia M, Mollenhauer B, Erro R, Picillo M, Palladino R, Barone P. Non-motor correlates of smoking habits in de novo Parkinson's disease. J Parkinsons Dis 2015;5:913–924. [DOI] [PubMed] [Google Scholar]
- 23.Pontieri FE, Tanda G, Orzi F, Di Chiara G. Effects of nicotine on the nucleus accumbens and similarity to those of addictive drugs. Nature 1996;382:255–257. [DOI] [PubMed] [Google Scholar]
- 24.Bilgic B, Bayram A, Arslan AB, et al. Differentiating symptomatic Parkin mutations carriers from patients with idiopathic Parkinson's disease: contribution of automated segmentation neuroimaging method. Parkinsonism Relat Disord 2012;18:562–566. [DOI] [PubMed] [Google Scholar]
- 25.Pavese N, Khan NL, Scherfler C, et al. Nigrostriatal dysfunction in homozygous and heterozygous parkin gene carriers: an 18F-dopa PET progression study. Mov Disord 2009;24:2260–2266. [DOI] [PubMed] [Google Scholar]
- 26.Varrone A, Pellecchia MT, Amboni M, et al. Imaging of dopaminergic dysfunction with [123I]FP-CIT SPECT in early-onset parkin disease. Neurology 2004;63:2097–2103. [DOI] [PubMed] [Google Scholar]
- 27.Rolls ET. The orbitofrontal cortex and reward. Cereb Cortex 2000;10:284–294. [DOI] [PubMed] [Google Scholar]
- 28.Jarbo K, Verstynen TD. Converging structural and functional connectivity of orbitofrontal, dorsolateral prefrontal, and posterior parietal cortex in the human striatum. J Neurosci 2015;35:3865–3878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Alcalay RN, Caccappolo E, Mejia-Santana H, et al. Cognitive and motor function in long-duration PARKIN-associated Parkinson disease. JAMA Neurol 2014;71:62–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.